ABSTRACT
Vascularization plays a significant role in treating nerve injury, especially to avoid the central necrosis observed in nerve grafts for large and long nerve defects. It is known that sufficient vascularization can sustain cell survival and maintain cell integration within tissue‐engineered constructs. Several studies have also shown that vascularization affects nerve regeneration. Motivated by these studies, vascularized nerve grafts have been developed using various different techniques, although donor site morbidity and limited nerve supply remain significant drawbacks. Tissue engineering provides an exciting alternative approach to prefabricate vascularized nerve constructs which could overcome the limitations of grafts. In this review article, we focus on the role of vascularization in nerve regeneration, discussing various approaches to generate vascularized nerve constructs and the contribution of tissue engineering and mathematical modeling to aid in developing vascularized engineered nerve constructs, illustrating these aspects with examples from our research experience. Anat Rec, 301:1657–1667, 2018. © 2018 The Authors. The Anatomical Record published by Wiley Periodicals, Inc. on behalf of American Association of Anatomists.
Keywords: peripheral nerve, regeneration, degeneration, therapies, vascularization
ROLE OF BLOOD VESSELS AND ENDOTHELIAL CELLS IN GUIDING NERVE REGENERATION
The vasculature plays a fundamental role in supporting the function of peripheral nerves through its supply of blood, oxygen and other nutrients to the cells comprising nerve tissue. Within the body, most cells reside within around 100–200 μm from the nearest vascular source to ensure sufficient delivery of oxygen by diffusion to meet the demands of cellular metabolism (Jain et al., 2005). The vasculature is also crucial in supporting nerve regeneration following injury (Ronald and Robert, 1995). The vascular system of the peripheral nerve, the vasa nervorum, can be categorized into two systems: extrinsic and intrinsic (Best and Mackinnon, 1994). The extrinsic system comprises a series of arteries and veins which run along the surface of a peripheral nerve and mainly supply the epineurial and perineurial regions (Fig. 1). By comparison, the intrinsic system operates independently from the extrinsic system. This system involves small arteries that supply blood and dissolved nutrients to the inner, endoneurial nerve compartment. Several features of the nerve vasculature mean that peripheral nerve tissues are prone to low oxygen conditions. First of all, the inter‐capillary distances in the vasa nervorum are larger than other tissues such as muscle (Low et al., 1989), while glial cell densities are relatively high. This can lead to a reduction in oxygen concentrations compared to other tissues, as a consequence of the balance between longer diffusion distances and significant metabolic demand for oxygen (Bell and Weddell, 1984). Further, the extrinsic vasculature is capable of regulation in response to changes in physiological conditions, (Appenzeller et al., 1984, Zochodne, 2002), whereas vessels of the intrinsic circulation lack the ability to autoregulate (Smith et al., 1977). As a result, if the systemic blood pressure drops, the intrinsic blood vessels fail to compensate for the associated perfusion changes, making the nerve susceptible to ischemia; this can lead to nerve hypoxia and damage.
Figure 1.
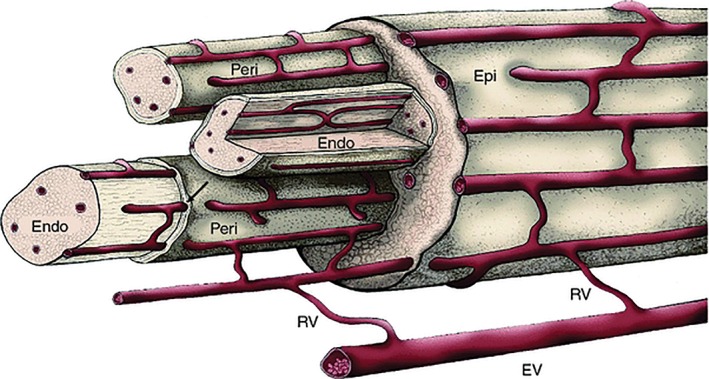
Microcirculation system of a peripheral nerve. The extrinsic vessels (EV) and branch radicular vessels (RV) supply the intrinsic circulation of the vasa nervorum. The intrinsic circulation consists of longitudinally oriented vessels that course to the perineurium (Peri) (Lundborg and Hansson, 1988).
The fundamental importance of vascularization during nerve regeneration is well‐established. A nerve transection event triggers the break up of the myelin sheath surrounding a nerve fiber, as well as the degeneration of axoplasm distally by the interactions of Schwann cells and macrophages during Wallerian degeneration. Schwann cells distal to the injury then proliferate and form bands of Büngner, which can guide axons growing from the proximal stump toward the distal end. Blood vessels are commonly found to precede Schwann cell migration as well as axonal extension, suggesting the important link between neurite growth and vascular growth (Hobson et al., 1997, 2000).
In a study undertaken in dogs, Tarlove and Epstein (1945) reported that the rate of vascularization seemed to limit the growth of axons into a peripheral nerve graft . In a separate study, cutaneous nerve plexuses of albino rabbits were crushed or transected to analyze the pattern of regeneration, and regions where axonal regeneration were prominent correlated with an abundance of larger blood vessels (Weddell, 1942). Indeed, there is extensive evidence that the architecture and functionality of capillaries in particular facilitate axonal regeneration. Changes in capillary number (Nukada, 1988) and capillary permeability (Weerasuriya, 1988, 1990) associated with successful axonal regeneration suggest that there is an interaction between blood vessels and regenerating axons. For example, the number of endoneurial capillaries increases during regeneration, and the permeability of these capillaries increases, which may encourage clearance of debris and aid elongating axons. Hobson et al. (1997) demonstrated the morphological inter‐relationships between angiogenesis and axonal regeneration in a rat sciatic nerve model (Hobson et al., 1997). The authors reported that Schwann cell migration and axon regeneration were greatest in well‐vascularized regions where vessels were aligned longitudinally, and also that the blood vessel front preceded Schwann cell penetration and axonal regeneration. Vascular endothelial growth factor (VEGF) added in a silicone chamber was shown to significantly increase vascularization and enhance axonal regrowth and Schwann cell proliferation in a rat sciatic nerve injury model, indicating the interdependence between the vascularization and nerve regeneration processes (Hobson et al., 2000).
Endothelial cells, which form the inner lining of blood vessels, are known to synthesize several factors that are supportive of nerve regeneration (Kokaia and Lindvall, 2003, Black et al., 1990). When subventricular zone (SVZ) explants of adult brain are co‐cultured with endothelial cells, neurite outgrowth and migration of neurons are enhanced (Leventhal et al., 1999). Further, vitronectin and heparin sulphate proteoglycans, glycoproteins expressed on the surface of endothelial cells, are involved in neurite activity and growth, as shown using dorsal root ganglion explants in culture (Isahara and Yamamoto, 1995). Human umbilical vein endothelial cells (HUVECs), a widely used source of endothelial cells in angiogenesis studies, have been shown to secrete brain‐derived neurotrophic factor (BDNF), a neurotrophic factor involved in promoting neural cell growth and survival (Nakahashi et al., 2000). Recent studies have shown that molecular cues from nerves influence the branching and network morphology of blood vessels and vice versa, suggesting cross‐talk between neural cells and vascular cells. This is illustrated by the fact that VEGF and Sema3A, which are proteins produced by endothelial cells and neurons respectively, have an opposite effect on neural cells and vascular cells (Bagnard et al., 2001, Miao et al., 1999). Sema3A inhibits both endothelial cell and axonal motility (which is mediated by Neuropilin1‐binding sites), whereas VEGF competes for the same binding sites to stimulate endothelial cell motility. In addition, VEGF antagonizes apoptosis induced by Sema3A. Conditions where neural activity is improved are also seen to trigger angiogenesis. For example, exercise induced increased synaptic activity and a greater density of blood vessels in the cerebellar cortex of adult rats (Black et al., 1990). These studies provide further data to support the relationship between blood vessels and nerves, as well as suggesting that endothelial cells could help promote neurite elongation by providing molecular cues in the early stage of regeneration.
There is also strong evidence that the surfaces of blood vessels directly support Schwann cell migration and hence axon growth (Cattin et al., 2015). This study was performed in a rat sciatic nerve model; when nerves were harvested, blood vessels, Schwann cells and axons were quantified using confocal microscopy. This demonstrated that Schwann cells migrated along the surface of blood vessels and consequently, Schwann cell migration was disrupted when blood vessel network architecture was disorganized.
In summary, the interlinkage between vascularization and nerve regeneration is well established. In the first place, the vasculature provides nutrients such as oxygen for regenerating axons and associated cells, and thus increases long‐term survival. Furthermore, endothelial cells secrete molecules that can be beneficial for neurogenesis and nerve regeneration. Finally, blood vessels also serve as tracks for Schwann cells to migrate along and thus guide axonal growth. As a result, vascularized artificial nerve conduits could be an exciting approach to explore for the repair of peripheral nerve injuries. In the following sections, vascularized nerve constructs are reviewed, then future technological directions including engineered tissues and mathematical modeling are discussed using examples from our current research.
VASCULARIZED NERVE SUBSTITUTES
Vascular substitutes can be categorized into four main groups: (1) Vascularized nerve grafts, (2) Vascularized grafts by vascular implantation, (3) Blood vessel‐including tubulation, and (4) Biogenic vascularized nerve conduits. Next we discuss these options in turn, including the features that limit their use as clinical repair options. A summary of all approaches is provided in Table 1.
Table 1.
Summary of the approaches to generate vascularized nerve grafts/conduits and some example studies.
| Method | Nerve type and its source of vascularization | Species | Nerve gap | Time point | Outcomes compared with controls | Reference |
|---|---|---|---|---|---|---|
| Vascularized nerve grafts | Vascularized sural nerve graft | Human | 50–80 mm | 2–41 months |
|
(Rose and Kowalski, 1985) |
| Sciatic nerve graft with proximal preserved pedicle | Rabbit | 45 mm | 5–15 weeks |
|
(Restrepo et al., 1985) | |
| Rabbit median nerve graft with brachial vessels | Rabbit | 30 mm | 10 and 24 weeks |
|
(Shibata et al., 1988) | |
| Rat sciatic nerve with caudal femoral vessels | Rat | 15 mm | 1–24 weeks |
|
(Koshima and Harii, 1985) | |
| Vascularized grafts by vascular implantation | Implanting an arteriovenous fistula into the sciatic nerve | Rat | N/A | N/A | N/A | (Cavadas and Vera‐Sempere, 1994) |
| Peripheral nerve graft was placed into the groove between the femoral artery and vein for 14 days | Rabbit | N/A | N/A | N/A | (Saray et al., 2002) | |
| PGA nerve conduit vascularized by host superficial inferior epigastric (SIE) for 14 days | Rat | 15 mm | 1–18 weeks |
|
(Iijima et al., 2016) | |
| Amnion tube placed in contact with the femoral artery and vein for 3 weeks | Rat | 10 mm | 3 months |
|
(Ozcan et al., 1993) | |
| Blood vessels‐including tubulation | Insertion of a subcutaneous artery and sciatic nerve in a silicone tube | Rat | 5 mm | 4, 8, and 15 weeks |
|
(Kosaka, 1990) |
| Silicone tube containing sural vessels | Rat | 25 mm | 12 and 24 weeks |
|
(Kakinoki et al., 1997) | |
| Silicone tube with a subcutaneous artery adjacent to the injured nerve | Human | 30–50 mm | 6–9 months |
|
(Yong‐xiang and Ti‐pei, 1992) | |
| Vascularized biogenic conduits | Silicone rod placed near a sciatic nerve for 8 weeks | Rat | 15 mm | 8 weeks |
|
(Yapici et al., 2017) |
| In vivo formation of biogenic conduit after 4 weeks of implantation parallel to the sciatic nerve | Rat | 15 mm | 1, 2, 3, and 4 weeks |
|
(Penna et al., 2011) | |
| Silicone rubber rod left in situ for at least 3 weeks | Rat | 10–12 mm | 3 months |
|
(Lundborg and Hansson, 1980) | |
| Pseudosheath formed around the silicone tube during the first stage is used as a tunnel to envelope the median nerve graft segment in the second stage | Rat | 15 mm | 3, 6, and 15 weeks |
|
(Zadegan et al., 2015) |
“N/A” indicates that there is no results of an in vivo assessment of the construct.
Abbreviations: CMAP, compound muscle action potential; SFI, sciatic functional index.
VASCULARIZED NERVE GRAFTS
Vascularized Nerve Grafts (VNGs) involve transplanting nerve grafts complete with vasculature to the site of nerve regeneration and have been developed to address numerous physiological challenges. VNGs could promote intra‐neural perfusion and nutrient delivery in poorly vascularized zones in humans (Schonauer et al., 2012), avoiding the early ischemia of conventional nerve grafts by restoring neural blood vessels.
In a nonvascularized nerve graft (NVNG), neovascularization, the onset of new blood vessel formation at the interface with host tissue, usually occurs by the third day after surgery under supportive conditions (Mani et al., 1992). Conversely, neovascularization in VNGs can occur before the onset of ischemia, due to the presence of vascular structures within the graft (Mani et al., 1992). As a result, VNGs are considered to be superior to NVNGs and have been successfully used in several clinical cases (Shibata et al., 1988, Rose and Kowalski, 1985, Restrepo et al., 1985, Koshima and Harii, 1985). An early example of VNGs can be seen in a study done by St. Clair Strange in 1947 (Strange, 1947). He successfully harvested the ulnar nerve together with its blood supply to graft the median nerve. In 1976, Taylor and Ham introduced a VNG from the superficial radial nerve (based on the radial artery) and used this to repair a median nerve (Taylor and Ham, 1976).
It has been shown that the ability to enhance nerve regeneration of VNGs is superior to NVNGs as length and size of the nerve injury increase, and as vascularization decreases, which can be seen in a scarred wound bed (Koshima and Harii, 1985, Restrepo et al., 1985, Shibata et al., 1988). In a rabbit sciatic nerve model, 45 mm of the nerve was transected and bridged with vascularized sciatic nerve graft with a vascular pedicle and conventional sciatic nerve grafts (NVNGs) (Restrepo et al., 1985). After 8 weeks, vascularized nerve grafts revealed better performances than the conventional graft in terms of number and diameter of nerve fibers. In the functional outcomes aspect, Kanaya et al. (1992) showed in their work that in a rat sciatic nerve model the vascularized sciatic nerve graft group exhibited an improved mean sciatic function index (SFI) compared with the nonvascularized group (Kanaya et al., 1992).
The criteria for selecting which nerves to use as VNGs were primarily based on whether there were dominant arterial pedicles or large supplying vessels that run for a distance outside the nerves. The vascularized radial or ulnar nerve graft has been demonstrated to be successful in several clinical cases by Taylor and Ham, which is ascribed to their dependable blood supply and suitable diameter for microsurgical transfer (El‐Barrany et al., 1999, Townsend and Taylor, 1984). However, due to the fact that a radial nerve's blood supply is the major limb artery (El‐Barrany et al., 1999) and the ulnar nerve is considered important (Townsend and Taylor, 1984), these limit their clinical uses in this context. In general, saphenous and sural nerve grafts are the most popular for grafting because of their dominant arterial pedicles as well as acceptable morbidity at the donor site (El‐Barrany et al., 1999, Staniforth and Fisher, 1978).
Although VNGs have the potential to improve nerve repair, the major limitation is the lack of donor sites. VNGs function well in large nerves, specifically in long gaps. However, to harvest such a large nerve, significant donor‐site morbidity and scarring is inevitably involved. Despite the fact that this hurdle can be solved in part by the use of cable grafting (i.e., several vascularized nerve graft strands), this leads to harvesting more nerve graft strands and thus increased subsequent donor site morbidity (D'Arpa et al., 2015).
VASCULARIZED GRAFTS BY VASCULAR IMPLANTATION
Several techniques have been studied to achieve the benefits of VNGs but avoid harvesting a nerve graft with its vascular supply to reduce the donor site‐related morbidity described above. One option is to fabricate a nerve graft and preimplant it between a host artery and vein to promote vascularization, then subsequently remove and implant to bridge the repair site (Falco et al., 1992).
Cavadas and Vera‐Sempere examined this approach in 1994 when they attempted to construct a vascularized nerve graft by implanting an arteriovenous fistula into the sciatic nerve in a rat model for 5 weeks (Cavadas and Vera‐Sempere, 1994). Such experimental surgeries have been performed, but their success in terms of promoting neural regeneration needs to be evaluated relative to the VNG approach. Using an arteriovenous bundle, Saray et al. (2002) vascularized sciatic nerve grafts from a rabbits by placing them into the groove between the femoral artery and vein and then removing after 3, 7, or 17 days to analyze. By day 3, the nerve graft was well vascularized and at day 14 the resident Schwann cells and graft integrity were still preserved (Saray et al., 2002). However, this study did not evaluate nerve regeneration using the prefabricated graft. In another study, Ozcan et al. vascularized an amnion tube by placing it between the femoral artery and vein in a rat model for 3 weeks (Ozcan et al., 1993). The microcirculation was successfully visualized at the repair site in all amnion tubes. At 3 months postoperatively, the vascularized amnion conduits showed comparable nerve regeneration to conventional vascularized nerve grafts in terms of number of myelinated axons, axon diameter and myelin thickness. This revealed the ability of vascularized amnion conduits to promote nerve regeneration and bridge the femoral nerve gap. Indeed, the authors concluded that vascularized amnion conduits were superior to their nonvascularized counterparts. More recently, nerve conduits made of polyglycolic acid (PGA) vascularized by host superficial inferior epigastric (SIE) vessels were successfully constructed and this facilitated the regeneration as well as re‐myelination of the rat sciatic nerve (Iijima et al., 2016).
Although prefabricated VNG through AV fistula implantation could be useful clinically for reducing donor‐site morbidity, this approach involves a two‐stage surgery which prolongs the delay before nerve repair and might increase the risk of complications. Therefore, there remain significant opportunities for improving on this approach in developing clinically‐feasible nerve repair solutions.
BLOOD VESSEL‐INCLUDING TUBULATION
This technique includes native blood vessels directly within a nerve conduit to promote the vascularization process. One of the first studies was conducted by Kakinoki et al. (1997) where a silicone tube containing the sural vessels implanted in a longitudinal orientation was used to bridge a sciatic nerve gap of 25 mm in a rat. The proximal and distal end of the sciatic nerve were sutured to the silicone tube. After 6 months, axons had regenerated across a 25 mm nerve gap in the rats and reinnervated the tibialis anterior muscle (Kakinoki et al., 1997); however, there was no control where tubes without blood vessels were tested, so it is difficult to determine the improvement that resulted from including blood vessels in the tube. In another study, a subcutaneous artery adjacent to the injured nerve was mobilized and then inserted into a silicone tube (Kosaka, 1990). This was used to bridge a 5 mm rat sciatic nerve gap, with the nerve stumps inserted alongside the artery into each end of the tube, and the repair analyzed after 4, 8, and 15 weeks. The results demonstrated more capillaries were present in the vessel‐containing conduits, with rapid capillary‐like structure formation taking place within the tube; by 4 weeks postoperatively, the total number of intraneural microvasculature vessels was almost four times greater than that in a control group. Also, the tube containing the artery exhibited greater morphological and functional recovery of the regenerating nerve.
Although results are promising, studies in this area have tended to use silicone tubes which are non‐biodegradable, may cause compression, negatively affect joint movement and induce synovitis (Lanzetta et al., 1994), so the tubes would need to be removed after several months if this approach were used clinically. To overcome this limitation, biodegradable or biological tube materials are available for clinical use, and it would be interesting to use these in future vascularization studies rather than silicone. Furthermore, their porosity and permeability to oxygen and nutrients may influence the outcomes. In addition, experiments using larger nerve gap lengths and longer follow‐up would be useful to investigate the performance of these approaches effectively.
VASCULARIZED BIOGENIC CONDUITS
Prefabricated vascularized biogenic nerve conduits can be generated by placing an artificial conduit near a donor nerve or blood supply. After a certain period of time (usually 1–3 weeks), the artificial conduit is enclosed by pseudosheath; in this stage, the tube is removed leaving intact pseudosheath as a vascularized biogenic nerve conduit. This conduit can then be used on its own, filled with biomaterials, or incorporated with a nerve graft.
In many studies, silicone tubes have been used to generate vascularized biogenic conduits. Due to the non‐absorbable characteristics of silicone, a pseudo‐synovial sheath forms around the silicone tube when implanted subcutaneously (Gu et al., 2011), and this was first used as a tendon graft (Culp, 1993). A study in a primate model conducted by Hunter et al. (1983) showed that the pseudo‐synovial sheath consists of three layers: (1) the intima layer which contains cells that provide a soft and sliding surface, (2) the media layer that was dense with collagen and vascularity, and (3) the adventitia layer which was composed of vascular fibrous tissue. This pseudo‐synovial sheath provides a highly vascular structure throughout all layers (Hunter et al., 1983). There is an evidence suggesting that this vascularized pseudo‐synovial sheath could be used as a nerve graft (Wolford and Stevao, 2003). Lundborg and Hansson used this pseudo‐synovial sheath as a biogenic nerve conduit to repair peripheral nerve injuries with gaps of 10 to 12 mm in rats (Lundborg and Hansson, 1980). Subsequently, several studies have reported success with biogenic conduits to repair nerve injuries in rat models (Penna et al., 2011, Yapici et al., 2017, Zadegan et al., 2015).
In one study, a polyvinyl chloride (PVC) tube was implanted parallel to the rat sciatic nerve (Penna et al., 2011). After 4 weeks, this PVC tube had been covered with pseudo‐synovial sheath and had a higher number of blood vessels per cross section compared to an autologous nerve graft. When used to repair a 15 mm nerve gap injury, there was successful regeneration with significantly higher axon area than an autologous nerve graft. Recently, Yapici et al. (2017) fabricated vascularized biological conduits by placing a silicon rod next to the sciatic nerve of the rat (Yapici et al., 2017). Without damaging the fibrovascular sheath formed around the rod, the silicone rod was removed at the 8‐week time point leaving the vascular sheath which was then used as a nerve conduit. This provided better nerve regeneration when compared to non‐vascularized conduits (connective tissue sheath from a silicone tube placed to the dorsum of the rat) and autografts, both histologically and electrophysiologically. This vascular sheath was also shown to reduce adhesion and scar formation compared to non‐vascularized conduits.
Vascularized biogenic conduits avoid sacrificing a donor nerve with its blood vessel supply (nerve tissue transfer), as well as decreasing the adverse effects of scarring at the nerve injury site. Furthermore, a biogenic conduit provides the soft lumen, good vascularization, and a stable structure that could be effectively used to envelop the nerve graft segment (Zadegan et al., 2015). However, these conduits require a two‐stage procedure for implementation clinically, and the delay associated with the prevascularization step would inevitably limit nerve regeneration capacity in a clinical setting.
Overall, vascularized nerve substitutes are generally accepted as a valuable reconstructive tool for nerve repair. However, the donor site morbidity associated with the vascular substitutes presents a significant limitation to clinical uptake. Therefore, several techniques have been developed to try to reduce this donor site‐related morbidity while enhancing nerve regeneration compared to the gold‐standard, as described in the four sections above. Despite better outcomes coming from those substitutes, problems associated with the long delay required to generate prevascularized nerve conduits, and the limited anatomical supply, still remain.
ENGINEERED NEURAL TISSUES
Tissue engineering using vascular endothelial cells and biomaterial scaffolds to prefabricate a vascularized nerve construct provides an alternative approach to vascularization in long gap nerve repair. There are not many studies that report specifically the engineering of vascularized nerve tissue constructs. One study demonstrated that by co‐culturing Schwann cells and vascular endothelial cells within fiber‐reinforced 3D composite scaffolds, vascularized nerve engineered constructs can be formed (Gao et al., 2013). According to that study, the combination of Schwann cells and vascular endothelial cells, both taken from rabbits and mixed at a ratio of 2:1, were cultured and then this mixed cell population was injected at both ends of the fiber‐reinforced scaffolds to form a vascularized tissue engineered nerve construct. These constructs were assessed in sciatic nerve injury rabbit models of 20 mm gap at three different postoperative periods (4, 8, and 16 weeks) and were beneficial in promoting nerve repair in terms of conduction velocity, number of nerve fibers and myelin thickness. In another study conducted by Gingras et al. (2003), a tissue‐engineered model of peripheral nerve regeneration was developed which consisted of collagen‐chitosan sponges populated with human endothelial cells and/or fibroblasts (Gingras et al., 2003). When the endothelial cells were present, there was a significant increase in neurite elongation after 14 days in culture, supporting the utility of having endothelial cells in an engineered nerve construct.
Combining the concept that vascularization of nerve grafts can improve their effectiveness with the observations that endothelial cell‐seeded constructs are beneficial and that vascular structures can guide Schwann cell migration, leads to the hypothesis that engineering vascular structures within scaffolds could help to improve nerve repair construct design. Several approaches have been studied to generate microvascular networks in vitro. Many studies attempted to form patterns with cells using techniques such as soft lithography (Whitesides et al., 2001), micropatterning (Folch and Toner, 2000), and photolithography (Kaihara et al., 2000). Another approach involves using the ability of endothelial cells to form networks within scaffolds. Endothelial cells can spontaneously form capillary‐like networks when cultured in 3D under permissive conditions in vitro. They have been demonstrated to be able to form tube‐like structures within hydrogels either by co‐culturing with other cell types like fibroblasts or pericytes, or by adding angiogenic growth factors (Berthod et al., 2006, Berthod et al., 2012). In our lab, we successfully created aligned tube‐like vascular structures within collagen hydrogels using human umbilical vein endothelial cells (HUVECs) (Fig. 2). The engineered vascular tissue was constructed using the same approach as previously applied to create aligned cellular collagen gels containing Schwann cells or other therapeutic cells for nerve repair termed Engineered Neural Tissue (EngNT) (Georgiou et al., 2013, Georgiou et al., 2015, Martens et al., 2014, O'Rourke et al., 2015, Sanen et al., 2017). In this process, cells embedded within tethered collagen hydrogels self‐align in response to tension generated through natural cell‐matrix interactions, then the aligned cellular gels are stabilized using plastic compression (Brown et al., 2005).
Figure 2.
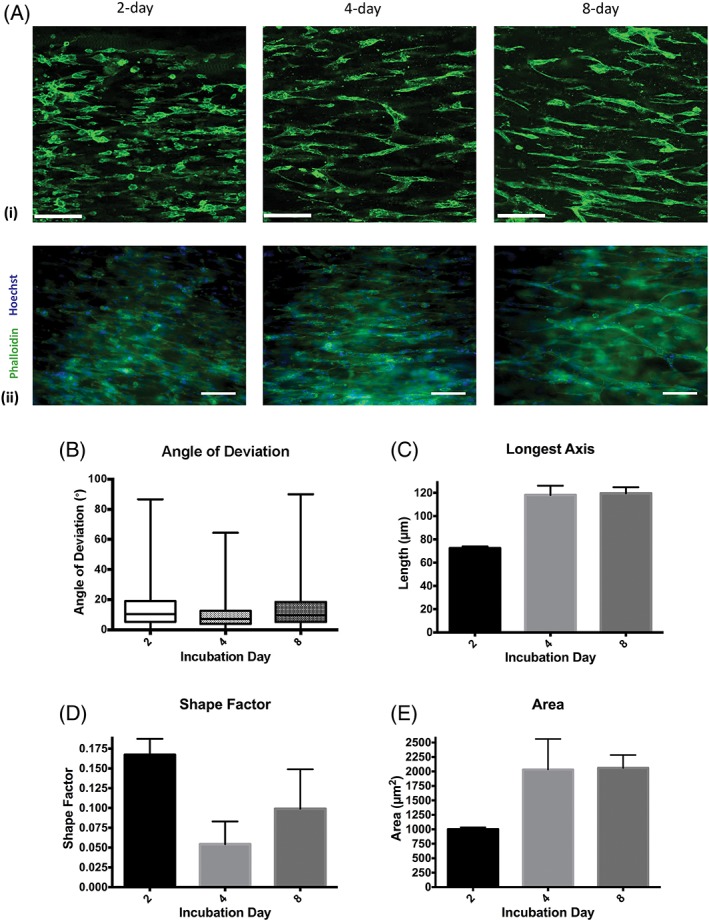
Self‐alignment of HUVECs and formation of tube‐like structures within tethered collagen gels. Confocal micrographs (A(i)) and immunofluorescence images (A(ii)) show aligned HUVECs forming vascular networks after 2, 4, and 8 days in culture, z‐distance 20 μm, step size 1 μm. Three‐dimensional image analysis was used to calculate the angle of deviation between HUVEC/tube alignment and the longitudinal axis of the gel (B). Boxes show interquartile range and median values, whiskers indicate maximum and minimum angles (N = 3 gels). The length of tube‐like structures (C), shape factor which determine how round the object is (values closer to 1 indicate more rounded shape) (D) and surface area (E) were compared in 2‐day, 4‐day, and 8‐day cultured gels. Graphs show mean value ± SEM. (N = 3 gels). Scale bars in (A(i)) = 120 μm and in (A(ii)) = 100 μm.
These collagen gels containing aligned tube‐like structures have been shown to promote neurite outgrowth in vitro when co‐cultured with dorsal root ganglion (DRG) explants (Fig. 3A) and dissociated DRGs (Fig. 3B), suggesting their ability to support and guide neuronal regeneration.
Figure 3.
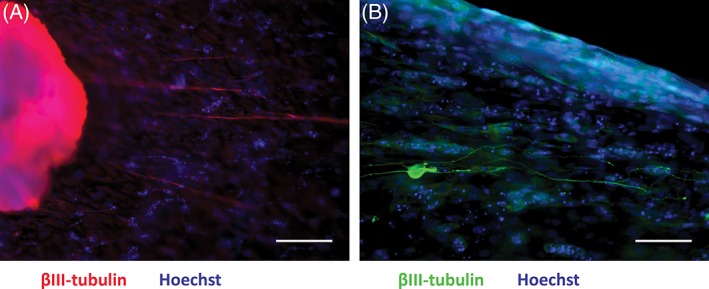
Aligned endothelial cells within collagen gels support and guide neurite growth in vitro. Neurites from explanted rat DRG (A) and from dissociated DRG neurons (B) elongated along the longitudinal axis of the gel and were associated with the aligned endothelial cells. Scale bars in A, B = 100 μm.
Besides using endothelial cells to generate vascular structures within tissue‐engineered nerve constructs, vascularization of engineered constructs can be promoted through embedding angiogenic factors. This can enhance local angiogenesis and promote integration of constructs with the host vasculature. In nerve tissue engineering studies, vascular endothelial growth factor (VEGF) has been added to nerve constructs and used to repair rat sciatic nerves, resulting in increased numbers of blood vessels as well as axons when compared to constructs without added VEGF (Hobson, 2002, Hobson et al., 2000, Mohammadi et al., 2013, Sondell et al., 1999). Furthermore, there are several growth factors used to induce and support vascularization for tissue engineering that can be applied for nerve constructs such as basic fibroblast growth factor (bFGF) and platelet‐derived growth factor (PDGF) (Carmeliet, 2000, Nomi et al., 2002). Novel angiogenic small molecules have also been investigated, such as in a study by Wieghaus et al. (2006) which developed SC‐3‐149, a non‐peptide‐based inducer, that can increase the proliferation of human microvascular endothelial cells and network formation in vitro (Wieghaus et al., 2006). Another approach is to genetically manipulate cells to overexpress a proangiogenic factor, the most widely used of which is VEGF. Geiger et al. (2007) used bone marrow stromal cells (BMSCs) transfected with the VEGF plasmid to enhance vascularization of a bone substitute. Their results showed that genetic modification of BMSCs significantly improved vascularization and osteogenesis for in vivo bone healing (Geiger et al., 2007).
MATHEMATICAL MODELING AS A TOOL TO AID IN THE FABRICATION OF VASCULARIZED NERVE CONSTRUCTS
Vascularized nerve constructs have potential to contribute to the next generation of living replacement tissues; however, there are numerous outstanding questions related to their optimal design and fabrication. For a vascular nerve repair construct to be successful, it must support angiogenesis in vivo while minimizing loss of valuable therapeutic cells, and also provide the physical and chemical cues to support axon regeneration. This requires sensitive consideration of the spatial and temporal distribution of material, cells, and chemical factors which is challenging to do in conventional experimental settings in isolation. For example, cell populations such as endothelial or therapeutic Schwann cells are usually added to constructs at standard densities, without exploring the effect of varying these seeding densities on regeneration outcomes. Similarly, the spatial distribution of seeded cells is rarely considered; typically cells are cultured on the luminal surface of a construct tube, distributed throughout the lumen of the tube in suspension, or grown on/in materials packed inside the construct. The seeded cell density and its spatial distribution is critically important, as they determine how gradients of oxygenation and vascular growth factors are established over time in vivo. In turn, these gradients regulate hypoxia, cell death rates, and the success of angiogenesis in vascularizing the construct.
These features are highly challenging to characterize experimentally due to the cost and time limitations of extensive in vitro and in vivo experimentation. However, combining mathematical modeling with the experimental programme has significant potential to streamline the design process and accelerate the pipeline toward clinical translation, and this is the approach taken in our lab (Coy et al., 2016). Such mathematical models should be developed in parallel with preliminary experimentation, so that iteration between model predictions and experimental measurements can inform parameters (e.g., cell proliferation or oxygen consumption rates, etc.) within the mathematical framework, and outputs of the mathematical models can inform the parameter range to explore experimentally. This parameterized framework can then be used to run virtual (or in silico) tests of design features (such as seeded cell densities and their spatial distribution), before prioritizing the most promising designs for more extensive experimental testing. In this way, the programme is focused on the experiments that will generate the most meaningful data and promising outcomes.
An experimental‐computational approach has the potential to capitalize on the diverse and advanced tissue engineering, biomaterial and cell technologies now available, and streamline the process of combining them to maximize regeneration using vascularized nerve constructs. It would also exploit a growing literature in computational modeling of blood flow, tissue oxygenation, and angiogenesis (Anderson and Chaplain, 1998, Secomb et al., 2013) for the benefit of peripheral nerve tissue engineering.
CONCLUSIONS
Vascularization is likely to be an important component in the development of successful new nerve repair strategies. It is required in order for the living components of cellular constructs to survive following implantation, as well as being involved directly in supporting and guiding Schwann cell migration and neuronal regeneration (Iijima et al., 2016, Auger et al., 2013, Cattin et al., 2015). Various techniques have been investigated including free and pedicled vascularized nerve grafts, vascular implantation, blood vessel‐including tubulation, and vascularized biogenic conduits. These in turn inform the development of vascularized tissue‐engineered nerve constructs, providing new opportunities to develop sophisticated living artificial tissue.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Royal Thai Government Scholarship and funding from the EPSRC via grants EP/R004463/1 and EP/N033493/1.
LITERATURE CITED
- Anderson AR, Chaplain MA. 1998. Continuous and discrete mathematical models of tumor‐induced angiogenesis. Bull Math Biol 60:857–899. [DOI] [PubMed] [Google Scholar]
- Appenzeller O, Dhital KK, Cowen T, Burnstock G. 1984. The nerves to blood vessels supplying blood to nerves: the innervation of vasa nervorum. Brain Res 304:383–386. [DOI] [PubMed] [Google Scholar]
- Auger FA, Gibot L, Lacroix D. 2013. The pivotal role of vascularization in tissue engineering. Annu Rev Biomed Eng 15:177–200. [DOI] [PubMed] [Google Scholar]
- Bagnard D, Vaillant C, Khuth ST, Dufay N, Lohrum M, Puschel AW, Belin MF, Bolz J, Thomasset N. 2001. Semaphorin 3A‐vascular endothelial growth factor‐165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J Neurosci 21:3332–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, Weddell AG. 1984. A descriptive study of the blood vessels of the sciatic nerve in the rat, man and other mammals. Brain 107:871–898. [DOI] [PubMed] [Google Scholar]
- Berthod F, Germain L, Tremblay N, Auger FA. 2006. Extracellular matrix deposition by fibroblasts is necessary to promote capillary‐like tube formation in vitro. J Cell Physiol 207:491–498. [DOI] [PubMed] [Google Scholar]
- Berthod F, Symes J, Tremblay N, Medin JA, Auger FA. 2012. Spontaneous fibroblast‐derived pericyte recruitment in a human tissue‐engineered angiogenesis model in vitro. J Cell Physiol 227:2130–2137. [DOI] [PubMed] [Google Scholar]
- Best TJ, Mackinnon SE. 1994. Peripheral nerve revascularization: a current literature review. J Reconstr Microsurg 10:193–204. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. 1990. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA 87:5568–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Wiseman M, Chuo CB, Cheema U, Nazhat SN. 2005. Ultrarapid engineering of biomimetic materials and tissues: Fabrication of nano‐ and microstructures by plastic compression. Adv Funct Mater 15:1762–1770. [Google Scholar]
- Carmeliet P. 2000. Mechanisms of angiogenesis and arteriogenesis. Nat Med 6:389–395. [DOI] [PubMed] [Google Scholar]
- Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, et al. 2015. Macrophage‐Induced Blood Vessels Guide Schwann Cell‐Mediated Regeneration of Peripheral Nerves. Cell 162:1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavadas PC, Vera‐Sempere FJ. 1994. Prefabrication of a vascularized nerve graft by vessel implantation: preliminary report of an experimental model. Microsurgery 15:877–881. [DOI] [PubMed] [Google Scholar]
- Coy RH, Evans OR, Phillips JB, Shipley RJ. 2016. An integrated theoretical‐experimental approach to accelerate translational tissue engineering. J Tissue Eng Regen Med 12:e53–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp RW. 1993. Reconstruction of flexor tendon with hunter tendon implant. Oper Tech Orthop 3:298–302. [Google Scholar]
- D'Arpa S, Claes KEY, Stillaert F, Colebunders B, Monstrey S, Blondeel P. 2015. Vascularized nerve “grafts”: just a graft or a worthwhile procedure? Plast Aesthet Res 2:183–194. [Google Scholar]
- El‐Barrany WG, Marei AG, Vallee B. 1999. Anatomic basis of vascularised nerve grafts: the blood supply of peripheral nerves. Surg Radiol Anat 21:95–102. [DOI] [PubMed] [Google Scholar]
- Falco NA, Pribaz JJ, Eriksson E. 1992. Vascularization of skin following implantation of an arteriovenous pedicle: Implications in flap prefabrication. Microsurgery 13:249–254. [DOI] [PubMed] [Google Scholar]
- Folch A, Toner M. 2000. Microengineering of cellular interactions. Annu Rev Biomed Eng 2:227–256. [DOI] [PubMed] [Google Scholar]
- Gao H, You Y, Zhang G, Zhao F, Sha Z, Shen Y. 2013. The use of fiber‐reinforced scaffolds cocultured with schwann cells and vascular endothelial cells to repair rabbit sciatic nerve defect with vascularization. Biomed Res Int 2013:362918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger F, Lorenz H, Xu W, Szalay K, Kasten P, Claes L, Augat P, Richter W. 2007. VEGF producing bone marrow stromal cells (BMSC) enhance vascularization and resorption of a natural coral bone substitute. Bone 41:516–522. [DOI] [PubMed] [Google Scholar]
- Georgiou M, Bunting SC, Davies HA, Loughlin AJ, Golding JP, Phillips JB. 2013. Engineered neural tissue for peripheral nerve repair. Biomaterials 34:7335–7343. [DOI] [PubMed] [Google Scholar]
- Georgiou M, Golding JP, Loughlin AJ, Kingham PJ, Phillips JB. 2015. Engineered neural tissue with aligned, differentiated adipose‐derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials 37:242–251. [DOI] [PubMed] [Google Scholar]
- Gingras M, Bergeron J, Dery J, Durham HD, Berthod F. 2003. In vitro development of a tissue‐engineered model of peripheral nerve regeneration to study neurite growth. FASEB J 17:2124–2126. [DOI] [PubMed] [Google Scholar]
- Gu X, Ding F, Yang Y, Liu J. 2011. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol 93:204–230. [DOI] [PubMed] [Google Scholar]
- Hobson MI. 2002. Increased vascularization enhances axonal regeneration within an acellular nerve conduit. Ann R Coll Surg Engl 84:47–53. [PMC free article] [PubMed] [Google Scholar]
- Hobson MI, Brown R, Green CJ, Terenghi G. 1997. Inter‐relationships between angiogenesis and nerve regeneration: A histochemical study. Br J Plast Surg 50:125–131. [DOI] [PubMed] [Google Scholar]
- Hobson MI, Green CJ, Terenghi G. 2000. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat 197(Pt 4):591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JM, Jaeger SH, Matsui T, Miyaji N. 1983. The pseudosynovial sheath; Its characteristics in a primate model. J Hand Surg 8:461–470. [DOI] [PubMed] [Google Scholar]
- Iijima Y, Ajiki T, Murayama A, Takeshita K. 2016. Effect of artificial nerve conduit vascularization on peripheral nerve in a necrotic bed. Plast Reconstr Surg Glob Open 4:e665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isahara K, Yamamoto M. 1995. The interaction of vascular endothelial cells and dorsal root ganglion neurites is mediated by vitronectin and heparan sulfate proteoglycans. Brain Res Dev Brain Res 84:164–178. [DOI] [PubMed] [Google Scholar]
- Jain RKAP, Tam J, Duda DG, Fukumura D. 2005. Engineering vascularized tissues. Nat Biotechnol 23:821–823. [DOI] [PubMed] [Google Scholar]
- Kaihara S, Borenstein J, Koka R, Lalan S, Ochoa ER, Ravens M, Pien H, Cunningham B, Vacanti JP. 2000. Silicon micromachining to tissue engineer branched vascular channels for liver fabrication. Tissue Eng 6:105–117. [DOI] [PubMed] [Google Scholar]
- Kakinoki R, Nishijima N, Ueba Y, Oka M, Yamamuro T, Nakamura T. 1997. Nerve regeneration over a 25 mm gap in rat sciatic nerves using tubes containing blood vessels: the possibility of clinical application. Int Orthop 21:332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya F, Firrell J, Tsai TM, Breidenbach WC. 1992. Functional results of vascularized versus nonvascularized nerve grafting. Plast Reconstr Surg 89:924–930. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Lindvall O. 2003. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol 13:127–132. [DOI] [PubMed] [Google Scholar]
- Kosaka M. 1990. Enhancement of rat peripheral nerve regeneration through artery‐including silicone tubing. Exp Neurol 107:69–77. [DOI] [PubMed] [Google Scholar]
- Koshima I, Harii K. 1985. Experimental study of vascularized nerve grafts: Multifactorial analyses of axonal regeneration of nerves transplanted into an acute burn wound. J Hand Surg 10:64–72. [DOI] [PubMed] [Google Scholar]
- Lanzetta M, Herbert TJ, Conolly WB. 1994. Silicone synovitis: A perspective. J Hand Surg [Br] 19:479–484. [DOI] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. 1999. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci 13:450–464. [DOI] [PubMed] [Google Scholar]
- Low PA, Lagerlund TD, McManis PG. 1989. Nerve blood flow and oxygen delivery in normal, diabetic, and ischemic neuropathy. Int Rev Neurobiol 31:355–438. [DOI] [PubMed] [Google Scholar]
- Lundborg G, Hansson HA. 1980. Nerve regeneration through preformed pseudosynovial tubes. J Hand Surg 5:35–38. [DOI] [PubMed] [Google Scholar]
- Mani GV, Shurey C, Green CJ. 1992. Is early vascularization of nerve grafts necessary? J Hand Surg 17:536–543. [DOI] [PubMed] [Google Scholar]
- Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I. 2014. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue‐engineered collagen construct in vitro. FASEB J 28:1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. 1999. Neuropilin‐1 mediates collapsin‐1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin‐1 and vascular endothelial growth factor‐165. J Cell Biol 146:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi R, Ahsan S, Masoumi M, Amini K. 2013. Vascular endothelial growth factor promotes peripheral nerve regeneration after sciatic nerve transection in rat. Chin J Traumatol 16:323–329. [PubMed] [Google Scholar]
- Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. 2000. Vascular endothelial cells synthesize and secrete brain‐derived neurotrophic factor. FEBS Lett 470:113–117. [DOI] [PubMed] [Google Scholar]
- Nomi M, Atala A, Coppi PD, Soker S. 2002. Principals of neovascularization for tissue engineering. Mol Aspects Med 23:463–483. [DOI] [PubMed] [Google Scholar]
- Nukada H. 1988. Post‐traumatic endoneurial neovascularization and nerve regeneration: a morphometric study. Brain Res 449:89–96. [DOI] [PubMed] [Google Scholar]
- O'Rourke C, Drake RA, Cameron GW, Jane Loughlin A, Phillips JB. 2015. Optimising contraction and alignment of cellular collagen hydrogels to achieve reliable and consistent engineered anisotropic tissue. J Biomater Appl 30:599–607. [DOI] [PubMed] [Google Scholar]
- Ozcan G, Shenaq S, Spira M. 1993. Vascularized Nerve Tube: An Experimental Alternative for Vascularized Nerve Grafts Over Short Gaps. J Reconstr Microsurg 9:405–413. [DOI] [PubMed] [Google Scholar]
- Penna V, Munder B, Stark GB, Lang EM. 2011. An in vivo engineered nerve conduit‐fabrication and experimental study in rats. Microsurgery 31:395–400. [DOI] [PubMed] [Google Scholar]
- Restrepo Y, Merle M, Michon J, Folliguet B, Barrat E. 1985. Free vascularized nerve grafts: an experimental study in the rabbit. Microsurgery 6:78–84. [DOI] [PubMed] [Google Scholar]
- Ronald JP, Robert RM. 1995. A diffusion‐reaction model of nerve regeneration. J Neurosci Methods 60:79–88. [DOI] [PubMed] [Google Scholar]
- Rose EH, Kowalski TA. 1985. Restoration of sensibility to anesthetic scarred digits with free vascularized nerve grafts from the dorsum of the foot. J Hand Surg Am 10:514–521. [DOI] [PubMed] [Google Scholar]
- Sanen K, Martens W, Georgiou M, Ameloot M, Lambrichts I, Phillips J. 2017. Engineered neural tissue with Schwann cell differentiated human dental pulp stem cells: potential for peripheral nerve repair? J Tissue Eng Regen Med 11:3362–3372. [DOI] [PubMed] [Google Scholar]
- Saray A, Teoman Tellioglu A, Altinok G. 2002. Prefabrication of a Free Peripheral Nerve Graft Following Implantation on an Arteriovenous Pedicle. J Reconstr Microsurg 18:281–288. [DOI] [PubMed] [Google Scholar]
- Schonauer F, Marlino S, Avvedimento S, Molea G. 2012. Peripheral nerve reconstruction with autologous grafts In: Rayegani DSM, editor. Basic principles of peripheral nerve disorders. Rijeka, Croatia: InTech. [Google Scholar]
- Secomb TW, Alberding JP, Hsu R, Dewhirst MW, Pries AR. 2013. Angiogenesis: an adaptive dynamic biological patterning problem. PLoS Comput Biol 9:e1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Tsai T, Firrell J, Breidenbach WC. 1988. Experimental comparison of vascularized and nonvascularized nerve grafting. J Hand Surg 13:358–365. [DOI] [PubMed] [Google Scholar]
- Smith DR, Kobrine AI, Rizzoli HV. 1977. Absence of autoregulation in peripheral nerve blood flow. J Neurol Sci 33:347–352. [DOI] [PubMed] [Google Scholar]
- Sondell M, Lundborg G, Kanje M. 1999. Vascular endothelial growth factor stimulates Schwann cell invasion and neovascularization of acellular nerve grafts. Brain Res 846:219–228. [DOI] [PubMed] [Google Scholar]
- Staniforth P, Fisher TR. 1978. The effects of sural nerve excision in autogenous nerve grafting. Hand 10:187–190. [DOI] [PubMed] [Google Scholar]
- Strange FG. 1947. An operation for nerve pedicle grafting: preliminary communication. Br J Plast Surg 34:423–425. [DOI] [PubMed] [Google Scholar]
- Tarlove IM, Epstein JA. 1945. Nerve grafts: the importance of an adequate blood supply. J Neurosurg 2:49–71. [Google Scholar]
- Taylor GI, Ham FJ. 1976. The free vascularized nerve graft. A further experimental and clinical application of microvascular techniques. Plast Reconstr Surg 57:413–426. [PubMed] [Google Scholar]
- Townsend PL, Taylor GI. 1984. Vascularised nerve grafts using composite arterialised neuro‐venous systems. Br J Plast Sur 37:1–17. [DOI] [PubMed] [Google Scholar]
- Weddell G. 1942. Axonal regeneration in cutaneous nerve plexuses. J Anat 77:49–62.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerasuriya A. 1988. Patterns of change in endoneurial capillary permeability and vascular space during Wallerian degeneration. Brain Res 445:181–187. [DOI] [PubMed] [Google Scholar]
- Weerasuriya A. 1990. Patterns of change in endoneurial capillary permeability and vascular space during nerve regeneration. Brain Res 510:135–139. [DOI] [PubMed] [Google Scholar]
- Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. 2001. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng 3:335–373. [DOI] [PubMed] [Google Scholar]
- Wieghaus KA, Capitosti SM, Anderson CR, Price RJ, Blackman BR, Brown ML, Botchwey EA. 2006. Small molecule inducers of angiogenesis for tissue engineering. Tissue Eng 12:1903–1913. [DOI] [PubMed] [Google Scholar]
- Wolford LM, Stevao EL. 2003. Considerations in nerve repair. Proc (Bayl Univ Med Cent) 16:152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici AK, Bayram Y, Akgun H, Gumus R, Zor F. 2017. The effect of in vivo created vascularized neurotube on peripheric nerve regeneration. Injury 48:1486–1491. [DOI] [PubMed] [Google Scholar]
- Yong‐xiang L, Ti‐pei W. 1992. A clinical application of artery‐including silicone tubing to peripheral nerve defect. J Tongji Med Univ 12:247–249. [DOI] [PubMed] [Google Scholar]
- Zadegan SA, Firouzi M, Nabian MH, Zanjani LO, Ashtiani AM, Kamrani RS. 2015. Two‐stage nerve graft in severe scar: A time‐course study in a rat model. Arch Bone Jt Surg 3:82–87. [PMC free article] [PubMed] [Google Scholar]
- Zochodne DW. 2002. Nerve and ganglion blood flow in diabetes: an appraisal. Int Rev Neurobiol 50:161–202. [DOI] [PubMed] [Google Scholar]


