Abstract
Failure to follow-up nonurgent, clinically significant test results (CSTRs) is an ambulatory patient safety concern. Tools within electronic health records (EHRs) may facilitate test result acknowledgment, but their utility with regard to nonurgent CSTRs is unclear. We measured use of an acknowledgment tool by 146 primary care physicians (PCPs) at 13 network-affiliated practices that use the same EHR. We then surveyed PCPs to assess use of, satisfaction with, and desired enhancements to the acknowledgment tool. The rate of acknowledgment of non-urgent CSTRs by PCPs was 78%. Of 73 survey respondents, 72 reported taking one or more actions after reviewing a CSTR; fewer (40–75%) reported that using the acknowledgment tool was helpful for a specific purpose. Forty-six (64%) were satisfied with the tool. Both satisfied and nonsatisfied PCPs reported that enhancements linking acknowledgment to routine actions would be useful. EHR vendors should consider enhancements to acknowledgment functionality to ensure follow-up of nonurgent CSTRs.
Keywords: test result acknowledgment, clinical significant test results, closed loop communication, patient safety
INTRODUCTION
Test result management is a multi-step process that involves ordering, reporting, reviewing, and acting. This process is critical for clinically significant test results (CSTRs), those results that require clinical action to avoid morbidity and mortality regardless of the urgency of that action.1 Life-threatening CSTRs (e.g., pneumothorax on chest X-ray) should be communicated directly to the responsible clinician. When consequences are immediate, verbal acknowledgment (“read-back”) is much more likely to result in timely follow-up. These CSTRs are the subject of standardized management at most institutions.
Nonurgent CSTRs (e.g., abnormal HbA1c, an incidental pulmonary nodule on chest imaging) require follow-up, but not immediately. Nonurgent CSTRs have received far less attention, but require standardized management nonetheless.2 Failure to follow-up nonurgent CSTRs may result in delays in diagnosis, missed treatment opportunities, additional healthcare utilization, and malpractice litigation.3–7 To ensure that appropriate follow-up occurs, authorities suggest establishing communication policies, clarifying roles and responsibilities, defining levels of urgency and expectations for follow-up, and implementing a process for escalating unacknowledged results after predefined time intervals for CSTRs.2,8
Health information technology has the potential to facilitate more seamless management of CSTRs in the ambulatory setting.9,10 Management of nonurgent CSTRs within electronic health records (EHRs), however, is less studied; patient safety concerns related to incomplete follow-up persist.7,8,11,12 Typically, CSTRs are acknowledged electronically to track whether the responsible clinician was aware of a significant abnormality. Limited data exist with regard to how clinicians actually use and perceive test result acknowledgment tools.
Poon et al.9 previously developed a Results Manager (RM) application within the EHR at Partners Healthcare, Inc. RM has been widely adopted by primary care physicians (PCPs) and includes an acknowledgement tool. The extent to which clinicians use and are satisfied with this feature is not known. The purpose of this study is to determine how often nonurgent CSTRs are acknowledged, verify typical actions taken after acknowledging test results, assess reported use and satisfaction with the acknowledgement tool, and evaluate the perceived utility of proposed enhancements.
METHODS
Setting and Participants: We conducted our study at Partners, Inc., an integrated healthcare delivery network in Boston, MA, USA. The study was approved by the Partners Institutional Review Board. All Partners affiliated primary care practices use the Longitudinal Medicine Record, the ambulatory EHR. PCPs affiliated with these practices from January 2011 through January 2012 were included.
RM Acknowledgment Tool (Figure 1a and 1b): See Figure 1a. RM is a web-based module accessible from Longitudinal Medicine Record with access to all data available in the Partners Clinical Data Repository.9 Partners’ PCPs typically use RM to manage ambulatory test results. Clinicians review results associated with a patient visit individually, and can choose from one of several actions: acknowledge result, forward result, and create or addend the patients’ results letter (see Figure 1b).
Figure 1a.
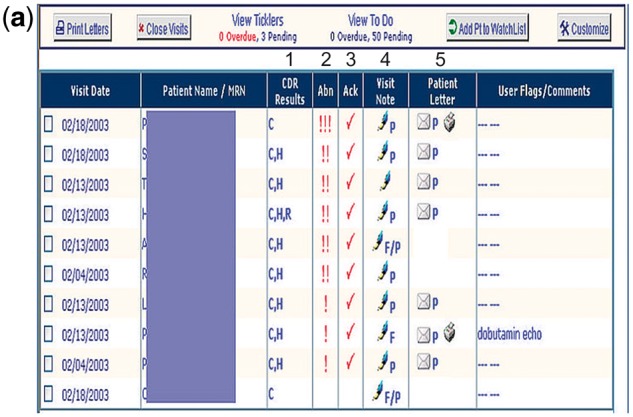
Results Manager.
1Clinicians can access chemistry (C), hematology (H), and radiology (R) results available in the Partners CDR.
2Abnormal results are automatically flagged by severity:!!! = life-threatening,!! = urgent,! = nonurgent.
3A checkmark under the “Ack” column appears after all results associated with a visit have been explicitly acknowledged.
4Clinicians can access visit notes associated with the ordered test, and can generate a prepopulated patient results letter to enhance and expedite communication of test results.
5Clinicians may record follow-up tasks in the “User Flags/Comments” column.
Figure 1b.
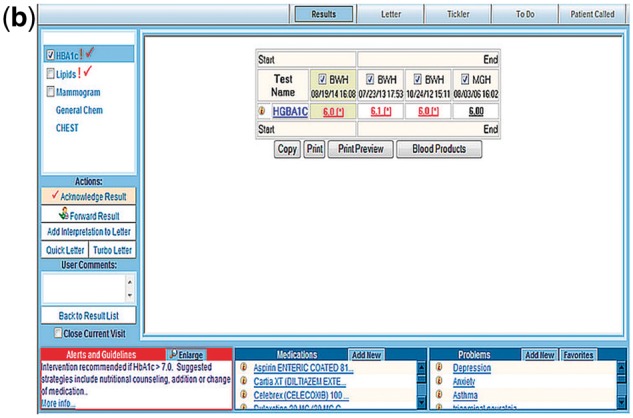
Results Manager: Acknowledgement Tool.
Query of nonurgent CSTRs: We defined nonurgent CSTRs as results that do not require immediate action to avoid morbidity and mortality (no action required within 48 h of reporting). Nonurgent CSTRs have a longer time interval for acknowledgment (see below).1 We selected six nonurgent CSTRs to determine the extent to which clinicians acknowledge results from within their RM queue. These included abnormal HbA1c, HbsAg, HCV Ab, LDL, urine HCG, and PSA results (level of urgency denoted by one (!) or two (!!) exclamation points). We queried the RM queues of all PCPs over a 12-month period to quantify use of the acknowledgement tool for these nonurgent CSTRs. A result was considered acknowledged when the “Acknowledge Result” button was clicked by the PCP.
Partners’ Communicating CCSTR Task Force: As part of a network-wide initiative to standardize test result communication and acknowledgment policies, we visited all PCPs at affiliated practices during 2011. Our goals were to (1) review standardized test result definitions and policies regarding CSTRs, (2) demonstrate how these definitions correlate with severity as designated by RM (!!!,!!,!), and (3) review the standardized timeframes for acknowledgment.1 The timeframe for acknowledging nonurgent CSTRs was 15 days.
Surveys: We developed a survey instrument to assess frequency of RM use (semi-quantitative), typical actions taken after reviewing a CSTR (all that apply), frequency of electronic acknowledgement (semi-quantitative), reasons for using electronic acknowledgment (all that apply), satisfaction with use (5 point Likert scale), and enhancements desired (all that apply). The survey questions were vetted and refined by members of the Partners communicating CSTR Task Force and a small group of practicing internists (<5) to ensure appropriate context and consistent understanding. We emailed surveys to PCPs in December 2011. Participants were provided space for comments and suggestions. All participants received a small financial incentive ($10 Amazon.com gift card) upon completion of the survey. All survey data were collected and managed using REDCap hosted by Partners, Inc.13
Statistical and qualitative analyses: We used descriptive statistics to report provider demographic data and to quantify reasons for use of, actions taken after, and desired enhancements to RM acknowledgment. We compared the proportion of nonurgent CSTRs acknowledged by survey respondents and nonrespondents using a chi-squared analysis (2-sided, p < 0.05). We dichotomized and compared user satisfaction data using Fisher’s Exact test (2-sided, p < 0.05). User comments were analyzed and grouped by themes using a two-person consensus approach. Responses from semi-quantitative questions were correlated with usage data from the RM query.
RESULTS
We sent surveys to 146 Partners PCPs at 13 primary care practices. We received 73 complete responses (response rate 50%). Surveyed PCPs had the following characteristics: 37 (51%) female, 50.2 ± 10.7 years of age, 22.9 ± 10.7 years of clinical experience, and 13.1 ± 8.9 years of work experience at Partners. We identified a total of 15 102 non-urgent CSTRs from our query of RM queues; of these, surveyed PCPs acknowledged 11 718 (acknowledgment rate 78%). PCP survey respondents and non-respondents acknowledged 6416 of 7639 (84%) and 5302 of 7463 (71%) nonurgent CSTRs, respectively (p < 0.0001).
Of the 73 PCPs who responded to the survey, 72 (99%) reported that they used RM. These 72 PCPs reported that they perform one or more actions after reviewing CSTRs; on average, they selected 6.5 ± 1.5 actions listed in the survey (Table 1).
Table 1:
Typical actions taken by PCPs after reviewing CSTRs
| Actions | N (%) of respondents, n = 72 |
|---|---|
| Contact patient | 72 (100) |
| Order additional test/study | 68 (94.4) |
| Contact another clinician | 60 (83.3) |
| Schedule follow-up | 60 (83.3) |
| Add issue to problem list | 58 (80.6) |
| Refer to specialist or other clinician | 54 (75.0) |
| Refer patient to emergency department | 45 (62.5) |
| Send clinical message | 41 (56.9) |
| Directly admit patient | 12 (16.7) |
Of the 72 PCPs who used RM, 48 (67%) “always” and 24 (33%) “sometimes” used the acknowledgement tool. When asked why they use the acknowledgment tool, 54 (75%) reported that it helps them review and acknowledge results as they return, 45 (62.5%) reported that it helps them track which CSTRs they have previously acknowledged, 29 (40.3%) reported that it helps them track results on which they have performed an action, 33 (45.8%) reported that it improves workflow efficiency, and 46 (63.9%) reported that it helps them systematically work through their RM results queue. Eleven (15.3%) reported that they use the acknowledgement function for other reasons, including five (45.0%) who commented that acknowledgment is required for legal/liability purposes (“required to lower liability risk,” “no idea why it is even there, suspect for legal reasons to cover their !%# of lab and radiology results so that the clinician can be blamed for anything missed”).
Of the 72 PCPs that used RM’s acknowledgment tool, 46 (63.9%) stated they were satisfied. On average, they selected 2.7 ± 1.9 suggested enhancements (Table 2). We did not observe a statistically significant difference for any enhancement by satisfied and nonsatisfied RM users (Table 2).
Table 2:
Suggested enhancements to RM’s acknowledgment tool
| Enhancement | N (%) of respondents (n = 72) | N (%) of satisfied users (n = 46) | N (%) of nonsatisfied users (n = 26) | p-value |
|---|---|---|---|---|
| Links to tools that facilitate routine actionsa | 36 (50) | 22 (48) | 14 (54) | 0.81 |
| Links to patient communication toolsb | 33 (45.8) | 25 (54) | 9 (35) | 0.14 |
| Ability to flag incorrectly routed results | 28 (38.9) | 21 (46) | 7 (27) | 0.14 |
| Links to other clinical information systems | 27 (37.5) | 17 (37) | 10 (38) | 1.0 |
| Ability to reconcile/acknowledge alerts from other systems | 27 (37.5) | 19 (41) | 8 (31) | 0.45 |
| Notify other clinicians of actions | 17 (23.6) | 13 (28) | 4 (15) | 0.26 |
| Notify other clinicians of acknowledgment | 9 (12.5) | 7 (15) | 2 (8) | 0.47 |
aRoutine actions include ordering additional tests/studies, updating the problem list, scheduling follow-up appointments, referring to a specialist or another provider, sending a clinical message
bMethods of communicating with the patient include phone, letters, and secure messaging.
Twenty-seven survey respondents (37.5%) provided written suggestions on which we conducted our qualitative analysis. We identified four major themes for enhancements: (1) configurable settings to view acknowledged and pending results, and to prompt acknowledgement when performing a task in which those results are reviewed (“ability to see at a glance which test results have been acknowledged”); (2) integration with follow-up reminders and to-do lists that prompt clinicians to perform future tasks (“it would be neat if you could create a ‘to do’ that would remind either yourself or anyone else caring for the patient what needed to be done”); (3) linkages to other components of the EHR, such as direct patient messaging (via patient portal), problem list, and appointment and scheduling tools; and, (4) reconfiguration to minimize duplication of activities (reviewing related documentation, reconciling alerts from other systems).
DISCUSSION
We observed that only 78% of nonurgent CSTRs were acknowledged electronically by surveyed PCPs. Moreover, we found a significantly lower rate of acknowledgement of nonurgent CSTRs by PCPs who did not respond to the survey. From our survey, we found that while nearly all PCPs used RM to manage test results, fewer (40–75%) reported that using the acknowledgment tool was helpful for a specific purpose despite taking one or more actions after reviewing a result. Sixty-three percent of surveyed PCPs reported that they were satisfied with the acknowledgement tool, but satisfied and nonsatisfied users alike thought suggested enhancements to current functionality would be desirable.
We believe that the lack of uniform use of RM’s acknowledgement tool is due to the following reasons: suboptimal integration of standard EHR tools that facilitate typical actions (securely messaging patients, ordering additional tests, scheduling follow-up, updating problem list, etc.); poor workflow integration (toggling between RM and other EHR modules, reviewing documentation of previously acknowledged results); poor transparency of test result acknowledgement and actions taken by other clinicians, lack of reconciliation of acknowledgment with other systems); perceptions of added work without added benefit; and misperceptions of its intended purpose (for legal/liability purposes).
Singh et al.11,14 previously reported that up to a third of test results remain unacknowledged, and that timely follow-up action is equally lacking for both acknowledged and unacknowledged results. While we report a similar finding, recent work demonstrates that result notification tools can improve rates of acknowledgement when designed effectively; however, the impact of these systems on rates of nonurgent CSTR follow-up remains unclear.15–18 In theory, closely tethering acknowledgment to other EHR functionality could enhance patient safety by ensuring that the closed loop communication process prompts timely action when necessary (linking acknowledgment to action). Interestingly, all surveyed PCPs in this study reported that they contacted patients after acknowledging results; this is not surprising given the close integration of the patient letter tool within RM. Thus, while acknowledging an abnormally elevated HbA1c flagged in a physician’s RM queue, an integrated decision support tool could prompt the physician to re-order HbA1c to be drawn in 3 months or create a follow-up reminder in 3 months. Similarly, if an indeterminate pulmonary nodule is identified in a radiology report and flagged in RM, then presenting evidenced-based recommendations for follow-up imaging to the responsible clinician, facilitating ordering of future imaging studies, or integrating tools that add “pulmonary nodule” to the active problem list could ensure that follow-up actions occur more reliably. Linking electronic acknowledgment functionality to components of the EHR in these ways could provide safety backstops for nonurgent CSTRs, thereby preventing morbidity related to delays in diagnosis or treatment.1
Our study has several limitations. First, because our sample size was small, our results may be subject to sampling bias. Second, responders were more likely than nonresponders to use RM’s acknowledgement tool; thus, our findings may be biased towards reporting the experience of those survey respondents who use RM more regularly, are more familiar with its functionality, and therefore, are more satisfied. However, this would suggest that satisfaction was actually worse than reported. Finally, our findings may not be generalizable as it took place at a single integrated healthcare network that uses a proprietary EHR and RM application.
In summary, results management applications should consider linking acknowledgment functionality to typical actions that clinicians take to facilitate follow-up, such as scheduling follow-up, reviewing evidence-based follow-up recommendations, setting reminders for future tasks, and messaging patients securely. Such enhancements would safeguard against non-urgent CSTRs “falling through the cracks,” perhaps allowing clinicians to perceive electronic acknowledgment as a value added task.5
FUNDING
This work was supported by the Agency for Healthcare Research and Quality (AHRQ), grant number R18 HS019603-03. AHRQ had no role in the design or conduct of the study; collection, analysis, or interpretation of data; or preparation or review of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of AHRQ.
COMPETING INTERESTS
None.
CONTRIBUTORS
All authors have contributed sufficiently and meaningfully to the conception, design, and conduct of the study; data acquisition, analysis, and interpretation; and/or drafting, editing, and revising the manuscript.
Supplementary Material
REFERENCES
- 1. Roy CL, Rothschild JM, Dighe AS, et al. An initiative to improve the management of clinically significant test results in a large health care network. Jt Comm J Qual Patient Saf. 2013;39:517–527. [DOI] [PubMed] [Google Scholar]
- 2. Singh H, Vij MS. Eight recommendations for policies for communicating abnormal test results. Jt Comm J Qual Patient Saf. 2010;36:226–232. [DOI] [PubMed] [Google Scholar]
- 3. Wahls T, Haugen T, Cram P. The continuing problem of missed test results in an integrated health system with an advanced electronic medical record. Jt Comm J Qual Patient Saf. 2007;33:485–492. [DOI] [PubMed] [Google Scholar]
- 4. Wahls TL, Cram PM. The frequency of missed test results and associated treatment delays in a highly computerized health system. BMC Fam Pract. 2007;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gandhi TK, Kachalia A, Thomas EJ, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Int Med. 2006;145:488–496. [DOI] [PubMed] [Google Scholar]
- 6. Callen J, Georgiou A, Li J, Westbrook JI. The safety implications of missed test results for hospitalised patients: a systematic review. BMJ Qual Saf. 2011;20:194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh H, Thomas EJ, Mani S, et al. Timely follow-up of abnormal diagnostic imaging test results in an outpatient setting: are electronic medical records achieving their potential? Arch Intern Med. 2009;169:1578–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Georgiou A, Lymer S, Forster M, et al. Lessons learned from the introduction of an electronic safety net to enhance test result management in an australian mothers’ hospital. J Am Med Inform Assoc. 2014;21:1104–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poon EG, Wang SJ, Gandhi TK, Bates DW, Kuperman GJ. Design and implementation of a comprehensive outpatient results manager. J Biomed Inform. 2003;36:80–91. [DOI] [PubMed] [Google Scholar]
- 10. Dalal AK, Schnipper JL, Poon EG, et al. Design and implementation of an automated email notification system for results of tests pending at discharge. J Am Med Inform Assoc. 2012;19:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh H, Thomas EJ, Sittig DF, et al. Notification of abnormal lab test results in an electronic medical record: do any safety concerns remain? Am J Med. 2010;123:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143:121–128. [DOI] [PubMed] [Google Scholar]
- 13. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh H, Arora HS, Vij MS, Rao R, Khan MM, Petersen LA. Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc. 2007;14:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dalal AK, Roy CL, Poon EG, et al. Impact of an automated email notification system for results of tests pending at discharge: a cluster-randomized controlled trial. J Am Med Inform Assoc. 2014;21:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Kareh R, Roy C, Williams DH, Poon EG. Impact of automated alerts on follow-up of post-discharge microbiology results: a cluster randomized controlled trial. J Gen Intern Med. 2012;27:1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lacson R, O'Connor SD, Andriole KP, Prevedello LM, Khorasani R. Automated critical test result notification system: architecture, design, and assessment of provider satisfaction. AJR Am J Roentgenol. 2014;203:W491–W496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lacson R, Prevedello LM, Andriole KP, et al. Four-year impact of an alert notification system on closed-loop communication of critical test results. AJR Am J Roentgenol. 2014;203:933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


