Abstract
Objective:
This article analyzed the clinical efficacy and tolerability of rivaroxaban and enoxaparin in patients undergoing total knee arthroplasty (TKA) surgery.
Methods:
Five randomized, controlled clinical trials on rivaroxaban versus enoxaparin in patients who underwent TKA were identified and included in this meta-analysis.
Results:
The meta-analysis indicated that rivaroxaban prophylaxis was associated with lower rates of symptomatic venous thromboembolism (VTE) (relative risk[RR]:0.55; 95% confidence interval [CI]: 0.35–0.86; P = .009), symptomatic deep vein thrombosis (DVT) (RR 0.44, 95% CI 0.25–0.80, P = .007), asymptomatic DVT (RR: 0.57; 95% CI: 0.37–0.89; P = .01), distal DVT (RR: 0.62; 95% CI: 0.45–0.85; P = .003) and proximal DVT (RR: 0.42; 95% CI: 0.24–0.75; P = .004). Compared with the enoxaparin group, the incidence of symptomatic pulmonary embolism (PE) (RR: 0.48; 95% CI: 0.19–1.24; P = .13) in the rivaroxaban group was not significantly different. A nonsignificant trend towards all-cause death (RR: 0.38; 95% CI: 0.03–4.92; P = .46) or major bleeding (RR: 1.59; 95% CI: 0.77–3.27; P = .21) risk between rivaroxaban and enoxaparin prophylaxis was found.
Conclusion:
Compared with the enoxaparin group, the group using rivaroxaban after TKA had a significantly lower rate of symptomatic VTE, symptomatic DVT, asymptomatic DVT, distal DVT, and proximal DVT. Our study shows that rivaroxaban after TKA is more effective than enoxaparin and did not increase major bleeding or all-cause mortality.
Level of evidence II
Keywords: enoxaparin, meta-analysis, rivaroxaban
1. Introduction
Venous thromboembolism (VTE) is a major healthcare problem that affects more than 1.6 million persons each year worldwide.[1] Patients undergoing major orthopedic surgery, total knee arthroplasty (TKA), and total hip arthroplasty (THA) are at high risk for developing VTE, which can manifest as deep vein thrombosis (DVT) or pulmonary embolism (PE), and PE can be life-threatening. The recommended pharmacologic therapy options for thromboprophylaxis after major orthopedic surgery include low-molecular-weight heparins (LMWHs; e.g., enoxaparin), direct factor-Xa inhibitors (e.g., rivaroxaban), vitamin K antagonists (VKAs; e.g., warfarin), and so on. Most hospitals use some kind of thromboprophylaxis routinely. TKA surgery is being performed with increasing regularity around the world. In addition, it is also one of the most frequent orthopedic procedures in the United States.[2] Rivaroxaban is approved in several countries and the European Union for the prevention of VTE in adult patients undergoing knee replacement surgery.[3] Rivaroxaban has the advantage of being an oral therapy that does not require laboratory monitoring, but it should also not be ignored that there is no effective antidote if major bleeding occurs. Rivaroxaban demonstrated a significant superiority to enoxaparin for the prevention of VTE after TKA or THA, without a remarkable increase in major bleeding rate.[4] Haas's study also showed that enoxaparin was significantly less effective than rivaroxaban in the prevention of VTE after total hip or knee replacement, with a similar safety profile.[5] However, some studies found that enoxaparin had a lower risk of bleeding than rivaroxaban.[6–9] Some studies also found no demonstrable differences between rivaroxaban and enoxaparin in rates of VTE, transfusion, reoperation, infection, or major bleeding after hip and knee arthroplasty.[10,11] Chahal et al[12] suggested that there is no consensus on the optimal form of VTE prophylactic treatment in knee arthroplasty patients or on the complication and safety profile of the available chemical prophylactic modalities.
2. Methods
2.1. Search strategy and eligibility and exclusion criteria
All eligible studies were obtained from Embase, PubMed, and the Cochrane Library databases. The following search terms were used, without any limitations, through December 2017: (Enoxaparin OR Enoxaparine OR EMT966 OR EMT967 OR Clexane OR Lovenox OR PK10169) AND (BAY 597939 OR Xarelto OR Rivaroxaban) AND knee. We manually screened the reference lists in the relevant studies. The following inclusion criteria were used to determine which trials were included in this study:
-
(1)
patients treated with TKA,
-
(2)
patients use random rivaroxaban or enoxaparin after TKA simultaneously,
-
(3)
outcomes included efficacy (symptomatic VTE, symptomatic DVT, symptomatic PE, asymptomatic DVT, distal DVT, and proximal DVT) and safety (major bleeding, all-cause mortality), and
-
(4)
the studies were randomized controlled clinical trials (RCTs). The search strategy is shown in Figure 1.
Figure 1.
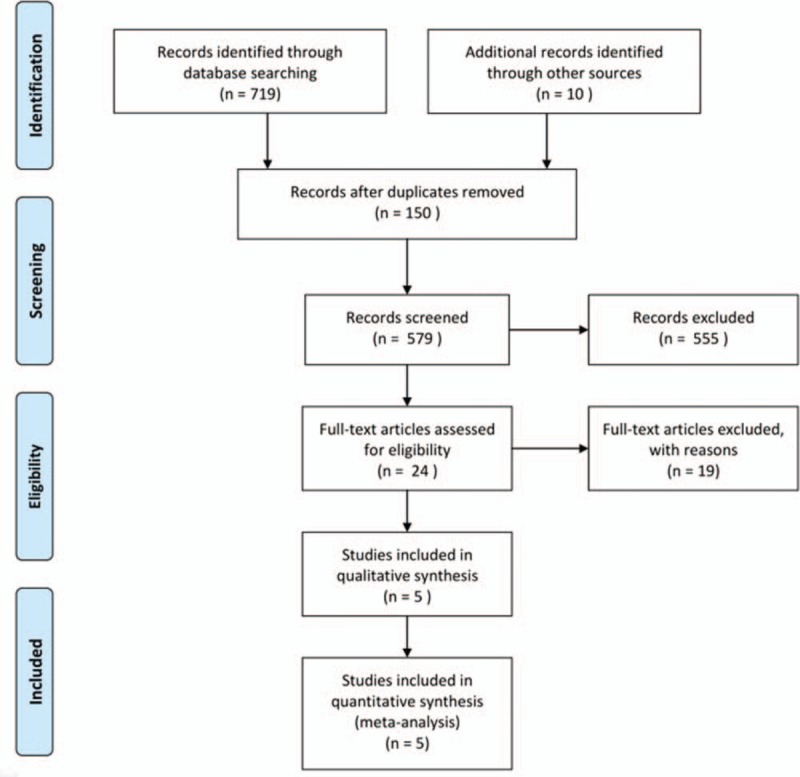
Flow diagram of the inclusion of studies for the meta-analysis.
2.2. Data extraction and quality assessment
Two independent authors extracted data from the included studies. Any disagreements were resolved by arbitration by a third team member or consensus. Data on study characteristics (study design, authors, year of publication, intervention, population, and average operation time) and outcomes (VTE, bleeding and all-cause mortality events) during treatment were extracted. We did not reclassify the events, and we accepted the authors’ definitions of the results. The primary efficacy outcomes were symptomatic VTE, symptomatic DVT and symptomatic PE in the studies. The secondary efficacy endpoint was asymptomatic DVT. The primary safety outcomes were defined as all-cause mortality or major bleeding. The Cochrane Collaboration tool was used to evaluate the selected studies. For every study, individual team authors judged the risk of bias and determined it to be “high,” “low,” or “unclear” (Fig. 2).
Figure 2.
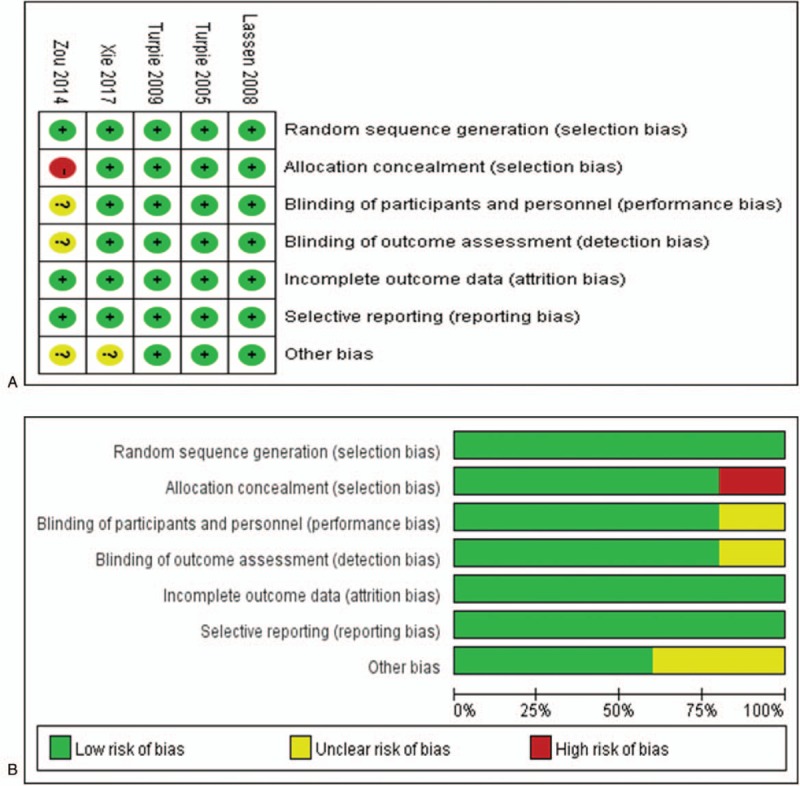
Risk of bias of the selected studies according to the Cochrane Collaboration tool. Panel A: Risk of bias graph; the judgment regarding each risk of bias item is presented as a percentage across all studies. Panel B: Risk of bias summary; the judgment regarding each risk of bias item for each study. (+), low risk of bias; (-), high risk of bias; (?), unclear risk of bias.
2.3. Statistical analysis
Review Manager version 5.3 software (Cochrane Collaboration, Copenhagen: The Nordic Cochrane Centre) was used to calculate 95% confidence intervals (CIs) and the relative risk (RR) for all the efficacy and safety outcomes throughout the meta-analysis. Heterogeneity between studies was considered low when 0% <I2 value <25%, moderate when 25% <I2 value <50%, and high when I2 value >50%. Random- or fixed-effects models were used based on the heterogeneity levels.
3. Results
3.1. Characteristics of the included studies
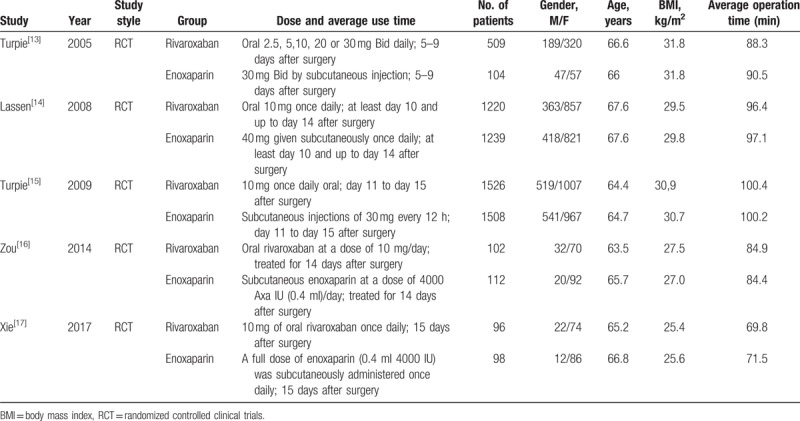
According to the search strategy, the electronic databases returned 729 potentially relevant records. One hundred fifty articles were duplicates; 555 citations were refused after review of the titles and abstracts; 24 articles were selected for further evaluation. Five randomized[13–17] controlled trials comparing rivaroxaban with enoxaparin for thromboprophylaxis were eligible for inclusion after application of the inclusion criteria. Table 1 shows the main characteristics of the 5 studies
Table 1.
Characteristics of the 5 selected clinical studies.
3.2. Efficacy outcomes
Data on the primary efficacy of outcomes (symptomatic VTE, symptomatic DVT, and symptomatic PE) were provided in all 5 relevant RCTs. Compared to enoxaparin, thromboprophylaxis with rivaroxaban was associated with significantly fewer instances of symptomatic VTE (6226 patients, RR 0.55, 95% CI 0.35–0.86, P = .009, Fig. 3) and symptomatic DVT (5116 patients, RR 0.44, 95% CI 0.25–0.80, P = .007, Fig. 4). Compared with the enoxaparin group, after TKA, the incidence of symptomatic PE in the rivaroxaban group was not significantly different (6226 patients, RR: 0.48, 95% CI: 0.19–1.24, P = .13, Fig. 5). Four studies included 2698 patients and reported the secondary efficacy outcome (asymptomatic DVT) after TKA in the 2 groups. Owing to the notable heterogeneity of the data (I2 value = 56%), we used a random-effects model to combine the asymptomatic DVT data. The meta-analysis demonstrated that the asymptomatic DVT rate was lower in the rivaroxaban group than in the enoxaparin group after TKA (RR: 0.57, 95% CI: 0.37–0.89, P = .01, Fig. 6). The third efficacy outcome (distal DVT, proximal DVT) was reported in 4186 TKA patients in these studies, in which data were reported from the 2 groups. Owing to the notable heterogeneity of the data (I2 value = 59%), we used a random-effects model to combine the distal DVT data. Compared with the enoxaparin group, the incidence of distal DVT in the rivaroxaban group was lower (RR: 0.62, 95% CI: 0.45–0.85, P = .003, Fig. 7). According to lower significant heterogeneity (I2 value = 0%), we used a fixed-effects model to combine the proximal DVT data. The proximal DVT rate was lower in the rivaroxaban group than in the enoxaparin group (RR: 0.42, 95% CI: 0.24–0.75, P = .004, Fig. 8).
Figure 3.
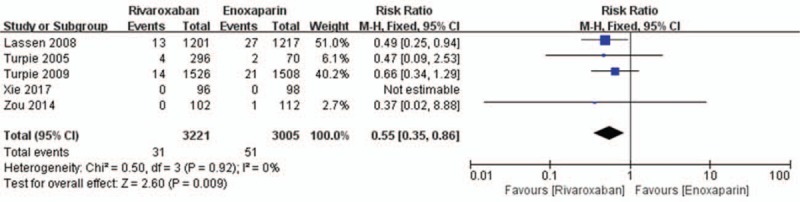
Rivaroxaban versus enoxaparin: symptomatic venous thromboembolism after total knee arthroplasty.
Figure 4.
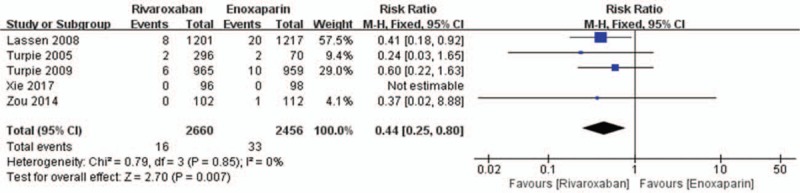
Rivaroxaban versus enoxaparin: symptomatic deep vein thrombosis after total knee arthroplasty.
Figure 5.
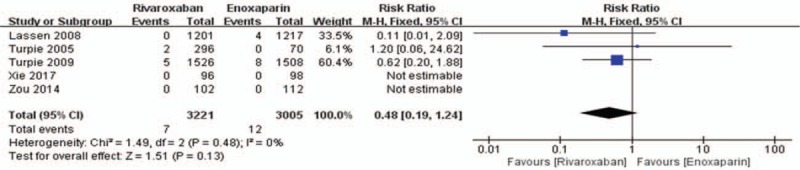
Rivaroxaban versus enoxaparin: symptomatic pulmonary embolism after total knee arthroplasty.
Figure 6.
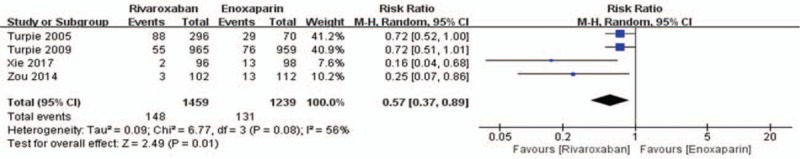
Rivaroxaban versus enoxaparin: asymptomatic deep vein thrombosis after total knee arthroplasty.
Figure 7.
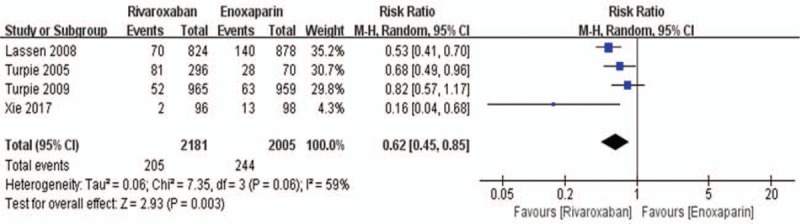
Rivaroxaban versus enoxaparin: distal deep vein thrombosis after total knee arthroplasty.
Figure 8.
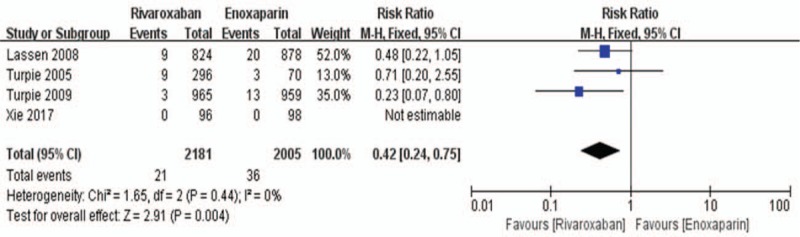
Rivaroxaban versus enoxaparin: proximal deep vein thrombosis after total knee arthroplasty.
3.3. Safety outcomes
All 5 randomized controlled trials reported the primary safety data (major bleeding and all-cause mortality) of using rivaroxaban or enoxaparin after total knee replacement. We used a fixed-effects model to combine the major bleeding data according to lower significant heterogeneity (I2 value = 0%). In the combined data, this model showed no significant difference between the rivaroxaban group and the enoxaparin group in major bleeding during the postoperative period (6108 patients, RR: 1.59; 95% CI: 0.77–3.27; P = .21, Fig. 9). Because of its high heterogeneity (I2 value = 66%), we used a random-effects model to combine the all-cause mortality data. The all-cause mortality was 0.19% (6/3221) in the rivaroxaban group versus 0.40% (12/3005) in the enoxaparin group. Similarly, there was no significant difference between the 2 groups in this model (6226 patients, RR: 0.38, 95% CI: 0.03–4.92, P = .46, Fig. 10).
Figure 9.
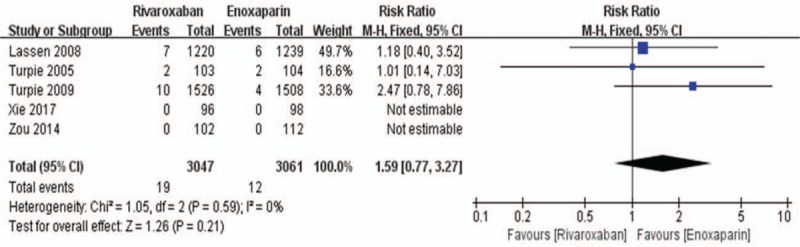
Rivaroxaban versus enoxaparin: major bleeding after total knee arthroplasty.
Figure 10.
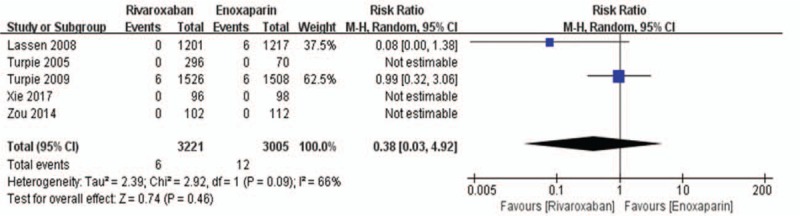
Rivaroxaban versus enoxaparin: all-cause mortality after total knee arthroplasty.
3.4. Sensitivity analysis
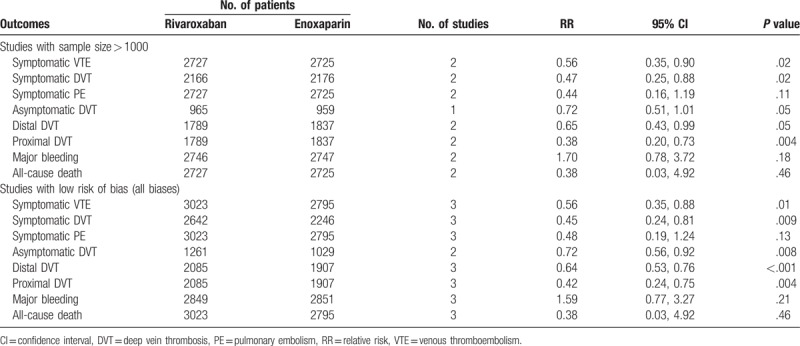
In the sensitivity analysis, 6 outcomes were included. The results are shown in Table 2. This analysis showed that the RR and the level of significance for the 8 outcomes (symptomatic VTE rates, symptomatic DVT rates, symptomatic PE rates, asymptomatic DVT rates, distal DVT rates, proximal DVT rates, major bleeding rates, and all-cause death rates) were not obviously altered between the 2 groups.
Table 2.
Sensitivity analysis of included studies.
4. Discussion
VTE events, either DVT or PE, are important complications in patients undergoing knee arthroplasty, as VTE is a major cause of death in hospitalized patients.
Patients undergoing total knee replacement surgery are at high risk of developing VTE in the postoperative period and after hospital discharge; and the health care costs of VTE are high, too. Hence, clinical guidelines recommend thromboprophylaxis for 10 to 35 days after TKA.[18] Although widespread use of anticoagulants and improved surgical techniques have substantially reduced the thromboembolic event rates, VTE is still a dangerous complication and PE remains the main cause of death. Rivaroxaban (Xarelto) is an oral, direct antithrombin-independent factor Xa inhibitor, which restricts thrombin generation both in vivo and in vitro, and is a safe and potent new compound for antithrombotic prophylaxis after orthopedic surgery.[19] Factor Xa is a coagulation factor leading to clot formation and thrombin generation.[20] Rivaroxaban has predictable pharmacokinetics, a rapid onset of action and high oral bioavailability.[21] Once-daily oral treatment of rivaroxaban could potentially improve adherence to extended-duration VTE treatment compared with enoxaparin in individuals with confirmed VTE[1] or in administration of thromboprophylaxis.[22] The oral administration of the treatment offers benefits on patient compliance and tolerability.[23] Chen et al believed that rivaroxaban has the major advantages of no required laboratory monitoring and once-daily oral dosing, giving it the opportunity to replace current antithrombotics on the market today.[24] Enoxaparin demonstrated budget overrun compared to rivaroxaban at TKA because of increasing thrombosis complications.[25,26] For VTE prophylaxis in patients undergoing total knee replacement in Canada, rivaroxaban is a cost-effective alternative to enoxaparin, providing more quality-of-life benefit at a lower cost.[27] Ryttberg et al[28] presented an economic model showing that rivaroxaban is a cost-effective alternative to 14 days of LMWH for VTE prophylaxis over a 5-year period in Sweden. Monreal et al[29] suggested that the use of rivaroxaban could result in a mass of healthcare cost savings and improved quality of life after total knee replacement surgery. Similarly, in Duran et al's decision-analytic model, rivaroxaban reduced the payers’ costs of total therapy versus enoxaparin for the prevention of VTE in TKA patients in the US[30] Loganathan et al[31] suggested that if a hospital patient received enoxaparin treatment of after knee arthroplasty, he/she should receive rivaroxaban when discharged.
In Eriksson et al's study,[32] the rate of major bleeding was very low for both rivaroxaban and enoxaparin at 2 weeks after elective knee replacement. However, enoxaparin was less effective than rivaroxaban in reducing the combined incidence of all-cause mortality and symptomatic VTE at 2 weeks. Turpie et al[33] found that rivaroxaban reduces all-cause mortality and symptomatic VTE compared with enoxaparin regimens after elective TKA. In our meta-analysis, our finding was the same as the above for the combined incidence between rivaroxaban group and enoxaparin group (1.15% vs 2.06%, P <.05) after TKA.
The direct Factor Xa inhibitor rivaroxaban showed a better anticoagulant effect than enoxaparin did.[34,35] And rivaroxaban had no adverse influence on liver function.[36] Compared with rivaroxaban, the risk of symptomatic VTE, which included symptomatic DVT and symptomatic PE, was higher with enoxaparin after knee replacement.[37] Our results showed that rivaroxaban could significantly reduce symptomatic VTE and symptomatic DVT compared with enoxaparin after TKA. The number of symptomatic PE events declined (P = .0084) following the introduction of rivaroxaban.[38] However, other literature also reported different results on PE. The rate of postoperative wound complications, PE, or death was no different after TKA.[39] Compared with enoxaparin, rivaroxaban did not significantly reduce the 30-day all-cause readmission rate in persons who had undergone TKA.[40] Complications such as PE or death were not significantly different between the 2 groups in this meta-analysis in patients undergoing TKA surgery. Our results also showed that rivaroxaban could not reduce symptomatic PE compared with enoxaparin after TKA. Asymptomatic DVT was a potential threat to patient health. Havé et al[41] suggested that the majority DVT of patients were asymptomatic and it would be interesting to identify DVT in a systematic manner in patients after knee arthroplasty. Rivaroxaban also showed a better anticoagulant effect of asymptomatic DVT, compared with enoxaparin in our study.
Proximal DVT was defined when the popliteal or femoral veins were included.[42] Therefore, distal DVT was DVT of the lower extremity. At present, there is a big debate about the clinical relevance of distal VTE, and therapy is currently not recommended.[43–47] Excluding distal DVT events and compared with rivaroxaban, the number needed to treat (NNT) for patients undergoing TKA treated with enoxaparin was 125 (1/[36/2005–21/2181]). However, regarding symptomatic DVT, the NNT of enoxaparin was 143 (1/[33/2456–16/2660]). The latter NNT is even larger than the former. Because symptomatic distal DVT is included in symptomatic DVT, it may be more accurate to use proximal DVT indicators to evaluate the efficacy.
Bleeding events were the most commonly reported adverse events across TKA clinical trials, and compared with enoxaparin, the greater efficacy of rivaroxaban was achieved without a remarkable increase in the rate of major bleeding episodes.[48] Ma G et al's research confirmed that direct Xa inhibitors were more effective for the prevention of VTE compared with enoxaparin after TKA, without increasing the risk of major bleeding.[49] The dominant blood loss of the rivaroxaban group was lower than that of enoxaparin group after TKA (P = .003).[50] In Francis et al's research, rivaroxaban was also superior to enoxaparin in the prevention of VTE and with no increase in bleeding complications.[51] Adverse surgical events occurred at a similar rate in the enoxaparin group compared with the rivaroxaban group after total knee replacement (2.69% vs 2.26%, respectively).[52] Similarly, there was also no significant difference in major bleeding between the 2 groups after TKA in our research.
In the coming years, the number of TKA surgeries was expected to increase significantly, and more effective and safer thromboprophylaxis was essential to mitigate the mortality and morbidity associated with VTE.[53] Our study shows that rivaroxaban is more effective than enoxaparin and with no increase in major bleeding or all-cause death rates after TKA. Rivaroxaban is the primary drug of choice after total knee replacement surgery.
4.1. Limitations
Several limitations cannot be neglected in our meta-analysis. First, heterogeneity between studies was present, such as in the all-cause death data, asymptomatic DVT data and distal DVT data in our meta-analysis. Second, the most significant drawback was the lack of RCTs, which increased the risk of bias in the meta-analysis. Third, the different doses of rivaroxaban and enoxaparin for patients were not considered in this research. Fourth, researchers’ experience might have also affected the outcomes, which was not considered in this study. We cannot explain the selection bias or unpublished data.
5. Conclusions
This study highlights some statistical advantages of rivaroxaban over enoxaparin, especially in quantifying the effective advantages. Our study showed that the rates of symptomatic VTE, symptomatic DVT, asymptomatic DVT, distal DVT, and proximal DVT were significantly lower in the rivaroxaban group. However, there were no distinct advantages in reducing symptomatic PE, major bleeding, or all-cause death rates for rivaroxaban after TKA. All of the studies in our meta-analysis were RCTs, but there were too few. Therefore, more multicenter, prospective RCTs are necessary.
Author contributions
Conceived and designed the article: XBT, QX. Literature search and data extraction: HFH, XTY. Analyzed the data: HFH, SSL. Wrote the paper: HFH, XTY. All of the authors read and approved the final manuscript.
Data curation: Shanshan Li.
Resources: Quan Xie, Xiaobin Tian.
Software: Xianteng Yang.
Writing – original draft: Haifeng Huang.
Quan Xie orcid: 0000-0002-2848-1745.
Footnotes
Abbreviations: CI = confidence interval, DVT = deep vein thrombosis, LMWHs = low-molecular-weight heparins, PE = pulmonary embolism, RCTs = randomized controlled clinical trials, RR = relative risk, THA = total hip arthroplasty, TKA = total knee arthroplasty, VTE = venous thromboembolism.
H-FH, S-SL contributed equally to this work.
This work was supported by grants from National Natural Science Foundation of China (No.81560356, No. 61264004), The Science and Technology Foundation of Guizhou Province (No.(2015) 2089, No.gzwjkj2018–1–040), and High-level Creative Talent Training Program in Guizhou Province of China (Grant No. [2015]4015). No other potential conflict of interests relevant to this paper were reported.
The authors have no conflicts of interest to disclose.
References
- [1].Cohen AT, Dobromirski M. The use of rivaroxaban for short- and long-term treatment of venous thromboembolism. Thromb Haemost 2012;107:1035–43. [DOI] [PubMed] [Google Scholar]
- [2].Brown TS, Huo MH. New anticoagulants for thromboprophylaxis after total knee arthroplasty. Am J Orthop (Belle Mead NJ) 2013;42:424–9. [PubMed] [Google Scholar]
- [3].Burghaus R, Coboeken K, Gaub T, et al. Evaluation of the efficacy and safety of rivaroxaban using a computer model for blood coagulation. PLoS One 2011;6:e17626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Borris LC. Rivaroxaban and dabigatran etexilate: two new oral anticoagulants for extended postoperative prevention of venous thromboembolism after elective total hip arthroplasty. Arch Orthop Trauma Surg 2010;130:583–9. [DOI] [PubMed] [Google Scholar]
- [5].Haas S. Rivaroxaban—an oral, direct Factor Xa inhibitor: lessons from a broad clinical study programme. Eur J Haematol 2009;82:339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huisman MV, Quinlan DJ, Dahl OE, et al. Enoxaparin versus dabigatran or rivaroxaban for thromboprophylaxis after hip or knee arthroplasty: Results of separate pooled analyses of phase III multicenter randomized trials. Circ Cardiovasc Qual Outcomes 2010;3:652–60. [DOI] [PubMed] [Google Scholar]
- [7].Hur M, Park SK, Koo CH, et al. Comparative efficacy and safety of anticoagulants for prevention of venous thromboembolism after hip and knee arthroplasty. Acta Orthop 2017;88:634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ning GZ, Kan SL, Chen LX, et al. Rivaroxaban for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis with trial sequential analysis of randomized controlled trials. Sci Rep 2016;6:23726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ricket AL, Stewart DW, Wood RC, et al. Comparison of postoperative bleeding in total hip and knee arthroplasty patients receiving rivaroxaban or enoxaparin. Ann Pharmacother 2016;50:270–5. [DOI] [PubMed] [Google Scholar]
- [10].Charters MA, Frisch NB, Wessell NM, et al. Rivaroxaban versus enoxaparin for venous thromboembolism prophylaxis after hip and knee arthroplasty. J Arthroplasty 2015;30:1277–80. [DOI] [PubMed] [Google Scholar]
- [11].Sindali K, Rose B, Soueid H, et al. Elective hip and knee arthroplasty and the effect of rivaroxaban and enoxaparin thromboprophylaxis on wound healing. Eur J Orthop Surg Traumatol 2013;23:481–6. [DOI] [PubMed] [Google Scholar]
- [12].Chahal GS, Saithna A, Brewster M, et al. A comparison of complications requiring return to theatre in hip and knee arthroplasty patients taking enoxaparin versus rivaroxaban for thromboprophylaxis. Ortop Traumatol Rehabil 2013;15:125–9. [DOI] [PubMed] [Google Scholar]
- [13].Turpie AG, Fisher WD, Bauer KA, et al. BAY 59-7939: an oral, direct factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement. A phase II dose-ranging study. J Thromb Haemost 2005;3:2479–86. [DOI] [PubMed] [Google Scholar]
- [14].Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776–86. [DOI] [PubMed] [Google Scholar]
- [15].Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009;373:1673–80. [DOI] [PubMed] [Google Scholar]
- [16].Zou Y, Tian S, Wang Y, et al. Administering aspirin, rivaroxaban and low-molecular-weight heparin to prevent deep venous thrombosis after total knee arthroplasty. Blood Coagul Fibrinolysis 2014;25:660–4. [DOI] [PubMed] [Google Scholar]
- [17].Xie J, Ma J, Huang Q, et al. Comparison of enoxaparin and rivaroxaban in balance of anti-fibrinolysis and anticoagulation following primary total knee replacement: a pilot study. Med Sci Monit 2017;23:704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guijarro Merino R, Villalobos Sánchez A. Prophylaxis of venous thromboembolism in orthopedic surgery. Role of the new anticoagulants. Med Clin (Barc) 2012;139:13–8. [DOI] [PubMed] [Google Scholar]
- [19].Borris LC. New compounds in the management of venous thromboembolism after orthopedic surgery: focus on rivaroxaban. Vasc Health Risk Manag 2008;4:855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Perzborn E, Roehrig S, Straub A, et al. Rivaroxaban: a new oral factor Xa inhibitor. Arterioscler Thromb Vasc Biol 2010;30:376–81. [DOI] [PubMed] [Google Scholar]
- [21].Perzborn E, Kubitza D, Misselwitz F, et al. A novel, oral, direct factor Xa inhibitor in clinical development for the prevention and treatment of thromboembolic disorders. Hamostaseologie 2007;27:282–9. [PubMed] [Google Scholar]
- [22].Kwong LM. Comparative safety and efficacy of antithrombotics in the management of venous thromboembolism after knee or hip replacement surgery: focus on rivaroxaban. Clin Pharmacol 2013;5:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gagliardi S, MacAione A, Boniforti F. Rivaroxaban versus enoxaparin sodium in the prevention of venous thromboembolism in major orthopaedic surgery. J Orthop Traumatol 2011;12Suppl. 1:S160. [Google Scholar]
- [24].Chen T, Lam S. Rivaroxaban: an oral direct factor Xa inhibitor for the prevention of thromboembolism. Cardiol Rev 2009;17:192–7. [DOI] [PubMed] [Google Scholar]
- [25].Krysanov I, Margieva A, Omelyanovsky VV, et al. Budget impact model of venous thromboembolism prevention after total hip and knee replacement. Value in Health 2010;13:A347. [Google Scholar]
- [26].Kwong LM. Cost-effectiveness of rivaroxaban after total hip or total knee arthroplasty. Am J Manag Care 2011;17:S22–6. [PubMed] [Google Scholar]
- [27].Diamantopoulos A, Lees M, Wells PS, et al. Cost-effectiveness of rivaroxaban versus enoxaparin for the prevention of postsurgical venous thromboembolism in Canada. Thromb Haemost 2010;104:760–70. [DOI] [PubMed] [Google Scholar]
- [28].Ryttberg L, Diamantopoulos A, Forster F, et al. Cost-effectiveness of rivaroxaban versus heparins for prevention of venous thromboembolism after total hip or knee surgery in Sweden. Expert Rev Pharmacoecon Outcomes Res 2011;11:601–15. [DOI] [PubMed] [Google Scholar]
- [29].Monreal M, Folkerts K, Diamantopoulos A, et al. Cost-effectiveness impact of rivaroxaban versus new and existing prophylaxis for the prevention of venous thromboembolism after total hip or knee replacement surgery in France. Italy and Spain Thromb Haemost 2013;110:987–94. [DOI] [PubMed] [Google Scholar]
- [30].Duran A, Sengupta N, Diamantopoulos A, et al. Cost and outcomes associated with rivaroxaban vs enoxaparin for the prevention of postsurgical venous thromboembolism from a US payer's perspective. J Med Econ 2011;14:824–34. [DOI] [PubMed] [Google Scholar]
- [31].Loganathan L, Hua A, Patel S, et al. Efficacy and safety of rivaroxaban thromboprophylaxis after arthroplasty of the hip or knee: retrospective cohort study. Ann R Coll Surg Engl 2016;98:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Eriksson BI, Kakkar AK, Turpie AG, et al. Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. J Bone Joint Surg Br 2009;91:636–44. [DOI] [PubMed] [Google Scholar]
- [33].Turpie AG, Lassen MR, Eriksson BI, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost 2011;105:444–53. [DOI] [PubMed] [Google Scholar]
- [34].Feng W, Wu K, Liu Z, et al. Oral direct factor Xa inhibitor versus enoxaparin for thromboprophylaxis after hip or knee arthroplasty: systemic review, traditional meta-analysis,dose-response meta-analysis and network meta-analysis. Thromb Res 2015;136:1133–44. [DOI] [PubMed] [Google Scholar]
- [35].Fisher WD. Impact of venous thromboembolism on clinical management and therapy after hip and knee arthroplasty. Can J Surg 2011;54:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Borris LC. Rivaroxaban, a new, oral, direct factor Xa inhibitor for thromboprophylaxis after major joint arthroplasty. Expert Opin Pharmacother 2009;10:1083–8. [DOI] [PubMed] [Google Scholar]
- [37].Gómez-Outes A, Terleira-Fernández AI, Suárez-Gea ML, et al. Dabigatran, rivaroxaban, or apixaban versus enoxaparin for thromboprophylaxis after total hip or knee replacement: systematic review, meta-analysis, and indirect treatment comparisons. BMJ 2012;344:e3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rath NK, Goodson MW, White SP, et al. The use of rivaroxaban for chemical thromboprophylaxis following total knee replacement. Knee 2013;20:397–400. [DOI] [PubMed] [Google Scholar]
- [39].Russell RD, Huo MH. Apixaban and rivaroxaban decrease deep venous thrombosis but not other complications after total hip and total knee arthroplasty. J Arthroplasty 2013;28:1477–81. [DOI] [PubMed] [Google Scholar]
- [40].Herschman MA, Rigelsky FS, Axtell SS. A pilot study comparing hospital readmission rates in patients receiving rivaroxaban or enoxaparin after orthopedic surgery. P T 2016;41:376–80. [PMC free article] [PubMed] [Google Scholar]
- [41].Havé L, Tondeur G, Fournel C, et al. Incidence of thromboembolic complications after hip and knee arthroplasty: interest of systematic post-operative Doppler exploration. Ann Phys Rehabil Med 2012;55:e56–8. [Google Scholar]
- [42].Marlovits S, Striessnig G, Schuster R, et al. Extended-duration thromboprophylaxis with enoxaparin after arthroscopic surgery of the anterior cruciate ligament: a prospective, randomized, placebo-controlled study. Arthroscopy 2007;23:696–702. [DOI] [PubMed] [Google Scholar]
- [43].Palareti G, Cosmi B, Lessiani G, et al. Evolution of untreated calf deep-vein thrombosis in high risk symptomatic outpatients: the blind, prospective CALTHRO study. Thromb Haemost 2010;104:1063–70. [DOI] [PubMed] [Google Scholar]
- [44].Galanaud JP, Sevestre MA, Genty C, et al. Incidence and predictors of venous thromboembolism recurrence after a first isolated distal deep vein thrombosis. J Thromb Haemost 2014;12:436–43. [DOI] [PubMed] [Google Scholar]
- [45].Barco S, Corti M, Trinchero A, et al. Survival and recurrent venous thromboembolism in patients with first proximal or isolated distal deep vein thrombosis and no pulmonary embolism. J Thromb Haemost 2017;15:1436–42. [DOI] [PubMed] [Google Scholar]
- [46].Garry J, Duke A, Labropoulos N. Systematic review of the complications following isolated calf deep vein thrombosis. Br J Surg 2016;103:789–96. [DOI] [PubMed] [Google Scholar]
- [47].Righini M, Galanaud JP, Guenneguez H, et al. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): arandomised, double-blind, placebo-controlled trial. Lancet Haematol 2016;3:e556–62. [DOI] [PubMed] [Google Scholar]
- [48].Duggan ST. Rivaroxaban: a review of its use for the prophylaxis of venous thromboembolism after total hip or knee replacement surgery. Am J Cardiovasc Drugs 2012;12:57–72. [DOI] [PubMed] [Google Scholar]
- [49].Ma G, Zhang R, Wu X, et al. Direct factor Xa inhibitors (rivaroxaban and apixaban) versus enoxaparin for the prevention of venous thromboembolism after total knee replacement: a meta-analysis of 6 randomized clinical trials. Thromb Res 2015;135:816–22. [DOI] [PubMed] [Google Scholar]
- [50].Li J, Jing J, Zhou Y, et al. Comparison of rivaroxaban and enoxaparin on blood loss after total knee arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2014;28:26–9. [PubMed] [Google Scholar]
- [51].Francis CW. New issues in oral anticoagulants. Hematology Am Soc Hematol Educ Program 2008;259–65. [DOI] [PubMed] [Google Scholar]
- [52].Lassen MR, Gent M, Kakkar AK, et al. The effects of rivaroxaban on the complications of surgery after total hip or knee replacement: results from the RECORD programme. J Bone Joint Surg Br 2012;94:1573–8. doi:10.1182/asheducation-2008.1.259. [DOI] [PubMed] [Google Scholar]
- [53].Friedman RJ. Benefits of novel oral anticoagulant agents for thromboprophylaxis after total hip or knee arthroplasty. Am Health Drug Benefits 2012;5:115–22. [PMC free article] [PubMed] [Google Scholar]


