Measurement of chromogranin B is more useful and helpful to detect pancreatic neuroendocrine tumor without liver metastasis and more differentiate pancreatic neuroendocrine tumor from other pancreatic diseases than chromogranin A.
Keywords: pancreatic neuroendocrine tumors, chromogranin A, chromogranin B, pancreatic diseases
Abstract
Objective
Currently, serum chromogranin A is a well-established biomarker for pancreatic neuroendocrine tumors; however, other pancreatic diseases, oral use of a proton pump inhibitor and renal impairment can affect chromogranin A. Meanwhile, chromogranin B, belonging to the same granin family as chromogranin A, is not fully examined in these conditions. The present study aimed to evaluate the utility of chromogranin B as a pancreatic neuroendocrine tumor biomarker.
Methods
Serum chromogranin B levels were determined by radioimmunoassay and serum chromogranin A levels by enzyme-linked immunosorbent assay in pancreatic neuroendocrine tumor (n = 91) and other pancreatic conditions, and in healthy people (n = 104), to assess the relationships with clinical features.
Results
The diagnostic ability of chromogranin B was as good as chromogranin A. The area under the curve was 0.79 for chromogranin B (sensitivity/specificity: 72%/77%), and 0.78 for chromogranin A (sensitivity/specificity: 79%/64%). Chromogranin B was not affected by proton pump inhibitor use and age, which affected chromogranin A. The number of cases without liver metastases was larger in pancreatic neuroendocrine tumor patients with positive chromogranin B and negative chromogranin A. Though chromogranin A significantly elevated cases with proton pump inhibitor treatment and had positive correlation with age, chromogranin B did not have the tendencies. However, both chromogranin B and chromogranin A elevated in the case with renal impairment. In addition, the logistic regression analysis showed that chromogranin B was superior to chromogranin A in differentiation of pancreatic neuroendocrine tumor from other pancreatic diseases.
Conclusions
Compared with chromogranin A, chromogranin B may be more useful during proton pump inhibitor treatment and can detect tumors without liver metastases. In addition, chromogranin B may be an excellent biomarker when differentiation of pancreatic neuroendocrine tumor from other pancreatic diseases is required.
Introduction
Pancreatic neuroendocrine tumor (pNET) is a relatively rare disease, but its prevalence has been rising (1). Occasionally, pNET progresses quickly and has a poor prognosis; therefore, pNET has recently been recognized as a malignant tumor. Among pNET cases, non-functional pNETs with very few clinical manifestations (2) are often found to have distant metastases at the time of diagnosis (3), and postoperative recurrence is not rare in non-functional pNETs (4). Owing to the current improvements in prognosis attributable to advancements in treatment and new drugs (5–7), the use of a biomarker with a higher diagnostic accuracy for pNET is necessary.
At present, serum chromogranin A (CgA) is the most useful diagnostic marker for pNET (3,8–11).
However, serum CgA values rise with oral use of proton pump inhibitors (PPIs) (12–14), and in patients with renal impairment (15), cardiovascular disease (16), inflammatory bowel disease (17), various malignant tumors (18) and other pancreatic diseases, showing false negative results (19,20), as described in previous studies (21,22).
Meanwhile, chromogranin B (CgB), a secretory protein in the same family as CgA, has been reported to be useful as a biomarker for pNET (8,23) and can be expected to replace CgA as a pNET marker that is not influenced by the above-mentioned variables that affect CgA (24,25). There are a few reports on CgB and CgA in pNET stating that no significant difference exists in the diagnostic ability between CgA and CgB (26), and that CgB is not likely to be affected by renal impairment or PPI treatment (24). Nevertheless, only a few reports describe a detailed comparison of utility between CgA and CgB as pNET biomarkers; therefore, this subject remains controversial.
Accordingly, in the present study, CgB was compared with CgA to evaluate its utility as diagnostic marker for Japanese patients with pNET.
Materials and methods
Collection of samples and measurements
We evaluated serum samples of patients with pNET (n = 91), neuroendocrine carcinoma (NEC) (n = 7), pancreatic cancer (PC) (n = 52), chronic pancreatitis (CP) (n = 54) and autoimmune pancreatitis (AIP) (n = 24). As per the World Health Organization 2010 classification, all patients in the pNET group had undergone tumor biopsies and were histologically diagnosed with well-differentiated tumors. Most (92.9%, 83/91) of the patients were classified into NET G1 with a Ki-67 index of no more than 2% or NET G2 with a Ki-67 index 2–20%. In the remaining 7.1% (8/91) of patients, a Ki-67 value was not determined. Each functional pNET was diagnosed on the basis of symptoms arising from over-secretion of hormones. Additionally, all patients with NEC were histologically diagnosed to determine poorly differentiated tumors with a Ki-67 index over 20% according to the World Health Organization 2010 classification. All patients with PC were histologically verified. All patients with CP or AIP were diagnosed using the standard diagnostic criteria in Japan. In addition, we excluded patients with cardiovascular disease, other cancers, inflammatory bowel disease or liver cirrhosis, because these diseases may cause elevation of serum CgA levels. We also collected serum from 104 healthy, age- and sex-matched controls. The study protocol was approved by the Ethics Committee at Kyushu University and all patients and healthy controls gave informed consent. We obtained clinical data retrospectively from our hospital information systems. Blood samples were collected from each patient while fasting, centrifuged to obtain serum samples and stored at −80°C until assayed. CgB was detected by the radioimmunoassay technique and CgA was detected by the enzyme-linked immunosorbent assay (ELISA) technique. For measurement of CgB and CgA values, we used EURIA-CgB (Eurodiagnostica, Malmö, Sweden), and CgA ELISA Chroma (CIS Bioassays, GIF-SUR-YVETTE, France).
Clinical features of patients
The characteristics of all patients are summarized in Table 1.
Table 1.
Patient characteristics in the study
| Characteristics | pNET | NEC | PC | CP | AIP | control | P value versus control |
|---|---|---|---|---|---|---|---|
| Total number | 91 | 7 | 52 | 54 | 24 | 104 | |
| Sex (%) | |||||||
| Male | 46 | 3 | 29 | 32 | 21 | 64 | 0.123 |
| Female | 45 (49) | 4 (57) | 23 (44) | 22 (40) | 3 (13) | 40 (38) | |
| Age (years old) | |||||||
| Mean ± SD | 57.1 ± 13.7 | 66.3 ± 9.5 | 63.6 ± 9.9 | 52.6 ± 14.1 | 64 ± 11.3 | 55.3 ± 13.7 | 0.389 |
| Range | 19–86 | 47–74 | 38–84 | 25–81 | 35–85 | 26–99 | |
| PPI use (%) | |||||||
| Yes | 35 (39) | 2 (29) | 20 (39) | 28 (52) | 13 (54) | 0 (0) | <0.0001 |
| eGFR (ml/min/1.73 m2) <60 | |||||||
| Yes | 15 (17) | 2 (29) | 8 (15) | 6 (11) | 4 (17) | 0 (0) | <0.0001 |
| Presence of liver metastasis | |||||||
| Yes | 43 (47) | 7 (100) | 20 (38) | ||||
| Histological grade | |||||||
| G1 | 47 (52) | ||||||
| G2 | 37 (40) | ||||||
| Undetermineda | 7 (8) | ||||||
| Size of the pancreatic tumor | |||||||
| ≤2 cm | 40 (44) | 0 (0) | |||||
| >2 cm | 22 (24) | 5 (71) | |||||
| Postoperativeb | 29 (32) | 2 (29) | |||||
| Presence of MEN-1 | |||||||
| Yes | 8 (9) | ||||||
| Type | |||||||
| Non-functioning | 55 (60) | ||||||
| Functioning | |||||||
| Gastrinoma | 19 (21) | ||||||
| Insulinoma | 12 (13) | ||||||
| Othersc | 5 (5) | ||||||
pNET, pancreatic neuroendocrine tumor; NEC, neuroendocrine carcinoma; PC, pancreatic cancer; CP, chronic pancreatitis; AIP, autoimmune pancreatitis; eGFR, estimated glomerular filtration rate. aDiagnosed to be NET G1 or G2 and Ki-67 was not evaluated. bPatients with liver metastases after resection of the pancreatic tumor. cOthers includes somatostatinoma, glucagonoma, VIPoma. P value was calculated using Chi-squared test. *Statistically significant.
In this study, renal impairment was defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2, which is equal to the Kidney Disease Outcomes Quality Initiative (KDOQI) kidney disease stages G3 and G4. The percentage of patients with renal impairment in the pNET, NEC, PC, CP, AIP and control groups were 17% (17/91), 29% (2/7), 39% (20/52), 52% (28/54), 13% (3/24) and 0%, respectively. The percentage of PPI users in the pNET, NEC, PC, CP, AIP and control groups were 39% (35/91), 29% (2/7), 39% (20/52), 29% (2/7), 52% (28/54), 54% (13/24) and 0%, respectively. In the AIP group, the ratio of male tended to be higher than in other groups. The age of the NEC group was higher than that of the other groups. The pNET group consisted of 47 G1, 37 G2, and seven not determined cases. Over two-third [68% (62/91)] of the pNET were non-functioning and the remaining were 19 gastrinomas, 12 insulinomas and 5 others, which included 1 somatostatinoma, 1 glucagonoma and 3 vasoactive intestinal polypeptide-omas (VIPoma). Among the patients with a primary tumor remaining in the pancreas, the maximum diameter of the primary tumor was within 2 cm in 40 patients (44%) and >2 cm in 22 patients (24%). The percentages of patients with liver metastases and multiple endocrine neoplasia type 1 (MEN-1) in the pNET group were 47% (43/91) and 9% (8/91), respectively.
Statistical analysis
We analyzed the differences of each biomarker between two group using the nonparametric Mann–Whitney's U test. P values < 0.05 were considered statistically significant. Construct receiver operating characteristics (ROC) curves was obtained to determine cut-off values and to compare the diagnostic accuracy. The point on the ROC curve which corresponding to Youden index, at which [sensitivity + specificity − 1] is maximized, was defined as cut-off values of CgB and CgA. Pearson's correlation coefficient was used to determine the relationship between each markers. In addition, we constructed single and multiple logistic regression models to assess the related factors for positive results of each marker and differentiation between pNET and another disease.
The software packages JMP v. 12 (SAS and JMP, Institute Inc., Cary, NY) and R version 3.2.3 (R Core Team, 2016) were employed for the statistical analysis.
Results
Comparison of serum CgB and CgA between pNET and controls
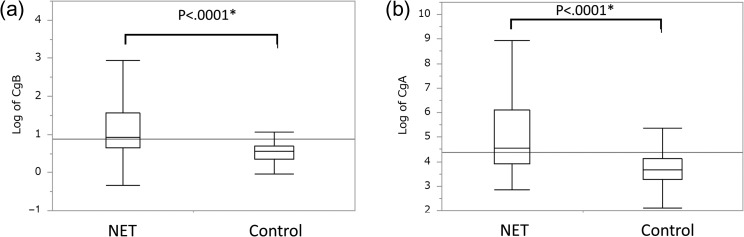
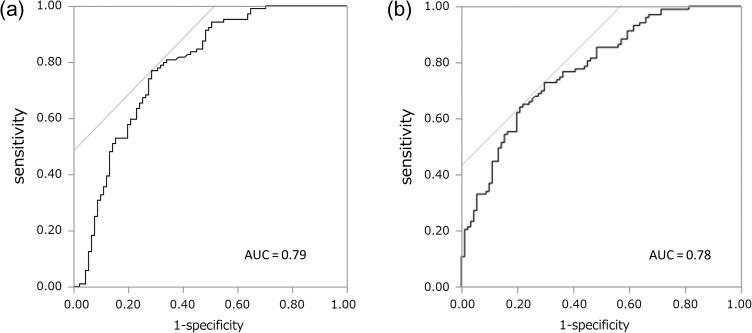
Figure 1 shows a box plot corresponding to the distributions of CgB and CgA, values in pNET cases and controls. The median serum CgB was 1.76 nmol/l in the controls and 2.34 nmol/l in the pNET group (P < 0.0001), and for CgA, it was 38.8 ng/ml in the controls and 92.9 ng/ml (P < 0.0001) in the pNET group, demonstrating a significant increase of both biomarkers. Aiming to evaluate their diagnostic ability, we formulated ROC curves for CgB (Fig. 2a) and CgA (Fig. 2b). Based on the ROC curves, the CgB cut-off value was 2.03 nmol/l (area under the curve, AUC: 0.79, sensitivity/specificity: 73%/77%), and the CgA cut-off value was 47.1 ng/ml (AUC: 0.78, sensitivity/specificity: 79%/64%) (Table 2). In addition, analysis of CgB and CgA values for non-functioning pNET (without functioning) showed the similar diagnostic performance (Supplementary Fig. 1).
Figure 1.
Box plot showing the distributions of marker levels. (a) Log of CgB and (b) log of CgA. The median is indicated with a line in the box, the end of the box indicates the 25th and 75th percentiles, and the10th and 90th percentiles are indicated with error bars. *Significant difference (P < 0.05) was calculated using a Mann–Whitney's U test.
Figure 2.
Receiver operating characteristic curve of (a) CgB value (pNET versus controls) and (b) CgA value (pNET versus controls). AUC, area under the curve.
Table 2.
Sensitivity and specificity of CgB and CgA values (cut-off value: CgB, 2.03 nmol/l; CgA, 47.1 ng/ml)
| Positive biomarker | Number (n = 91) | AUC (95% CI) | Sensitivity | Specificity | P value |
|---|---|---|---|---|---|
| CgB | 66 | 0.79 (0.71–0.85) | 0.73 | 0.77 | <0.0001* |
| CgA | 72 | 0.78 (0.71–0.84) | 0.79 | 0.64 | <0.0001* |
CgA, chromogranin A; CgB, chromogranin B; CI, confidence interval; AUC, area under the curve; *Statistically significant using Chi-square test.
Correlation between each marker and gastrin
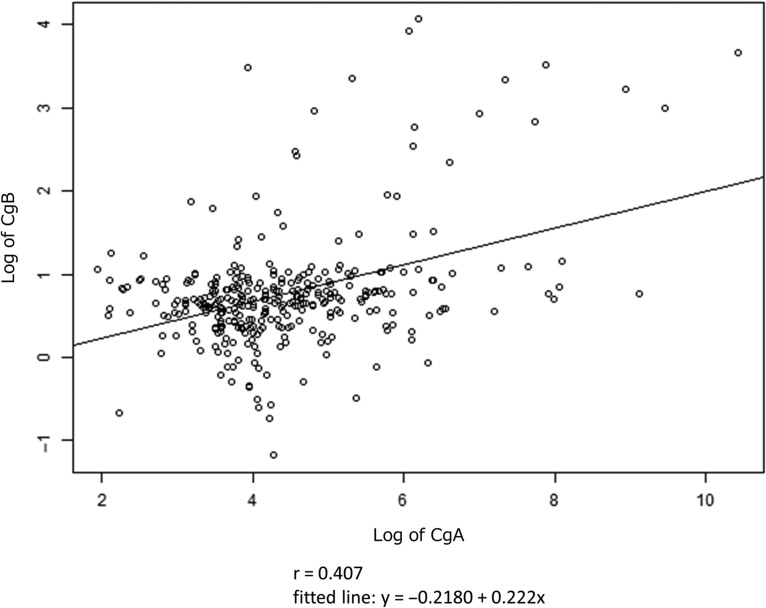
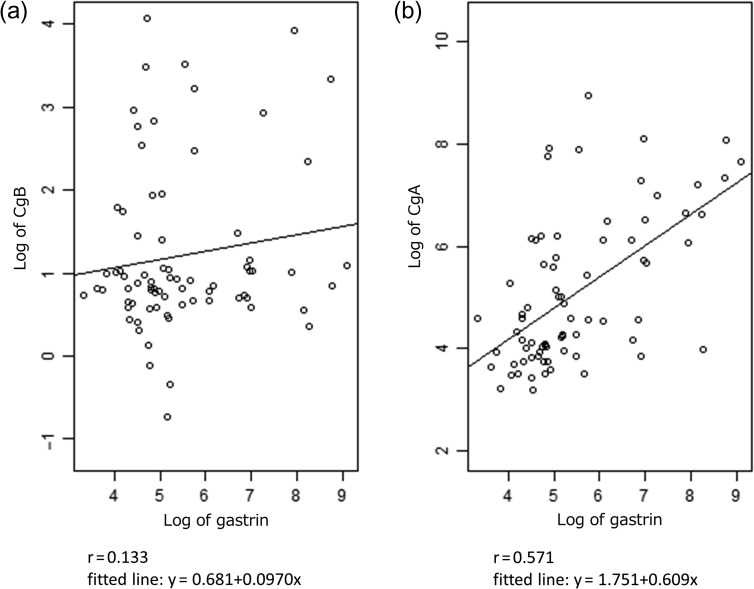
Next, we calculated correlation coefficient for biomarkers in pNET. Pearson's correlation coefficient r between CgB and CgA was 0.407 (Fig. 3). Additionally, correlation coefficient for CgB and gastrin (r = 0.133) was lower than CgA and gastrin (r = 0.571) (Fig. 4a and b).
Figure 3.
Correlation between log of CgB and CgA. r means Pearson's correlation coefficient.
Figure 4.
Correlation between log of gastrin and (a) log of CgB, (b) log of CgA. r means Pearson's correlation coefficient.
Related factors with positive serum CgB and CgA in pNET
Furthermore, the subjects were divided into positive and negative groups by CgB and CgA. Age, sex, functioning/non-functioning, primary (pancreatic) tumor size, histological grade (G1/G2), presence/absence of multiple endocrine neoplasia type 1 (MEN-1) and presence/absence of liver metastases were examined by univariate logistic regression analysis to find the factors related to positive results (Table 3). The factors that were significantly related to positive results (P value < 0.05) were primary tumor size >2 cm (P = 0.048, odds ratio [OR] 3.6) and the presence of liver metastases (P = 0.003, OR 4.3) for CgB, and primary tumor size >2 cm (P = 0.002, OR 12.8), histological grade G2 (P = 0.07, OR 2.7) and the presence of liver metastases (P < 0.0001, OR 27.5) for CgA.
Table 3.
Univariate and multivariate analysis in patients with pNET
| Univariate analysis | Multivariate analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Size | CgB | CgA | Size | CgB | Size | CgA | ||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||||
| Age (years old) | 91 | 0.98 (0.94–1.01) | 0.16 | 1.02 (0.99–1.06) | 0.25 | – | |||||
| Sex, male versus female | 91 | 0.93 (0.39–2.4) | 0.89 | 2.26 (0.81–6.35) | 0.12 | – | |||||
| Functioning tumor, yes versus no | 91 | 1.14 (0.47–2.96) | 0.78 | 0.98 (0.35–2.70) | 0.96 | – | |||||
| Primary tumor size more than 2 cm, yes versus no | 62 | 3.6 (1.03–15.9) | 0.048* | 12.8 (1.56–104.9) | 0.002* | 61 | 1.79 (0.099–2.7) | 0.47 | 56 | 4.43 (0.46–111.6) | 0.21 |
| Historical grade, G2 versus G1 | 83 | 2.17 (0.85–6.0) | 0.12 | 2.72 (0.88–8.41) | 0.07 | 56 | 4.20 (0.51–89.2) | 0.19 | |||
| Presence of MEN-1, yes versus no | 91 | 0.81 (0.15–4.35) | 0.79 | 2.08 (0.24–18.0) | 0.51 | – | |||||
| Presence of liver metastases, yes versus no | 91 | 4.29 (1.4–10.4) | 0.003* | 27.5 (3.49–217.2) | <0.0001* | 61 | 4.20 (0.79–33.5) | 0.10 | 56 | 866 066 5776.68- | 0.0004* |
OR, odds ratio; CI, confidence interval; variables with P < 0.1 (bold figure) in univariate analysis were retained in multivariate regression analysis (available-case analysis). *Statistically significant (P < 0.05); size, the number of the case excluded for missing data.
Furthermore, multiple regression analysis of these factors showed that there were no significant differences between any items for CgB, while in the presence of liver, metastases (P = 0.0004) have a significant tendency to show the elevation for CgA. Additionally, we analyzed 11 cases that were positive for CgB and negative for CgA (Supplementary Table 1). Among the 11 cases, 3 had insulinomas and 8 had non-functioning pNETs. Seven had histological grade G1 and three had G2 (1, not determined). And among 10 pre-operative cases of them, 8 cases had primary tumor with the size <2 cm. Ten of 11 cases with isolated elevation of CgB (with normal CgA) did not have liver metastases. Therefore, CgB has a higher sensitivity in the cases with no liver metastases compared with CgA.
Effects of oral PPI, renal impairment and age on serum CgB and CgA values
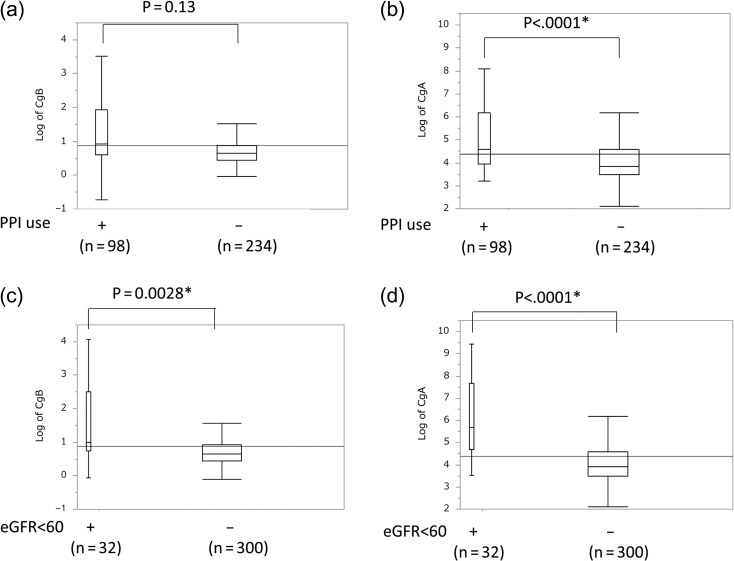
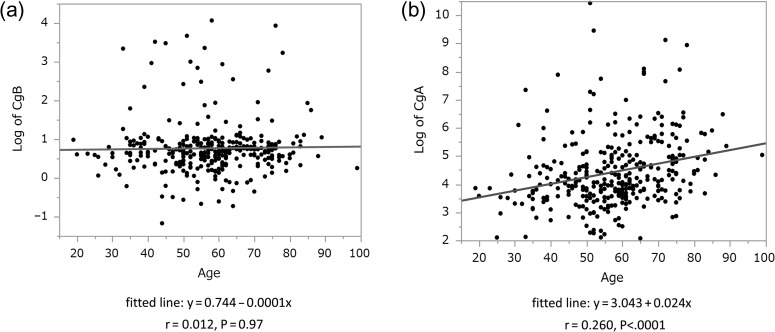
Next, to analyze the related factor of CgB and CgA in whole cases, we examined the difference of CgB and CgA between two groups divided according to following: sex, PPI use (Fig. 5a and b) and the presence of renal impairment (Fig. 5c and d). We also calculated the correlations between each marker and age (Fig. 6). Both markers were increased in the presence of renal impairment and have no difference in gender. CgA was significantly higher in patient using PPI; however, CgB did not have the tendency. In addition, CgA and age are related to positive correlation.
Figure 5.
Box plot showing the distributions of marker levels. (a) Log of CgB and (b) log of CgA in case with or without PPI treatment. (c) Log of CgB and (d) log of CgA in cases with or without renal impairment. *Significant difference (P < 0.05) was calculated using a Mann–Whitney's U test.
Figure 6.
Relationship between age and (a) CgB, (b) CgA. r means Pearson's correlation coefficient.
We analyzed the distribution of both markers divided by the presence of PPI use and renal impairment in each disease (Supplementary Figs 2 and 3 and Supplementary Tables 2 and 3) and calculated the differences by using Mann–Whitney's U test. According to the analysis, they showed that CgA was significantly higher in PC (P = 0.002) and AIP (P = 0.047) but CgB was not significantly different in any group under PPI use. In terms of association with renal impairment, CgA was higher in pNET; however, CgB did not show any significant difference.
Serum CgA and CgB values in pNET and other pancreatic diseases
To evaluate the utility for differential diagnosis, we analyzed the results of serum CgB and CgA values in different diseases (Table 4). Compared with the controls, CgA was significantly higher in any of the pNET groups (P < 0.0001), NEC (P = 0.0032), PC (P = 0.0007), CP (P < 0.0001) and AIP (P < 0.0001). Meanwhile, significantly higher values of CgB were only observed in pNET (P < 0.0001) and PC (P < 0.0001) compared with the controls. As for NEC (n = 7), CgA was higher as in the case of NET, but CgB was as low as controls.
Table 4.
Serum CgA and CgB of each group
| pNET (n = 91) | NEC (n = 7) | PC (n = 52) | CP (n = 54) | AIP (n = 24) | Control (n = 104) | ||
|---|---|---|---|---|---|---|---|
| CgB (nmol/l) | Median (25–75 percentile) | 2.34 (1.86–4.24) | 1.84 (0.89–2.13) | 2.20 (1.85–2.55) | 1.91 (1.38–2.23) | 1.78 (1.38–2.20) | 1.76 (1.43–2.02) |
| P value | <0.0001* | 0.54 | <0.0001* | 0.9401 | 0.9998 | ||
| CgA (ng/ml) | Median (25–75 percentile) | 92.85 (50.62–433.5) | 94.93 (57.85–320.4) | 92.09 (42.29–387.22) | 76.47 (43.37–137.23) | 94.93 (51.84–262.7) | 38.77 (26.19–61.29) |
| P value | <0.0001* | 0.0032* | 0.0007* | <0.0001* | <0.0001* |
P value (*statistically significant < 0.05) was calculated using Mann–Whitney's U test comparing to controls.
Furthermore, we evaluated the differential potential of both markers. To adjust the related factors, as we have shown former, we constructed single and multiple logistic regression analysis (Table 5a and b). According to the single regression analysis, CgB was significantly increased in pNET in comparison with all group. However, there was no significant difference of CgA in two pairs; pNET and NEC (P = 0.72) and pNET and PC (P = 0.30). In addition, multiple regression analysis showed that CgA is not significantly increased even between pNET and CP, pNET and AIP; however, CgB is significantly higher than any group, that were pNET versus controls (<0.0001, OR 4.5), versus NEC (P = 0.0014, OR 5.3), versus PC (P < 0.0001 OR 1.4), versus CP (P < 0.0001, OR 2.3), AIP (P = 0.0003, OR 1.6).
Table 5.
Logistic regression analysis comparing NET and other groups: (a) univariate analysis and (b) multivariate analysis
| (a) Univariate analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | pNET versus controls | pNET versus NEC | pNET versus PC | pNET versus CP | pNET versus AIP | |||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (years old) | 1.02 (0.99–1.04) | 0.26 | 0.94 (0.86–1.02) | 0.07* | 0.96 (0.92–0.99) | 0.005* | 1.03 (1.00–1.05) | 0.04* | 0.96 (0.92–0.99) | 0.03* |
| Sex: male or female | 0.67 (0.37–1.18) | 0.16 | 1.42 (0.30–7.57) | 0.65 | 0.81 (0.40–1.61) | 0.55 | 0.73 (0.39–1.45) | 0.37 | 0.153 (0.034–0.48) | 0.0007* |
| CgB (nmol/l) | 3.57 (2.09–6.68) | <0.0001* | 4.21 (1.36–16.5) | 0.002* | 1.39 (1.12–1.99) | 0.029* | 2.63 (1.58–4.90) | <0.0001* | 1.71 (1.13–3.19) | 0.0002* |
| CgA (ng/ml) | 1.006 (1.003–1.01) | <0.0001* | 1.00 (1.00–1.00) | 0.72 | 1.00 (0.99–1.01) | 0.30 | 1.003 (1.00–1.005) | <0.0001* | 1.001 (1.00–1.003) | 0.028* |
| PPI use: yes or no | 14 936945 6.89e+56– |
<0.0001* | 1.56 (0.32–11.3) | 0.60 | 1.00 (0.50–2.03) | 1.00 | 0.58 (0.29–1.14) | 0.23 | 0.529 (0.21–1.31) | 0.17 |
| Renal impairment: yes or no | 41 029 32110.65– | <0.0001* | 0.28 (0.06–1.56) | 0.18 | 1.02 (0.42–2.59) | 0.97 | 1.70 (0.65–5.03) | 0.29 | 10.07 (0.345–4.03) | 0.92 |
| (b) Multivariate analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | NET versus controls | NET versus NEC | NET versus PC | NET versus CP | NET versus AIP | |||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (years old) | 0.91 (0.80–0.99) | 0.047* | 0.96 (0.93–0.99) | 0.023* | 1.02 (1.00–1.06) | 0.07 | 0.95 (0.90–0.99) | 0.022* | ||
| Sex: male versus female | 0.130 (0.03–0.45) | 0.0008 | ||||||||
| CgB (nmol/l) | 4.5 (2.21–10.9) | <0.0001* | 5.3 (1.52–27.0) | 0.0014* | 1.4 (1.11–2.04) | <0.0001* | 2.3 (1.40–4.53) | 0.006* | 1.6 (1.11–2.97) | 0.003* |
| CgA (ng/ml) | 1.002 (1.00–1.007) | 0.037* | 1.00 (0.99–1.004) | 0.16 | 1.00 (1.00–1.003) | 0.10 | ||||
| PPI use: yes versus no | 2.3e+1047.9– | <0.0001* | ||||||||
| Renal impairment: yes versus no | 4.3e+94.76– | 0.0007* | ||||||||
Variables with P < 0.1 (bold figures) in univariate analysis were retained in multivariate regression analysis. *Statistically significant equals to P < 0.05.
Discussion
We examined utility of CgB as a diagnostic marker of pNET by comparing with CgA, which has been shown as a well-established marker. The diagnostic potential of serum CgB was as same extent as CgA. Furthermore, CgB was not affected by age and PPI use and was significant different between pNET and other pancreatic disease. This result indicated that CgB can overcome the drawback of CgA as a diagnostic marker of pNET.
CgB, also known as secretogranin I, belongs to the same granin family as CgA. CgB is a secretory protein consisting of 657 amino acids, localized in the healthy pancreas and within secretory granules of neuroendocrine cells, and it regulates hormone production via intracellular processing (27–29). Stridsberg et al. (25) measured the blood CgB in pNET and gastrointestinal NET by region-specific CgB assays using 13 different epitopes. According to their results, the detection rate of the CgB 439–451 antibody was the best. In the present study, we conducted experiments using an antibody that recognizes CgB 439–451, and confirmed the utility of CgB as a biomarker for pNET.
Many reports have indicated that CgB is different from CgA in its level/mode of expression (30). In the present study, the correlation between serum CgB and CgA was not high, probably reflecting the difference in expression mechanisms between CgB and CgA. This indicates that combination of CgB and CgA measurement may be useful.
While both biomarkers are equally capable of pNET diagnosis, CgA tended to increase with the presence of liver metastasis as previous paper showed (8,9), CgB did not have the tendency. Additionally, many of the cases with positive CgB and negative CgA were characterized by no liver metastasis. These findings indicated that CgB may have better utility than CgA in diagnosing pNETs without liver metastasis.
In the whole population, a problem of CgA increasing in response to PPI use was observed in the present study, as Fig. 5 showed (12,14). In the present study, CgB values did not go up in response to PPI treatment. The correlation coefficient between CgA and gastrin was 0.571, but only 0.133 between CgB and gastrin (Fig. 4a and b), which did not contradict the finding that CgB was not likely to be increased during PPI treatment. This suggests that serum CgB could be useful as a biomarker for pNET even during PPI treatment, because CgB is not likely to be influenced by PPI use.
As for the effect of renal impairment, both CgB and CgA tended to increase with renal impairment, contrary to the previous report that CgB is not likely to go up in renal impairment (24,31).
Interestingly, we found that CgA has a positive correlation with age for the first time. There is a possibility of pseudo-positive among elderly patients, so further study is required.
There is a strength of this study. We evaluated CgB and CgA levels of not only pNET but also other pancreatic diseases, because it has been shown that CgA is influenced by PC and AIP (19,20) and it may be a drawback of CgA. On the other hand, there is a limitation that we could not correct samples of well-matched cases and controls. So we conducted logistic regression analysis to adjust the related factors. As the result, the results were consistent with the previous reports. CgA in pNET was not significantly different from CgA in other groups. On the other hand, CgB was significantly elevated in pNET compared with all groups. This indicates that CgB is superior in differentiating pNET from other pancreatic diseases.
To summarize the results of the present study, CgB has more tendency to increase even in early stages without liver metastasis. But, further study is required because the size of the cases of this study is small. Compared with CgA, the advantage of CgB is that it is less likely to be influenced by PPI treatment and in its usefulness for differentiation between pNET and other pancreatic diseases. In future, CgB is expected to be useful as a diagnostic marker for pNET management.
Supplementary Material
Acknowledgments
The authors are most grateful to Sceti Medical Labo.
Supplementary data
Supplementary data are available at Japanese Journal of Clinical Oncology online.
Funding
This research received no funding.
Conflict of interest statement
The authors have no conflict of interest to declare.
References
- 1. Ito T, Igarashi H, Kazuhiko K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol 2015;50:58–64. [DOI] [PubMed] [Google Scholar]
- 2. Modlin IM, Moss SF, Chung DC, Jensen RT, Snyderwine E. Priorities for improving the management of gastroenteropancreatic neuroendocrine tumors. J Natl Cancer Inst 2008;100:1282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;61–72. [DOI] [PubMed] [Google Scholar]
- 4. Kimura W, Tezuka K, Hirai I. Surgical management of pancreatic neuroendocrine tumors. Surg Today 2011;41:1332–42. [DOI] [PubMed] [Google Scholar]
- 5. Ito T, Igarashi H, Jensen RT. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): recent insights and advances. J Gastroenterol 2012;47:941–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee L, Igarashi H, Fujimori N, et al. Long-term outcomes and prognostic factors in 78 Japanese patients with advanced pancreatic neuroendocrine neoplasms: a single-center retrospective study. Jpn J Clin Oncol 2015;45:1131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito T, Hijioka S, Masui T, et al. Advances in the diagnosis and treatment of pancreatic neuroendocrine neoplasms in Japan. J Gastroenterol 2016;DOI:10.1007/s00535-016-1250-9. [DOI] [PubMed] [Google Scholar]
- 8. Hijioka M, Ito T, Igarashi H, et al. Serum chromogranin A is a useful marker for Japanese patients with pancreatic neuroendocrine tumors. Cancer Sci 2014;105:1464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seregni E, Ferrari L, Bajetta E, Martinetti A, Bombardieri E. Clinical significance of blood chromogranin A measurement in neuroendocrine tumours. Ann Oncol 2001;12:69–72. [DOI] [PubMed] [Google Scholar]
- 10. Bajetta E, Ferrari L, Martinetti A, et al. Chromogranin A, neuron specific enolase, carcinoembryonic antigen, and hydroxyindole acetic acid evaluation in patients with neuroendocrine tumors. Cancer 1999;86:858–65. [DOI] [PubMed] [Google Scholar]
- 11. Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A—biological function and clinical utility in neuro endocrine tumor disease. Annl Surg Oncol 2010;17:2427–43. [DOI] [PubMed] [Google Scholar]
- 12. D'herbomez M, Do Cao C, Vezzosi D, Borzon-Chasot F, Baudin E. Chromogranin A assay in clinical practice. Ann Endocrinol 2010;71:274–80. [DOI] [PubMed] [Google Scholar]
- 13. Korse CM, Muller M, Taal BG. Discontinuation of proton pump inhibitors during assessment of chromogranin A levels in patients with neuroendocrine tumours. Br J Cancer 2011;105:1173–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raines D, Chester M, Diebold AE, et al. A prospective evaluation of the effect of chronic proton pump inhibitor use on plasma biomarker levels in humans. Pancreas 2012;41:508–11. [DOI] [PubMed] [Google Scholar]
- 15. Hsiao RAYJ, Mezger MS, Connor DTO. Chromogranin A in uremia: progressive retention of immunoreactive fragments. Elsevier Masson SAS 1990;37:955–64. [DOI] [PubMed] [Google Scholar]
- 16. Ceconi C, Ferrari R, Bachetti T, et al. Chromogranin A in heart failure: a novel neurohumoral factor and a predictor for mortality. Eur Heart J 2002;23:967–74. [DOI] [PubMed] [Google Scholar]
- 17. Sciola V, Massironi S, Conte D, et al. Plasma chromogranin a in patients with inflammatory bowel disease. Inflamm Bowel Dis 2009;15:867–71. [DOI] [PubMed] [Google Scholar]
- 18. Bofin AM, Qvigstad G, Waldum C, Waldum HL. Neuroendocrine differentiation in carcinoma of the breast. Tyramide signal amplification discloses chromogranin A-positive tumour cells in more breast tumours than previously realized. Apmis 2002;110:658–64. [DOI] [PubMed] [Google Scholar]
- 19. Malaguarnera M, Cristaldi E, Cammalleri L, et al. Elevated chromogranin A (CgA) serum levels in the patients with advanced pancreatic cancer. Arch Gerontol Geriatr 2009;48:213–7. [DOI] [PubMed] [Google Scholar]
- 20. Nobilia E, Pezzillib R, Santinic D, et al. Autoimmune pancreatitis associated with high levels of chromogranin A, serotonin and 5-hydroxyindoleacetic acid. Case Rep Gastroenterol 2008;2:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marotta V, Nuzzo V, Ferrara T, et al. Limitations of chromogranin A in clinical practice.bmed_result. Biomarkers 2012;17:186–91. [DOI] [PubMed] [Google Scholar]
- 22. Ito T, Igarashi H, Jensen RT. Serum pancreastatin the long sought universal, sensitive, specific tumor marker for neuroendocrine tumors. Pancreas 2012;41:505–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y, Yang Q, Lin Y, Xue L, Chen M, Chen J. Chromogranin A as a marker for diagnosis, treatment, and survival in patients with gastroenteropancreatic neuroendocrine neoplasm. Medicine (Baltimore) 2014;93:e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stridsberg M, Eriksson B, Fellström B, Kristiansson G, Tiensuu Janson E. Measurements of chromogranin B can serve as a complement to chromogranin A. Regul Pept 2007;139:80–3. [DOI] [PubMed] [Google Scholar]
- 25. Stridsberg M, Eriksson B, Öberg K, Janson ET. A panel of 13 region-specific radioimmunoassays for measurements of human chromogranin B. Regul Pept 2005;125:193–9. [DOI] [PubMed] [Google Scholar]
- 26. Monaghan PJ, Lamarca A, Valle JW, et al. Routine measurement of plasma chromogranin B has limited clinical utility in the management of patients with neuroendocrine tumours. Clin Endocrinol 2016;84:348–52. [DOI] [PubMed] [Google Scholar]
- 27. Portela-Gomes GM, Grimelius L, Wilander E, Stridsberg M. Granins and granin-related peptides in neuroendocrine tumours. Regul Pept 2010;165:12–20. Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- 28. Conlon JM. Granin-derived peptides as diagnostic and prognostic markers for endocrine tumors. Regul Pept 2010;165:5–11. [DOI] [PubMed] [Google Scholar]
- 29. Portela-Gomes GM, Grimelius L, Wilander E, Stridsberg M. Granins and granin-related peptides in neuroendocrine tumours. Regul Pept 2010;165:12–20. [DOI] [PubMed] [Google Scholar]
- 30. Portela-Gomes GM, Grimelius L, Stridsberg M. Immunohistochemical and biochemical studies with region-specific antibodies to chromogranins A and B and secretogranins II and III in neuroendocrine tumors. Cell Mol Neurobiol 2010;30:1147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Di Rienzo M, Fdg B, Girard A, Julien C. Quantifying the effects of renal impairment on plasma concentrations of the neuroendocrine neoplasia biomarkers chromogranin A, chromogranin B, and cocaineand amphetamine-regulated transcript. Clin Chem 2012;950:941–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.