Abstract
Aim
To compare the efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin with the sodium‐glucose transporter‐2 inhibitor dapagliflozin in patients with type 2 diabetes and mild renal insufficiency.
Materials and Methods
Patients with HbA1c ≥7.0 to ≤9.5% (≥53 to ≤80 mmol/mol) and estimated glomerular filtration rate ≥60 to <90 mL/min/1.73m2 on metformin (≥1500 mg/d) ± sulfonylurea were randomized to sitagliptin 100 mg (n = 307) or dapagliflozin 5 mg titrated to 10 mg (n = 306) once daily for 24 weeks. A longitudinal data analysis model was used to test the primary hypothesis that sitagliptin is non‐inferior to dapagliflozin in reducing HbA1c at Week 24, with superiority to be tested if non‐inferiority is met. https://ClinicalTrials.gov NCT02532855.
Results
Baseline mean HbA1c (% [mmol/mol]) was 7.7 (60.9) and 7.8 (61.2), and mean eGFR (mL/min/1.73m2) was 79.4 and 76.9 for the sitagliptin and dapagliflozin groups, respectively. After 24 weeks, the between‐group difference in least squares mean (95% CI) changes from baseline in HbA1c was −0.15% (−0.26, −0.04) (−1.67 mmol/mol [−2.86, −0.48]), P = 0.006, meeting the prespecified criteria for declaring both non‐inferiority and superiority of sitagliptin versus dapagliflozin. The HbA1c goal of <7% (<53 mmol/mol) was met by 43% (sitagliptin) and 27% (dapagliflozin) of patients. No meaningful between‐group difference was observed in a pre‐specified analysis of 2‐hour incremental postprandial glucose excursion. A review of adverse events (AEs) was notable for a lower incidence of drug‐related AEs with sitagliptin compared with dapagliflozin.
Conclusions
In patients with type 2 diabetes, mild renal insufficiency and inadequate glycaemic control on metformin ± sulfonylurea, sitagliptin treatment resulted in greater improvement in glycaemic control compared with dapagliflozin and was generally well tolerated.
Keywords: clinical trial, dapagliflozin, sitagliptin, type 2 diabetes
1. INTRODUCTION
Chronic kidney disease (CKD) is a common complication of type 2 diabetes. While the prevalence of moderate or severe renal insufficiency (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73m2, CKD stages 3‐5) in patients with type 2 diabetes has been estimated at approximately 22%, a larger proportion (38%) have mild renal insufficiency (eGFR ≥60 and <90 mL/min/1.73m2, stage 2).1 These rates increased in those with type 2 diabetes ≥65 years of age, among whom 43% have moderate or severe renal insufficiency and 48% have mild renal insufficiency.1 While it is widely recognized that the choice of an antihyperglycaemic agent (AHA) for treatment of type 2 diabetes should be influenced by renal function, only CKD stages 3‐5 are usually considered relevant to that decision.2 Additionally, clinical studies in patients with CKD are usually focused on patients with eGFR <60 mL/min/1.73m2. While patients with type 2 diabetes and mild renal insufficiency are typically included in Phase III studies of antihyperglycaemic therapies, the specific impact of mild renal insufficiency on the efficacy and safety of most AHAs has not generally been prospectively evaluated in clinical trials.
Among the classes of oral AHAs available for treatment of type 2 diabetes, dipeptidyl peptidase‐4 (DPP‐4) inhibitors are considered both efficacious and well tolerated across the range of renal function, although dose adjustment to control drug exposure is sometimes required.3 In contrast, the sodium/glucose transporter‐2 (SGLT‐2) inhibitors have reduced efficacy in patients with moderate to severe renal insufficiency due to their mechanism of action.4
The CompoSIT‐R (comparison of sitagliptin with dapagliflozin in mild renal impairment) study was a prospective, randomized clinical trial comparing the efficacy and safety of the DPP‐4 inhibitor sitagliptin with the SGLT‐2 inhibitor dapagliflozin in patients with type 2 diabetes and mild renal insufficiency.
2. METHODS
2.1. Participants
Eligible patients were male or female, ≥25 years of age, with type 2 diabetes and mild renal insufficiency (eGFR ≥60 and <90 mL/min/1.73m2, calculated by the Chronic Kidney Disease Epidemiology Collaboration [CKD‐epi] serum creatinine equation5). Eligible patients were on a stable dose of metformin (≥1500 mg/d) alone or in combination with a sulfonylurea (SU) (at a dose of ≥50% of the maximum labelled dose in the country of the investigational site) for ≥8 weeks, with an HbA1c ≥7.0% and ≤9.5% (≥53 mmol/mol to ≤80 mmol/mol) at screening, and a fasting finger‐stick glucose >6.1 mmol/L and <14.4 mmol/L at randomization.
Patients were excluded from the study if they had type 1 diabetes, a history of ketoacidosis, active liver disease, significant cardiovascular disease, malignancy or haematological disorders, or if, in the opinion of the investigator, they were at high risk for volume depletion, hypotension and/or electrolyte imbalances. Patients were also excluded if they had been previously treated with any AHAs other than metformin or, if on dual therapy, metformin in combination with an SU, within 12 weeks prior to screening. Laboratory exclusion criteria included serum alanine aminotransferase or aspartate aminotransferase levels >2 times the upper limit of normal (ULN), haemoglobin <120 g/L (male) or <110 g/L (female), triglycerides >6.8 mmol/L or thyroid‐stimulating hormone outside the central laboratory normal range.
Written informed consent was obtained from all study participants.
2.2. Study design
This was a multinational, randomized, double‐blind, active comparator‐controlled, parallel‐group trial, including a 2‐week screening period, a 2‐week single‐blind placebo run‐in period, a 24‐week double‐blind treatment period and a post‐treatment telephone or in‐person contact 14 days after the last dose of blinded study drug (Figure 1).
Figure 1.
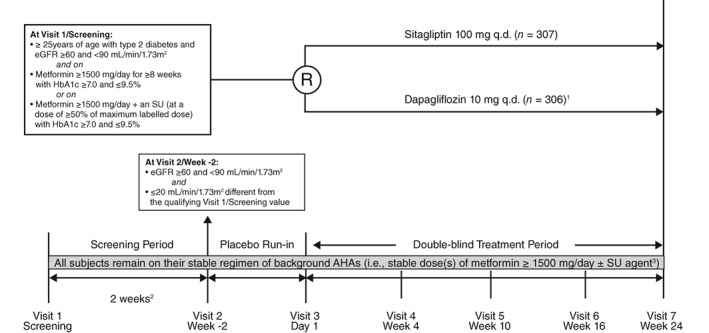
Study design. eGFR, estimated glomerular filtration rate; q.d., once daily; SU, sulfonylurea; R, randomization.1Subjects initiated dapagliflozin 5 mg q.d. at Randomization/Visit 3/Day 1 and were to uptitrated to dapagliflozin 10 mg q.d. at Visit 4/Week 4. 2The interval between Visit 1 and Visit 2 for eligible subjects was to be at least 2 weeks and no more than approximately 6 weeks. 3Subjects entering the study on metformin remained on a stable dose of metformin; subjects entering on metformin + an SU remained on stable doses of both agents
After the run‐in period, patients were randomized centrally using an interactive voice response system, in a 1:1 ratio, to sitagliptin 100 mg/d and placebo matching dapagliflozin or dapagliflozin and placebo matching sitagliptin. Dapagliflozin/matching placebo was initiated at 5 mg and titrated up to 10 mg at Week 4, unless, in the opinion of the investigator, a patient was unable to tolerate uptitration to 10 mg, in which case the dose was to remain at 5 mg. Dapagliflozin 10 mg/matching placebo was downtitrated to dapagliflozin 5 mg/matching placebo if the higher dose was not tolerated. Patients with eGFR persistently <50 mL/min/1.73m2 (CKD‐epi formula) from Visit 3/Day 1 through 1 day prior to Visit 5/Week 10 or persistently <60 mL/min/1.73m2 (calculated by the CKD‐epi formula) from Visit 5/Week 10 through Visit 7/Week 24 were discontinued from study medication. Other discontinuation criteria for hyperglycaemia, hypoglycaemia, and liver function are listed in the supporting information for this article.
A mixed‐meal tolerance test (MMTT) was performed at randomization (Day 1), prior to the first dose of double‐blind study drug and background medication, and at Week 24 (or discontinuation visit). At Week 24 (or discontinuation visit) patients took study drug and background medication approximately 1 hour before consuming the standard meal for the MMTT. The MMTT meal consisted of 2 nutrition bars and 1 nutrition drink and had a nutrient content of 660 kcal (96 g carbohydrate, 20 g fat and 28 g protein).
This clinical trial (MK‐0431‐838; http://clinicaltrials.gov Identifier: NCT02532855; EudraCT: 2014‐005525‐13) was conducted in accordance with the principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies.
2.3. Study evaluations
The primary objectives of this study were: (i) after 24 weeks, to assess the effect of the addition of sitagliptin compared with the addition of dapagliflozin on HbA1c; and (ii) over 24 weeks, to assess the overall safety and tolerability of sitagliptin in comparison to that of dapagliflozin. The primary study hypothesis was that after 24 weeks, the change from baseline in HbA1c in subjects treated with the addition of sitagliptin is non‐inferior to that in subjects treated with the addition of dapagliflozin.
Secondary objectives were assessment of 2‐hour incremental postprandial glucose excursion (PPGE), 2‐hour postprandial glucose (PPG), fasting plasma glucose (FPG), 2‐hour postprandial area under the curve (AUC0‐120) for insulin and glucagon, the postprandial insulin AUC0‐120 to glucagon AUC0‐120 ratio, and the proportion of subjects at the HbA1c goal of <7.0% (<53 mmol/mol) after 24 weeks of treatment. An exploratory objective was to assess the effect of the addition of sitagliptin compared with dapagliflozin on medical resource utilization (i.e. unplanned telephone contacts or visits to healthcare providers) during the 24‐week treatment period plus the 2‐week safety follow‐up period.
2.4. Efficacy endpoints
Glycaemic efficacy endpoints were change from baseline in HbA1c, 2‐hour incremental PPGE, 2‐hour PPG, FPG, postprandial insulin, glucagon, and insulin:glucagon ratio at Week 24, and proportion of patients who achieved an HbA1c goal of <7% (<53 mmol/mol) at Week 24.
2.5. Safety endpoints
Safety endpoints were incidences of adverse events (AEs), percentages of patients meeting predefined limits of change (PDLC) in laboratory parameters, and change from baseline at Week 24 in laboratory parameters, vital signs and body weight. AEs of hypoglycaemia, documented hypoglycaemia and severe hypoglycaemia were predefined AEs of interest. In subjects completing the clinical trial on study medication, safety data were collected from the initiation of treatment until 2 weeks after discontinuation of treatment. For subjects who discontinued study medication prematurely, safety data were collected from the initiation of treatment until Week 24.
2.6. Statistical analyses
The full analysis set ([FAS], all randomized subjects who received at least one dose of study treatment and had a baseline or a postbaseline measurement) served as the primary analysis population for the change from baseline HbA1c, percentages of individuals at HbA1c goal, 2‐hour incremental PPGE, 2‐hour PPG, and FPG. The per protocol (PP) population (all randomized subjects who received at least one dose of study treatment and had a baseline and a Week 24 measurement, without a protocol deviation that would affect or confound the measure of efficacy) was used as a primary analysis population for glucagon AUC0‐120, insulin AUC0‐120, and insulin AUC0‐120 to glucagon AUC0‐120 ratio. A longitudinal data analysis (LDA) model6 was used to evaluate the continuous endpoints with fixed effects for treatment, time, background AHA (metformin alone, or metformin in combination with an SU), the interaction of time by background AHA, and the interaction of time by treatment, with a constraint that the true mean at baseline is common to all treatment groups (which is valid due to randomization). Data after the last dose of study medication plus an offset of 5 days was excluded from primary LDA analyses. Missing data were handled implicitly by the LDA model.The primary hypothesis regarding the non‐inferiority of sitagliptin versus dapagliflozin in decreasing HbA1c was assessed using the estimated between‐treatment difference from the LDA model. If the upper bound of the two‐sided 95% confidence interval (CI) for the mean difference between sitagliptin and dapagliflozin was less than the non‐inferiority margin (δ = 0.3%), then sitagliptin would be declared non‐inferior to dapagliflozin; if the upper bound was <0.0%, sitagliptin would be declared superior to dapagliflozin. Evaluation of the percentage of individuals at the HbA1c goal of <7.0% (<53 mmol/mol) at Week 24 was based on estimated rates and CIs for between‐group rate differences computed using the Miettinen and Nurminen method.7 For this analysis, multiple imputations of missing Week 24 data, using techniques proposed by Rubin,8 were based on the LDA model used for analysis of HbA1c. After imputations, patients were categorized as either at, or not at, the HbA1c goal at Week 24.
Analysis of safety data used the population of all randomized patients who received at least one dose of study treatment. Safety and tolerability were assessed by clinical review of all relevant parameters, including AEs, laboratory tests, ECG, vital signs and body weight, during the treatment period and through 14 days after treatment ended. Point estimates with 95% CIs were calculated using the method of Miettinen and Nurminen7 for between‐group comparisons for AEs with incidence of ≥4 patients in either treatment group, and for AEs of hypoglycaemia, documented hypoglycaemia and severe hypoglycaemia. Analysis of hypoglycaemia was performed separately by background AHA, i.e. metformin alone and metformin in combination with an SU. Descriptive statistics were calculated for all other safety endpoints.
A sample size of 278 participants per treatment group was estimated to provide >99% power to establish that sitagliptin is non‐inferior to dapagliflozin in lowering HbA1c at an overall one‐sided, 2.5% α‐level, assuming an underlying treatment difference of 0%, and 90% power to demonstrate the superiority of sitagliptin versus dapagliflozin in lowering HbA1c at an overall one‐sided, 2.5% α‐level, if the underlying treatment difference in HbA1c is −0.2%.
The study‐wise type I error rate was controlled using an ordered testing procedure. First, non‐inferiority of sitagliptin compared with dapagliflozin for change from baseline in Hba1c was tested. When the success criterion for non‐inferiority was met, superiority was assessed. When the test for superiority was successful, the secondary hypothesis for postprandial glucose excursion was tested. All three tests were conducted at α = 0.025 (one‐sided).
3. RESULTS
3.1. Patient disposition and characteristics and dapagliflozin doses
The study was conducted at 185 sites in 24 countries (a list of participating investigators can be found in Table S1). A total of 2770 patients were screened and 614 were randomized, 307 to sitagliptin and 307 to dapagliflozin. The study was initiated on October 21, 2015 and completed on October 10, 2017. Of the 614 randomized patients, 595 (96.9%) completed the study, and 494 (80.5%) completed on study medication (Table S2). One patient in the dapagliflozin group, associated with a protocol violation, was randomized but did not take a dose of study medication; this patient was included in the population of randomized patients for the disposition table but was excluded from all efficacy and safety analyses.
Baseline demographics and clinical parameters were similar between the treatment groups (Table 1). The mean age of patients in the study was 67.1 years, approximately 42% were female, mean BMI was 31.6 kg/m2, mean HbA1c was 7.7% (61.1 mmol/mol), and mean duration of diabetes was 10.6 years. All patients in the dapagliflozin group initiated treatment at a dose of 5 mg/d; 94.8% (n = 290) uptitrated to 10 mg/d (Table S3).
Table 1.
Baseline demographic, anthropometric and disease characteristics of study treatment groups
| Sitagliptin n = 307 | Dapagliflozin n = 306 | |
|---|---|---|
| Age, years | 67.7 ± 8.5 | 66.6 ± 8.6 |
| Female, n (%) | 138 (45.0) | 120 (39.2) |
| Race, n (%) | ||
| White | 240 (78.2) | 234 (76.5) |
| Multiple | 30 (9.8) | 39 (12.7) |
| American Indian/Alaska native | 18 (5.9) | 14 (4.6) |
| Asian | 11 (3.6) | 7 (2.3) |
| Black or African American | 8 (2.6) | 11 (3.6) |
| Native Hawaiian or other Pacific Islander | 0 (0.0) | 1 (0.3) |
| Ethnicity, n (%) | ||
| Neither Hispanic nor Latino | 195 (63.5) | 194 (63.4) |
| Hispanic or Latino | 109 (35.5) | 109 (35.6) |
| Not reported | 3 (1.0) | 2 (0.7) |
| Unknown | 0 (0.0) | 1 (0.3) |
| Body weight, kg | 87.4 ± 20.2 | 88.7 ± 18.0 |
| BMI, kg/m2 | 31.8 ± 5.7 | 31.5 ± 5.3 |
| HbA1c, % (mmol/mol) | 7.7 ± 0.7 (60.9 ± 7.9) | 7.8 ± 0.7 (61.2 ± 8.0) |
| FPGa, mmol/L | 9.0 ± 2.2 | 9.2 ± 2.3 |
| eGFR, mL/min/1.73 m2 | 79.4 ± 11.3 | 76.9 ± 12.3 |
| Duration of type 2 diabetes, years | 10.5 ± 7.0 | 10.7 ± 7.4 |
| Background medication | ||
| Metformin alone | 212 (69.1) | 225 (73.5) |
| Metformin + SU | 95 (30.9) | 81 (26.5) |
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; SU, sulfonylurea. Values are mean ± standard deviation unless otherwise noted.
To convert to mg/dL multiply mmol/L value by 18.
3.2. Efficacy
After 24 weeks of treatment, the least squares (LS) mean change from baseline in HbA1c (95% CI) was significantly greater with sitagliptin 100 mg (−0.51% [−0.60, −0.43] [−5.58 mmol/mol {−6.52, −4.65}]) compared with dapagliflozin (−0.36% [−0.45, −0.27] [−3.92 mmol/mol {−4.88, −2.95}]) (Table 2 and Figure 2A); the between‐group difference was −0.15% (−0.26, −0.04) (−1.67 mmol/mol [−2.86, −0.48]); P = 0.006, thus meeting the primary efficacy hypothesis that sitagliptin is non‐inferior to dapagliflozin. In both treatment groups, a near maximum reduction in HbA1c was observed by Week 10 (the first on‐treatment measurement time‐point) and improved glycaemic efficacy continued through the end of treatment (Figure 2A). At Week 24, the between‐group difference (sitagliptin ‐ dapagliflozin) in patients (95% CI) with HbA1c <7.0% (53 mmol/mol) was 15.5% (7.7, 23.2) (Figure 2B).
Table 2.
Efficacy endpoints at week 24
| Parameter | Sitagliptin | Dapagliflozin |
|---|---|---|
| HbA1c, % (mmol/mol) | (n = 307) | (n = 306) |
| Baseline | 7.7 ± 0.7 (60.9 ± 7.9) | 7.8 ± 0.7 (61.2 ± 8.0) |
| Week 24 | 7.1 ± 0.7 (54.1 ± 7.7) | 7.3 ± 0.6 (56.1 ± 7.0) |
| Change from baselinea | −0.51 (−0.60, −0.43) (−5.58 [−6.52, −4.65]) | −0.36 (−0.45, −0.27) (−3.92 [−4.88, −2.95]) |
| Change vs. dapagliflozinb | −0.15* (−0.26, −0.04) (−1.67* [−2.86, −0.48]) | ‐ |
| 2 h‐incremental PPGEc, mmol/L | (n = 295) | (n = 290) |
| Baseline | 5.3 ± 3.1 | 5.3 ± 2.6 |
| Week 24 | 4.0 ± 2.8 | 4.3 ± 2.6 |
| Change from baselinea | −1.3 (−1.7, −1.0) | −1.0 (−1.4, −0.7) |
| Change vs. dapagliflozinb | −0.3 (−0.7, 0.1) | ‐ |
| 2 h PPGc, mmol/L | (n = 296) | (n = 292) |
| Baseline | 14.3 ±3.7 | 14.4 ±3.6 |
| Week 24 | 12.0 ±3.3 | 12.2 ±3.1 |
| Change from baselinea | −2.4 (−2.8, −2.0) | −2.2 (−2.6, −1.8) |
| Change vs. dapagliflozinb | −0.2 (−0.7, 0.3) | ‐ |
| FPGc, mmol/L | (n = 307) | (n = 306) |
| Baseline | 9.0 ±2.2 | 9.2 ±2.3 |
| Week 24 | 8.0 ±1.8 | 7.8 ±1.6 |
| Change from baselinea | −0.9 (−1.1,‐0.7) | −1.1 (−1.3, −0.9) |
| Change vs. dapagliflozinb | 0.2 (−0.1, 0.5) | ‐ |
| Insulin AUC0‐120, mIU.hr/L | (n = 96) | (n = 94) |
| Baseline | 158.5 ± 118.8 | 148.4 ± 98.9 |
| Week 24 | 132.6 ± 103.8 | 121.0 ± 80.1 |
| Change from baselinea | −23.4 (−36.8, −9.9) | −28.2 (−42.1, −14.4) |
| Change vs. dapagliflozinb | 4.9 (−12.2, 22.0) | ‐ |
| Glucagon AUC0‐120, pmol.hr/L | (n = 85) | (n = 88) |
| Baseline | 45.5 ± 18.6 | 52.3 ± 47.1 |
| Week 24 | 42.5 ± 16.9 | 48.0 ± 22.8 |
| Change from baselinea | −4.2 (−8.8, 0.4) | 0.2 (−4.4, 4.8) |
| Change vs. dapagliflozinb | −4.4 (−10.1, 1.4) | ‐ |
| Insulin AUC0‐120: Glucagon AUC0‐120 | (n = 83) | (n = 81) |
| Baseline | 4.1 ± 3.7 | 3.7 ± 3.2 |
| Week 24 | 3.6 ± 3.4 | 2.8 ± 1.9 |
| Change from baselinea | −0.6 (−1.1, −0.0) | −1.2 (−1.8, −0.7) |
| Change vs. dapagliflozinb | 0.6 (−0.1, 1.3) | ‐ |
Abbreviations: AUC, area under the curve; FPG, fasting plasma glucose; PPG, postprandial glucose; PPGE, postprandial glucose excursion.
Values are mean ± standard deviation unless otherwise noted.
*P = 0.006.
Least squares (LS) mean (95% CI).
Difference in LS means (95% CI).
To convert to mg/dL multiply mmol/L value by 18.
Figure 2.
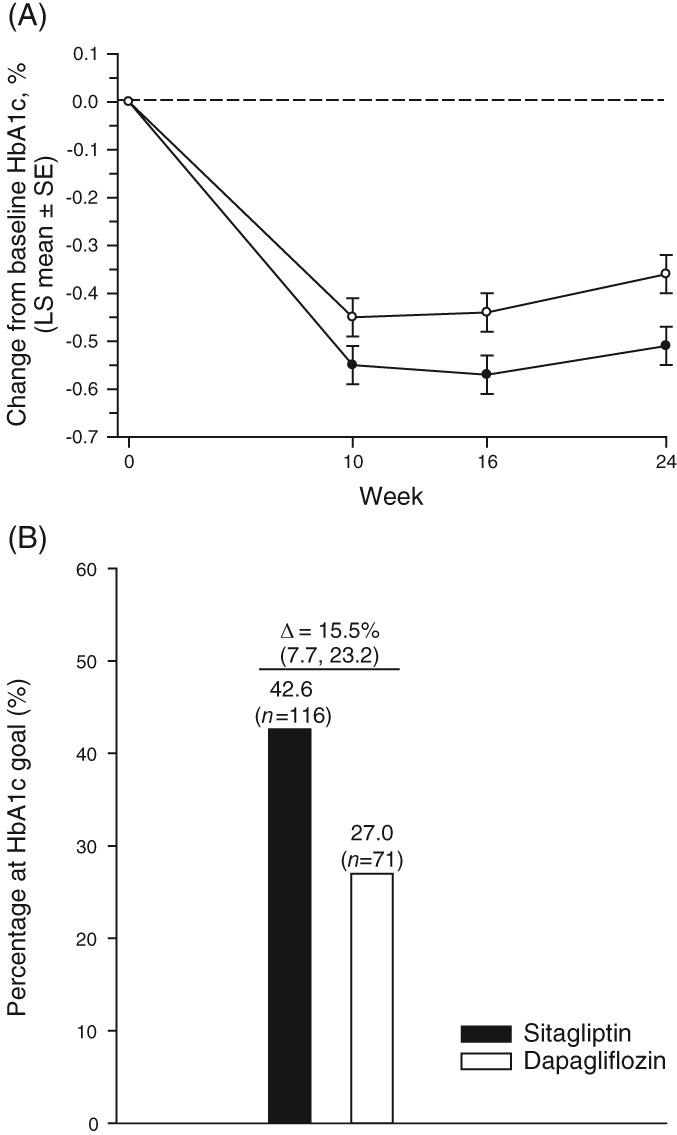
HbA1c measures through week 24: A, LS mean ± SE change from baseline HbA1c (%); black circles = sitagliptin, open circles = dapagliflozin; B, percentage of patients at goal of HbA1c <7% at week 24. For both A and B, results were calculated using the LDA model described in Methods
At Week 24, there were no significant between‐group differences in changes from baseline in LS mean 2‐hour incremental PPGE, 2‐hour PPG, FPG, or in changes from baseline in postmeal glucagon AUC0‐120, insulin AUC0‐120, or the ratio of insulin AUC0‐120 to glucagon AUC0‐120 (Table 2).
Throughout the trial (the 24‐week treatment period plus the 2‐week safety follow‐up period), a lower percentage of patients in the sitagliptin group had one or more telephone contacts associated with personal health concerns (not study‐scheduled contacts) compared with the dapagliflozin group (6.8% vs. 11.8%, respectively). The total number of telephone contacts was also lower in the sitagliptin group than in the dapagliflozin group (30 vs. 58, respectively). The percentage of patients with one or more in‐person visits associated with personal health concerns (not scheduled study visits) was similar in the sitagliptin and dapagliflozin groups (35.2% and 35.6%, respectively); the total number of in‐person visits was 247 in the sitagliptin group and 272 in the dapagliflozin group.
3.3. Safety and tolerability
During the study, 48.9% of patients in the sitagliptin group experienced one or more AEs compared with 51.6% in the dapagliflozin group. No patients died. With the exception of drug‐related AEs (7.8% in the sitagliptin group and 13.7% in the dapagliflozin group, between‐group difference [95% CI] −5.9 [−11.0, −1.0]), summary measures of AEs were similar between groups (Table 3).
Table 3.
Adverse events (AEs) summary and AEs of hypoglycaemia
| Patients, n (%) | Sitagliptin n = 307 | Dapagliflozin n = 306 | Differencea |
|---|---|---|---|
| With one or more | |||
| AEs | 150 (48.9) | 158 (51.6) | −2.8 (−10.1, 5.1) |
| Drug‐relatedb AEs | 24 (7.8) | 42 (13.7) | −5.9 (−11.0, −1.0) |
| Serious AEs | 10 (3.3) | 13 (4.2) | −1.0 (−4.3, 2.2) |
| Serious drug‐relatedb AEs | 0 (0.0) | 1 (0.3) | −0.3 |
| Who died | 0 (0.0) | 0 (0.0) | 0.0 |
| Who discontinued due to | |||
| An AE | 10 (3.3) | 10 (3.3) | −0.0 (−3.0, 3.0) |
| A drug‐relatedb AE | 5 (1.6) | 6 (2.0) | −0.3 (−2.8, 2.0) |
| A serious AE | 3 (1.0) | 3 (1.0) | −0.0 |
| A serious drug‐relatedb AE | 0 (0.0) | 1 (0.3) | −0.3 |
| Patients on metformin alone | (n = 212) | (n = 225) | |
| With one or more AE of hypoglycaemia | 7 (3.3) | 8 (3.6) | −0.3 (−4.0, 3.5) |
| Symptomaticc | 5 (2.4) | 7 (3.1) | −0.8 (−4.2, 2.7) |
| Documentedd | 5 (2.4) | 7 (3.1) | −0.8 (−4.2, 2.7) |
| Severee | 1 (0.5) | 2 (0.9) | −0.4 |
| Asymptomaticf | 2 (0.9) | 2 (0.9) | 0.1 |
| Patients on metformin and a sulfonylurea | (n = 95) | (n = 81) | |
| With one or more AE of hypoglycaemia | 15 (15.8) | 13 (16.0) | −0.3 (−11.6, 10.7) |
| Symptomaticc | 13 (13.7) | 10 (12.3) | 1.3 (−9.2, 11.5) |
| Documentedd | 13 (13.7) | 9 (11.1) | 2.6 (−7.8, 12.6) |
| Severee | 0 (0.0) | 0 (0.0) | 0.0 |
| Asymptomaticf | 6 (6.3) | 4 (4.9) | 1.4 (−6.5, 8.9) |
Difference in % vs. dapagliflozin; estimate (95% CI) was computed only for AE summary and hypoglycaemia endpoints with at least 4 patients having events in one or more treatment groups.
Assessed by the investigator as related to study drug.
Symptomatic hypoglycaemia: episode with clinical symptoms attributed to hypoglycaemia, without regard to glucose level.
Documented symptomatic hypoglycaemia: episode with clinical symptoms attributed to hypoglycaemia with a documented glucose level of ≤3.9 mmol/L (≤70 mg/dL).
Severe hypoglycaemia: episode that required assistance, either medical or non‐medical. Episodes with a markedly depressed level of consciousness, a loss of consciousness, or seizure were classified as having required medical assistance, whether or not medical assistance was obtained.
Asymptomatic hypoglycaemia: finger‐stick glucose values ≤3.9 mmol/L (≤70 mg/dL) without symptoms.
The incidences of AEs and of specific AEs by system organ class (SOC) reported for ≥4 patients in at least one treatment group were generally similar between the treatment groups (Table S4). Infections and infestations was the only SOC in which the 95% CI for the between‐group difference in incidence excluded 0; in this SOC the incidence of AEs was higher in the dapagliflozin group (n = 66 [21.6%]) than in the sitagliptin group (n = 46 [15.0%]), between‐group difference (in %) = −6.6 [−12.7, −0.5], in part due to a higher observed incidence of genital mycotic infections in the dapagliflozin group. The incidences of specific AEs were generally similar between the sitagliptin and dapagliflozin groups during the treatment period. The only specific AEs that occurred at a higher observed incidence in one group compared with the other (95% CI for the between‐group difference in incidence excluded 0) were abdominal pain and vomiting (higher in the sitagliptin group than in the dapagliflozin group ‐ abdominal pain: sitagliptin n = 5 [1.6%], dapagliflozin n = 0 [0.0%], difference [in %] = 1.6 [0.4, 3.8]; vomiting: sitagliptin n = 4 [1.3%], dapagliflozin n = 0 [0.0%], difference [in %] = 1.3 [0.1, 3.3]) and edema peripheral (higher in the dapagliflozin group n = 4 [1.3%] than in the sitagliptin group, n = 0 [0.0%]; difference [in %] = −1.3 [−3.3, −0.1]).
The incidences of patients with documented symptomatic hypoglycaemia, severe hypoglycaemia and asymptomatic hypoglycaemia were similar between the two treatment groups (Table 3). There were higher incidences of patients with AEs of hypoglycaemia in the population whose background medication included an SU (15.8% and 16.0% in the sitagliptin and dapagliflozin groups, respectively) compared with the population not using an SU (3.3% and 3.6% in the sitagliptin and dapagliflozin groups, respectively) (Table 3).
There was a lower incidence of genital mycotic infection‐related AEs in the sitagliptin group compared with the dapagliflozin group in both men (0.6% vs. 4.3%, respectively) and women (0.0% vs. 5.0%, respectively). The incidences of volume depletion events were low in both groups (0.7% and 1.3% in the sitagliptin and dapagliflozin groups, respectively). The proportions of participants who met PDLC criteria for laboratory parameters were similar between the sitagliptin and dapagliflozin groups, and no clinically meaningful between‐group differences were observed. The proportion of participants with at least one eGFR decrease from baseline >30% was similar between the sitagliptin and dapagliflozin groups (4.3% and 5.6%, respectively), while three participants (1.0%) in the sitagliptin group and none in the dapagliflozin group had at least one eGFR decrease from baseline >50%. Greater decreases from baseline in mean systolic blood pressure and body weight were observed in the dapagliflozin group than in the sitagliptin group through Week 24 (Figure S1).
4. DISCUSSION
In the clinical trial described here, in subjects with type 2 diabetes and mild renal insufficiency, sitagliptin improved glycaemic control to a greater extent than dapagliflozin. These data provide clinical trial evidence that can inform patient‐centered decisions for the treatment of patients with type 2 diabetes and mild renal insufficiency.9 While clinical trials have been conducted to study the safety and efficacy of various AHAs in patients with moderate or severe renal insufficiency (eGFR <60 mL/min/1.73 m2), there are limited data defining the safety and efficacy profile of these agents in the cohort of patients with type 2 diabetes and mild renal insufficiency (eGFR ≥60 to <90 mL/min/1.73 m2).
The overall prevalence of patients with type 2 diabetes and mild renal insufficiency is estimated to be nearly 40% and prevalence increases with age (estimated to be nearly 50% in patients with type 2 diabetes ≥65 years of age).1 Improved glycaemic control has been shown to reduce the risk of diabetic complications and to slow progression of renal impairment.10 While metformin is the standard first‐line pharmacologic intervention for the management of hyperglycaemia in type 2 diabetes, additional therapies, including oral agents such as an SU, a DPP‐4 inhibitor, or an SGLT‐2 inhibitor are often prescribed in patients with mild renal insufficiency to achieve glycaemic control. This paper provides data on the efficacy and safety of sitagliptin and dapagliflozin, in combination with other commonly used AHAs, in patients with type 2 diabetes and mild renal insufficiency.
DPP‐4 inhibitors are often used in patients with type 2 diabetes and renal disease because these agents maintain efficacy and demonstrate good tolerability across the spectrum of renal disease.3 On the other hand, the efficacy of SGLT‐2 inhibitors is reduced in patients with moderate renal insufficiency and is contraindicated in patients with severe renal insufficiency.11 These clinical observations are consistent with the distinct mechanisms of action of the two classes of agents. DPP‐4 inhibitors stabilize the incretins GLP‐1 and GIP, two peptides which stimulate the release of insulin in a glucose‐dependent manner,12 while the mechanism of action of SGLT‐2 inhibitors depends on renal function.13 However, until now, these two classes of AHAs have not been prospectively evaluated in a study limited to a population of patients with mild renal insufficiency.
In the current study, 24 weeks of treatment with the DPP‐4 inhibitor sitagliptin was associated with greater reduction from baseline in HbA1c compared with the SGLT‐2 inhibitor dapagliflozin; in addition, after 24 weeks, more patients met the HbA1c goal of <7% with sitagliptin than with dapagliflozin. Both treatments resulted in reductions from baseline in the 2‐hour incremental PPGE and 2‐hour PPG. While the reductions with sitagliptin were slightly larger than with dapagliflozin for both of these postprandial glycaemic endpoints, there were no significant between‐group differences in these parameters.
Both treatments were generally well tolerated. The treatment groups had similarly low rates of hypoglycaemia when the background medication was metformin alone. When the background medication included a sulfonylurea, the rates of hypoglycaemia were higher and similar in both groups, as expected due to the influence of this class of agent.14, 15, 16 With regard to the small increase in the observed incidences of abdominal pain and vomiting with sitagliptin compared with dapagliflozin, and of edema peripheral with dapagliflozin compared with sitagliptin, imbalances of this type have not previously been noted in pooled safety analyses of either treatment.17, 18
Overall, the incidence of drug‐related AEs was higher in the dapagliflozin group than in the sitagliptin group. Most of the drug‐related AEs that occurred at a higher observed incidence with dapagliflozin compared with sitagliptin were in categories of events that have been associated with SGLT‐2 inhibitor treatment, in particular, genital mycotic infections.19 The increased risk of genital mycotic infections is typical of the class of SGLT‐2 inhibitors and is likely to be related to the mechanism of action of the class, which results in increased glycosuria. Other between‐group differences observed were related to blood pressure and body weight. Greater mean decreases from baseline in systolic blood pressure and body weight were observed in the dapagliflozin group than in the sitagliptin group, as expected with SGLT‐2 inhibitors.20 No meaningful changes from baseline in mean blood pressure or body weight were observed in the sitagliptin group.
The results of this clinical trial may be of particular interest to physicians treating older patients with type 2 diabetes. Age is associated with reduction in renal function,21 and together with potentially longer duration of diabetes, older patients are likely to be at increased risk of microvascular complications22 including renal impairment and other co‐morbidities.23
A limitation of this study is that the results are relevant to the population studied and not necessarily to patients at other stages of CKD. Another limitation is that the study evaluated sitagliptin and dapagliflozin, and results cannot be extrapolated to other DPP‐4 or SGLT‐2 inhibitors. The strengths of this study are: its large sample size, which allowed a robust estimate of between‐group differences in efficacy; the requirement for duplicate eGFR measurements during screening that ensured a population with stable renal status; and uptitration of dapagliflozin to its maximal approved dose (achieved in approximately 95% of patients). Lastly, the CKD‐epi formula used to estimate GFR in this study is considered more accurate and less likely to underestimate GFR at higher levels of renal function (≥ 60 mL/min/1.73 m2) than the Modification of Diet in Renal Disease estimate.24, 25
In summary, in patients with type 2 diabetes and mild renal insufficiency who were inadequately controlled on metformin ± sulfonylurea, treatment with sitagliptin compared with dapagliflozin demonstrated greater glycaemic efficacy, a greater percentage of patients at glycaemic goal, and a good safety profile. Additional prospective studies, evaluating the safety and efficacy of other AHAs in patients with mild renal impairment, could be of value to prescribing physicians. Physicians and patients should consider the level of a patient's renal function, including mild renal insufficiency, when choosing antihyperglycaemic therapy.
Supporting information
Appendix S1. Supplementary Text.
Figure S1. Mean systolic blood pressure (A) and body weight (B) through Week 24; black circles = sitagliptin, open circles = dapagliflozin.
Table S1. List of participating investigators
Table S2. Patient disposition; *one patient in the dapagliflozin group, associated with a protocol violation, was randomized but did not take a dose of study medication. The subject is included in this table but excluded from all efficacy and safety analyses; eGFR = estimated glomerular filtration rate.
Table S3. Summary of dapagliflozin placebo and dapagliflozin titration status
Table S4. Incidences of adverse events by system organ class, occurring in ≥4 patients in one or more treatment group.
ACKNOWLEDGMENTS
The authors wish to thank Elizabeth Ommen, Mahesh Patel, and Lei Xu (Merck & Co., Inc., Kenilworth, NJ) for contributing to the study design and/or execution of this clinical trial. The authors also thank Jennifer Rotonda, Michele McColgan, and Alan Meehan (Merck & Co., Inc., Kenilworth, NJ) for their assistance in preparing this paper for publication.
Conflict of interest
J. M., Z. Z., R. L. H. L., E. A. O., K. D. K., S. S. E. and A. R. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, and may hold stock and/or stock options in the company. R. S. has no conflicts of interest to declare.
Author contributions
All of the authors are responsible for the work described in this paper. J. M., Z. Z., R. L. H. L., K. D. K., S. S. E. and A. R. conceived, designed and/or planned the study. A. R. acquired the data. Z. Z. and A. R. analyzed the data. R. S., J. M., R. L. H. L., E. A. O., K. D. K., S. S. E. and A. R. interpreted the results. Z. Z., E. A. O. and A. R. drafted the manuscript. R. S., J. M., R. L. H. L., K. D. K., S. S. E. and A. R. critically reviewed and/or revised the manuscript for important intellectual content. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data availability
Merck & Co., Inc.’s data sharing policy, including restrictions, is available at http://engagezone.merck.com/ds_documentation.php. Requests for access to the study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Scott R, Morgan J, Zimmer Z, et al. A randomized clinical trial of the efficacy and safety of sitagliptin compared with dapagliflozin in patients with type 2 diabetes mellitus and mild renal insufficiency: The CompoSIT‐R study. Diabetes Obes Metab. 2018;20:2876–2884. 10.1111/dom.13473
Funding information This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
REFERENCES
- 1. Bailey RA, Wang Y, Zhu V, Rupnow MF. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on kidney disease: improving global outcomes (KDIGO) staging. BMC Res Notes. 2014;7:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hahr AJ, Molitch ME. Management of diabetes mellitus in patients with chronic kidney disease. Clin Diabetes Endocrinol. 2015;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deacon CF. A review of dipeptidyl peptidase‐4 inhibitors. Hot topics from randomized controlled trials. Diabetes Obes Metab. 2018;20(Suppl 1):34‐46. [DOI] [PubMed] [Google Scholar]
- 4. Arnouts P, Bolignano D, Nistor I, et al. Glucose‐lowering drugs in patients with chronic kidney disease: a narrative review on pharmacokinetic properties. Nephrol Dial Transplant. 2014;29:1284‐1300. [DOI] [PubMed] [Google Scholar]
- 5. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liang KY, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre‐post designs. Indian J Statist. 2000;62:134‐148. [Google Scholar]
- 7. Miettinen O, Nurminen M. Comparative analysis of two rates. StatMed. 1985;4:213‐226. [DOI] [PubMed] [Google Scholar]
- 8. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 9. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of Hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2012;35:1364‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977‐986. [DOI] [PubMed] [Google Scholar]
- 11. Kohan DE, Fioretto P, Tang W, List JF. Long‐term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plosker GL. Sitagliptin: a review of its use in patients with type 2 diabetes mellitus. Drugs. 2014;74:223‐242. [DOI] [PubMed] [Google Scholar]
- 13. Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. SGLT2 inhibitors and the diabetic kidney. Diabetes Care. 2016;39(Suppl 2):S165‐S171. [DOI] [PubMed] [Google Scholar]
- 14. Rosenstock J, Einhorn D, Hershon K, Glazer NB, Yu S. Efficacy and safety of pioglitazone in type 2 diabetes: a randomised, placebo‐controlled study in patients receiving stable insulin therapy. Int J Clin Pract. 2002;56:251‐257. [PubMed] [Google Scholar]
- 15. Raskin P, Rendell M, Riddle MC, Dole JF, Freed MI, Rosenstock J. A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin‐treated type 2 diabetes. Diabetes Care. 2001;24:1226‐1232. [DOI] [PubMed] [Google Scholar]
- 16. Sheffield CA, Kane MP, Busch RS, Bakst G, Abelseth JM, Hamilton RA. Safety and efficacy of exenatide in combination with insulin in patients with type 2 diabetes mellitus. Endocr Pract. 2008;14:285‐292. [DOI] [PubMed] [Google Scholar]
- 17. Engel SS, Round E, Golm GT, Kaufman KD, Goldstein BJ. Safety and tolerability of sitagliptiin in type 2 diabetes: pooled analysis of 25 clinical studies. Diabetes Ther. 2013;4:119‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, Langkilde AM. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab. 2018;20:620‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scheen AJ. SGLT2 inhibition: efficacy and safety in type 2 diabetes treatment. Expert Opin Drug Saf. 2015;14:1879‐1904. [DOI] [PubMed] [Google Scholar]
- 20. Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium‐glucose co‐transporter‐2 inhibitors in type 2 diabetes mellitus: systematic review and network meta‐analysis. Diabetes Obes Metab. 2016;18:783‐794. [DOI] [PubMed] [Google Scholar]
- 21. Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis. 2010;17:302‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nathan DM, Singer DE, Godine JE, Harrington CH, Perlmuter LC. Retinopathy in older type II diabetics. Association with glucose control. Diabetes. 1986;35:797‐801. [DOI] [PubMed] [Google Scholar]
- 23. Del Prato S, Heine RJ, Keilson L, Guitard C, Shen SG, Emmons RP. Treatment of patients over 64 years of age with type 2 diabetes: experience from nateglinide pooled database retrospective analysis. Diabetes Care. 2003;26:2075‐2080. [DOI] [PubMed] [Google Scholar]
- 24. Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749‐2757. [DOI] [PubMed] [Google Scholar]
- 25. Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5‐18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary Text.
Figure S1. Mean systolic blood pressure (A) and body weight (B) through Week 24; black circles = sitagliptin, open circles = dapagliflozin.
Table S1. List of participating investigators
Table S2. Patient disposition; *one patient in the dapagliflozin group, associated with a protocol violation, was randomized but did not take a dose of study medication. The subject is included in this table but excluded from all efficacy and safety analyses; eGFR = estimated glomerular filtration rate.
Table S3. Summary of dapagliflozin placebo and dapagliflozin titration status
Table S4. Incidences of adverse events by system organ class, occurring in ≥4 patients in one or more treatment group.
Data Availability Statement
Merck & Co., Inc.’s data sharing policy, including restrictions, is available at http://engagezone.merck.com/ds_documentation.php. Requests for access to the study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.


