Abstract
OBJECTIVES: Prospective analysis of left ventricular (LV) morphological/functional parameters in patients with bicuspid versus tricuspid aortic valve (TAV) stenosis undergoing aortic valve replacement (AVR) surgery.
METHODS: A total of 190 consecutive patients with BAV (n = 154) and TAV stenosis (n = 36) (mean age 61 ± 8 years, 65% male) underwent AVR ± concomitant aortic surgery from January 2012 through May 2015. All patients underwent preoperative cardiac magnetic resonance imaging in order to evaluate: (i) left ventricular outflow tract (LVOT) dimensions, (ii) length of anterior mitral leaflet (AML), (iii) end-systolic and end-diastolic LV wall thickness, (iv) LV area, (v) LV end-systolic and end-diastolic diameters (LVESD, LVEDD), (vi) LV end-diastolic and end-systolic volumes (LVEDV, LVESV) and (vii) maximal diameter of aortic root. These parameters were compared between the two study groups.
RESULTS: The LVOT diameter was significantly larger in BAV patients (21.7 ± 3 mm in BAV vs 18.9 ± 3 mm in TAV, P < 0.001). Moreover, BAV patients had significantly longer AML (24 ± 3 mm in BAV vs 22 ± 4 mm in TAV, P = 0.009). LVEDV and LVESV were significantly larger in BAV patients (LVEDV: 164.9 ± 68.4 ml in BAV groups vs 126.5 ± 53.1 ml in TAV group, P = 0.037; LVESV: 82.1 ± 57.9 ml in BAV group vs 52.9 ± 25.7 ml in TAV group, P = 0.008). A strong linear correlation was found between LVOT diameter and aortic annulus diameter in BAV patients (r = 0.7, P < 0.001), whereas significantly weaker correlation was observed in TAV patients (r = 0.5, P = 0.006, z = 1.65, P = 0.04). Presence of BAV morphology was independently associated with larger LVOT diameters (OR 9.0, 95% CI 1.0–81.3, P = 0.04).
CONCLUSIONS: We found relevant differences in LV morphological/functional parameters between BAV and TAV stenosis patients. Further investigations are warranted in order to determine the cause of these observed differences.
Keywords: Bicuspid aortic valve, Cardiomyopathy, Aortic valve stenosis, Ascending aorta
INTRODUCTION
There is an ever-growing interest in bicuspid aortic valve (BAV) pathology and its association with the anatomical structures distal to the aortic valve (i.e. BAV-associated aortopathy) [1]. BAV accounts for about one-half of isolated aortic stenosis cases requiring surgical aortic valve replacement (AVR) [2]. Asymmetrical flow patterns and turbulence can be observed in the ascending aorta even in patients with an “echocardiographically normal” BAV [3]. Despite an increasing amount of research in this area, the clinical presentation of BAV disease is multifaceted and remains insufficiently defined.
One-half of BAV patients present with non-valvular manifestations of BAV disease [4]. Distal non-valvular manifestations may be summarized under the term “BAV-associated aortopathy” [5–7]. It begins early in life and involves a wide range of manifestations [8] which are a subject of intense clinical and basic research. On the contrary, there is a scarcity of data on proximal non-valvular manifestations of BAV disease. BAV-associated valvular cardiomyopathy is insufficiently explored and might be potentially different as compared with tricuspid aortic valve (TAV) disease. Moreover, to date no prospective study has been conducted in order to analyse and compare the preoperative left ventricular (LV) functional and anatomical attributes in patients with BAV vs TAV disease.
Cardiovascular magnetic resonance imaging (cMRI) is a non-invasive imaging tool that has the potential to simultaneously assess functional and morphological characteristics of the aortic valve, adjacent distal and proximal anatomical structures [9] as well as functional parameters of the LV, especially in patients with heart failure [10].
The aim of this study was to prospectively assess the morphological and functional LV parameters by means of preoperative cMRI in BAV-stenosis patients, and to compare these metrics with those of TAV stenosis patients. Furthermore, we aimed to search for any significant correlations between valvular and subvalvular parameters in both study subgroups.
MATERIALS AND METHODS
The data for this study were collected prospectively by enrolling all patients with congenital BAV who were referred for AVR surgery to our institution (Central Hospital, Bad Berka, Germany) with or without concurrent replacement of the proximal thoracic aorta, from January 2012 through May 2015. Approval of our institutional ethics committee was obtained and all patients gave written informed consent. Patients in the study had the following inclusion criteria: (i) presence of BAV as identified by means of preoperative transthoracic and/or transoesophageal echocardiography and cardiac MRI; (ii) indication for conventional AVR surgery due to a severely stenotic aortic valve. The presence of congenital BAV was confirmed intraoperatively by visual inspection by the attending surgeon.
A total of 201 patients with BAV disease underwent elective AVR surgery with or without concurrent replacement of the proximal aorta. We excluded BAV patients who presented with isolated/predominant aortic valve regurgitation (n = 32), and those with comparable degree of regurgitation and stenosis (i.e. mixed disease) (n = 2). Patients with mixed BAV disease were enrolled only if valve stenosis was the prevailing lesion. Furthermore, in 13 BAV patients no MRI could be performed preoperatively due to contraindications (i.e. presence of implanted cardiac pacemaker/defibrillator or claustrophobia). We excluded all patients who presented with connective tissue disorders (e.g. Marfan syndrome) as well as those who underwent urgent/emergent surgery and/or combined cardiac surgical procedures other than concurrent proximal aortic surgery. Based on the inclusion and exclusion criteria, a total of 154 consecutive BAV patients with predominant stenosis (mean age 61 ± 9 years, 66% male) were identified and served as our study cohort.
During the same study period, a group of patients undergoing AVR for TAV stenosis were entered in our study database in order to enable a between-group comparison. TAV patients over 70 years of age were excluded in order to achieve a similar age profile to BAV patients. Identical inclusion/exclusion criteria were applied in TAV as in BAV patients. A total of 90 TAV patients requiring AVR were screened. Patients with isolated/predominant aortic valve regurgitation (n = 52) as well as those with a contraindication for MRI (n = 2) were excluded. A total of 36 consecutive TAV-stenosis patients (mean age 64 ± 5 years, 61% male) therefore formed our control group.
The primary purpose of this study was to systematically compare morphological/functional LV parameters between BAV and TAV patients, and to identify correlations between valvular, LV (i.e. subvalvular) and aortic root parameters. In particular, we aimed to prospectively address whether morphological/functional LV differences exist between BAV and TAV stenosis patients.
Definitions and measurements
Morphology and function of the aortic valve (AV) was determined by means of echocardiography and cardiac MRI in all patients. If only two commissures and two AV cusps were observed, with or without the presence of a raphe and cusp redundancy, a BAV was suspected and all such patients underwent subsequent cardiac MRI. Nevertheless, the intraoperative description of the AV morphology by the attending surgeon was used as the final decision regarding the bicuspidality of the AV. Results of transthoracic echocardiography were in accordance with the intraoperative valve description in 91 (59%) study patients, whereas cardiac MRI demonstrated a rather high sensitivity and specificity in identifying BAV disease (i.e. 98%). TAV stenosis was diagnosed by means of echocardiography and was later confirmed by intraoperative inspection. Severe aortic stenosis for both study groups was determined in accordance with previously published guidelines [11]. Morphological/functional LV parameters were assessed by preoperative cMRI (see below).
Preoperative magnetic resonance imaging examination
All patients with suspected BAV stenosis as well as the TAV patients who met the above-mentioned inclusion criteria underwent a preoperative cardiac phase-contrast cine MRI examination in order to properly visualize the AV and all adjacent proximal and distal structures. MRI examination was conducted according to the previously described standards [12].
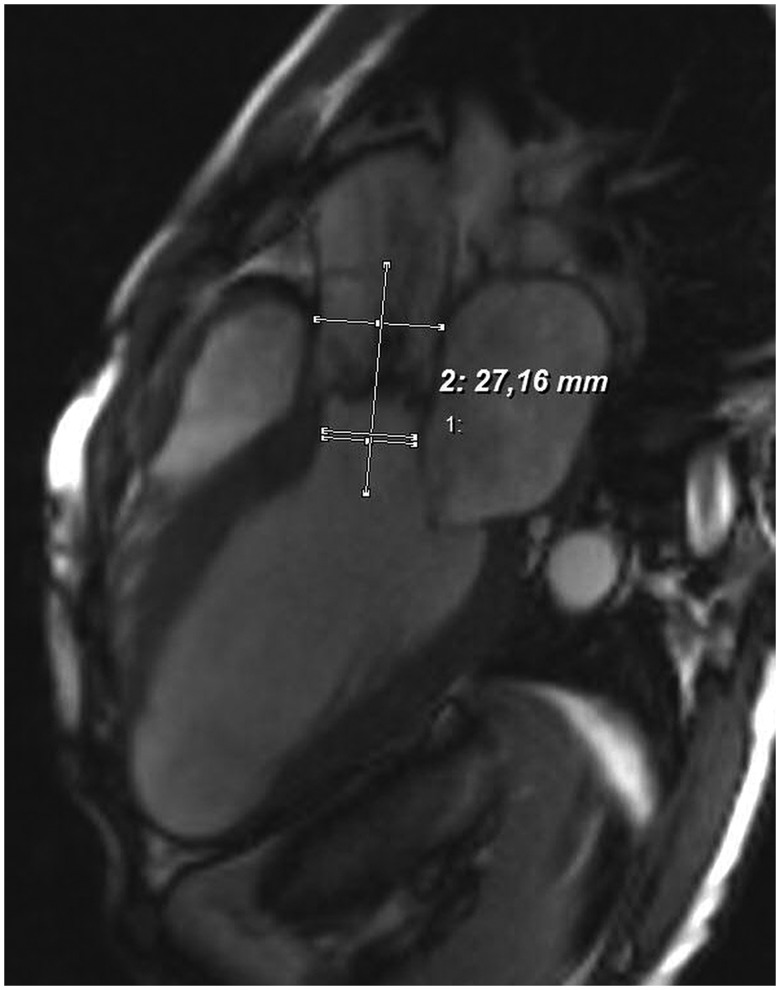
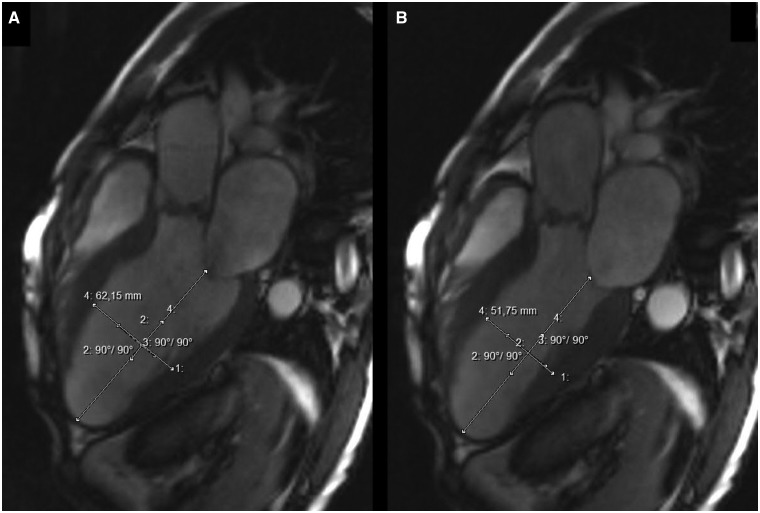
The maximal cross-sectional diameters of the proximal aorta were assessed at the level of the aortic annulus, sinuses of Valsalva, sinotubular junction and tubular mid-ascending aorta. As previously recommended [13], aortic diameters were measured as the greatest observed cross-sectional diameter perpendicular to the aortic axis in a mid-vessel slice at end-diastole using inner edge to inner edge approach. The virtual basal ring was found at the level of the basal insertions of the aortic cusps at the left ventricle. The aortic annulus was sized at the same timeframe of the cardiac cycle as LVOT diameter (i.e. early-systole). Furthermore, anatomical structures proximal to the BAV [i.e. LVOT, LV and anterior mitral leaflet (AML)] were visualized using a breath-hold steady-state free precision cine images (tf2D), using the LV inflow–outflow tract view. LVOT diameter (LVOTd) was measured at the early systole, beneath the physiological aorto-ventricular junction, perpendicular to the imaginary mid-aortic to mid-LVOT line (Fig. 1). LV volumes were calculated at the end-diastole (LVEDV) and end-systole (LVESV) in the long-axis views using the biplane ellipsoid model. LV myocardial wall thickness was measured at the anteroseptal and posterolateral walls during end-diastole and end-systole, in the mid-ventricular position. Of note, the short-axis view was used for orientation purposes. LV end-diastolic and end-systolic diameters (LVEDD, LVESD) were assessed in the mid-ventricular portion, perpendicular to the longitudinal LV axis. A virtual longitudinal LV axis line was drawn from the mitral valve level to the LV apex. LVEDD and LVESD were measured using the transversal mid-ventricular line, which was drawn perpendicularly to the above mentioned longitudinal LV axis line (Fig. 2A and B). AML length was measured in the mid-A2 segment at end-diastole using the view identical to the echocardiographic parasternal long-axis view, from the hinge point to the tip of the leaflet. Due to the fibrous nature of the leaflet, bright-blood imaging was used in order to distinguish the mitral leaflet from the attached chordae tendinae at the free edge of the leaflet. MRI data were measured by two investigators (K.D. and G.D.). A total of three consecutive measurements were repeated and averaged thereafter for every single variable.
Figure 1.
Assessment of the left ventricular outflow tract diameter (LVOTd) in the LV inflow–outflow tract view during early systole, as identified by cardiac MRI. Note the two parallel lines in LVOT. The upper line represents the LVOTd, whereas the lower one represents an orientation line to designate the mid-point of the lower border of LVOT. The transversal line connects the mid-points of LVOT and aortic root and is perpendicular to the line of assessment of LVOTd.
Figure 2.
LVOT view of cardiac MRI. (A) Assessment of the LVEDD and (B) assessment of the LVESD.
Study population
Demographic and clinical characteristics of the study population are displayed in Table 1. BAV patients were significantly younger and presented with larger proximal aortic diameter as compared with the TAV population. All 190 patients underwent conventional AVR with or without concomitant replacement of ascending aorta. Proximal aortic surgery for co-existent aortic aneurysm was performed in 18 patients (12%), and all of them were in the BAV group. Two further patients (1%) underwent concomitant replacement of the aortic root due to aneurysmal dilatation.
Table 1.
Demographic and clinical characteristics of the study population
| Study population |
|||
|---|---|---|---|
| Variable | BAV-AS (n = 154) | TAV-AS (n = 36) | P-value |
| Mean age (years) | 60.7 ± 8.8 (34–70) | 64.3 ± 5.4 (52–70) | 0.002 |
| Male sex | 101 (66) | 22 (61) | 0.613 |
| BSA (m2) | 2.00 ± 0.2 (1.50–2.60) | 1.95 ± 0.2 (1.53–2.43) | 0.224 |
| Baseline LVEF (%)a | 56 ± 9 (25–70) | 57 ± 8 (35–70) | 0.553 |
| Peak aortic valve gradient (mmHg)a | 65 ± 36 (52–119) | 56 ± 38 (42–102) | 0.021 |
| Mean aortic valve gradient (mmHg)a | 41 ± 26 (31–72) | 35 ± 24 (25–64) | 0.037 |
| Concomitant ARb | 55 (36) | 11 (30) | 0.769 |
| Mean MR (degree)a | 0.6 ± 0.5 | 0.3 ± 0.5 | 0.165 |
| No/trivial MR | 94 (61) | 26 (71) | 0.255 |
| Mild MR | 60 (39) | 10 (29) | 0.317 |
| NYHA class III or IV | 36 (23) | 8 (22) | 0.375 |
| Ascending aorta (mm)c | 42 ± 8 (25–65) | 36 ± 8 (24–70) | <0.001 |
| Diabetes mellitus | 26 (17) | 10 (28) | 0.133 |
| Smoking | 36 (23) | 8 (22) | 0.882 |
| Arterial hypertension | 128 (83) | 34 (94) | 0.084 |
| β-blocker therapy | 51 (33) | 20 (56) | 0.058 |
| Peripheral arterial disease | 4 (3) | 0 (0) | 0.272 |
| COPD | 10 (7) | 4 (11) | 0.340 |
| Endocarditis | 1 (1) | 0 (0) | 0.588 |
| CPB time (min) | 84 ± 24 (51–188) | 74 ± 17 (57–114) | 0.129 |
| Cross-clamp time (min) | 58 ± 15 (36–130) | 56 ± 16 (40–85) | 0.657 |
| Mechanical valve prosthesis | 35 (23) | 4 (11) | 0.023 |
| Mean biological-prosthesis size (mm) | 23.8 ± 2.2 (21–29) | 21.8 ± 1.4 (21–25) | <0.001 |
| Mean mechanical-prosthesis size (mm) | 24.2 ± 1.7 (21–29) | 22.2 ± 1.1 (21–25) | <0.001 |
| Ascending aortic replacement | 16 (10) | 0 (0) | 0.017 |
| Aortic root replacement | 2 (1) | 0 (0) | 0.272 |
| Hemiarch replacement | 3 (2) | 0 (0) | 0.229 |
Data presented as numbers (%) or as mean ± SD (range).
BSA: body surface area; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; NYHA: New York Heart Association; COPD: chronic obstructive pulmonary disease; CPB: cardiopulmonary bypass.
Measured by preoperative echocardiography.
Nondominant aortic regurgitation in patients with mixed lesions.
Maximal diameter of tubular ascending aorta as measured by magnetic resonance imaging.
Statistical analysis
Categorical variables are presented as numbers with percentage and continuous variables as mean value ± standard deviation (range). Continuous variables between the study subgroups were compared using unpaired two-sided t-test. Categorical variables were analysed by χ2 test or Fisher’s exact test as appropriate. P-values of <0.05 were considered statistically significant. Data were tested for normal distribution using the Kolmogorov–Smirnov test. A Cox-regression analysis was conducted to identify independent predictors of LVOTd >21 mm. A cut-off value of 0.1 at the univariate analysis was set as condition to incorporate variables into the Cox regression model. Correlation analyses were performed using Pearson’s correlation. Fisher r-to-z transformation was implemented to calculate a z-value to assess the significance of the difference between correlation coefficients.
Intra-rater reliability of LVOT diameter and AML length measurements was evaluated by duplicate measurements conducted by the same observer. Inter-rater reliability was evaluated for the same measurements conducted by the two study members. Reliability statistics consisted of Lin’s concordance correlation coefficient, coefficient of variation and Bland-Altman 95% confidence interval (CI) of agreement (Table 2).
Table 2.
Intra- and inter-rater reliability of left ventricular outflow tract diameter (LVOTd) and anterior mitral leaflet (AML)
| Parameter | Mean value (mm) | SD (mm) | CV (%) | CCC | Bland-Altman 95% CI |
|---|---|---|---|---|---|
| Intrarater | |||||
| LVOTd | 20.3 | 3.2 | 1.1 | 0.99 | −0.3 to 0.2 |
| AML length | 23.6 | 3.3 | 1.3 | 0.98 | −0.6 to 0.5 |
| Inter-rater | |||||
| LVOTd | 21.1 | 2.8 | 5.8 | 0.92 | −2.7 to 1.9 |
| AML length | 23.1 | 3.2 | 3.3 | 0.96 | −0.9 to 1.7 |
SD: standard deviation; CV: coefficient of variation; CCC: concordance correlation coefficient.
RESULTS
Morphological left ventricular parameters
Baseline MRI measurements in both study groups are displayed in Table 3. BAV patients demonstrated significantly larger LVOTd as compared with their TAV counterparts (21.7 ± 3.0 mm in BAV group and 18.9 ± 2.7 mm in TAV group, P < 0.001). Systolic LV area was also found to be significantly larger in BAV subjects (1960 ± 873 mm2 in BAV group versus 1699 ± 449 mm2 in TAV group, P = 0.039). However, there was no statistically significant difference between the groups in terms of diastolic LV area. BAV patients presented with significantly larger LV end-diastolic volume (LVEDV) compared with their TAV counterparts (164.9 ± 68.4 ml in BAV groups versus 126.5 ± 53.1 ml in TAV group, P = 0.037). Similarly, the LV end-systolic volume (LVESV) was significantly larger in BAV subjects (82.1 ± 57.9 ml in BAV group versus 52.9 ± 25.7 ml in TAV group, P = 0.008). With regard to LV wall thickness, significant differences were observed only for diastolic anterior LV wall thickness (i.e. 15.2 ± 3.0 mm in BAV group and 14.1 ± 1.8 mm in TAV patients, P = 0.026). There were no significant between-group differences for LVEDD and LVESD dimensions. The AML was significantly larger in BAV patients (24 ± 3 mm in BAV group versus 22 ± 3 in TAV group, P = 0.009). Proximal aortic diameters as assessed by MRI were also found to be significantly larger in BAV vs TAV stenosis patients (42 ± 8 mm in BAV group versus 36 ± 8 mm in TAV group, P < 0.001). Of note, echocardiographically defined LV ejection fraction (LVEF) was not significantly different between study groups.
Table 3.
Baseline magnetic resonance imaging measurements of the study population
| Study population |
|||
|---|---|---|---|
| Variable | BAV-AS (n = 154) | TAV-AS (n = 36) | P-value |
| LVOT diameter (mm) | 21.7 ± 3.0 | 18.9 ± 2.7 | <0.001 |
| Ventricular area—systolic (mm2) | 1960 ± 873 | 1699 ± 449 | 0.039 |
| Ventricular area—diastolic (mm2) | 3369 ± 953 | 3175 ± 563 | 0.191 |
| LV end-systolic volume (ml) | 82.1 ± 57.9 | 52.9 ± 25.7 | 0.008 |
| LV end-diastolic volume (ml) | 164.9 ± 68.4 | 126.5 ± 53.1 | 0.037 |
| LV anterior wall thickness-diastolic (mm) | 15.2 ± 3.0 | 14.1 ± 1.8 | 0.026 |
| LV posterior wall thickness-diastolic (mm) | 14.0 ± 2.3 | 13.5 ± 1.8 | 0.291 |
| LV end-diastolic diameter (mm) | 44.3 ± 8.2 | 42.1 ± 7.8 | 0.225 |
| LV end-systolic diameter (mm) | 27.9 ± 9.3 | 25.3 ± 7.9 | 0.169 |
| AML length (mm) | 24.0 ± 3.2 | 22.0 ± 3.5 | 0.009 |
| Aortic annulus (mm) | 27.0 ± 3.1 | 25.0 ± 2.2 | <0.001 |
| Sinus of Valsalva (mm) | 36.4 ± 5.7 | 32.6 ± 3.4 | <0.001 |
| Sinotubular junction (mm) | 31.3 ± 5.1 | 28.0 ± 2.6 | <0.001 |
Data are presented as mean ± SD, LV: left ventricular.
Intra- and inter-rater reliability for assessment of LVOT diameter and AML length are summarized in Table 2. The degree of agreement of both observers was good, with a concordance correlation coefficient above 0.92. Furthermore, there was no evidence of observer-associated bias, as Bland-Altman analysis revealed that all confidence intervals included a zero value.
Correlation analysis between aortic, valvular and ventricular parameters
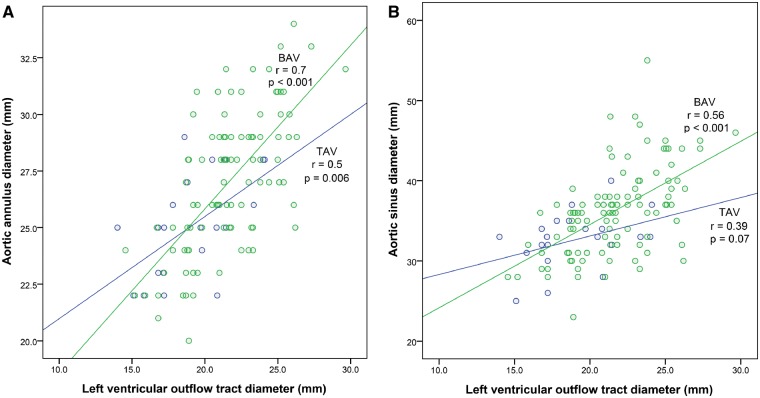
Correlation analyses were performed between LV parameters (i.e. LVOT diameter, LVEF, LVEDD, LVESD, AML length) and valvular/aortic root metrics for both study groups. BAV patients showed a significant correlation between the LVOTd and maximal aortic root diameters at all levels. In particular, LVOTd correlated strongly with the aortic annulus diameter (r = 0.7, P < 0.001 in BAV group versus r = 0.5, P = 0.006 in TAV group) (Fig. 3A). Significant correlations were similarly observed with the sinus of Valsalva diameter (r = 0.56, P < 0.001 in BAV group versus r = 0.39, P = 0.07 in TAV group) (Fig. 3B) and sinotubular junction diameter (r = 0.47, P < 0.001 in BAV group versus r = 0.33, P = 0.124 in TAV group) in BAV group. Only weak correlation was observed between LVOTd and mid-ascending aortic diameter in both study subgroups (r = 0.26, P = 0.006 in BAV group versus r = 0.41, P = 0.04 in TAV group). However, a significant difference in correlation coefficients was found only for the correlation between LVOTd and aortic annulus diameter (z = 1.65, P = 0.04). The remaining correlation patterns of LVOTd and aortic root diameters were comparable between the BAV and TAV groups (z = 1.15, P = 0.12 for aortic sinus; z = 0.85, P = 0.19 for aortic sinotubular junction). Furthermore, a significant strong correlation of LVOTd and the LVEDV was found only in the BAV group (r = 0.55, P < 0.001 in BAV group versus r = 0.26, P = 0.45 in TAV group; z = 1.8, P = 0.03). Similarly, LVOTd and LVESV correlated strongly only in BAV patients (r = 0.55, P < 0.001 in BAV group versus r = 0.31, P = 0.36 in TAV group; z = 1.5, P = 0.06).
Figure 3.
Correlation analyses between LVOT diameter (LVOTd) and proximal aortic diameters. (A) Aortic annulus diameter; (B) Sinus of Valsalva diameter.
Predictors of left ventricular outflow tract dilatation
Cox-regression analysis was performed in order to identify independent predictors of LVOT enlargement in the whole study cohort. Based on the median LVOTd value, patients were divided into those having LVOTd ≤21 mm vs those with LVOTd >21 mm. Univariate analysis revealed that 61% of BAV patients versus 21% TAV patients had LVOTd >21 mm (P < 0.001). Ascending aortic diameter (OR 1.2, P = 0.007) and the presence of BAV (OR 9.0, P = 0.04) were independently associated with LVOTd >21 mm (Table 4). Of note, body surface area and end-systolic LV diameters were not significantly associated with LVOT diameter >21 mm.
Table 4.
Independent predictors of LVOT diameter >21 mm (as determined by Cox-regression analysis)
| Variable | Odds ratio | P-value | 95% CI | |
|---|---|---|---|---|
| Proximal aortic diametera | 1.148 | 0.007 | 1.039 | 1.268 |
| Body surface area | 0.998 | 0.775 | 0.986 | 1.010 |
| Baseline LVEF | 1.017 | 0.746 | 0.919 | 1.125 |
| LV end-systolic diameter | 0.993 | 0.993 | 0.849 | 1.163 |
| Ventricular area – systolic | 1.002 | 0.152 | 0.999 | 1.004 |
| BAVb | 9.031 | 0.041 | 1.003 | 81.291 |
LVEF: left ventricular ejection fraction; CI: confidence interval.
Maximal diameter of ascending aorta (as defined by cardiac MRI).
When compared with tricuspid aortic valve (i.e. categorical variable).
DISCUSSION
In this study, we aimed to prospectively assess the subvalvular differences observed in BAV and TAV stenosis patients undergoing AVR. Our aim was to test the hypothesis that presence of BAV morphology is associated with a more severe LV remodelling than TAV morphology. To the best of our knowledge, this study is the first to prospectively report MRI-based quantitative assessment of LV parameters in BAV patients undergoing AVR surgery.
What are the possible explanations of our observed LV differences between BAV and TAV stenosis patients? First of all, BAV patients require AVR surgery at a significantly younger age when compared with their TAV counterparts. Younger patients may not develop symptoms until later in their disease process and therefore may have signs of more advanced valvular cardiomyopathy. Second, BAV patients experience relevant aortic valvular lesions earlier in life [3], which in turn, may promote earlier morphological changes of LV architecture. As evidence of this statement, some recent studies detected subclinical impairment of LV systolic and diastolic function even in patients with a “normally” functioning BAV [14]. Third, BAV patients are known to have marked eccentricity of systolic BAV opening, resulting in a functional severity of given anatomic orifice that is always greater in BAV vs TAV-stenosis [15]. This situation leads to a more severe “afterload mismatch” in BAV patients. Long-standing and potentially underestimated valvular dysfunction in BAV patients may subsequently lead to irreversible myocardial damage and valvular cardiomyopathy. Finally, considering the baseline differences between BAV and TAV patients with regard to LV systolic and diastolic function and LV mechanics [14, 16], we hypothesize that BAV might be associated with a more severe valvular cardiomyopathy. Of interest was the observation of our study that BAV patients presented with significantly larger LVEDV and LVESV compared with TAV patients. Our results prove the profound discrepancy regarding the preload and afterload between the both study groups. Specifically, the long-standing valvular stenosis in BAV patients as compared with TAV patients may be the cause of the larger LVEDV and consecutively greater distension of the ventricle, which might in turn induce a greater preload.
In this study, patients with BAV stenosis showed significantly larger LVOT dimensions as compared with their TAV counterparts, with a mean LVOTd that was 15% larger. To the best of our knowledge, such comparative data of LV metrics have not been published before. An echocardiographic study by Shiran et al. [17] found a larger mean LVOTd in patients with Marfan syndrome as compared with a control group. As expected, the LVOTd of Marfan patients in their study was larger than the BAV stenosis population in this study. One contributing factor for the increased LVOTd in Marfan patients from the Shiran study [17] was their increased BSA compared with controls. In contrast, we did not observe any significant difference in BSA between BAV and TAV patients in this study.
We found a strong correlation between LVOT and aortic annulus diameters in BAV patients, and this correlation was significantly weaker in TAV patients (i.e. z-value showed significant difference in correlation patterns between study subgroups). Of note, correlation patterns between LVOT and sinus of Valsalva/sinotubular junction diameters were not significantly different between both study subgroups.
Anatomic and functional aorto-ventricular junction play a crucial role in the complex interaction between the aortic root and LVOT, which is even more complex in BAV patients, due to the unmethodical spatial arrangement of aortic sinuses and the co-existence of heterogeneous BAV morphotypes. Although rare, the “pure” BAV with two equal sized cusps and without raphe (i.e. Sievers Type 0), represents the most intriguing phenotype in terms of its relation to the LVOT. It might be assumed that along with the diversiform spatial cusp orientation (i.e. antero-posterior or lateral), the triangular intersinusal fibrous extensions and LVOT muscular extensions may become subject to various intraplanar shifting and rearrangements. This assumption is further supported by the observation that from the sub-valvular view, the “commissural area presents rather as an indentation and not as a space” [18]. Moreover, one intersinusal triangle may present either as rudimentary (if there is a BAV phenotype with a raphe) or absent (in the rare BAV phenotype without a raphe) [19]. Thus, BAV patients present with a variety of aortic root deformations which may affect commissures and the aortic annulus as a whole.
The causal chain of interaction between all aortic root components in BAV morphology remains to be further elucidated. The larger LVOTd and strong correlation between LVOTd and aortic root diameter in the setting of BAV morphology may indicate a congenital component of BAV-associated cardiomyopathy. The fact that the cusps and their supporting sinuses are formed from a part of the developing outflow tract [20] along with their anatomical and rheological bond, turns out to affect the postnatal interdependency of the aortic root and LVOT. The combination of valvular and subvalvular characteristics of BAV patients may have an impact on echocardiographic findings [15], that BAV stenosis is more severe for a given anatomic orifice as compared with TAV stenosis. Furthermore, these structural alterations may promote stenosis early in a patient’s life. Hence, LV architecture of BAV patients may be exposed to a greater “afterload mismatch” which begins at much earlier stage as compared with TAV patients. It may therefore be assumed that long-standing BAV dysfunction may promote rheologically-triggered BAV-associated valvular cardiomyopathy. In order to address this question, a prospective histological study comparing BAV and TAV cohorts, with and without aortic root dilatation, would be required.
By adding another piece to the puzzle of BAV disease, our prospective study confirms that the “BAV syndrome” consists not only of the valvular impairment and aortic aneurysm/dissection, but with subannular and ventricular features as well, be it subclinical or occult. It is widely accepted that BAVs produce eccentric jets which might contribute in the pathogenesis of BAV aortopathy [12]. By producing different types of eccentric jets, cusp fusion patterns are related to different types of aortopathy (i.e. root phenotype and mid-ascending aortic dilation). As previously shown by Richards et al. [21], in eccentric BAVs with smaller LVOT, there is a reduced overall pressure gradient. Moreover, the larger the LVOT, the larger the pressure gradient and the peak velocity, and therefore the greater the eccentricity. It may be postulated that in BAV patients with larger LVOT, due to the greater jet eccentricity, more malign forms of aortopathy may be induced. However, this needs to be verified by further rheological studies involving the analysis of jet eccentricity and the LVOT geometry in BAV and TAV patients.
Another important finding from this study is the significant difference in the length of the AML between BAV and TAV stenosis patients. Similar findings were previously published by Charitos et al. [22]. Our results support the previous hypothesis that an elongated AML is an important morphological characteristic of the BAV-associated malformation spectrum. However, none of our study patients in the BAV cohort had significant mitral regurgitation. Therefore, the functional relevance of this finding has to still be clarified.
Study limitations
The most relevant limitation is the small sample size, especially in the TAV group. However, the small sample size is a result of excluding all TAV-stenosis patients >70 years of age in order to achieve a homogeneous and comparable subgroup of TAV patients. Another limitation is our decision to include only those patients presenting with severe aortic valve stenosis referred for AVR surgery. Our findings are therefore not necessarily applicable to the whole spectrum of BAV patients. Although the shape of the LVOT cannot be depicted to be circular, all measurements were conducted in a standard orientation (LV inflow–outflow view) in order to minimize variations. Finally, we did not perform cMRI measurements in control BAV and TAV patients without significant aortic stenosis. It is impossible to determine, therefore, whether our observed differences between BAV and TAV patients precede the development of severe aortic stenosis.
CONCLUSION
Our findings demonstrate that BAV stenosis may be associated with distinct morphological/functional features of valvular cardiomyopathy when compared with TAV stenosis. Moreover, BAV morphology is independently associated with LVOT dilation. Comparable baseline characteristics between the two study groups (i.e. gender, BSA, LVEF and NYHA class) enable us to exclude confounding effect of these variables. Further studies are required to confirm if BAV morphology is associated with a more severe valvular cardiomyopathy when compared with TAV patients.
Conflict of interest: none declared.
REFERENCES
- 1. Della Corte A. Phenotypic heterogeneity of bicuspid aortopathy: a potential key to decode the prognosis? Heart 2014;100:96–7. [DOI] [PubMed] [Google Scholar]
- 2. Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol 1970;26:72–83. [DOI] [PubMed] [Google Scholar]
- 3. Robicsek F, Thubrikar MJ, Cook JW, Fowler B.. The congenitally bicuspid aortic valve: how does it function? Why does it fail? Ann Thorac Surg 2004;77:177–85. [DOI] [PubMed] [Google Scholar]
- 4. Siu SC, Silversides CK.. Bicuspid aortic valve disease. J Am Coll Cardiol 2010;55:2789–800. [DOI] [PubMed] [Google Scholar]
- 5. Hahn RT, Roman MJ, Mogtader AH, Devereux RB.. Association of aortic dilation with regurgitant, stenotic and functionally normal bicuspid aortic valves. J Am Coll Cardiol 1992;19:283–88. [DOI] [PubMed] [Google Scholar]
- 6. Nistri S, Sorbo MD, Marin M, Palisi M, Scognamiglio R, Thiene G.. Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart 1999;82:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cecconi M, Manfrin M, Moraca A, Zanoli R, Colonna PL, Bettuzzi MG. et al. Aortic dimensions in patients with bicuspid aortic valve without significant valve dysfunction. Am J Cardiol 2005;95:292–4. [DOI] [PubMed] [Google Scholar]
- 8. Beroukhim RS, Kruzick TL, Taylor AL, Gao D, Yetman AT.. Progression of aortic dilation in children with a functionally normal bicuspid aortic valve. Am J Cardiol 2006;98:828–30. [DOI] [PubMed] [Google Scholar]
- 9. Wassmuth R, von Knobelsdorff-Brenkenhoff F, Gruettner H, Utz W, Schulz-Menger J.. Cardiac magnetic resonance imaging of congenital bicuspid aortic valves and associated aortic pathologies in adults. Eur Heart J Cardiovasc Imaging 2014;15:673–9. [DOI] [PubMed] [Google Scholar]
- 10. Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG. et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J 2000;21:1387–96. [DOI] [PubMed] [Google Scholar]
- 11. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP. et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr 2009;10:1–25. [DOI] [PubMed] [Google Scholar]
- 12. Girdauskas E, Rouman M, Disha K, Espinoza A, Dubslaff G, Fey B. et al. Aortopathy in patients with bicuspid aortic valve stenosis: role of aortic root functional parameters. Eur J Cardiothorac Surg 2016;49:635–44. [DOI] [PubMed] [Google Scholar]
- 13. Burman ED, Keegan J, Kilner PJ.. Aortic root measurement by cardiovascular magnetic resonance: specification of planes and lines of measurement and corresponding normal values. Circ Cardiovasc Imaging 2008; 1:104–13. [DOI] [PubMed] [Google Scholar]
- 14. Demir M. Left ventricular systolic and diastolic function in subjects with a bicuspid aortic valve without significant valvular dysfunction. Exp Clin Cardiol 2013;18:e1–4. [PMC free article] [PubMed] [Google Scholar]
- 15. Donal E, Novaro GM, Deserrano D, Popovic ZB, Greenberg NL, Richards KE. et al. Planimetric assessment of anatomic valve area overestimates effective orifice area in bicuspid aortic stenosis. J Am Soc Echocardiogr 2005;18:1392–8. [DOI] [PubMed] [Google Scholar]
- 16. Kurt M, Tanboga IH, Bilen E, Isik T, Kaya A, Karakas MF. et al. Abnormal left ventricular mechanics in isolated bicuspid aortic valve disease may be independent of aortic distensibility: 2D strain imaging study. J Heart Valve Dis 2012;21:608–14. [PubMed] [Google Scholar]
- 17. Shiran H, Haddad F, Miller DC, Liang D.. Comparison of aortic root diameter to left ventricular outflow diameter vs body surface area in patients with Marfan syndrome. Am J Cardiol 2012;110:1518–22. [DOI] [PubMed] [Google Scholar]
- 18. Sievers HH, Schmidtke C.. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133:1226–33. [DOI] [PubMed] [Google Scholar]
- 19. Anderson RH, Devine WA, Ho SY, Smith A, McKay R.. The myth of the aortic annulus: the anatomy of the subaortic outflow tract. Ann Thorac Surg 1991;52:640–6. [DOI] [PubMed] [Google Scholar]
- 20. Anderson RH, Webb S, Brown NA, Lamers W, Moorman A.. Development of the heart: (3) formation of the ventricular outflow tracts, arterial valves, and intrapericardial arterial trunks. Heart 2003;89:1110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richards KE, Deserranno D, Donal E, Greenberg NL, Thomas JD, Garcia MJ.. Influence of structural geometry on the severity of bicuspid aortic stenosis. Am J Physiol Heart Circ Physiol 2004;287:H1410–6. [DOI] [PubMed] [Google Scholar]
- 22. Charitos EI, Hanke T, Karluss A, Hilker L, Stierle U, Sievers HH.. New insights into bicuspid aortic valve disease: the elongated anterior mitral leaflet. Eur J Cardiothorac Surg 2013;43:367–70. [DOI] [PubMed] [Google Scholar]