Abstract
This study aims to analyze the clinical and imaging features of vertebrobasilar dolichoectasia (VBD) combined with posterior circulation infarction, and to explore risk factors for the occurrence of posterior circulation infarction in VBD patients.
VBD patients were divided into 2 groups, according to the results of the imaging examination: posterior circulation infarction group and nonposterior circulation infarction group. The demographics, vascular risk factors, imaging, and other clinical data of the VBD patients were collected and retrospectively compared, and the risk factors for the occurrence of posterior circulation infarction in VBD patients were analyzed. The relationship between imaging features of the VBD blood supply artery and the infarct site was also analyzed.
A total of 56 VBD patients were included into the analysis. Among these patients, 26 patients had posterior circulation infarction. Infarction occurred in the blood supply area of the posterior cerebral artery in 14 patients. The difference in the height of the basilar artery bifurcation between patients with vertebrobasilar artery blood supply area infarction and patients with posterior cerebral artery supply area infarction was statistically significant. Hypertension and posterior circulation intracranial atherosclerosis were the risk factors for posterior circulation infarction in VBD patients.
Elevated basilar artery bifurcation is a risk factor for infarction in the posterior cerebral artery supply area in VBD patients. Posterior circulation infarction in VBD may be the comprehensive result of multiple factors, such as congenital defects of the basilar artery wall, hypertension, and atherosclerotic lesions.
Keywords: basal arteries, brain stroke, cerebral apoplexy, risk factor, vertebral artery
1. Introduction
Vertebrobasilar dolichoectasia (VBD) refers to the significant expansion, extension, distortion, or angulation of the vertebral basilar artery caused by various factors. The incidence of the disease is low, and most patients have no clinical symptoms, but the mortality and disability rate of symptomatic patients of this disease are high.[1] Furthermore, the etiology and pathogenesis of VBD remain unknown, and it remains controversial whether VBD is congenital, acquired, or both.[2] A present study revealed that acquired factors are mainly associated with atherosclerosis in VBD, and male gender, age, hypertension, obesity, hyperlipidemia, diabetes, long-term smoking, old myocardial infarction, lacunar infarction, and sedentary may all be risk factors for VBD.[3]
The clinical manifestations of VBD patients are varied and atypical. Symptomatic patients can be characterized by ischemic stroke, cranial nerve symptoms and brainstem compression, hydrocephalus, arterial rupture and bleeding, obstructive hydrocephalus, and other nonspecific symptoms.[3–6] The diagnosis of VBD depends mainly on imaging examination. A study revealed that ischemic stroke is the most common clinical manifestation of VBD, and is the most important cause of death in VBD patients.[7] In VBD patients, the main subtype of ischemic stroke is posterior circulation infarction, and VBD is an independent risk factor for posterior circulation infarction.[8] However, the mechanism of VBD leading to posterior circulation infarction remains unknown, and it remains controversial whether posterior circulation infarction is associated with atherosclerotic lesions after VBD. This study aims to retrospectively analyze the clinical and imaging features of VBD patients with posterior circulation infarction, in order to investigate the risk factors and pathogenesis of VBD-related posterior circulation ischemic events.
2. Subjects and methods
2.1. Subjects
2.1.1. Inclusion criteria for subjects
From January 2010 to December 2016, patients diagnosed with VBD by head magnetic resonance imaging (MRI) and head magnetic resonance angiography (MRA) in the Department of Neurology, the People's Hospital of Wuxi, or during hospitalization, were included. Exclusion criteria: Patients with symptoms caused by other clear diseases (such as severe stenosis or occlusion of the posterior circulation vessels, arterial dissection, cardiogenic stroke, peripheral vertigo, hypertensive intracerebral hemorrhage, and posterior fossa tumor) and patients combined with anterior circulation infarction were excluded. All patients or their family members provided a signed informed consent. The present study was approved by the Ethics Committee of the People's Hospital of Wuxi.
2.1.2. Clinical data acquisition
The patient's age, gender, smoking and alcohol consumption, body weight, height, blood pressure, blood glucose, blood lipids (total cholesterol, triacylglycerol, high-density lipoprotein, and low-density lipoprotein), carotid intima-media thickness, neurological symptoms and signs at admission and doctor visits, previous stroke or transient ischemic attack, hypertension, diabetes, and heart disease were recorded.
2.1.3. Assessment of cardiovascular risk factors
Obesity: body mass index (BMI) ≥25 kg/m2; smoking: smoking was currently in process; hypertension: has a systolic pressure of ≥140 mm Hg (1 Hg = 0.133 kPa) or a diastolic blood pressure of >90 mm Hg, or receiving antihypertensive treatment; diabetes: fasting blood glucose was ≥7.0 mmol/L or glycated hemoglobin was >6.5%, or receiving hypoglycemic treatment; hyperlipidemia: serum low-density lipoprotein cholesterol (LDL-C) was ≥3.62 mmol/L and high-density lipoprotein cholesterol (HDL-C) was <1.03 mmol/L; hyperhomocysteinemia: homocysteine was ≥10 μmol/L.
2.2. Imaging examination and diagnostic criteria
Routine head scanning was performed using a dual source computed tomography (CT) (Somatoma Definition, Siemens Medical Solutions, Forehheim, Germany) with a slice thickness set to 5 mm for the posterior fossa and supratentorial scans (120 kV, 350 or 400 mAs). Magnetic resonance imaging (MRI) was performed on a 3.0-Tesla MR system (Magnetom Trio, Siemens Medical Solutions, Erlangen, Germany) for all patients. A standardized MRI protocol was used in all patients, including transverse, coronal, and sagittal localizing sequences followed by transverse oblique contiguous images with a slice thickness of 5 mm aligned with the inferior borders of the corpus callosum (applied on sequences 2–6); T1-weighted images; T2-weighted images; diffusion-weighted images; FLAIR images; T2∗-weighted images. Furthermore, all patients were examined by three-dimensional time-of-flight magnetic resonance angiography (3D-TOF-MRA) with a slice thickness of 1 mm. Experienced neuroradiologists reviewed the brain CT, MRI, and MRA images.
The CT diagnostic criteria for VBD were based on the Smoker criteria.[9] The height of the BA bifurcation and the lateral displacement of the BA were graded into 4 levels. BA height was classified as grade 0 (showing the bifurcation within the dorsum sellae), 1 (showing the bifurcation within the suprasellar cistern), 2 (showing the bifurcation at the level of the third ventricle floor), or 3 (showing the bifurcation indenting the third ventricle floor). BA lateral displacement also was classified as grade 0 (BA located in the middle of the dorsum sellae or clivus), 1 (BA located in the medial to lateral margin of the clivus or dorsum sellae), grade 2 (BA located lateral to those landmarks), or grade 3 (BA located in the cerebellopontine angle). Finally, if the height was ≥grade 2 or the lateral displacement was ≥grade 2, and the diameter of BA was >4.5 mm, a diagnosis of VBD was determined. The MRI diagnostic criteria were based on the criteria proposed by Giang et al.[10] The classification criteria of height and the VBD diagnostic criteria are consistent with the Smoker[9] method. The MRA diagnostic criteria were based the on semiquantitative criteria proposed by Ubogu and Zaidat.[11] When the length of the BA was >29.5 mm and the transverse deviation exceeds the vertical connecting line between the starting point and the bifurcation of the BA by 10 mm, it was defined as abnormal; when the length of the intracranial segment of the vertebral artery (VA) was >23.5 mm and any of the vertebral arteries deviates from the connecting line between the intracranial entrance of the VA and the starting point of the BA by 10 mm, it was defined as abnormal. VBD was defined as a BA diameter >4.5 mm along the entire course and an intracranial VA diameter >4.0 mm. Segmental and fusiform enlargement of the BA and the VA were excluded from this study. Six patients underwent digital subtraction angiography (DSA).
2.3. Measurement methods of the degree of vascular stenosis and the diameter of VBD vessels
The measurement and classification of intracranial and extracranial artery stenosis were based on the North American Symptomatic Carotid Endarterectomy Trial (NASCET).[12] Stenosis rate (%) = (1 − stenosis diameter/narrow distal normal diameter) × 100%. Measurement of the VBD blood vessel diameter: On the maximum intensity projection (MIP) reconstruction images or original MRA images, the BA diameter was measured at the midpons level, while the dilated VA diameter was measured at the rostral medulla oblongata. The measurement of the length of the VA and the maximum distance between 2 points in the head MRA data of all patients was completed using the auxiliary measuring software or scale equipped in the MR machine.
2.4. Statistical analysis
General data and clinical and imaging features of VBD patients with or without posterior circulation infarction were compared. The relationship between the imaging features of the VBD blood supply artery and the infarct site in VBD patients with posterior circulation infarction was analyzed. All data were statistically analyzed by statistical software SPSS 20.0. Measurement data were expressed as mean ± standard deviation (x ± SD), and compared between 2 groups using independent sample t test. Count data were expressed as percentage, and compared between 2 groups using Fisher exact test. All tests were 2-sided tests. The risk factors for the occurrence of posterior circulation infarction in VBD patients were analyzed using multivariate logistic regression analysis.
3. Results
3.1. Clinical characteristics
3.1.1. General information
A total of 56 VBD patients were included in the analysis. Among these patients, 26 patients were assigned into the posterior circulation infarction group, while 30 patients were assigned into the nonposterior circulation infarction group. The age of onset of these 2 groups of patients was 45 to 85 years old, with an average age of 64.76 ± 5.34 years old. Among these patients, 35 patients were male and 21 patients were female. The comparison of the general clinical data and the characteristics of risk factors for cerebrovascular disease between these 2 groups are presented in Table 1. The statistics revealed that all patients with posterior circulation infarction had more than 1 risk factor for arteriosclerosis. These 2 groups were compared, and the differences in hypertension and posterior circulation intracranial atherosclerosis were statistically significant (Tables 1, 2). However, the differences in other cardiovascular risk factors between the 2 groups were not statistically significant.
Table 1.
Clinical data and risk factors of CVD were compared in patients with or without posterior circulation infarction VBD (n, %).
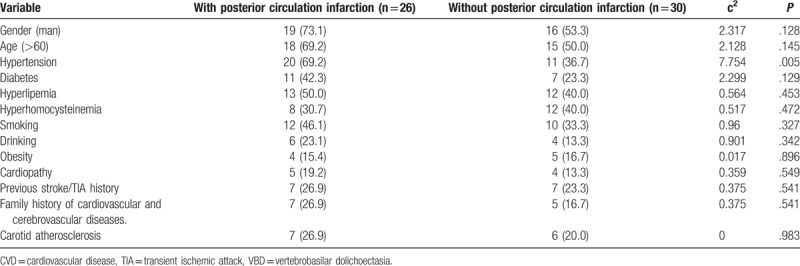
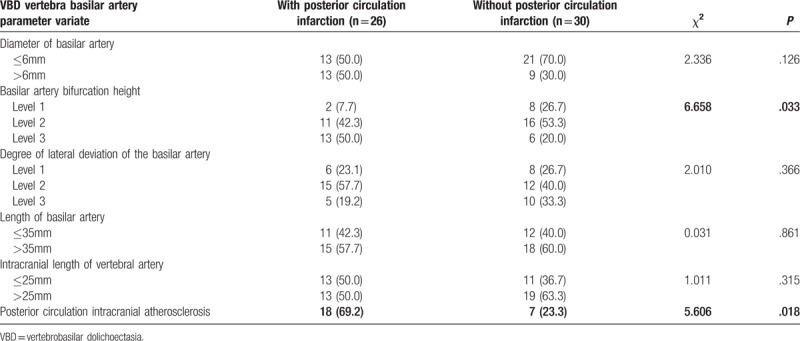
Table 2.
Risk factors of recurrent cerebral infarction after VBD (count data) (n, %).
3.1.2. Types of onset
Forty-two patients had posterior circulation ischemia. Among these patients, 16 patients had transient ischemic attacks of the VA system, while 26 patients had posterior circulation infarction. Furthermore, 2 patients had cerebellar bleeding and transformation, while 2 patients had cranial nerve paralysis, including 1 patient with trigeminal nerve paralysis and 1 patient with oculomotor nerve paralysis.
3.1.3. Clinical manifestations
Fourteen patients were asymptomatic. Among those symptomatic patients with posterior circulation ischemia or infarction, dizziness and vertigo were frequent presenting symptoms and occurred in 22 cases. Clinical features of patients with posterior circulation infarction are also manifested by other symptoms and different degrees of dizziness as follows. Three patients had different degrees of headache, 6 patients had dizziness and unsteadiness of walking, 3 patients had vertigo, 4 patients had adynamia and numbness of the unilateral extremities, 2 patients had dizziness, hemianopsia and numbness and weakness of the extremities, 2 patients had dizziness, hemiplegia and choking with drinking water, 1 patient had dizziness, hemianopsia, hemiplegia, epilepsy and mental abnormality, 1 patient had diplopia and eyeball dyskinesia.
3.2. Imaging findings
3.2.1. Head CT and MRI
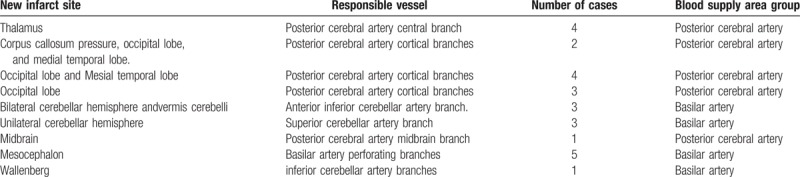
In all the patients, the height of the BA bifurcation was graded as follows: 10 patients were grade 1, 27 patients were grade 2, and 19 patients were grade 3. The degree of lateral migration of the BA was graded as follows: 14 patients were grade 1, 27 patients were grade 2, and 15 patients were grade 3. The BA shifted left in 21 patients and shifted right in 17 patients, and presented with an “S or inverted S” vascular loop straddling in 18 patients. Compression deformation of the medulla oblongata occurred in 10 patients, and the compression deformation of the pons occurred in 10 patients. The calcification of the BA occurred in 18 patients, while white matter degeneration occurred in 15 patients. Among these VBD patients, 26 patients were diagnosed with new cerebral infarction through head MRI. The infarct sites were as follows: the thalamus in 4 patients, the pad of corpus callosum, occipital lobe, and mesial temporal lobe in 2 patients, the occipital lobe and mesial temporal lobe in 4 patients, the occipital lobe in 3 patients, the bilateral cerebellar hemisphere and vermis cerebelli in 3 patients, the unilateral cerebellar hemisphere in 3 patients, the mesencephalon in 1 patient, the pons in 5 patients, and the dorsolateral medulla oblongata in 1 patient. According to the infarct site and the offending vessels, these patients were divided into 2 groups: VA blood supply area infarction group and posterior cerebral artery blood supply area infarction group. The specific infarct sites and offending vessels in the 2 groups are presented in Table 3.
Table 3.
The infarction site and its responsible blood vessel distribution in 26 patients with posterior circulation cerebral infarction.
3.2.2. Head MRA
The diameter of the BA was 4.50 to 7.20 mm in all the patients, with an average of 5.68 ± 0.67 mm, the length of the BA was 30.20 to 39.35 mm, with average of 33.02 ± 2.10 mm, and the distance of the transverse deviation exceeding the vertical connecting line between the starting point and the bifurcation of the BA was 10.10 to 13.50 mm, with an average of 11.35 ± 0.26 mm. The mean length of the intracranial segment of the vertebral artery was 25.41 ± 0.89 mm (range: 24.00–27.50 mm), and the mean length of vertebral arteries deviating from the connecting line between the intracranial entrance of the vertebral artery and the starting point of the BA was 11.33 ± 0.57 mm (range: 10.50–15.70 mm). Arteriosclerotic plaques at the BA or the intracranial length of the vertebral artery (the degree of stenosis was <50%) occurred in 15 patients. Furthermore, unilateral or bilateral posterior cerebral arteriosclerotic plaques (the degree of stenosis was <50%) occurred in 10 patients. Moreover, carotid atherosclerosis occurred in 13 patients, and intracranial arteriosclerosis in the anterior circulation occurred in 3 patients.
3.3. Analysis of risk factors in related imaging features for posterior circulation infarction in VBD
First, the relationship between the imaging features of the VBD blood supply artery and the infarct site in VBD patients with posterior circulation infarction was analyzed, and the results are presented in Tables 4 and 5 As shown in these 2 tables, the difference in height of the BA bifurcation between patients with VA blood supply area infarction and patients with posterior cerebral artery supply area infarction was statistically significant, while the differences in other indicators between the 2 types of patients were not statistically significant. These results revealed that the elevation of the height of the BA bifurcation was associated with the occurrence of infarction in the blood supply area of the posterior cerebral artery.(Fig. 1) Second, the imaging features of the 56 VBD patients with or without posterior circulation infarction were compared to analyze the risk factors in the imaging characteristics for posterior circulation infarction in VBD, and results are presented in Tables 2 and 6. These tables demonstrate that the differences in height of the BA bifurcation and the presence of posterior circulation arteriosclerostic plaque (the degree of stenosis was <50%) between the 2 groups were statistically significant (P<.05), while the differences in other indicators between the 2 types of patients were not statistically significant. These results revealed that the height of the BA bifurcation and the presence of posterior circulation atherosclerosis were associated with posterior circulation infarction in VBD (Fig. 2) The diameter of the BA and the degree of its deviation were not correlated to the occurrence of cerebral infarction in VBD.
Table 4.
The arterial characteristics and infarct location of cerebral infarction after VBD occurred (count data) (mm,  ).
).

Table 5.
The arterial characteristics and infarct location of cerebral infarction after VBD occurred (count data) (n, %).
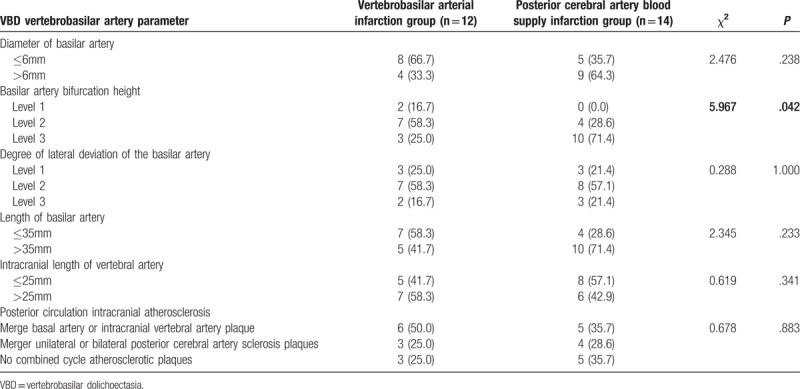
Figure 1.
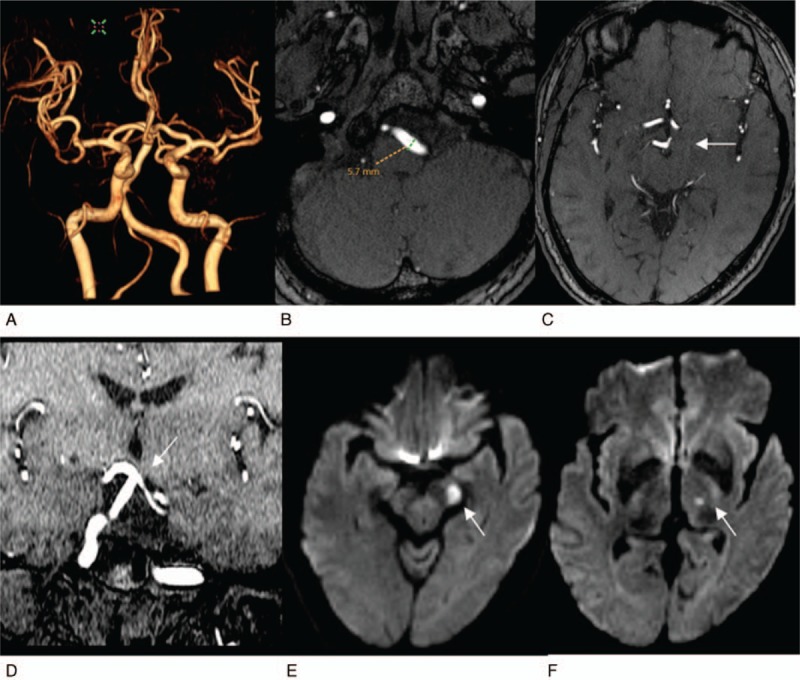
It reveals a high bifurcation of the basilar artery complicated with infarction in the blood supply area of the posterior cerebral artery. The patient was a 55-year-old woman. A, 3D VR MRA reconstruction image of cerebral vessels, from the anterior-to-posterior view. The vertebrobasilar artery significantly deviates to right, showing an S shape (grade 2), and has a high bifurcation. B, C, Cross-sectional TOF images. (B) It reveals that the diameter of the left vertebral artery is 5.7 mm, (C) it reveals that the basilar artery bifurcation reaches the level of third ventricle (white arrow), at grade 3. D, TOF reconstruction imaging of coronary surface confirms that the basilar artery bifurcation reaches the lower edge of the third ventricle (white arrow) and is slightly compressed and changed. E, F, Cross-sectional images of DWI, respectively, reveal fresh lacunar cerebral infarctions of left cerebral peduncle and left thalamus (white arrows).
Table 6.
Risk factors of recurrent cerebral infarction after VBD (count data) (mm,  ).
).

Figure 2.
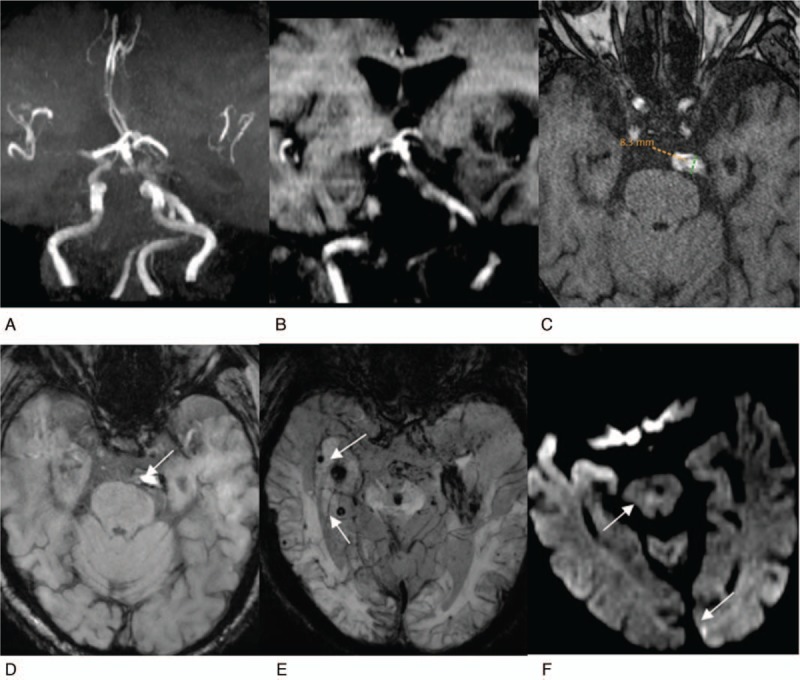
Reveals a high bifurcation of the basilar artery complicated with atherosclerosis and posterior circulation infarction. The patient is 85-year-old man. A, Reconstruction MRA image of maximum density projection of cerebral vessels, from the anterior-to-posterior view. The cerebral artery presents with atherosclerotic changes, the vertebrobasilar artery significantly deviates to right, showing an S shape, reaching the left CP corner area (grade 3). B, TOF reconstruction image of the coronal surface, the basilar artery bifurcation reaches the level of the middle of the third ventricle (grade 3). C, Cross-sectional TOF image. The basilar artery signals are uneven, suggesting mural macro thrombi and atherosclerosis, with a diameter of 8.3 mm. D, E, Cross-sectional SWI images. D, It reveals low mural signal, suggesting mural thrombi (white arrow). E, It reveals the scattered distribution of “black spots” on the bilateral temporal occipital lobe of the brain stem (white arrow), suggesting micro hemorrhagic foci. F, Cross-sectional DWI image, suggesting fresh lacunar cerebral infarction of brainstem and left occipital lobe (white arrow).
With posterior circulation infarction as a dependent variable, and with age, gender, smoking and alcohol consumption, body weight, height, blood pressure, blood glucose, blood lipid, homocysteine, carotid intima-media thickness, previous stroke or transient ischemic attack, hypertension, diabetes and heart disease, posterior circulation intracranial atherosclerosis, BA diameter, BA bifurcation height score and BA shifting score, BA length, and length intracranial segment of the vertebral artery as independent variables, a multivariate logistic regression analysis was conducted. The results revealed that hypertension (OR: 6.745, 95% CI: 1.792–6.089, P = .005) and posterior circulation intracranial atherosclerosis (OR: 5.205, 95% CI: 1.427–18.985, P = .012) were risk factors for posterior circulation infarction in VBD patients.
4. Discussion
VBD is a variant-induced cerebrovascular disease, which is at the stage of research and exploration. When vertebrobasilar dilatation and tortuosity are not obvious, the change in brain tissue compression and hemodynamics are not significant, and patients may have no clinical symptoms and signs. Once the excessive dilation and circuity of the VA occurs, it may cause symptoms of cerebral ischemia, and present with complex clinical manifestations.[13] A literature reported that the common symptoms of VBD patients with posterior circulation stroke were dizziness, cranial nerve paralysis, gait abnormalities, ataxia, and pyramidal tract symptoms.[13] In the present study, among the 56 VBD patients, 14 patients were asymptomatic, and 42 patients had posterior circulation ischemia. Among these patients, 16 patients had transient ischemic attack of the VA system, 26 patients had posterior circulation infarction (46.4%), and 30 patients did not have posterior circulation infarction (53.6%). Symptomatic patients mostly presented with varying degrees of dizziness (22 patients, 40%), or combined with brainstem or cerebellar damage symptoms, and their clinical manifestations were mainly correlated to the ischemic and infarct sites of the blood supply area of the posterior circulation artery. The risk factors for posterior circulation infarction in VBD patients were analyzed by comparing the images of the 2 groups of VBD patients with or without posterior circulation infarction (56 patients), and the results revealed that the height of the basilar artery bifurcation and the presence of posterior circulation atherosclerosis were correlated to the occurrence of posterior circulation infarction in VBD. This suggests that the pathomorphological abnormalities of the BA may be the basis of ischemic stroke in VBD patients, and traditional cerebral atherosclerotic lesions are more likely to promote and induce the occurrence of infarction.
The etiology of VBD remains unclear. It remains disputed whether it is congenital, acquired, or both. The risk factors for VBD include male, hypertension, smoking, drinking, and history of cerebrovascular disease in the family. Its onset peak is mainly at the age group of 60 to 80 years old.[14] A report pointed out that the acquired factors for the occurrence and development of the disease were mainly correlated to atherosclerotic factors.[15] A comparative study of stroke patients with or without VBD also confirmed that the severity of hypertension, VBD morphological changes, and the imaging progress could promote the occurrence of stroke in VBD patients.[16] This was also confirmed in patients in the present study. In the present study, the male-to-female ratio was approximately 1.6:1.0, and the average age was 64.7 years old. Furthermore, 57% of patients had hypertension, all patients had posterior circulation infarction and had more than 1 risk factor for arteriosclerosis, and more than 40% patients were complicated with posterior circulation intracranial arteriosclerosis plaques. In the present study, the common vascular risk factors were found in both the infarction group and noninfarction group. The comparison between these 2 groups revealed that the differences in hypertension and posterior circulation intracranial atherosclerosis were statistically significant, while the differences in other risk factors, including obesity, gender, history of smoking and drinking, hyperglycemia, hyperlipidemia, homocysteine, carotid intima-media thickness, previous history of stroke and heart disease, were not statistically significant. The risk factors for the occurrence of posterior circulation infarction in VBD patients were analyzed using multivariate logistic regression analysis. The results revealed that hypertension (OR: 6.745, 95% CI: 1.792–16.089; P = .005) and posterior circulation intracranial atherosclerosis (OR: 5.205, 95% CI: 1.427–18.985; P = .012) were the risk factors for posterior circulation infarction in VBD patients.
At present, it has been considered that VBD is an independent risk factor for stroke.[8,17] In the present study, 42 patients had posterior circulation ischemia. Among these patients, posterior circulation cerebral infarction occurred in 26 patients. Furthermore, supratentorial infarction mainly affected the blood supply area of the posterior cerebral artery, including the thalamus, the pad of the corpus callosum, the medial temporal lobe, and the occipital lobe, while subtentorial infarction mainly affected the brainstem and cerebellum. Patients with an affected brainstem commonly had pontine infarction, and had both perforating infarction and cortical branch blood supply area infarction. The relationship between cerebral blood supply artery imaging features and infarct sites in 26 patients with VBD combined with posterior circulation infarction was analyzed. The results revealed that the height of the BA bifurcation was correlated to infarction in the posterior cerebral artery supply area. In the present study, infarction in the posterior cerebral artery blood supply area accounted for 54% (14/26) of posterior circulation infarction. The reason may be that the blood flow in the dilated and tortuous BA was slow,[18] and the micro thrombus easily forms and falls off, which obstructs the tiny distal and deep perforating branch artery. The reason may also be that the distributions of the posterior cerebral artery and branches are tortuous, which more significantly affects the hemodynamics caused by VBD. Brainstem infarction is more common in the pons. The pathogenesis may be as follows: the direction of the blood flow in the dilated and tortuous pontine branch of the BA was reverse to that in the BA, and the diameters of these branches are smaller; when blood flows from the thicker BA to the smaller pontine branches with larger resistance, the blood flow is likely to produce a vortex, causing a site prone to inadequacy of perfusion. Passcro et al considered that ischemia and stroke in the distal BA areas (thalamus, the blood supply area of cerebral branch of the posterior cerebral artery and the cerebellum) may be correlated to hemodynamic changes and arterial embolism. Furthermore, some ischemic strokes in the brainstem and cerebellum may be correlated to the occurrence of atherosclerotic thrombosis at the beginning of the BA.[16,19] Kumral et al considered that the influence of BA hemodynamic changes, such as slower speed, on the blood supply area of the thalamus, cerebellum and posterior cerebral artery, is greater than that on the blood supply area of the direct branch of the BA.[20]
In summary, patients were retrospectively analyzed and studied in the present study. These results reveal that the height of the BA bifurcation is an important imaging feature associated with the occurrence of posterior circulation infarction in VBD, hypertension, and atherosclerotic lesions of the posterior circulation are risk factors for VBD combined with infarction. Posterior circulation infarction in VBD may be the comprehensive result of multiple congenital and acquired factors, such as congenital defects of the posterior circulation cerebral artery wall, especially the BA wall, hypertension, and atherosclerotic lesions. Indeed, there were limitations in the present study. For example, the number of patients was small, these patients were enrolled from a single center, and these patients were mostly the elderly and had a variety of risk factors for arteriosclerosis. The vast majority of these patients were characterized by posterior circulation ischemia or infarction, and patients with anterior circulation infarction were excluded. There was a certain bias in the inclusion of these cases. The imaging changes of the VB pathology of VBD should be further analyzed through high-resolution MRI of the intracranial arterial vascular wall and other techniques.
Author contributions
Conceptualization: Feng Wang.
Formal analysis: XiaoYun Hu, Tao Wang, XiangMing Fang, Zheng Dai, XuQiang Mao, Zhi-Ming Cui.
Investigation: Dao-liu Guo.
Methodology: Feng Wang, Dao-liu Guo, Zhi-Ming Cui.
Writing – original draft: Feng Wang.
Writing – review & editing: Feng Wang, XiaoYun Hu, Tao Wang, XiangMing Fang, Zheng Dai, XuQiang Mao.
Footnotes
Abbreviations: 3D-TOF-MRA = three-dimensional time-of-flight magnetic resonance angiography, BA = basilar artery, BMI = body mass index, CT = computed tomography, DSA = digital subtraction angiography, MIP = maximum intensity projection, MRA = magnetic resonance angiography, MRI = magnetic resonance imaging, NASCET = North American Symptomatic Carotid Endarterectomy Trial, VA = vertebral artery, VBD = vertebrobasilar dolichoectasia.
FW and X-YH are co-author and contributed equally to this work.
It was supported by grants from 333 High-level Talents Training Project of Jiangsu (No. 2016III-0603) and Youth Talents Program of Science-education Rejuvenating Healthy in Jiangsu Provincial Commission of Health and Family Planning (No. QNRC 2016181).
The authors have no conflicts of interest to disclose.
References
- [1].Passero SG, Rossi S. Natural history of vertebrobasilar dolichoectasia. Neurology 2008;70:66–72. [DOI] [PubMed] [Google Scholar]
- [2].Pico F, Labreuche J, Amarenco P. Pathophysiology, presentation, prognosis, and management of intracranial arterial dolichoectasia. The Lancet. Neurology 2015;14:833–45. [DOI] [PubMed] [Google Scholar]
- [3].Ken Ikeda, Yoshikazu Nakamura, Takehisa Hirayama, et al. Cardiovascular risk and neuroradiological profiles in asymptomatic vertebrobasilar dolichoectasia. Cerebrovasc Dis 2010;30:23–8. [DOI] [PubMed] [Google Scholar]
- [4].Samim M, Goldstein A, Schindler J, et al. Multimodality imaging of vertebrobasilar dolichoectasia: clinical presentations and imaging spectrum. Radiographics 2016;36:1129–46. [DOI] [PubMed] [Google Scholar]
- [5].Del Brutto VJ, Ortiz JG, Biller J. Intracranial arterial dolichoectasia. Front Neurol 2017;8:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Siddiqui A, Chew NS, Miszkiel K. Vertebrobasilar dolichoectasia: a rare cause of obstructive hydrocephalus: case report. Br J Radiol 2008;81:123–6. [DOI] [PubMed] [Google Scholar]
- [7].Nakamura Y, Hirayama T, Ikeda K. Clinicoradiologic features of vertebrobasilar dolichoectasia in stroke patients. J Stroke Cerebrovasc Dis 2012;21:5–10. [DOI] [PubMed] [Google Scholar]
- [8].Pico F, Labreuche J, Touboul PJ, et al. Intracranial arterial dolichoectasia and small-vessel disease in stroke patients. Ann Neurol 2005;57:472–9. [DOI] [PubMed] [Google Scholar]
- [9].Smoker WR, Corbett JJ, Gentry LR, et al. High-resolution computed tomography of the basilar artery: 2. Vertebrobasilar dolichoectasia: clinical-pathologic correlation and review. AJNR Am J Neuroradiol 1986;7:61–72. [PMC free article] [PubMed] [Google Scholar]
- [10].Giang DW, Perlin SJ, Monajati A, et al. Vertebrobasilar dolichoectasia: assessment using MR. Neuroradiology 1988;30:518–23. [DOI] [PubMed] [Google Scholar]
- [11].Ubogu EE, Zaidat OO. Vertebrobasilar dolichoectasia diagnosed by magnetic resonance angiography and risk of stroke and death: a cohort study. J Neurol Neurosurg Psychiatry 2004;75:22–6. [PMC free article] [PubMed] [Google Scholar]
- [12].North American Symptomatic Carotid Endarterectomy Trial Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–53. [DOI] [PubMed] [Google Scholar]
- [13].Gutierrez J, Sacco RL, Wright CB. Dolichoectasia: an evolving arterial disease. Nat Rev Neurol 2011;7:41–50. [DOI] [PubMed] [Google Scholar]
- [14].Wolters FJ, Rinkel GJ, Vergouwen MD. Clinical course and treatment of vertebrobasilar dolichoectasia: a systematic review of the literature. Neurol Res 2013;35:131–7. [DOI] [PubMed] [Google Scholar]
- [15].Pico F, Labreuche J, Touboul PJ, et al. Intracranial arterial dolichoectasia and its relation with atherosclerosis and stroke subtype. Neurology 2003;61:1736–42. [DOI] [PubMed] [Google Scholar]
- [16].Passero S, Filosomi G. Posterior circulation infarcts in patients with vertebrobasilar dolichoectasia. Stroke 1998;29:653–9. [DOI] [PubMed] [Google Scholar]
- [17].Yuan YJ, Xu K, Luo Q, et al. Research progress on vertebrobasilar dolichoectasia. Int J Med Sci 2014;11:1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Forster A, Kerl HU, Wenz H, et al. Fluid attenuated inversion recovery vascular hyperintensities possibly indicate slow arterial blood flow in vertebrobasilar dolichoectasia. J Neuroimaging 2015;25:608–13. [DOI] [PubMed] [Google Scholar]
- [19].Passero SG, Calchetti B, Bartalini S. Intracranial bleeding in patients with vertebrobasilar dolichoectasia. Stroke 2005;36:1421–5. [DOI] [PubMed] [Google Scholar]
- [20].Kumml E, Kisabay A, Atae C, et al. The mechanism of ischemic stroke in patients with doliehoectatic basilar artery. Eur J Neurol 2005;12:437–44. [DOI] [PubMed] [Google Scholar]


