Supplemental Digital Content is available in the text
Keywords: alcoholic liver cirrhosis, FIB-4 index, hepatocellular carcinoma, modified FIB-4 index
Abstract
Recently, modified fibrosis-4 index (mFIB-4) and the easy liver fibrosis test (eLIFT) were developed for predicting liver fibrosis in chronic liver disease patients. We evaluated whether the 2 tests can predict hepatocellular carcinoma (HCC) risk in alcoholic liver cirrhosis (ALC) patients.
A retrospective cohort of 924 ALC patients was assessed for HCC development. Four non-invasive serum biomarkers, mFIB-4, the eLIFT score, fibrosis-4 index (FIB-4), and aspartate aminotransferase to platelet ratio index (APRI) were tested using time-dependent analysis of areas under receiver operating characteristic curve (AUROC), DeLong, and log-rank tests.
During a median 4.8 years of follow-up, HCC occurred in 83 patients (9.0%). For predicting HCC development at 3 years, the mFIB-4 showed a significantly higher AUROC than APRI and eLIFT scores (0.71 vs 0.61 and 0.56, respectively, all P < .05). The AUROCs of the mFIB-4 for HCC development were not significantly different from those of the FIB-4. According to the mFIB-4, the risk of HCC development was significantly stratified by low index (≤4)/high index (>4) (P < .001 by log-rank test).
The mFIB-4 showed better predictability of HCC development than APRI and eLIFT scores, and significantly stratified HCC risk in Asian ALC patients.
1. Introduction
Alcoholic liver disease is a complex disease encompassing a wide spectrum of progressive conditions, ranging from simple steatosis to alcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma (HCC).[1] Although only a minority of patients (15–40%) develop liver fibrosis and cirrhosis,[2,3] the incidence of HCC among patients with alcoholic liver cirrhosis (ALC) is very high, ranging from 7% to 16% after 5 years of the disease to as much as 29% after 10 years.[4] A 5-year cirrhosis risk of 16% was reported in patients with alcoholic hepatitis versus 6.9% in patients with a simple fatty liver.[5] Alcoholic liver disease is estimated to account for 13% to 14.5% of the total number of liver diseases in South Korea. According to the 2016 National Statistical Office's “Cause of Death Statistics for 2015”, the number of deaths due to alcohol-related illnesses increased by 5.8% year-over-year to 4,746, with a mortality rate of 9.3 per 100,000.[6]
Although various non-invasive tests such as the fibrosis-4 (FIB-4) index, the aspartate aminotransferase (AST)-to-platelet ratio index (APRI), gamma-glutamyl transferase to platelet counts ratio index, AST to alanine aminotransferase (ALT) ratio, BARD, Forn's index, the enhanced liver fibrosis score, Lok index, the Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) model, and the non-alcoholic fatty liver disease (NAFLD) fibrosis score have been introduced to date, such tests were developed and validated in patients with chronic hepatitis B or C, or NAFLD,[7] but not alcoholic liver disease. In NAFLD patients, non-invasive scoring systems such as FIB-4 index, APRI, and NAFLD fibrosis score were good predictors of morbidity and mortality and had an additive value in predicting the development of hepatic and extra-hepatic cancers.[8] In addition, APRI and FIB-4 index were reported in a previous study as reasonable tools to distinguish NAFLD patients with advanced fibrosis and FIB-4 might better discriminate between intermediate fibrosis stages.[9] However, studies are lacking that validate the predictabilities of the non-invasive tests that estimate HCC development in patients with alcoholic liver disease.[7] Although in the last few years, different biochemical abnormalities indicative of ALD have been identified, currently, there is no laboratory test that can diagnose ALD in clinical settings.[10]
Thus, we aimed to evaluate the predictabilities of non-invasive tests in forecasting HCC development in patients with alcoholic cirrhosis.[11] In the last years, 2 non-invasive tests were studied to enhance the predictabilities of tests associated with hepatic fibrosis because FIB-4 and APRI exhibited moderate sensitivity and accuracy. The modified fibrosis-4 (mFIB-4) index was developed as a tool to assess the liver fibrosis stage in patients with chronic hepatitis B or C,[12] and easy liver fibrosis test (eLIFT) scores were developed as tools for the detection of advanced liver fibrosis in all chronic liver disease patients.[13] In a study describing the eLIFT, the main etiologies for chronic liver disease were chronic hepatitis C (45.5%) and non-alcoholic liver disease (34.2%); only 7.7% of patients had an etiology of alcoholic liver disease.[13] Although the 2 tests derived from large cohorts showed the highest predictive performance levels for liver cirrhosis in the current literature,[12,13] the predictive performance of the 2 tests still needs to be validated in independent cohorts.
Because studies relating to the predictabilities of non-invasive tests for HCC development in patients with alcoholic liver disease are non-existent, there is a great need to study the predictabilities of such tests in patients with alcoholic cirrhosis. In this study, we evaluated the predictabilities of newly introduced non-invasive tests (mFIB-4 index and eLIFT score) for HCC development in patients with ALC.
2. Materials and methods
2.1. Patients
This study population was obtained from the inpatient and outpatient database at Kangwon National University Hospital (Chuncheon, Korea) filed between January 1, 2007 and December 31, 2015 and consisted of a cohort of 924 consecutive Asian patients with compensated ALC (Fig. 1). Patients were excluded from this study if they met any of the following criteria:
Figure 1.
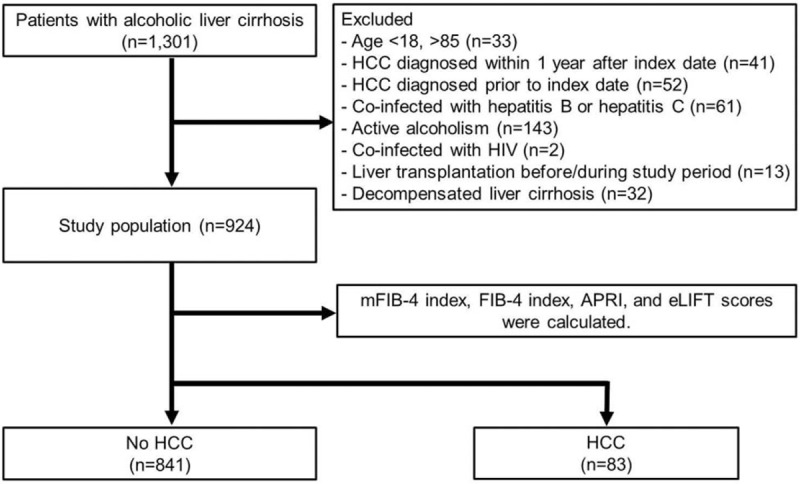
Patient flowchart of this study.
-
1.
aged <18 or >85 years;
-
2.
developed HCC within 12 months from the date of cirrhosis diagnosis;
-
3.
diagnosed with HCC before study enrollment;
-
4.
diagnosed with infection of hepatitis B, hepatitis C, and other hepatotropic viruses or human immunodeficiency virus; or
-
5.
had a medical history indicating active alcoholism, liver transplantation, or decompensated cirrhosis.
The time limit of abstinence accepted for inclusion criteria was 2 years. We included the patients who had compensated cirrhosis when the non-invasive fibrosis tests were performed at baseline; patients with decompensated cirrhosis at baseline (n = 32) were excluded. This study was approved by the Institutional Review Board of Kangwon National University Hospital, and the requirement for informed consent from patients was waived.
2.2. Outcomes and follow-up evaluation
The primary outcome in this study was to compare the performance of the mFIB-4 index, eLIFT score, FIB-4 index, and APRI for HCC development at 3 years of follow-up. Calculations of mFIB-4, FIB-4, APRI, and eLIFT scores are shown in Supplementary Table 1.[12–15] The index date was defined as the date that a patient was enrolled in this study. The censored date was defined as the date of a patient's death, the last date of follow-up, or the data cut-off date (December 31, 2016). Patients regularly underwent clinical examinations and liver function tests every 6 months. The primary modality for HCC surveillance in this study was ultrasonography in combination with serum alpha-fetoprotein levels in accordance with current guidelines of South Korea.[16]
2.3. Definitions
Clinical diagnosis of liver cirrhosis was determined as follows:
-
1.
platelet count of < 100,000/mL and ultrasonography findings suggestive of cirrhosis, including a blunted, nodular liver edge accompanied by splenomegaly (>12 cm); or
-
2.
other clinical signs of portal hypertension such as ascites, varix, or hepatic encephalopathy.[17,18]
The patients diagnosed with ALC had a history of alcohol consumption >10 years, exceeding 60 g/day for males and 40 g/day for females.
A diagnosis of HCC was established based on the guidelines of the American Association for the Study of Liver Diseases.[19] Regarding the application of risk scores, the mFIB-4 index, eLIFT score, FIB-4 index, and APRI were calculated using biochemical values at baseline. Cut-off values of each test were used as suggested in the original articles; cut-off values of mFIB-4 index, FIB-4 index, APRI, and eLIFT scores were 4, 3.25, 1, and 8, respectively. Scores higher than cut-off values indicate a high-risk group.[12–15]
2.4. Statistical analysis
Comparisons of baseline characteristics were performed using the t test, analysis of variance, Mann–Whitney U test, or Kruskal–Wallis test for continuous variables and the χ2 test or Fisher exact test for categorical variables. The cumulative incidence rate of HCC was calculated using the Kaplan–Meier method and compared by log-rank tests among the patient groups. Univariable and multivariable Cox proportional hazards regression models were used to estimate the effect of various variables on the hazard of HCC occurrence. Hazard ratios (HR) and their 95% confidence intervals (CIs) along with corresponding P values are presented. The performance of predicting HCC development at 3 years was assessed using time-dependent areas under receiver operating characteristic curve (AUROC). Pairwise comparison of AUROC values between each prediction score was performed by the DeLong test.[20] All statistical analyses were conducted using the R statistical programming environment (v3.0.1; http://www.r-project.org), with P < .05 reflecting statistical significance.
3. Results
3.1. Baseline characteristics
The baseline clinical characteristics of this study cohort are described in Table 1. In this study cohort, the median age was 59 years, and the predominant gender was male (n = 581, 62.9%) (Table 1). The median aspartate, alanine aminotransferase, and platelet count levels were 32 U/L, 19 U/L, and 175 × 109/L, respectively. The median serum creatinine and total bilirubin levels were both 0.8 mg/dL. The median value of Child-Turcotte-Pugh (CTP) scores was 5 (IQR 5–5); MELD scores, 8 (IQR 6–10); mFIB-4 index, 5.57 (IQR 3.58–8.97), FIB-4 index, 2.49 (IQR 1.63–3.77); APRI, 0.47 (IQR 0.32–0.71); and eLIFT scores, 8 (IQR 6–11).
Table 1.
Baseline characteristics.
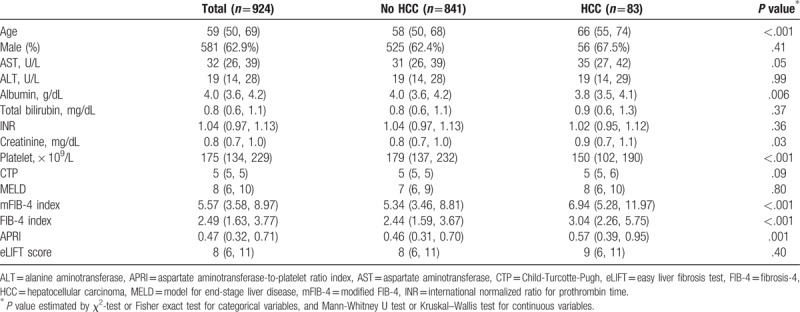
The baseline characteristics of patients who developed HCC (n = 83, 9.0%) and those who did not (n = 841, 91.0%) are compared in Table 1. Age, serum creatinine levels, mFIB-4 index, FIB-4 index, and APRI were significantly higher in patients who developed HCC than in those without HCC (all values: P < .05). However, serum albumin levels and platelet counts were significantly lower in patients who developed HCC than in those without HCC (all values: P < .05).
3.2. Predictive performances of four risk prediction models for HCC development
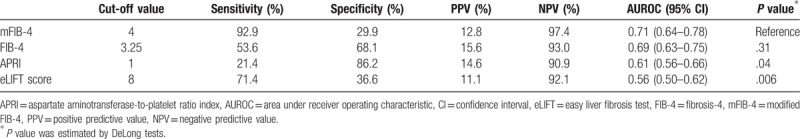
During the follow-up period (median 58 months, IQR: 31–94 months), HCC developed in 83 patients (9.0%). The 3-year cumulative incidence rates of HCC were 3.0%. The AUROC of each risk prediction model was calculated to predict HCC development at 3 years (Fig. 2A). The mFIB-4 index showed the highest performance for predicting HCC development at 3 years (AUROC = 0.71, 95% confidence interval [CI]: 0.64–0.78), followed by the FIB-4 index (AUROC = 0.69, 95% CI: 0.63–0.75), APRI (AUROC = 0.61, 95% CI: 0.56–0.66), and eLIFT score (AUROC = 0.56, 95% CI: 0.50–0.62). The AUROCs of the mFIB-4 index were significantly higher than those of APRI and eLIFT scores at 3 years (all P < .05; Table 2). No significant difference in AUROCs was found between the mFIB-4 and FIB-4 indexes at 3 years (P = .31).
Figure 2.
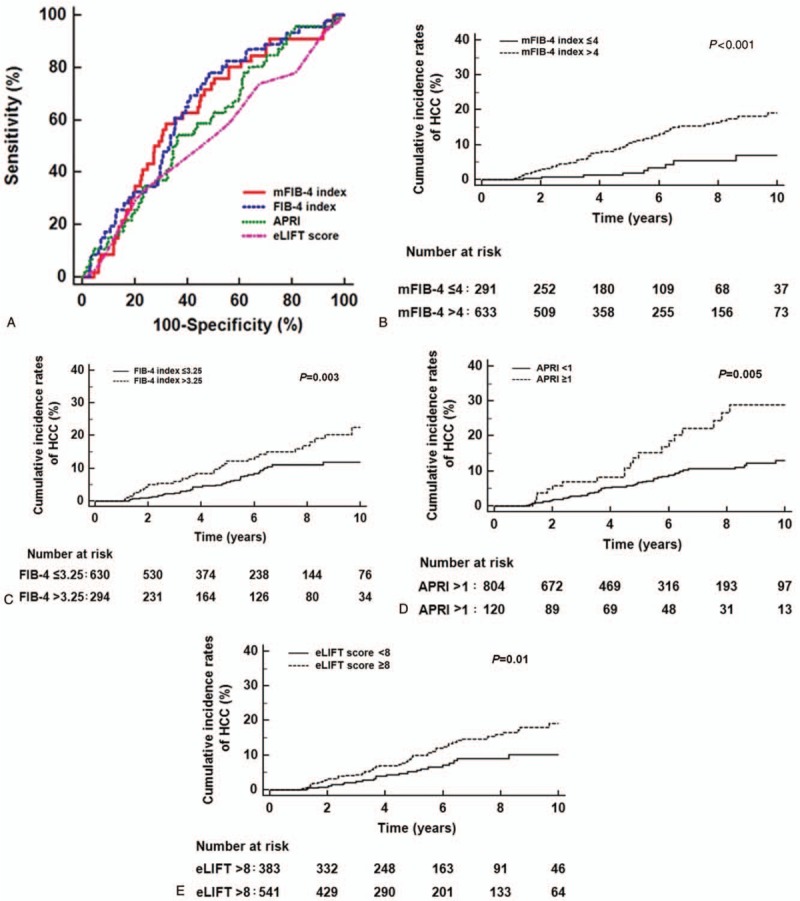
Cumulative incidence rates of HCC development according to risk groups. (A) The AUROCs of the mFIB-4 index, FIB-4 index, APRI, and eLIFT score in predicting HCC development at 3 years were 0.71, 0.69, 0.61, and 0.56, respectively. (B) Patients with an mFIB-4 index >4 had a significantly higher HCC development than those with an mFIB-4 index ≤4 (P value < 0.001 by log-rank test). (C) Patients with a FIB-4 index >3.25 had a significantly higher HCC development than those with a FIB-4 index ≤3.25 (P = .003 by log-rank test). (D) Patients with APRI scores >1 had a significantly higher HCC development than those with a APRI scores ≤1 (P = .005 by log-rank test). (E) Patients with eLIFT scores ≤ 8 had a significantly higher HCC development than those with eLIFT scores > 8 (P = .01 by log-rank test). APRI = aspartate aminotransferase-to-platelet ratio index, AUROC = areas under receiver operating characteristics curves, eLIFT = easy liver fibrosis test, FIB-4 = fibrosis-4, HCC = hepatocellular carcinoma, mFIB-4 = modified Fibrosis-4.
Table 2.
Cut-off values of each score for prediction of HCC development at 3 years.
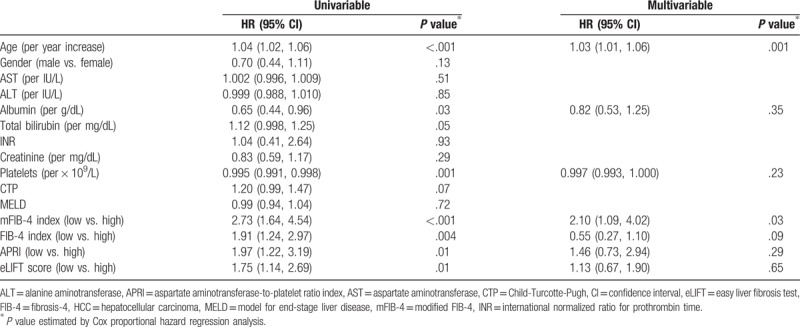
In univariable analysis, older age, lower serum albumin levels, lower platelet counts, higher mFIB-4, FIB-4, APRI, and eLIFT scores significantly predicted HCC development (all, P < .05; Table 3). A subsequent multivariable analysis revealed that older age (HR: 1.03; 95% CI: 1.01–1.06; P = .001) and mFIB-4 index (HR: 2.10; 95% CI: 1.09–4.02; P = .03) were independent predictors of HCC development (Table 3).
Table 3.
Cox proportional hazards regression analysis for HCC development.
In the prediction of HCC development at 5 years, AUROCs of dynamic changes (Δ) in non-invasive tests between baseline and 3 years were assessed (n = 642). The AUROCs of ΔmFIB-4, ΔFIB-4, ΔAPRI, and ΔeLIFT scores were 0.51 (95% CI, 0.47–0.55), 0.54 (95% CI, 0.50–0.58), 0.56 (95% CI, 0.52–0.60), and 0.52 (95% CI, 0.48–0.56), respectively. The dynamic value of the mFIB-4 index did not show significantly different predictive performance as compared to that of three non-invasive tests such as ΔFIB-4, ΔAPRI, and ΔeLIFT scores (P = .19, P = .23, and P = .78, respectively). In univariable analysis for HCC development at 5 years (n = 642), dynamic change in the four non-invasive tests did not show a significant association with HCC development (Supplementary Table 2).
3.3. Sensitivities of non-invasive tests based on tumor size
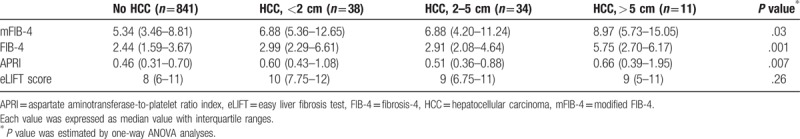
Sensitivities of non-invasive tests based on tumor size (<2 cm, 2–5 cm, >5 cm) in patients (n = 83) who developed HCC were analyzed. In this study, among HCC patients (n = 83), 38 patients had a tumor <2 cm in size, 34 patients had a tumor 2 to 5 cm in size, and 11 patients had a tumor >5 cm in size. The median tumor size was 2.0 cm (IQR 1.5–3.0 cm). Based on tumor size, the value of non-invasive tests tended to increase (Table 4).
Table 4.
Values of non-invasive tests according to tumor size of HCC.
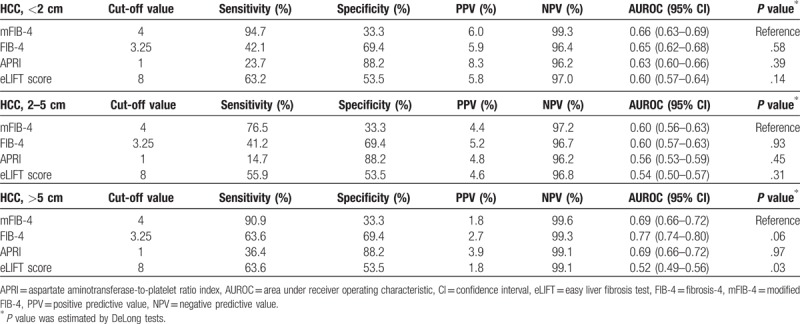
Regarding sensitivities of mFIB-4 index and other tests for HCC detection based on tumor size (<2 cm, 2–5 cm, and > 5 cm), the mFIB-4 index tended to show higher sensitivities irrespective of tumor size compared with other non-invasive tests (Table 5); sensitivities in HCC patients based on tumor size were 94.7%, 76.5%, and 90.9% in tumors <2 cm, 2 to 5 cm, and >5 cm in size, respectively. Regarding predictive performance of mFIB-4 index and other tests for HCC detection based on tumors <2 cm, 2 to 5 cm, and >5 cm in size, mFIB-4 index tended to better predict HCC development in patients with a tumor ≤5 cm in size than other non-invasive tests (Table 5). Conversely, in patients with a tumor >5 cm in size, the FIB-4 index tended to better predict HCC development than other non-invasive tests.
Table 5.
Sensitivities of non-invasive tests for diagnosis of HCC according to tumor size.
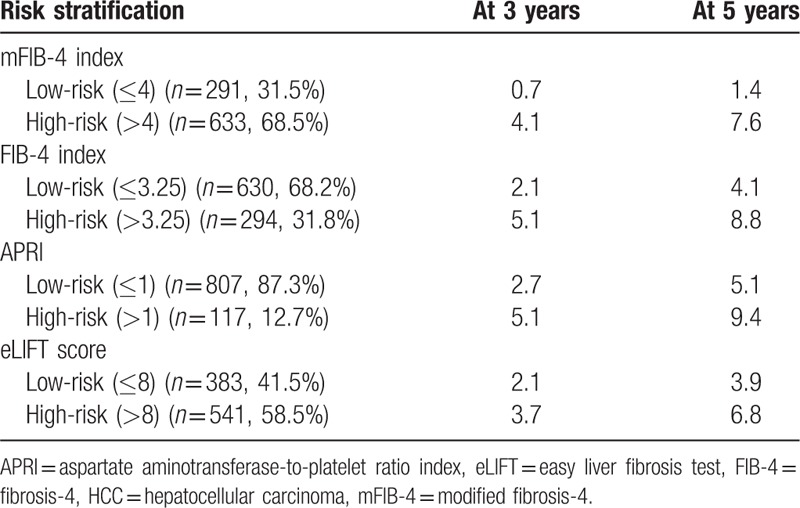
3.4. Risk stratification for HCC development using fibrosis models
Among the four non-invasive tests, mFIB-4 showed the highest sensitivities of 92.9% (Table 2). The study population was stratified into low- and high-risk groups according to cut-off values from this study for risk prediction models (Table 6). In the stratification for the 3-year cumulative HCC incidence according to cut-off values from original studies, patients (n = 633) with a high mFIB-4 index had a significantly higher HCC risk than those (n = 291) with a low mFIB-4 index (HR: 3.14, 95% CI: 1.98–4.97, P < .001; Fig. 2B). Regarding the FIB-4 index, patients (n = 294) with a high FIB-4 index had a significantly higher HCC risk than those (n = 630) with a low FIB-4 index (HR: 1.71, 95% CI: 1.08–2.71, P = .01; Fig. 2C). Regarding APRI, patients (n = 117) with high APRI scores had a significantly higher HCC risk than those (n = 807) with low APRI scores (HR: 1.94, 95% CI: 1.01–3.73, P = .01; Fig. 2D). In stratification for HCC risk using eLIFT scores, patients (n = 541) with high eLIFT scores had a significantly higher HCC risk than those (n = 383) with low eLIFT scores (HR: 1.71, 95% CI: 1.11–2.64, P = .02; Fig. 2E).
Table 6.
Cumulative incidence rates of HCC development according to the risk stratification.
According to mFIB-4 risk stratification that showed highest sensitivities among the four non-invasive tests, HCC developed in 24 patients who were identified among the low-risk patients (n = 291) for a median follow-up period of 62 months. Low-risk patients who developed HCC (n = 24) showed significantly lower serum platelet counts than those who did not show HCC development (n = 267; P = .01; Supplementary Table 3).
4. Discussion
This is the first study to validate newly introduced non-invasive fibrosis tests, the mFIB-4 index and the eLIFT score, with regard to the performance of predictabilities and risk stratification on HCC development in patients with compensated ALC. In this study, the mFIB-4 index showed significantly higher predictabilities for HCC development at 3 years than APRI and eLIFT scores; the mFIB-4 index significantly stratified the individual HCC risk. In a previous study, mFIB-4 index exhibited the highest diagnostic performance for cirrhosis in patients with chronic viral hepatitis B or C with an AUROC of 0.85 compared with other non-invasive tests such as APRI, FIB-4, or Lok index.[12] Because cirrhosis is an important risk factor for HCC development and mFIB-4 index is an excellent diagnostic tool for cirrhosis of viral etiologies, good predicitive performance of mFIB-4 index for HCC development in patients with alcoholic cirrhosis can be in agreement with previous studies.
These results suggest that the mFIB-4 index can help identify ALC patients at high risk of developing HCC. When the mFIB-4 index was ≤4 points, the cumulative incidence rates of HCC at 5 years were very low (1.4%). Thus, patients with an mFIB-4 index ≤4 can be regarded as low-risk patients for HCC development. Because these low incidence rates of HCC suggest that 6-month surveillance may not be cost-effective,[21] low-risk patients can instead be monitored less frequently at 12-month intervals. Of note, although mFIB-4 showed high sensitivities of 92.3% for HCC development at 3 years in this study, HCC developed in 0.7% of patients who were identified among low-risk patients for 3 years. Given the low-risk patients showing HCC development had low platelet counts, patients with low platelet counts may be diagnosed with HCC over long-term periods and therefore need more cautious surveillance for HCC even in low-risk patients stratified by mFIB-4. In contrast, patients with an mFIB-4 index >4 had a 4.1% cumulative incidence rate of HCC at 3 years. Thus, patients with an mFIB-4 index >4 may be regarded as high-risk patients for HCC development. These patients should be followed with caution every 6 months. Furthermore, the mFIB-4 index can be used in resource-limited settings because it does not require the use of rarely available tests for biochemical markers (such as α2-macroglobulin or apolipoprotein A1) or a costly assessment of fibrotic burden, such as transient elastography, which is not universally available. Thus, risk stratification based on the mFIB-4 index can be used widely, especially in developing countries, where the detrimental effects of alcohol are proportionally higher than in developed countries.[22]
This study is the first to validate the eLIFT score in Asian patients with ALC, which was recently developed using a cohort of Caucasian patients with chronic liver disease.[13] In this study, the eLIFT score was not highly accurate in predicting HCC. In a recent validation study of the eLIFT score for patients with severe hepatic fibrosis and chronic hepatitis B,[23] the eLIFT score also showed low predictability compared to that of the FIB-4 index and APRI. Given that hepatic fibrosis is an important factor for HCC development, the low accuracy of the eLIFT score for severe hepatic fibrosis can induce low predictabilities for HCC development.
To avoid potential bias from active alcoholism when evaluating the performance of non-invasive fibrosis tests for predicting HCC development, we included patients who did not consume alcohol for at least 2 years. Although patients with active alcoholism were excluded, ongoing alcohol consumption might not affect HCC development in patients with alcoholic cirrhosis. In a recent study, multivariable analyses showed that alcohol consumption was a non-significant independent risk factor for HCC development in patients with alcoholic cirrhosis.[24] Therefore, although we arbitrarily set “2 years” as the abstinence period to exclude patients with active alcoholism, this period may not affect the results in this study; based on a previous study,[24] active or inactive alcoholism did not influence HCC development.
However, several limitations exist in this study. First, cirrhosis was clinically defined by gastro-endoscopic findings, blood test profiles, ultrasonographic findings, or clinical symptoms, but not by histological evaluation. However, even if a liver biopsy is available, the sensitivity and specificity of a liver biopsy are not 100%, and it can have substantial inter-observer and intra-observer variations, particularly in daily clinical practice.[25,26] The clinical approach to diagnosing cirrhosis is closer to that found in routine clinical practice. Second, fibrosis can be assessed non-invasively by Fibroscan,[27–29] which has shown potential for identifying patients at risk of HCC.[30] Our cohort lacked Fibroscan data, so a comparison of Fibroscan with APRI and FIB-4 could not be made. Third, most patients in this study had early cirrhosis rather than advanced cirrhosis: median MELD scores were 8 and CTP scores were 5. Thus, a selection bias may exist in our results for all stages of cirrhosis, particularly advanced cirrhosis. Although our study mainly included patients with early rather than advanced cirrhosis, it may be more beneficial to prevent HCC development in the former rather than latter patients with regard to clinical aspects: early intervention may be required to prevent HCC in early cirrhotic patients. Fourth, although our study enrolled patients who did not drink alcohol as determined by a meticulous review of medical records, laboratory parameters were not available to prove abstinence in study patients. However, practical biomarkers with high sensitivity and specificity to screen ongoing alcohol consumption are not presently available.[11] Lastly, selection bias could exist because different surveillance intervals can affect late detection of HCC development. However, in this study, the goal was not to detect the early stages of HCC, but to evaluate the performance of non-invasive fibrosis tests for predicting HCC development. Therefore, the bias might have only minimally affected the study results. Further studies are warranted to assess the performance of non-invasive fibrosis tests for predicting HCC development in patients followed up on a regular basis.
In conclusion, the mFIB-4 index, a newly developed non-invasive marker of liver fibrosis, can predict HCC and stratify HCC risk in patients with ALC. This result indicates that this index can help clinicians make surveillance strategies based on individual risk.
Author contributions
Conceptualization: Minjong Lee.
Data curation: Ji Hyun Kim, Minjong Lee, Seung Woo Park, Myungho Kang, Sang Hoon Lee, Tae Suk Kim, and Jin Myung Park.
Formal analysis: Ji Hyun Kim and Minjong Lee.
Funding acquisition: Minjong Lee and Dae Hee Choi.
Investigation: Ji Hyun Kim and Minjong Lee.
Methodology: Minjong Lee and Myungho Kang.
Resources: Dae Hee Choi.
Supervision: Minjong Lee.
Validation: Minjong Lee and Minjeong Kim.
Visualization: Minjong Lee and Minjeong Kim.
Writing – original draft: Minjong Lee.
Writing – review & editing: Minjong Lee and Dae Hee Choi.
Minjong Lee orcid: 0000-0002-3159-5444.
Supplementary Material
Footnotes
Abbreviations: ALC = alcoholic liver cirrhosis, APRI = aspartate aminotransferase to platelet ratio index, AUROC = area under the receiver operating characteristic curve, CTP = child-turcotte-pugh, FIB-4 = fibrosis-4, HCC = hepatocellular carcinoma, INR = international normalized ratio for prothrombin time, MELD = model for end-stage liver disease, mFIB-4 = modified FIB-4.
Sources of support: This study was supported by a grant from 2017 Kangwon National University Hospital, a fund from Gangwon branch of the Korean Association for the Study of the Liver, 2015 Research Grant from the Kangwon National University (grant number: 520150354), a research grant from Hanmi Pharmaceutical Co, Ltd, and a grant from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: 2017R1D1A1B03031499).
No potential conflict of interest relevant to this article was reported.
Supplemental Digital Content is available for this article.
References
- [1].European Association for the Study of Liver EASL-ALEH clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012;57:399–420. [DOI] [PubMed] [Google Scholar]
- [2].Naveau S, Raynard B, Ratziu V, et al. Biomarkers for the prediction of liver fibrosis in patients with chronic alcoholic liver disease. Clin Gastroenterol Hepatol 2005;3:167–74. [DOI] [PubMed] [Google Scholar]
- [3].Ho AM, Contardi LH. Pure alcoholic fatty liver and progression to cirrhosis or fibrosis. Lancet 1995;346:1562–3. [DOI] [PubMed] [Google Scholar]
- [4].N’Kontchou G, Paries J, Htar MT, et al. Risk factors for hepatocellular carcinoma in patients with alcoholic or viral C cirrhosis. Clin Gastroenterol Hepatol 2006;4:1062–8. [DOI] [PubMed] [Google Scholar]
- [5].Masarone M, Rosato V, Dallio M, et al. Epidemiology and natural history of alcoholic liver disease. Rev Recent Clin Trials 2016;11:167–74. [DOI] [PubMed] [Google Scholar]
- [6].Jang JY, Kim DJ. Epidemiology of alcoholic liver disease in Korea. Clin Mol Hepatol 2018;24:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].European Association for Study of Liver EASL-ALEH clinical practice guidelines: noninvasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237–64. [DOI] [PubMed] [Google Scholar]
- [8].Peleg N, Sneh Arbib O, Issachar A, et al. Noninvasive scoring systems predict hepatic and extra-hepatic cancers in patients with nonalcoholic fatty liver disease. PLoS One 2018;13:e0202393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Peleg N, Issachar A, Sneh-Arbib O, et al. AST to Platelet Ratio Index and fibrosis 4 calculator scores for non-invasive assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease. Dig Liver Dis 2017;49:1133–8. [DOI] [PubMed] [Google Scholar]
- [10].Peta V, Elaribi D, Abenavoli L. Laboratory tests for diagnosis of alcoholic liver disease. Rev Recent Clin Trials 2016;11:180–4. [DOI] [PubMed] [Google Scholar]
- [11].Singal AK, Bataller R, Ahn J, et al. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol 2018;113:175–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang HW, Peng CY, Lai HC, et al. New noninvasive index for predicting liver fibrosis in Asian patients with chronic viral hepatitis. Sci Rep 2017;7:3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Boursier J, de Ledinghen V, Leroy V, et al. A stepwise algorithm using an at-a-glance first-line test for the non-invasive diagnosis of advanced liver fibrosis and cirrhosis. J Hepatol 2017;66:1158–65. [DOI] [PubMed] [Google Scholar]
- [14].Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011;53:726–36. [DOI] [PubMed] [Google Scholar]
- [15].Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- [16].Korean Liver Cancer Study Group, National Cancer Center Korea 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver 2015;9:267–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim DY, Kim SU, Ahn SH, et al. Usefulness of FibroScan for detection of early compensated liver cirrhosis in chronic hepatitis B. Dig Dis Sci 2009;54:1758–63. [DOI] [PubMed] [Google Scholar]
- [18].Jung KS, Kim SU, Ahn SH, et al. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology 2011;53:885–94. [DOI] [PubMed] [Google Scholar]
- [19].Bruix J, Sherman M. American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- [21].European Association For The Study Of The Liver, European Organisation For Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–43. [DOI] [PubMed] [Google Scholar]
- [22].Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009;373:2223–33. [DOI] [PubMed] [Google Scholar]
- [23].Li Q, Lu C, Li W, et al. Evaluation of eLIFT for non-invasive assessment of liver fibrosis and cirrhosis in patients with chronic hepatitis B virus infection. Sci Rep 2017;7:5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ganne-Carrie N, Chaffaut C, Bourcier V, et al. Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis. J Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- [25].Scheuer PJ. Liver biopsy size matters in chronic hepatitis: bigger is better. Hepatology 2003;38:1356–8. [DOI] [PubMed] [Google Scholar]
- [26].Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344:495–500. [DOI] [PubMed] [Google Scholar]
- [27].Wong GL, Espinosa WZ, Wong VW. Personalized management of cirrhosis by non-invasive tests of liver fibrosis. Clin Mol Hepatol 2015;21:200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jung KS, Kim SU. Clinical applications of transient elastography. Clin Mol Hepatol 2012;18:163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Castera L, Pinzani M. Non-invasive assessment of liver fibrosis: are we ready? Lancet 2010;375:1419–20. [DOI] [PubMed] [Google Scholar]
- [30].Kim MN, Kim SU, Kim BK, et al. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology 2015;61:1851–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


