Abstract
Rationale:
The presence of purpura is a compulsory criteria for the diagnosis of Henoch-Schönlein purpura (HSP). Typical purpura of HSP is distributed symmetrically over the extensor surfaces of the lower limbs, buttocks, and forearms with the occasional involvement of trunk and face in children. It occurs only involving the bottom of the feet has never been reported.
Patient concerns:
A 7-year-old girl was admitted to the hospital with abdominal pain, vomiting, and fever.
Diagnoses:
Combining clinical manifestations with results of radiologic examinations, acute appendicitis was suspected and a laparotomy was considered. Purpura was found on the bottom of her feet when she was in the operating room and HSP was diagnosed.
Interventions:
The patient was treated with glucocorticosteroids, antibiotics, cimetidine, and restriction of feeding.
Outcomes:
The abdominal pain and purpura resolved at discharge and there were no recurrences in the subsequent 3-, 6-, and 12-month follow-ups.
Lessons:
Careful examination of skin including the bottom of the feet can help to direct the diagnostic workup for children with abdominal pain.
Keywords: abdominal pain, Henoch-Schölein purpura, purpura location, vasculitis
1. Introduction
Henoch-Schönlein purpura (HSP) is a well-characterized immune complex-mediated small vessel vasculitis of childhood with an incidence of 6.1 to 20.4 per 100,000 children per year,[1,2] presenting most often as nonthrombocytopenic purpura, arthritis/arthralgia, abdominal pain, and nephritis. The cutaneous manifestation of HSP is present in all patients.[3] Typical purpura is distributed symmetrically over the extensor surfaces of the lower limbs, buttocks, and forearms with the occasional involvement of trunk and face in children.[4] Here, we report an unusual case in which the palpable purpura was only on the bottom of the feet.
2. Case report
A 7-year-old girl came to our emergency department with complaints of abdominal pain, vomiting, and fever for 5 days. On physical examination, the vital signs were as follows: temperature, 37.2°C; heart rate, 100/min; respiratory rate, 24/min; blood pressure, 110/73 mm Hg. The abdominal examination was significant for pain in the right lower quadrant upon palpation. There was no rebound tenderness or muscle rigidity. The remainder of the physical examination was normal. Laboratory testing revealed the following: white blood cell count, 4.49 × 109/L; neutrophilic granulocytes, 66.5%; hemoglobin, 129 g/L; platelet count, 225 × 109/L, C-reactive protein, 34 mg/L. Urinalysis was negative. Blood urea nitrogen and creatinine were normal. The renal functions were normal. Immunoglobulin A (IgA) level was normal. Antinuclear antibody by immunofluorescence assay was negative. Other biochemical parameters were normal. Abdominal ultrasonography showed echo enhancement in the right lower quadrant. Computed tomography (CT) scan showed left upper abdominal small bowel wall thickening and fluid around duodenum. Based on the above findings, a diagnosis of acute appendicitis was suspected and a laparotomy was considered. Palpable purpura (Fig. 1) was found on the bottom of her feet when she was in the operating room and HSP was diagnosed. Diagnosis of HSP is based on the presence of purpura plus abdominal pain. According to the European League Against Rheumatism, Paediatric Rheumatology European Society, and Paediatric Rheumatology International Trials Organisation classification of childhood vasculitis, HSP belongs to the group of nongranulomatous, predominantly small vessel vasculitides.[5] Laparotomy was cancelled due to the new diagnosis of HSP. Thus, gastroscopy (Fig. 2) was performed and petechiae were found on the esophagus, gastric antrum, and duodenal bulbar mucosa, consistent with the diagnosis of HSP. Pathologic analysis suggested chronic interstitial inflammation on the duodenal mucosa. She was treated with intravenous methylprednisolone at 2 mg/kg/d, antibiotics, cimetidine, and restriction of feeding. The abdominal pain and purpura resolved at discharge and she continued a 6-week prednisolone taper at home. There were no recurrences in the subsequent 3-, 6-, and 12-month follow-ups.
Figure 1.
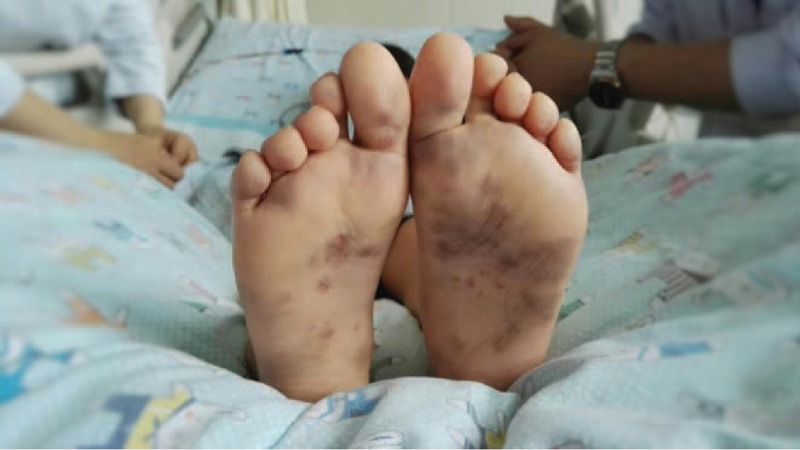
Purpura on the bottom of her feet.
Figure 2.
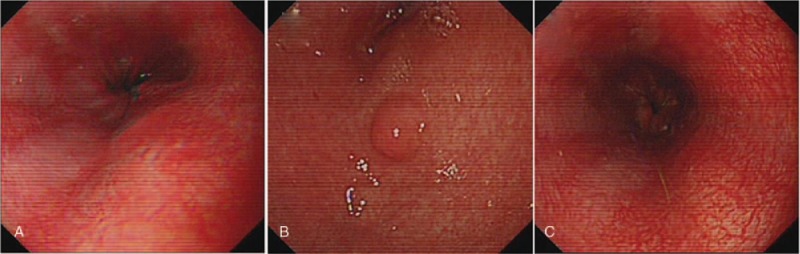
Petechiae on the esophagus (A), gastric antrum (B), and duodenal bulbar mucosa (C) under gastroscopy.
3. Discussion
To our best knowledge, this is the 1st case of HSP involving purpura only on the bottom of the feet, where is easily ignored. This case was diagnosed as HSP according to European League Against Rheumatism/Paediatric Rheumatology International Trials Organization/Paediatric Rheumatology European Society (EULAR/PRINTO/PRES) criteria.[5]
Skin involvement is present in all children with HSP. The typical rash of HSP is palpable purpura symmetrically distributed over the extensor surfaces of the lower limbs and buttocks. It may also involve the arms, face, and ears but usually spares the trunk. The location of purpura lesion is shown to correlate with renal involvement in adults.[6] Patients with purpura lesions restricted to extremities might be at increased risk for long-term renal involvement.[6] Whether skin lesion distribution is a predictor of renal involvement remains to be studied in children. In this patient, whose purpura located on the bottom of her feet, renal functions were normal at the 3-, 6-, and 12-month follow-ups.
Studies have shown that biopsy of the affected skin reveals leukocytoclastic vasculitis with deposition of IgA-containing immune complexes, predominantly in small vessels in the papillary dermis.[7] Neutrophils undergo destruction with destructive fragmentation of the nuclei of dying cells during apoptosis or necrosis. Deposits of IgA and C3 in the dermal capillaries of purpuric lesions and uninvolved skin by immune-fluorescent staining are considered valid diagnostic criteria, with 100% specificity in combination with leukocytoclastic vasculitis.[8] IgA plays an important role in HSP and is elevated in serum. The IgA level is normal in this case. One reasonable explanation is that many serologic studies revealed increased serum levels of IgA in only 50% of patients with HSP during the acute stage.[9]
The use of early glucocorticosteroids (GCSs) may shorten the duration of abdominal pain, decrease the risks of intussusception and surgical intervention,[9] but may not prevent renal disease.[10,11] In most cases, the outcome of HSP is excellent with spontaneous resolution of symptoms and signs. HSP recurs in approximately one-third of patients, typically within 4 months of the initial presentation. The long-term morbidity of HSP is related to the degree of HSP nephritis. In this report, the patient responded well to GCS and the clinical outcome was satisfactory.
In summary, the abdominal pain in children should be considered as HSP. The presence of purpura is a compulsory criterion for the diagnosis of HSP. Careful examination of skin including the bottom of the feet can help to direct the diagnostic workup for children with abdominal pain.
Author contributions
Conceptualization: Fanhui Zhang.
Resources: Fanhui Zhang, Lihua Chen.
Supervision: Shiqiang Shang, Kewen Jiang.
Writing – original draft: Fanhui Zhang, Lihua Chen.
Writing – review & editing: Shiqiang Shang, Kewen Jiang.
Fanhui Zhang orcid: 0000-0002-1870-0668.
Footnotes
Abbreviations: CT = computed tomography, GCSs = glucocorticosteroids, HSP = Henoch-Schönlein purpura, IgA = immunoglobulin A.
Written informed consent was obtained from the guardian of the patient in this case report.
The authors have no conflicts of interest to disclose.
Funding: This publication was supported by the National Natural Science Foundation of China 81571263, 81871012 and 81300975), the Zhejiang Provincial Technology Plan (2015C37105), and by the Key Laboratory of Reproductive Genetics (Zhejiang University), Ministry of Education, and the Key Laboratory for Diagnosis and Therapy of Neonatal Diseases of Zhejiang Province.
References
- [1].Gardner-Medwin JM, Dolezalova P, Cummins C, et al. Incidence of Henoch-Schonlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002;360:1197–202. [DOI] [PubMed] [Google Scholar]
- [2].Aalberse J, Dolman K, Ramnath G, et al. Henoch Schonlein purpura in children: an epidemiological study among Dutch paediatricians on incidence and diagnostic criteria. Ann Rheum Dis 2007;66:1648–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Calvo-Rio V, Loricera J, Mata C, et al. Henoch-Schonlein purpura in northern Spain: clinical spectrum of the disease in 417 patients from a single center. Medicine 2014;93:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Trnka P. Henoch-Schonlein purpura in children. J Paediatr Child Health 2013;49:995–1003. [DOI] [PubMed] [Google Scholar]
- [5].Yang YH, Yu HH, Chiang BL. The diagnosis and classification of Henoch-Schonlein purpura: an updated review. Autoimmun Rev 2014;13:355–8. [DOI] [PubMed] [Google Scholar]
- [6].St John J, Vedak P, Garza-Mayers AC, et al. Location of skin lesions in Henoch-Schonlein purpura and its association with significant renal involvement. J Am Acad Dermatol 2018;78:115–20. [DOI] [PubMed] [Google Scholar]
- [7].Eleftheriou D, Batu ED, Ozen S, et al. Vasculitis in children. Nephrol Dial Transplant 2015;30:i94–103. [DOI] [PubMed] [Google Scholar]
- [8].Gonzalez LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schonlein purpura. Int J Dermatol 2009;48:1157–65. [DOI] [PubMed] [Google Scholar]
- [9].Audemard-Verger A, Terrier B, Dechartres A, et al. Characteristics and management of IgA vasculitis (Henoch-Schonlein) in adults: data from 260 patients included in a French Multicenter Retrospective Survey. Arthritis Rheumatol 2017;69:1862–70. [DOI] [PubMed] [Google Scholar]
- [10].Chartapisak W, Opastiraku S, Willis NS, et al. Prevention and treatment of renal disease in Henoch-Schonlein purpura: a systematic review. Arch Dis Child 2009;94:132–7. [DOI] [PubMed] [Google Scholar]
- [11].Dudley J, Smith G, Llewelyn-Edwards A, et al. Randomised, double-blind, placebo-controlled trial to determine whether steroids reduce the incidence and severity of nephropathy in Henoch-Schonlein Purpura (HSP). Arch Dis Child 2013;98:756–63. [DOI] [PubMed] [Google Scholar]


