Abstract
Close to 30 forms of gonadotropin releasing hormone (GnRH) and at least five GnRH receptors have been identified in a wide variety of vertebrates and some invertebrates. One form, now called GnRH II, has the broadest distribution and the most ancient and conserved phylogeny. The distribution of the neurons that produce this peptide are completely nonoverlapping with any other GnRH forms. Fibers that project from these neurons overlap with GnRH I cells and/or fibers in a few regions, but are primarily divergent. The musk shrew (Suncus murinus) continues to be the most tractable mammalian species to use for studies of the function of GnRH II. The brain of the musk shrew has two GnRH genes (I and II), two GnRH receptors (types-1 and -2), and two different behaviors can be influenced by central infusion of GnRH II, but not by GnRH I; receptivity and feeding. Here, we summarize research on the musk shrew relative to the behavioral functions of GnRH II. First, female musk shrews are continually sexually receptive by virtue of their lack of an ovarian and/or behavioral estrus cycle. This feature of their reproductive ecology may be related to their semi-tropical distribution and their breeding season is highly dependent on changes in the availability of food. When food is not abundant, females stop mating, but brief bouts of feeding reinstate reproductive behavior. Likewise, intake of food is related to GnRH II mRNA and peptide content in the brain; after mild food restriction both decline. When GnRH II is infused centrally, at times when its content is low, it can both enhance receptivity and inhibit food intake. Simultaneous administration of a type-1 antagonist does not change the effect of GnRH II and use of an analog (135-18) that is a specific GnRH II agonist as well as a type-1 antagonist has the same effect as the endogenous GnRH II peptide. We propose that GnRH II plays a critical role by orchestrating the coordination of reproduction with the availability of nutritional support for these activities. Humans are bombarded with copious nutritional opportunities and at present obesity is a larger threat to health in many parts of the world than is under nutrition. It is our hope that understanding neuropeptides such as GnRH II that regulate food intake can ultimately lead to products that may curb appetite and thus decrease obesity and related risks to health.
Introduction to GnRH and GnRH II
Gonadotropin releasing hormone (GnRH) I was first identified in mammals (Matsuo et al. 1971; Burgus et al. 1972) and was subsequently isolated and sequenced from all major vertebrate classes. Further studies revealed that almost 30 different variants of GnRH I exist and most vertebrates have multiple forms, usually in distinct but overlapping regions (Yu et al. 1988; Amano et al. 1991; Sherwood et al. 1993; Lescheid et al. 1997; White et al. 1998). GnRH II is a widely expressed variant present in every vertebrate species examined except jawless fish (Miyamoto et al. 1984; Millar 2003). Originally named chicken GnRH II after the first species from which it was isolated, GnRH II has been reported in the brain of a wide range species including marsupials (King et al. 1989, 1990), mammals (Dellovade et al. 1993; Kasten et al. 1996; Chen et al. 1998; Montaner et al. 2002; Millar 2003), and primates, including humans (Lescheid et al. 1997; White et al. 1998; Urbanski et al. 1999; Latimer et al. 2000). The presence of GnRH II across vertebrate species suggests that GnRH II is evolutionarily conserved and likely to have an important biological function.
The exact biological role of GnRH II has remained elusive, however. Since the peptide was first discovered in the brain, it was hypothesized that its function would be similar to GnRH I and it would play a key regulatory role in gonadotropin release from the pituitary. Initial studies in chickens seemed to confirm this with the finding that GnRH II stimulates luteinizing hormone (LH) release both in vitro and in vivo (Millar et al. 1986; Nakamura et al. 1991). This observation was repeated in a variety of mammalian species, however, GnRH II's potency to stimulate LH release is much lower than GnRH I (∼2% as effective) (Millar and King 1983; Millar et al. 1986). Similarly, additional studies found that GnRH II infusions stimulate ovulation but with a lower potency than GnRH I (∼10% as effective) (Rissman et al. 1995). Because exogenous GnRH II is only effective at stimulating LH release and ovulation at high doses, these effects may be contributed to the peptide acting through type-1 GnRH receptors (that it binds with low affinity) rather than activation of GnRH I-independent pathways. This has been supported by studies in musk shrews, sheep, and primates. Administration of type-1 GnRH receptor antagonists will completely block GnRH II's ability to stimulate the pituitary despite the presence of type-2 receptors on gonadotropes (Densmore and Urbanski 2003; Gault et al. 2003; Okada et al. 2003). Additionally, administration of a type-1 antagonist will block GnRH II's ability to stimulate ovulation in musk shrews (Kauffman et al. 2005). Thus, GnRH II most likely has a minimal role, if any, in the normal secretion of LH and stimulation of ovulation.
Likewise, data suggest that GnRH II's role, if any, in the stimulation of follicle stimulating hormone (FSH) is minimal. Initial studies suggested that GnRH II may have a modest preferential ability to induce FSH release (Millar 1986; Yu 1990; Millar et al. 2001). However, more recent studies have found that GnRH II has no significant ability to release LH or FSH, except at very high doses, and no selective releasing activity of FSH (Yu et al. 1990, 1997; Densmore and Urbanski 2003; Gault et al. 2003; Okada et al. 2003). Neuroanatomical data also fail to support a major role for GnRH II in stimulating gonadotropin release. In mammals, the majority of GnRH II cell bodies reside in the midbrain, with few cells present in the hypothalamic and forebrain regions (Dellovade et al. 1993; Kasten et al. 1996; White et al. 1998). GnRH II fibers are present only scarcely in the hypothalamic/pituitary regions known to regulate gonadotropin secretion (Rissman et al. 1995). Collectively, the data indicate that the primary function of GnRH II is distinct from GnRH I and does not involve stimulation of gonadotropin hormone release.
GnRH II's function more likely involves coordination of reproduction. A functional role for mediating sex behavior has been implicated in both birds and mammals (Maney et al. 1997; Kauffman 2004; Bentley et al. 2006). In house sparrows and Japanese quail, both GnRH I and GnRH II exhibit seasonal changes in association with other changes in reproductive physiology (Hahn and Ball 1995; Teruyama and Beck 2000; Stevenson and MacDougall-Shackleton 2005). Housing females with courting males significantly decreases immunoreactive GnRH II fibers in regions of the brain associated with reproductive behavior (Stevenson et al. 2008). There is a growing body of evidence that suggests GnRH II functions to coordinate energy availability and reproduction. Using data primarily derived from studies using the musk shrew, a unique role for GnRH II as a permissive regulator of female reproductive behavior based on energy status as well as an inhibitor of short-term food intake has been elucidated.
Background on ecology and reproduction in musk shrews
Musk shrews (Suncus murinus) are a semi-tropical species with a very wide distribution in Asia, including India, Sri Lanka, Nepal, Thailand, Cambodia, Vietnam, Indonesia, and Japan (Yamagata et al. 1995; Kurachi et al. 2007a). Within this large range they vary in size, from adult males weighing 33.3 g in the Philippines to 118.6 g on average in Bangladesh, in coloration of the fur from light to very dark gray and even in chromosome number, between 30 and 40. Musk shrews are excellent colonizers of new habitats and have as high a degree of genetic variability as wild mouse (Mus musculus) populations (Kurachi et al. 2007b). Work on variance in mitochondrial mDNA and variation in blood protein in wild trapped members of the species shows 53 mtDNA haplotypes, 14 genetically distinct populations, and 2–3 major geographic groups of populations; South Asia, Southeast Asia and Malay (Malaysian) (Kurachi et al. 2007a, 2007b). Unfortunately, genetic data on the Indian musk shrews are lacking, but data from other parts of the range suggest that the species originated in India and migrated into southeastern Asia via Malaysia prior to the arrival of humans, but continuing to migrate along with humans after they arrived (Yamagata et al. 1995; Macaulay et al. 2005). The population in our laboratory came from the island of Guam where it was introduced, likely via US Navy ships in the early 1950's, from the Philippines (Hasler et al. 1977).
Reproduction in this species in equatorial regions is not seasonal and pregnant females have been trapped year round (Louch et al. 1966; Barbehenn 1962). Small peaks in pregnancy rates are correlated the hatching of insects in some parts of the range (Advani and Rana 1981). Not surprisingly, given their semi-tropical distribution musk shrews have very mild responses to photoperiod (Rissman et al. 1990). Males kept from the time of weaning for at least 20 days in short day lengths (10L:14D) have reduced testes weight compared with males in long days (14L:10D or 18L:6D). However, the antigonadal effect of short day lengths on male maturation can be overridden by simultaneous exposure to a female (Wayne and Rissman 1990). Interestingly, males reared in contact with females not only mature faster, but also they eat more and when food intake is regulated and ad libitum eating is eliminated, sexual maturation is delayed (Wayne and Rissman 1991; Wayne et al. 1991). In the laboratory, females are capable of reproduction soon after weaning and are able to mate and have offspring as early as 25 days of age. Food restriction delays puberty in females, but the interaction between food and exposure to a potential mate has yet to be examined (Gill and Rissman 1997).
Puberty in females is not spontaneous but is induced by mating, which in turn induces ovulation (Clendenon and Rissman 1990). Unlike other species with induced ovulation, musk shrews have very low circulating levels of estradiol when they begin to mate; the ovaries are immature at this point and contain only small primary and secondary follicles (Fortune et al. 1992). Moreover, multiple mating bouts that include ejaculations, over the course of at least 3 days are required to facilitate ovulation (Clendenon and Rissman 1990; Tai et al. 1997). This observation initiated our studies on the stimulatory effects of mating on GnRH I activity. Both the number of GnRH I immunoreactive (ir) neurons and the amount of peptide (assayed by radioimmunoassay) are elevated after mating, cell numbers increased rapidly (within minutes) and peptide takes at least a day to accumulate (Dellovade et al. 1995a, 1995b). During the original studies of the shrew GnRH system, we found an anatomically distinct group of cells in the midbrain that subsequently were identified as containing GnRH II (Dellovade et al. 1993).
GnRH II and the type-2 receptor
GnRH II has been identified in vertebrates ranging from mammals to fishes (Millar 2005). At the time that we identified GnRH II in musk shrew brains the only other mammals known to have the peptide were marsupials (King et al. 1990). In the few years that followed, GnRH II was discovered in other shrews, as well as in moles, rodents, sheep, and primates (King et al. 1994; Quanbeck et al. 1997; White et al. 1998; Chen et al. 1998; Morgan et al. 2006). In most cases the location of the cell bodies that make GnRH II is in the midbrain; however, in a few species cells are detected outside this region (Dellovade et al. 1993; Lescheid et al. 1997; Chen et al. 1998). Work on the ontogeny of the GnRH II cells also demonstrates that they do not migrate into the brain alongside the GnRH I cells but arise from the ventricle (Parhar 2002). In addition, the projections of the GnRH II cells are unique in that the major terminal field is the medial habenula (mHB) (Rissman et al. 1995) with fibers present in a number of regions in the hypothalamus, preoptic area (POA), olfactory bulb, hippocampus (Hip), midbrain central gray, and the spinal cord (Yamamoto et al. 1995; Stevenson et al. 2007).
Along with multiple forms of the GnRH peptide, three distinct GnRH receptors have been identified in mammals (Reinhart et al. 1992; Tsutsumi et al. 1992; King et al. 2000; Millar et al. 2001; Neill et al. 2001; Wang et al. 2001). The type-2 GnRH receptor is a G-protein-coupled receptor and shares 40% sequence homology with the type-1 GnRH receptor (Millar et al. 2001). While the two GnRH peptides can bind either receptor, GnRH I binds type-1 receptors with higher affinity than GnRH II, whereas GnRH II has a higher affinity for type-2 receptors (Millar et al. 2001; Neill 2002; Millar 2003; Morgan and Millar 2004). Molecular analysis has shown that the human type-2 GnRH receptor contains a stop codon and is thought to be inactive (Faurholm et al. 2001; Neill 2002). This is odd in light of the wide distribution of GnRH II mRNA in many human tissues including the brain, prostate, ovary, kidney, and bone marrow (White et al. 1998; Choi et al. 2001; Kang et al. 2001). The high affinity of GnRH II for the type-1 GnRH receptor suggests that, despite the type-2 GnRH receptor in humans being nonfunctional, GnRH II could act through the type-1 GnRH receptor (Millar 2003). Through differential stabilization of the type-1 GnRH receptor by different ligands distinct signaling pathways can be activated. This phenomenon, called ligand-induced signal selectivity, predicts that both agonists and classical antagonists selectively signal through the same receptor. This has been observed in the type-1 GnRH receptor; GnRH I activates while GnRH II inhibits Src at the type-1 GnRH receptor (Millar et al. 2004). Furthermore, the possibility that a partial type-2 GnRH receptor mediates GnRH II signaling or that posttranscriptional editing creates a functional receptor by editing out a stop codon could explain GnRH II's actions in humans (Millar 2003). Regardless of the mechanism, the conservation of GnRH II structure and its wide tissue distribution suggests this decapeptide serves an important physiological function. However, the role of GnRH II in humans remains undetermined.
Role for GnRH II in mating behavior
The first paper to show an effect of GnRH II on receptivity was conducted in female sparrows given central GnRH II infusions, which enhanced their receptivity responses to male song (Maney et al. 1997). Shortly thereafter, work in our laboratory turned to this question. Female musk shrews go from being completely unreceptive and actively preventing the males from approaching them to cooperating with them and soliciting copulations within a matter of minutes (Rissman et al. 1997). The fact that the transition is so fast suggested a neurotransmitter or neuropeptide. Adult females were cannulated and substances delivered into the lateral ventricles. Neither GnRH peptide (I or II) enhanced receptivity in female shrews (Schiml and Rissman 2000). Because receptivity is difficult to suppress, and thus enhancements are difficult to measure, we selected another approach and employed a food restriction paradigm that we had found previously would reduce the incidence of receptivity (Gill and Rissman 1997). Food intake was monitored for at least 4 days and each female received only 60% of her preferred baseline intake, next a subset of the animals was given food ad libitum to determine how much time was required to shift them from being unreceptive versus receptive based on available energy (Temple and Rissman 2000). Females that were underfed for 2 days but received ad libitum access to food for just 90 min returned to the ad libitum levels of receptivity. Next females were cannulated to facilitate central infusions, and were either maintained on ad libitum food or restricted for 2 days to 60% of their ad libitum levels. Instead of using food to reinstate sexual behavior, we infused either GnRH I or II (or vehicle). Food restriction did not inhibit sexual behavior in GnRH II-infused females but did so in all other groups (Temple et al. 2003). Recently, this enhancement effect of GnRH II has also been demonstrated in marmoset monkeys (Barnett et al. 2006).
Most effects of GnRH II are mediated by the type-2 GnRH receptor in shrews (Kauffman et al. 2005) and nonhuman primates (Millar et al. 2001; Neill et al. 2001). In the experiments above administration of Antide, a type-1 GnRH receptor antagonist had no effect on GnRH II stimulation of receptivity or food intake. Furthermore, administration of a peptide that is both a type-2 GnRH receptor agonist and a type-1 GnRH receptor antagonist (analog 135–18) stimulated sexual behavior in the female musk shrew (Kauffman et al. 2005), suggesting the effect is mediated specifically by the type-2 GnRH receptor.
GnRH II and nutrition
Of interest is that GnRH II's stimulatory effects on reproductive behavior are only observed after food restriction. This led to our investigations into the effects of alterations in energy balance on the GnRH II system. In food-restricted female musk shrews, the amount of GnRH II mRNA in its cell bodies and the protein concentrations in several target regions are significantly decreased. The targets, including the mHB, ventromedial nucleus (VMN) midbrain GnRH II cells and periaqueductal gray (PAG), had significantly decreased levels of GnRH II peptide compared with controls that were fed ad libitum (Kauffman et al. 2006). Refeeding for 90 min restored mating behavior and GnRH II concentrations to levels similar to ad libitum conditions in the VMN and mHB, but not in the midbrain or PAG (Fig. 1). These findings indicate that alterations in energy balance can regulate both the transcription of the GnRH II gene and the translation and/or protein processing of the GnRH II peptide. Male shrews also express abundant GnRH II peptide in many brain areas. Some of these regions overlap with expression patterns in females while others are unique to the male. This sexually dimorphic expression pattern may be why, despite abundant expression of GnRH II peptide, mild food restriction does not seem to affect peptide levels in the male. Since males are 50% larger than females, they may only respond to more severe reduction of food. Together, these results suggest a critical relationship between GnRH II regulation and energy balance.
Fig. 1.
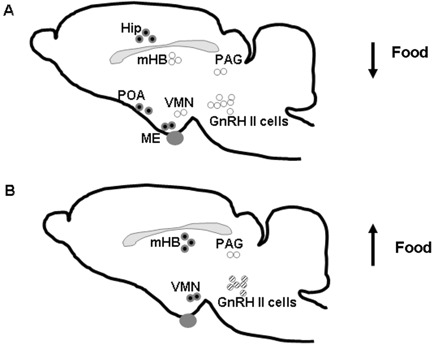
Sagittal view of a shrew brain showing the sites where restriction of food decreases GnRH II (A) and acute (90 min) of refeeding increases GnRH II (B). GnRH II peptide is present in many regions including: the Hip, median eminence (ME), POA, mHB, VMN, PAG, and GnRH II containing cells (GnRH II cells). Regions with open circles (in A) display a drop in GnRH II peptide after underfeeding. Refeeding (B) increased GnRH II levels in the mHB and VMN (as designated by filled circles). The cell bodies producing GnRH II have elevated mRNA levels, but low GnRH II peptide levels after 90 min of refeeding (designated by striped circles in B). Because mating behavior is reinstated by 90 min of refeeding, we suggest that the VMN and mHB are regions involved in regulation of sexual behavior in these species.
In times of decreased availability of energy, and lower expression of GnRH II, females will typically inhibit reproductive behaviors and increase feeding behaviors. Conversely, in times of sufficient availability of energy and increased expression of GnRH II, females will engage in reproductive behaviors. Thus, it has been hypothesized that GnRH II is a neurotransmitter that is permissive to mating when energy is highly available and inhibitory to mating when availability of energy is low (Kauffman 2004). However, the effects of GnRH II on food intake, specifically whether GnRH II decreases food intake, and whether its effects are dependent on energetic status, have only recently been determined. Intracerebroventricular infusions of GnRH II in underfed female shrews resulted in a significant reduction in food intake. Unlike GnRH II's effects on sexual behavior, which are only observed in food-restricted animals, GnRH II's effects on feeding behavior are observed in females fed ad libitum (Kauffman and Rissman 2004). Under both conditions, decreased food intake was acute—the effects of infusing GnRH II were observed within 90 min and persisted for ∼3 h. These findings suggest metabolic and caloric cues associated with energy balance may regulate feeding behavior of females by modifying GnRH II mRNA production and protein release. Thus, GnRH II inhibits feeding and stimulates reproductive behavior, suggesting a role in integrating energetic status and reproductive behavior in response to fluctuating changes in environmental availability of energy. As noted earlier, the effects of GnRH II on intake of food were not blocked by preadministration of Antide (Kauffman et al. 2005). The GnRH II analog, 135-18, likewise mimicked the actions of GnRH II on feeding (Kauffman et al. 2005). Recently GnRH II has been shown to have a highly selective effect on suppressing food intake in male and female goldfish, a species that does not possess the type 2 receptor. Importantly, these data show that the effects of the peptide on food intake generalize to other vertebrates and to both sexes (Matsuda et al. submitted for publication).
Future research directions
Maintaining energy balance is critical to the fitness of any individual. Ideally, energy balance should be well regulated with total energy expenditure equal to, or less than, dietary intake. Dysregulation of feeding behaviors can lead to serious conditions, including eating disorders and obesity. Given GnRH II's dramatic regulation of short-term intake of food, independent of energetic status, we are interested in expanding these studies to assess the role of GnRH II in the regulation of binge-type eating disorders.
Binge eating disorder (BED) is characterized by repeated, intermittent ingestion of very large amounts of high fat, and high calorie food in a brief period of time with a sense of loss of control over eating that is not accompanied by any compensatory mechanisms (i.e., fasting, excessive exercise, or purging) (American Psychiatric Association 1994; Yanovski 1993). The absence of inappropriate compensatory mechanisms is what makes BED distinct from other purge-type eating disorders, such as bulimia nervosa. Eating episodes, or binges, occur at least 2 days a week over a period of at least 6 months (American Psychiatric Association 1994). The musk shrew is a convenient animal model for the study of energy balance and hormonal regulation of eating disorders. The expression of GnRH II and type-2 GnRH receptors in the shrew facilitates examination of the decapeptide's role in binge eating. Additionally, musk shrews, unlike mice and rats, are capable of emesis (Andrews et al. 1996, 2000; Ueno et al. 1987). While distinct disorders, BED and bulimia nervosa share some common characteristics and thus an animal model of both disorders in the same species could provide useful insights into the neural mechanisms underlying the behaviors.
Studies in mice and rats have not been conducted to determine what role, if any, GnRH II has on energy balance whereas the effect of GnRH II on energy balance in shrews is more fully characterized. Although a functional human type-2 GnRH receptor has not yet been identified, the study of GnRH II function can still provide exciting insight into novel physiological roles in humans. The GnRH II peptide is able to bind to the type-1 receptor and signal in a manner distinct from GnRH I (Maudsley et al. 2004; Millar et al. 2004). Thus, despite the presumed silenced receptor, GnRH II may still have an important function in energy regulation in humans and studies in the musk shrew may provide valuable insight.
Acknowledgments
The research reported here is supported by National Institutes of Health grant R01MH068729. J.S.S. was supported by National Institutes of Health grant T32 HD07382.
References
- Advani R, Rana B. Food of the house shrew, Suncus murinus sindensis, in the Indian desert. Acta Theriologica. 1981;27:133–134. [Google Scholar]
- Amano M, Oka Y, Aida K, Okumoto N, Kawashima S, Hasegawa Y. Immunocytochemical demonstration of salmon GnRH and chicken GnRH-II in the brain of masu salmon, Oncorhynchus masou. J Comp Neurol. 1991;314:587–597. doi: 10.1002/cne.903140313. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagonistic and statistical manual of mental disorders (DSM-IV), 4th edition. Washington (DC): American Psychiatric Press, Inc; 1994. [Google Scholar]
- Andrews P, Dovey E, Hockaday J, Hoyle CH, Woods AJ, Matsuki N. The development of the emetic reflex in the house musk shrew, Suncus murinus. Brain Res Dev Brain Res. 2000;121:29–34. doi: 10.1016/s0165-3806(00)00022-5. [DOI] [PubMed] [Google Scholar]
- Andrews P, Torii Y, Saito H, Matsuki N. The pharmacology of the emetic response to upper gastrointestinal tract stimulation in Suncus murinus. Eur J Pharmacol. 1996;307:305–313. doi: 10.1016/0014-2999(96)00275-0. [DOI] [PubMed] [Google Scholar]
- Barbehenn K. The house shrew on Guam. In: Bernice P, editor. Pacific island rat ecology. Honolulu: Bishop Museum Bulletin; 1962. pp. 248–256. [Google Scholar]
- Barnett DK, Bunnell TM, Millar RP, Abbott DH. Gonadotropin-releasing hormone II stimulates female sexual behavior in marmoset monkeys. Endocrinology. 2006;147:615–623. doi: 10.1210/en.2005-0662. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Jensen JP, Kaur GJ, Wacker DW, Tsutsui K, Wingfield JC. Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH) Horm Behav. 2006;49:550–555. doi: 10.1016/j.yhbeh.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Burgus R, Butcher M, Amoss M, Ling N, Monahan M, Rivier J, Fellows R, Blackwell R, Vale W, Guillemin R. Primary structure of the ovine hypothalamic luteinizing hormone-releasing factor (LRF) (LH-hypothalamus-LRF-gas chromatography-mass spectrometry-decapeptide-Edman degradation) Proc Natl Acad Sci USA. 1972;69:278–282. doi: 10.1073/pnas.69.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Yahalom D, Ben-Aroya N, Kaganovsky E, Okon E, Koch Y. A second isoform of gonadotropin-releasing hormone is present in the brain of human and rodents. FEBS Lett. 1998;435:199–203. doi: 10.1016/s0014-5793(98)01064-3. [DOI] [PubMed] [Google Scholar]
- Choi KC, Auersperg N, Leung PC. Expression and antiproliferative effect of a second form of gonadotropin-releasing hormone in normal and neoplastic ovarian surface epithelial cells. J Clin Endocrinol Metab. 2001;86:5075–5078. doi: 10.1210/jcem.86.10.8100. [DOI] [PubMed] [Google Scholar]
- Clendenon AL, Rissman EF. Prolonged copulatory behavior facilitates pregnancy success in the musk shrew. Physiol Behav. 1990;47:831–835. doi: 10.1016/0031-9384(90)90005-o. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Hunter E, Rissman EF. Interactions with males promote rapid changes in gonadotropin-releasing hormone immunoreactive cells. Neuroendocrinology. 1995;62:385–395. doi: 10.1159/000127028. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, King JA, Millar RP, Rissman EF. Presence and differential distribution of distinct forms of immunoreactive gonadotropin-releasing hormone in the musk shrew brain. Neuroendocrinology. 1993;58:166–177. doi: 10.1159/000126529. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Ottinger MA, Rissman EF. Mating alters gonadotropin-releasing hormone cell number and content. Endocrinology. 1995;136:1648–1657. doi: 10.1210/endo.136.4.7895675. [DOI] [PubMed] [Google Scholar]
- Densmore VS, Urbanski HF. Relative effect of gonadotropin-releasing hormone (GnRH)-I and GnRH-II on gonadotropin release. J Clin Endocrinol Metab. 2003;88:2126–2134. doi: 10.1210/jc.2002-021359. [DOI] [PubMed] [Google Scholar]
- Faurholm B, Millar RP, Katz AA. The genes encoding the type II gonadotropin-releasing hormone receptor and the ribonucleoprotein RBM8A in humans overlap in two genomic loci. Genomics. 2001;78:15–18. doi: 10.1006/geno.2001.6650. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Eppig JJ, Rissman EF. Mating stimulates estradiol production by ovaries of the musk shrew (Suncus murinus) Biol Reprod. 1992;46:885–891. doi: 10.1095/biolreprod46.5.885. [DOI] [PubMed] [Google Scholar]
- Gault PM, Maudsley S, Lincoln GA. Evidence that gonadotropin-releasing hormone II is not a physiological regulator of gonadotropin secretion in mammals. J Neuroendocrinol. 2003;15:831–839. doi: 10.1046/j.1365-2826.2003.01065.x. [DOI] [PubMed] [Google Scholar]
- Gill CJ, Rissman EF. Female sexual behavior is inhibited by short- and long-term food restriction. Physiol Behav. 1997;61:387–394. doi: 10.1016/s0031-9384(96)00449-0. [DOI] [PubMed] [Google Scholar]
- Hahn TP, Ball GF. Changes in brain GnRH associated with photorefractoriness in house sparrows (Passer domesticus) Gen Comp Endocrinol. 1995;99:349–363. doi: 10.1006/gcen.1995.1119. [DOI] [PubMed] [Google Scholar]
- Hasler MJ, Hasler JF, Nalbandov AV. Comparative breeding biology of musk shrews (Suncus murinus) from Guam and Madagascar. J Mammal. 1977;58:285–290. [PubMed] [Google Scholar]
- Kang SK, Tai CJ, Nathwani PS, Leung PC. Differential regulation of two forms of gonadotropin-releasing hormone messenger ribonucleic acid in human granulosa-luteal cells. Endocrinology. 2001;142:182–192. doi: 10.1210/endo.142.1.7895. [DOI] [PubMed] [Google Scholar]
- Kasten TL, White SA, Norton TT, Bond CT, Adelman JP, Fernald RD. Characterization of two new preproGnRH mRNAs in the tree shrew: first direct evidence for mesencephalic GnRH gene expression in a placental mammal. Gen Comp Endocrinol. 1996;104:7–19. doi: 10.1006/gcen.1996.0135. [DOI] [PubMed] [Google Scholar]
- Kauffman AS. Emerging functions of gonadotropin-releasing hormone II in mammalian physiology and behaviour. J Neuroendocrinol. 2004;16:794–806. doi: 10.1111/j.1365-2826.2004.01229.x. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Bojkowska K, Wills A, Rissman EF. Gonadotropin-releasing hormone-II messenger ribonucleic acid and protein content in the mammalian brain are modulated by food intake. Endocrinology. 2006;147:5069–5077. doi: 10.1210/en.2006-0615. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Rissman EF. The evolutionarily conserved gonadotropin-releasing hormone II modifies food intake. Endocrinology. 2004;145:686–691. doi: 10.1210/en.2003-1150. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Wills A, Millar RP, Rissman EF. Evidence that the type-2 gonadotrophin-releasing hormone (GnRH) receptor mediates the behavioural effects of GnRH-II on feeding and reproduction in musk shrews. J Neuroendocrinol. 2005;17:489–497. doi: 10.1111/j.1365-2826.2005.01334.x. [DOI] [PubMed] [Google Scholar]
- King JA, Fidler A, Lawrence S, Adam T, Millar RP, Katz A. Cloning and expression, pharmacological characterization, and internalization kinetics of the pituitary GnRH receptor in a metatherian species of mammal. Gen Comp Endocrinol. 2000;117:439–448. doi: 10.1006/gcen.1999.7418. [DOI] [PubMed] [Google Scholar]
- King JA, Hinds LA, Mehl AE, Saunders NR, Millar RP. Chicken GnRH II occurs together with mammalian GnRH in a South American species of marsupial (Monodelphis domestica) Peptides. 1990;11:521–525. doi: 10.1016/0196-9781(90)90053-8. [DOI] [PubMed] [Google Scholar]
- King JA, Mehl AE, Tyndale-Biscoe CH, Hinds L, Millar RP. A second form of gonadotropin-releasing hormone (GnRH), with chicken GnRH II-like properties, occurs together with mammalian GnRH in marsupial brains. Endocrinology. 1989;125:2244–2252. doi: 10.1210/endo-125-5-2244. [DOI] [PubMed] [Google Scholar]
- King JA, Steneveld AA, Curlewis JD, Rissman EF, Millar RP. Identification of chicken GnRH II in brains of metatherian and early-evolved eutherian species of mammals. Regul Pept. 1994;54:467–477. doi: 10.1016/0167-0115(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Kurachi M, Chau BL, Dang VB, Dorji T, Yamamoto Y, Nyunt MM, Maeda Y, Chhum-Phith L, Namikawa T, Yamagata T. Population structure of wild musk shrews (Suncus murinus) in Asia based on mitochondrial DNA variation, with research in Cambodia and Bhutan. Biochem Genet. 2007;45:165–183. doi: 10.1007/s10528-006-9051-0. [DOI] [PubMed] [Google Scholar]
- Kurachi M, et al. Phylogeography of wild musk shrew (Suncus murinus) populations in Asia based on blood protein/enzyme variation. Biochem Genet. 2007;45:543–563. doi: 10.1007/s10528-007-9096-8. [DOI] [PubMed] [Google Scholar]
- Latimer VS, Rodrigues SM, Garyfallou VT, Kohama SG, White RB, Fernald RD, Urbanski HF. Two molecular forms of gonadotropin-releasing hormone (GnRH-I and GnRH-II) are expressed by two separate populations of cells in the rhesus macaque hypothalamus. Brain Res Mol Brain Res. 2000;75:287–292. doi: 10.1016/s0169-328x(99)00316-2. [DOI] [PubMed] [Google Scholar]
- Lescheid DW, Terasawa E, Abler LA, Urbanski HF, Warby CM, Millar RP, Sherwood NM. A second form of gonadotropin-releasing hormone (GnRH) with characteristics of chicken GnRH-II is present in the primate brain. Endocrinology. 1997;138:5618–5629. doi: 10.1210/endo.138.12.5592. [DOI] [PubMed] [Google Scholar]
- Louch C, Ghosh A, Pal B. Seasonal changes in weight and reproductive activity of Suncus murinus in West Bengal, India. J Mammal. 1966;47:73–78. [PubMed] [Google Scholar]
- Macaulay V, et al. Single, rapid coastal settlement of Asia revealed by analysis of complete mitochondrial genomes. Science. 2005;308:1034–1036. doi: 10.1126/science.1109792. [DOI] [PubMed] [Google Scholar]
- Maney DL, Richardson RD, Wingfield JC. Central administration of chicken gonadotropin-releasing hormone-II enhances courtship behavior in a female sparrow. Horm Behav. 1997;32:11–18. doi: 10.1006/hbeh.1997.1399. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Nakamure K, Shimakura S-I, Miura T, Kageyama H, Uchiyama M, Shioda S, Ando H. Inhibitory effect of chicken gonadotropin-releasing hormone II on food intake in the goldfish, Carassius auratus. Horm Beh. doi: 10.1016/j.yhbeh.2008.01.011. submitted for publication. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV. Structure of the porcine LH- and FSH-releasing hormone-I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43:1334–1339. doi: 10.1016/s0006-291x(71)80019-0. [DOI] [PubMed] [Google Scholar]
- Maudsley S, Davidson L, Pawson AJ, Chan R, Lopez de MR, Millar RP. Gonadotropin-releasing hormone (GnRH) antagonists promote proapoptotic signaling in peripheral reproductive tumor cells by activating a Galphai-coupling state of the type I GnRH receptor. Cancer Res. 2004;64:7533–7544. doi: 10.1158/0008-5472.CAN-04-1360. [DOI] [PubMed] [Google Scholar]
- Millar RP. GnRH II and type II GnRH receptors. Trends Endocrinol Metab. 2003;14:35–43. doi: 10.1016/s1043-2760(02)00016-4. [DOI] [PubMed] [Google Scholar]
- Millar RP. GnRHs and GnRH receptors. Anim Reprod Sci. 2005;88:5–28. doi: 10.1016/j.anireprosci.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Millar RP, King JA. Synthesis, luteinizing hormone-releasing activity, and receptor binding of chicken hypothalamic luteinizing hormone-releasing hormone. Endocrinology. 1983;113:1364–1369. doi: 10.1210/endo-113-4-1364. [DOI] [PubMed] [Google Scholar]
- Millar R, et al. A novel mammalian receptor for the evolutionarily conserved type II GnRH. Proc Natl Acad Sci USA. 2001;98:9636–9641. doi: 10.1073/pnas.141048498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- Millar RP, Milton RC, Follett BK, King JA. Receptor binding and gonadotropin-releasing activity of a novel chicken gonadotropin-releasing hormone ([His5, Trp7, Tyr8]GnRH) and a D-Arg6 analog. Endocrinology. 1986;119:224–231. doi: 10.1210/endo-119-1-224. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Hasegawa Y, Nomura M, Igarashi M, Kangawa K, Matsuo H. Identification of the second gonadotropin-releasing hormone in chicken hypothalamus: evidence that gonadotropin secretion is probably controlled by two distince gonadotropin-releasing hormones in avian species. Proc Natl Acad Sci USA. 1984;81:3874–3878. doi: 10.1073/pnas.81.12.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner AD, Mongiat L, Lux-Lantos VA, Warby C, Chewpoy B, Bianchi MS, Libertun C, Rivier JE, Sherwood NM, Somoza GM. Guinea pig gonadotropin-releasing hormone: expression pattern, characterization and biological activity in rodents. Neuroendocrinology. 2002;75:326–338. doi: 10.1159/000057342. [DOI] [PubMed] [Google Scholar]
- Morgan K, Millar RP. Evolution of GnRH ligand precursors and GnRH receptors in protochordate and vertebrate species. Gen Comp Endocrinol. 2004;139:191–197. doi: 10.1016/j.ygcen.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Morgan K, Sellar R, Pawson AJ, Lu ZL, Millar RP. Bovine and ovine gonadotropin-releasing hormone (GnRH)-II ligand precursors and type II GnRH receptor genes are functionally inactivated. Endocrinology. 2006;147:5041–5051. doi: 10.1210/en.2006-0222. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nagata T, Tanabe Y, Yanaihara N, Hasegawa Y. Comparison of in vivo biological activities of luteinizing hormone releasing hormone (LHRH) analogues in 60-day-old cockerels. Gen Comp Endocrinol. 1991;83:290–296. doi: 10.1016/0016-6480(91)90033-3. [DOI] [PubMed] [Google Scholar]
- Neill JD. GnRH and GnRH receptor genes in the human genome. Endocrinology. 2002;143:737–743. doi: 10.1210/endo.143.3.8705. [DOI] [PubMed] [Google Scholar]
- Neill JD, Duck LW, Sellers JC, Musgrove LC. A gonadotropin-releasing hormone (GnRH) receptor specific for GnRH II in primates. Biochem Biophys Res Commun. 2001;282:1012–1018. doi: 10.1006/bbrc.2001.4678. [DOI] [PubMed] [Google Scholar]
- Okada Y, Murota-Kawano A, Kakar SS, Winters SJ. Evidence that gonadotropin-releasing hormone (GnRH) II stimulates luteinizing hormone and follicle-stimulating hormone secretion from monkey pituitary cultures by activating the GnRH I receptor. Biol Reprod. 2003;69:1356–1361. doi: 10.1095/biolreprod.103.016162. [DOI] [PubMed] [Google Scholar]
- Parhar IS. Cell migration and evolutionary significance of GnRH subtypes. Prog Brain Res. 2002;141:3–17. doi: 10.1016/S0079-6123(02)41080-1. [DOI] [PubMed] [Google Scholar]
- Quanbeck C, Sherwood NM, Millar RP, Terasawa E. Two populations of luteinizing hormone-releasing hormone neurons in the forebrain of the rhesus macaque during embryonic development. J Comp Neurol. 1997;380:293–309. [PubMed] [Google Scholar]
- Reinhart J, Mertz LM, Catt KJ. Molecular cloning and expression of cDNA encoding the murine gonadotropin-releasing hormone receptor. J Biol Chem. 1992;267:21281–21284. [PubMed] [Google Scholar]
- Rissman EF, Alones VE, Craig-Veit CB, Millam JR. Distribution of chicken-II gonadotropin-releasing hormone in mammalian brain. J Comp Neurol. 1995;357:524–531. doi: 10.1002/cne.903570404. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Li X, King JA, Millar RP. Behavioral regulation of gonadotropin-releasing hormone production. Brain Res Bull. 1997;44:459–464. doi: 10.1016/s0361-9230(97)00226-8. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Taymans SE, Wayne NL. Social cues influence growth and sexual maturation of the male musk shrew (Suncus murinus) J Reprod Fertil. 1990;89:697–706. doi: 10.1530/jrf.0.0890697. [DOI] [PubMed] [Google Scholar]
- Schiml PA, Rissman EF. Effects of gonadotropin-releasing hormones, corticotropin-releasing hormone, and vasopressin on female sexual behavior. Horm Behav. 2000;37:212–220. doi: 10.1006/hbeh.2000.1575. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Grier HJ, Warby C, Peute J, Taylor RG. Gonadotropin-releasing hormones, including a novel form, in snook Centropomus undecimalis, in comparison with forms in black sea bass Centropristis striata. Regul Pept. 1993;46:523–534. doi: 10.1016/0167-0115(93)90253-5. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Arckens L, MacDougall-Shackleton SA. Distribution of gonadotropin releasing-hormone-II in the house sparrow brain (Passer domesticus) Gen Comp Endocrinol. 2007;150:96–105. doi: 10.1016/j.ygcen.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Bentley GE, Ubuka T, Arckens L, Hampson E, MacDougall-Shackleton SA. Effects of social cues on GnRH-I, GnRH-II, and reproductive physiology in female house sparrows (Passer domesticus) Gen Comp Endocrinol. 2008;156:385–394. doi: 10.1016/j.ygcen.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, MacDougall-Shackleton SA. Season- and age-related variation in neural cGnRH-I and cGnRH-II immunoreactivity in house sparrows (Passer domesticus) Gen Comp Endocrinol. 2005;143:33–39. doi: 10.1016/j.ygcen.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Tai VC, Schiml PA, Li X, Rissman EF. Behavioral interactions have rapid effects on immunoreactivity of prohormone and gonadotropin-releasing hormone peptide. Brain Res. 1997;772:87–94. doi: 10.1016/s0006-8993(97)00878-0. [DOI] [PubMed] [Google Scholar]
- Temple JL, Millar RP, Rissman EF. An evolutionarily conserved form of gonadotropin-releasing hormone coordinates energy and reproductive behavior. Endocrinology. 2003;144:13–19. doi: 10.1210/en.2002-220883. [DOI] [PubMed] [Google Scholar]
- Temple JL, Rissman EF. Acute re-feeding reverses food restriction-induced hypothalamic-pituitary-gonadal axis deficits. Biol Reprod. 2000;63:1721–1726. doi: 10.1095/biolreprod63.6.1721. [DOI] [PubMed] [Google Scholar]
- Teruyama R, Beck MM. Changes in immunoreactivity to anti-cGnRH-I and -II are associated with photostimulated sexual status in male quail. Cell Tissue Res. 2000;300:413–426. doi: 10.1007/s004410000218. [DOI] [PubMed] [Google Scholar]
- Tsutsumi M, Zhou W, Millar RP, Mellon PL, Roberts JL, Flanagan CA, Dong K, Gillo B, Sealfon SC. Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol. 1992;6:1163–1169. doi: 10.1210/mend.6.7.1324422. [DOI] [PubMed] [Google Scholar]
- Ueno S, Matsuki N, Saito H. Suncus murinus: a new experimental model in emesis research. Life Sci. 1987;41:513–518. doi: 10.1016/0024-3205(87)90229-3. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, White RB, Fernald RD, Kohama SG, Garyfallou VT, Densmore VS. Regional expression of mRNA encoding a second form of gonadotropin-releasing hormone in the macaque brain. Endocrinology. 1999;140:1945–1948. doi: 10.1210/endo.140.4.6779. [DOI] [PubMed] [Google Scholar]
- Wang L, et al. Three distinct types of GnRH receptor characterized in the bullfrog. Proc Natl Acad Sci USA. 2001;98:361–366. doi: 10.1073/pnas.011508498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne NL, Rissman EF. Effects of photoperiod and social variables on reproduction and growth in the male musk shrew (Suncus murinus) J Reprod Fertil. 1990;89:707–715. doi: 10.1530/jrf.0.0890707. [DOI] [PubMed] [Google Scholar]
- Wayne NL, Rissman EF. Tropical photoperiods affect reproductive development in the musk shrew, Suncus murinus. Physiol Behav. 1991;50:549–553. doi: 10.1016/0031-9384(91)90544-x. [DOI] [PubMed] [Google Scholar]
- Wayne NL, Wade GN, Rissman EF. Effects of food restriction and social cues on sexual maturation and growth in male musk shrews (Suncus murinus) J Reprod Fertil. 1991;91:385–392. doi: 10.1530/jrf.0.0910385. [DOI] [PubMed] [Google Scholar]
- White RB, Eisen JA, Kasten TL, Fernald RD. Second gene for gonadotropin-releasing hormone in humans. Proc Natl Acad Sci USA. 1998;95:305–309. doi: 10.1073/pnas.95.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T, Ohishi K, Faruque MO, Masangkay JS, Ba-Loc C, Vu-Binh D, Mansjoer SS, Ikeda H, Namikawa T. Genetic variation and geographic distribution on the mitochondrial DNA in local populations of the musk shrew, Suncus murinus. Jpn J Genet. 1995;70:321–337. doi: 10.1266/jjg.70.321. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Oka Y, Amano M, Aida K, Hasegawa Y, Kawashima S. Multiple gonadotropin-releasing hormone (GnRH)-immunoreactive systems in the brain of the dwarf gourami, Colisa lalia: immunohistochemistry and radioimmunoassay. J Comp Neurol. 1995;355:354–368. doi: 10.1002/cne.903550303. [DOI] [PubMed] [Google Scholar]
- Yanovski S. Binge eating disorder: current knowledge and future directions. Obes Res. 1993;1:306–324. doi: 10.1002/j.1550-8528.1993.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Yu KL, Sherwood NM, Peter RE. Differential distribution of two molecular forms of gonadotropin-releasing hormone in discrete brain areas of goldfish (Carassius auratus) Peptides. 1988;9:625–630. doi: 10.1016/0196-9781(88)90174-x. [DOI] [PubMed] [Google Scholar]
- Yu W, Millar R, Milton S, Milton RC, McCann S. Selective FSH-releasing activity of [D-Trp9]Gap1-13: comparison with gonadotropin-releasing abilities of analogs of GAP and natural LHRHs. Brain Res Bull. 1990;25:867–873. doi: 10.1016/0361-9230(90)90182-y. [DOI] [PubMed] [Google Scholar]
- Yu WH, Karanth S, Walczewska A, Sower SA, McCann SM. A hypothalamic follicle-stimulating hormone-releasing decapeptide in the rat. Proc Natl Acad Sci USA. 1997;94:9499–9503. doi: 10.1073/pnas.94.17.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]


