Abstract
The aim of this study is to observe the therapeutic effect of percutaneous endoscopic discectomy and its influencing factors for lumbar disc herniation and compare the advantages and disadvantages of transforaminal and interlaminar of percutaneous endoscopy.
Data from 143 patients with lumbar disc herniation were respectively collected, including demographic and clinical data. Study population were divided into curative effect group and poor curative effect group, and logistic regression was used to explore the influencing factors of curative effect. The operation data and pre-and post-operation scores were compared to explore the effect of transforaminal and interlaminar approach on surgery efficacy.
The rate of curative effect was 93.7%. 120 patients were classified as curative group and 23 patients were categorized as poor effective group. Univariate analysis found that the patients in the curative effect group tended to receive the interlaminar approach (58.3% vs 34.8%, P = .038). Multivariate logistic regression did not find operation approach was not related to curative effect of operation (transforaminal and interlaminar). But age ≥45 (odd risk (OR) = 6.43, P = .016), course of disease >12 month (OR = 3.77, P = .003), back and leg pain (OR = 3.46, P = .026), history of trauma (OR = 3.88, P = .014), Pfirrmann level IV (OR = 4.84, P = .004), and pre-Visual Analogue Scale (VAS) <5.3 (OR = 3.63, P = .015) were associated with operation efficacy. Compared with transforaminal group, the interlaminar group has less operative time (P = .000), less fluoroscopy time (P = .000), less puncture time (P = .000), less blood loss (P = .011).
The transforaminal or interlaminar did not affect the treatment efficacy of percutaneous endoscopic discectomy for lumbar disc herniation. The selection of surgery approach depended on anatomical structure and physiological characteristics. It should be noted that 45 years of age or older, in the course of more than 12 months, both lumbocrural pain and lumbar disc herniation with grade IV, with history of trauma, may have impact on the efficacy of surgery.
Keywords: lumbar disc herniation, percutaneous transforaminal endoscopic, retrospective study
1. Introduction
With the rapid development of medical science, much attention has recently been given to surgical techniques that result in less trauma, faster postoperative rehabilitation, and lower postoperative bedrest requirements.[1] With the increasing prevalence of lumbar spine injuries and cervical spondylosis, and the inability of simple surgical treatment to achieve the above outcomes in the case of spinal surgery, there is a clear demand for greater refinement of surgical techniques to treat problems of the back and neck. After half a century of continuous development,[2] open surgery has reached the limits of its possible refinement. Traditional open surgery carries a high risk of complications, including severe trauma, excessive bleeding, severe postoperative pain, and intraoperative low back muscle injury, and it requires extensive soft tissue stripping. These complications can lead to chronic low back pain due to soft tissue injury.[3] Recently developed minimally invasive surgical techniques have obvious advantages over traditional surgical methods: they entail less trauma, reduced intraoperative blood loss, soft tissue injury, and postoperative pain, and faster recovery time. Minimally invasive techniques have been applied in spinal surgery for a number of years.[4,5] In the early stage, doctors invented the small window technique, based on open surgery. This had obvious advantages over open surgery but was rapidly surpassed by other surgical microscopy techniques. With the wide application of endoscopic systems in surgical fields, spinal endoscopic surgery has come to be the standard procedure in clinical practice.[6]
Lumbar disc degenerative diseases (DDDs) are extremely common in the current era. They include lumbar disc herniation, lumbar spinal stenosis, lumbar degenerative spondylolisthesis, lumbar discogenic low back pain, lumbar spondylolysis, and lumbar degenerative scoliosis (DS), among others.[7] Lumbar disc herniation is the most common and complicated type of DDD. Minimally invasive treatments for lumbar disc herniation include non-surgical intervention, endoscopic surgery, and open surgery.[8] Currently, the main types of minimally invasive surgeries used for treating lower lumbar DDDs include laparoscopic anterior lumbar fusion surgery (anterior lumbar intervertebrae fusion [ALIF]), axial lumbar fusion (AxiaLIF), lumbar artificial disc replacement surgery (lumbar disc arthroplasty [LDA]), lateral percutaneous endoscopic lumbar discectomy (PLED), direct lumbar inter-vertebrae fusion (DLIF), posterior lumbar discectomy (microendoscopic discectomy [MED]), and full-endoscopic lumbar-discectomy (FLD).[9] The lateral percutaneous endoscopic lumbar discectomy combines YESS and TYSYSS technology. The aim of this study is to examine the therapeutic efficacy of percutaneous endoscopic discectomy, and the factors influencing its effectiveness in treating lumbar disc herniation and to compare the advantages and disadvantages of the transforaminal and interlaminar approaches in percutaneous endoscopy.
2. Materials and methods
2.1. Study population
We retrospectively collected clinical data on 143 patients with lumbar disc herniation who underwent interlaminar or transforaminal percutaneous endoscopic discectomy in our hospital between November 2014 and July 2016. All patients had typical back pain, leg pain, or both, and were diagnosed with lumbar disc herniation, which was confirmed by imaging. This study was approved by the Institutional Review Board of The Affiliated Changzhou No. 2 Hospital of Nanjing Medical University.
2.2. Criteria for inclusion and exclusion
Patients included in the study met the following criteria:
-
(1)
definitively diagnosed with lumbar disc herniation in accordance with the accepted diagnostic definition;
-
(2)
no evident benefits within 3 months of non-operative treatment, including physiotherapy, therapeutic traction, and medication; and
-
(3)
obvious symptoms accompanied by back and/or leg pain.
Patients were excluded if:
-
(1)
they did not meet the above inclusion criteria;
-
(2)
they had a history of operation on the same lumbar vertebral segment;
-
(3)
there was adhesion between the thecal sac and spinal nerve roots;
-
(4)
back and/or leg pain were caused by non-lumbar disc herniation;
-
(5)
disc herniation was accompanied by lumbar instability or slippage;
-
(6)
spinal tumor infection or tuberculosis were present; or
-
(7)
skin damage and/or ulcers occurred at the puncture site after surgery.
2.3. Operative procedure
Patients were given basal anesthesia plus a local infiltration anesthesia and requested to assume a lateral position. The physician marked the midline of the lumbar spine and the vertical line under a C-arm X-ray machine. The puncture was made at a 15° angle with the central line as the starting point. The distance from midline to puncture point was 6 to 8 cm for L2-L3, 8 to 10 cm for L3-L4, and 12 to 14 cm for L4-5 and L5-S1. The puncture needle entered the lumbar disc via either a transforaminal or interlaminar approach, creating an incision 0.7 cm wide in the skin. Dilation tubes and casing pipes were inserted into the path made by the needle. The height and depth of the insertions were confirmed by C-arm machine fluoroscopy. Methylene blue (1 mL) was injected into the lumbar disc to stain the nucleus pulposus. Tissue pieces were cleared from around the operation field. The surgeon located and completely removed the nucleus pulposus and cleaned up any remnants. The thecal sac was then decompressed, the operation area was repeatedly rinsed with saline, and the surgeon closed the incision.
2.4. Data collection
We collected the following data on each subject: age, gender, course of disease, history of trauma, mode of work (sedentary vs active), location of surgery, symptom type, Lee level, Pfirrmann level, type of nucleus pulposus (central vs other types), operative approach (transforaminal vs interlaminar), and preoperative visual analog scale (VAS) pain, Oswestry Disability Index (ODI), and Japanese Orthopedic Association (JOA) scores. The operation parameters included operative time, fluoroscopy time, puncture time, volume of blood loss, postoperative bedrest time, postoperative time to discharge, and VAS, ODI, and JOA after operation.[10]
2.5. Assessment of efficacy
Operation outcomes were rated according to modified MacNab criteria as “best” (patient totally symptom-free, able to resume normal life and work), “better” (slight symptoms remain, activity slightly limited, no effect on life and work[11]), “good” (some symptom relief, activity significantly limited, life and work affected) or “bad” (post-operation symptoms the same or worse as pre-operation symptoms).
2.6. Statistical analysis
All analyses were performed using SPSS Statistics software (ver. 21.0;SPSS Inc., Chicago, IL). Quantitative data are expressed as mean ± standard deviation. Student t test was used for comparisons between 2 groups. For quantitative data with a non-normal distribution, we provide the median and range and used a non-parametric test for the analysis. Qualitative data are expressed as count and percentage and were evaluated by chi-square test. Cases with outcomes of “best” or “better” were included in the “curative effect” group, while those with outcomes of “good” or “bad” were classed as “noncurative effect.” We conducted multivariate logistic regression with bidirectional elimination to identify factors that influenced surgical effectiveness. We conducted multivariate logistic regression to identify the influence factors of effect by bidirectional elimination. Logistic regression model includes the following variables: age, gender, course of disease, mode of work, location of surgery, symptoms, Pfirrmann level, type of nucleus pulposus, pre-VAS, ODI, and JOA, and operation approach (transforaminal vs interlaminar). Post-operation scores on the above-described instruments were used to compare the relative efficacy of transforaminal and interlaminar approaches for percutaneous endoscopic interlaminar discectomy to treat lumbar disc herniation. The threshold for significance was set at P < .05.
3. Results
3.1. General characteristics of the study population
We enrolled 143 patients with lumbar disc herniation who underwent percutaneous endoscopic discectomy; 54 were male and 89 were female. The mean age of patients was 45.4 years. The disease course ranged from 2 to 16 months, and 22 (15.4%) patients had a history of trauma. The transforaminal approach was used in 65 patients, and the interlaminar approach was used in 78 patients. Mean preoperative VAS, ODI, and JOA scores were 6.5 ± 1.8, 53.0 ± 16.5, and 16.0 ± 5.7, respectively. The mean operative time was 94.5 minutes, and the mean blood loss was 23.6 ml. According to the MacNab criteria, 59 surgeries resulted in “best” outcomes, 61 in “better” outcomes, 14 in “good” outcomes, and 9 in “bad” outcomes. The rate of surgical effectiveness was 93.7%, meaning that 120 surgeries were classified as curative and 23 as noncurative.
3.2. Comparison of general characteristics between the curative and noncurative surgery groups
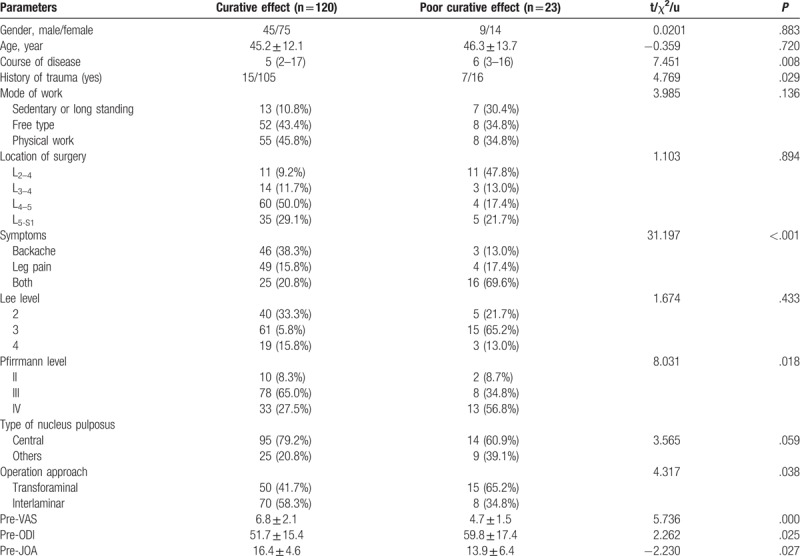
The results of a comparison of patient parameters between the curative and noncurative groups are presented in Table 1. There were no significant differences between the 2 groups in age (P = .883), gender (P = .720), modes of work (P = .136), location of surgery (P = .894), Lee level (P = .433), or type of nucleus pulposus (P = .059). Compared with patients in the noncurative group, the curative group had a shorter course of disease (P = .008) and lower levels of back/leg pain (P < .001). The ratio of patients with a Pfirrmann level of IV was higher in the noncurative group than in the curative group (P = .018). Preoperative VAS pain, ODI, and JOA scores were better in the curative group than in the noncurative group (P = .000, P = .025, P = .027). The patients in the curative group were more likely to have undergone the interlaminar approach (58.3% vs 34.8%, P = .038).
Table 1.
Comparisons of general characteristics between curative and poor curative effect group.
3.3. Multivariate logistic regression of operation efficacy
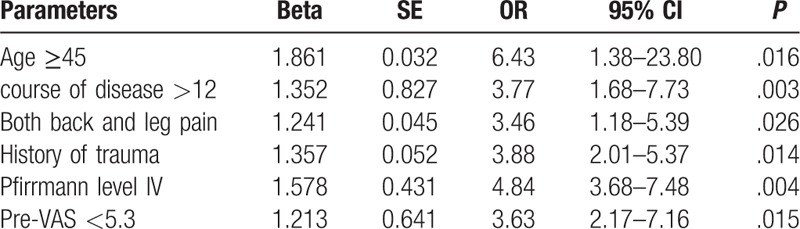
A stepwise logistic regression was conducted (Table 2) and confounding factors were adjusted for in the regression analysis (age, gender, course of disease, history of trauma, mode of work, location of surgery, symptoms, Lee level, Pfirrmann level, type of nucleus pulposus, operative approach, pre-VAS, pre-ODI, pre-JOA). A multivariate logistic regression indicated that age ≥45 years (odd risk (OR) = 6.43, 95% confidence interval (CI): 1.38–23.80, P = .016), course of disease >12 months (OR = 3.77, 95% CI: 1.68–7.73, P = .003), presence of both back and leg pain (OR = 3.46, 95% CI: 1.18–5.39, P = .026), history of trauma (OR = 3.88, 95% CI: 2.01–5.37, P = .014), Pfirrmann level of IV (OR = 4.84, 95% CI: 3.68–7.48, P = .004) and a preoperative VAS pain score < 5.3 (OR = 3.63, 95% CI: 2.17–7.16, P = .015) were all associated with lower operation efficacy. The operative approach (transforaminal or interlaminar) was not related to the efficacy of the operation.
Table 2.
logistic analysis affecting curative effect after surgery.
3.4. Assessment of operation efficacy in transforaminal and interlaminar approaches
As shown in Table 3, the preoperative VAS, ODI, and JOA scores of the transforaminal and interlaminar groups were equivalent (P >.05). No significant differences were observed in the post-operative scores of the transforaminal and interlaminar groups on these 3 indices (P >.05). However, in both groups, VAS pain and ODI scores were higher before than after surgery (P <.05).
Table 3.
Comparisons of efficacy after operation between transforaminal and interlaminar approach.

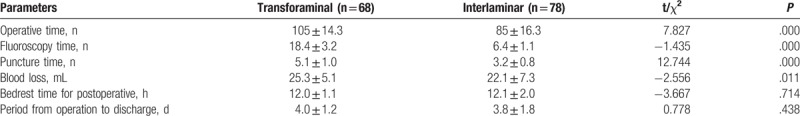
Table 4 gives the results of comparisons of intraoperative parameters between the transforaminal and interlaminar approaches. Compared with the transforaminal group, the interlaminar group had shorter operative (P = .000), fluoroscopy (P = .000), and puncture times (P = .000), and less blood loss (P = .011). No significant differences were observed in bedrest time (P = .714) or postoperative time to discharge (P = .438).
Table 4.
Comparisons of parameters during operation between transforaminal and interlaminar approach.
4. Discussion
The results of this study indicate that age (45 years or older), length of disease (more than 12 months), presence of lumbocrural pain, degree of lumbar disc herniation (Pfirrmann level IV), and a history of trauma all have negative effects on the efficacy of percutaneous endoscopic interlaminar discectomy in patients with lumbar disc herniation. We found no difference in effectiveness between the transforaminal and interlaminar approaches. However, operative time, fluoroscopy time, puncture time, and blood loss were all lower in the interlaminar group.
We first explored independent and related factors affecting the clinical efficacy of transforaminal percutaneous endoscopic lumbar discectomy for lumbar disc herniation. Our results indicated that age was independently associated with clinical efficacy. The incidence of degenerative disc disease is closely related to age;[12] intervertebral disc degradation is far more likely after the age of 45 years and is often accompanied by lumbar stenosis, intervertebral joint hyperplasia, nerve root adhesion, and local pathological changes.[13] These changes render surgery extremely difficult. Xu et al reported that lumbar disc surgery is more effective in patients under 45 years old than in those aged 45 years or above, a finding that concurs with the results of this study.[14] A history of trauma influenced surgical efficacy in the same manner as age. Intervertebral disc degradation also increases with age. Theoretically, the higher the Pfirrmann level, the more difficult surgery is to perform.[15,16] Our results showed that the proportion of patients with a Pfirrmann level of IV was significantly lower in the curative group than in the noncurative group. Logistic regression suggested that a Pfirmmann score of IV was an independent factor related to surgical efficacy. Manchinkanti reported that a longer history of disease usually predicts greater latency to functional recovery and lower surgical efficacy.[17] He suggested that surgery is most effective when performed after symptoms have been present for less than 6 months, as the degree of lumbar disc degeneration and nerve injury are still limited at that time. Patients with a disease course of more than 12 months had severely damaged lumbar discs, limiting the effectiveness of surgery. Similarly, we found that patients with a disease course of 12 months or more before surgery were less likely to show optimal surgical outcomes than those whose disease course was less than 12 months. Lumbar disc herniation is usually accompanied by back and/or leg pain. We found that the presence of lumbocrural pain affected surgical efficacy, possibly due to the different mechanisms underlying back and leg pain: back pain in lumbar disc herniation is usually caused by inflammatory stimulation and immune reaction, while leg pain is largely attributable to mechanical compression. Patients with both back and leg pain may thus be affected by 2 factors. Previous studies also reported that surgical outcomes are poorer in patients with both back and leg pain.[18,19] We also found that pre-VAS was related to curative effective, which means that the basic physical status affects the operation efficacy.
Our results indicate that the transforaminal and interlaminar methods of percutaneous endoscopic discectomy have equivalent efficacy rates. Multivariate analysis showed that operative approach did not affect curative outcomes. The success rate of percutaneous endoscopic discectomy with either approach was 93.7%, which falls within the previously reported range of 85.4% to 95.1%. However, the transforaminal surgeries examined in this study took longer, required more time for fluoroscopy and puncture, and entailed greater blood loss. The selection of operative approach was determined by patients’ anatomical structure, including the height of the iliac crest and the location of pensions. The transforaminal approach is often inappropriate for patients with L5-S1 lesions, as the intervertebral space may be blocked by the iliac crest and the L5 transverse process, preventing the puncture needle from reaching the edge of the intervertebral foramen.[20] Under these conditions, an interlaminar approach must be used. The L5-S1 is the widest of the intervertebral spaces; it is about 9.95 to 13.24 mm high and about 25.75 to 31.89 mm wide.[21] The outlet edge of the S1 nerve root is more vertical than that of other lumbar vertebral segments, and the dilation tube will enter the intervertebral disc via the nerve root shoulder. This path is consistent with the puncture path reported by a previous study.[22] According to the findings described above, the interlaminar approach is not recommended in patients with narrow intervertebral spaces, obvious facet hypertrophy, or severe lumbar disc hypertrophy. We also found that pre-VAS was related to curative effective, which means that the basic physical status affects the operation efficacy.
Based on the results of this study, we have several recommendations regarding the choice of approach in percutaneous endoscopic discectomy. The interlaminar approach is not recommended in patients with any of the following:
-
(1)
central spinal canal stenosis confirmed by computed tomography or magnetic resonance imaging (less than 10 mm);
-
(2)
narrow intervertebral space (less than 7.5 mm);
-
(3)
prolapse, drift, or calcification of the lumbar intervertebral disc;
-
(4)
a history of a previous operation on the same segment; or
-
(5)
less than 7.5 mm intervertebral space or less than 3 mm between dural sacs and the lateral wall of the spinal canal.
In the latter case, the interlaminar approach would increase the risk of damage to the dural sac and nerve root. Some advice for interlaminar approach during operation:
-
(1)
try to avoid excessive resection of intervertebral disc tissue as much as possible, and keep the intervertebral disc as much as possible, which is conducive to postoperative functional recovery.
-
(2)
during the operation, effective release of nerve roots should be paid attention to, decompression of lateral crypt should be conducted, and damage to blood vessels, nerve roots, dura, and other tissues should be avoided.
-
(3)
during the operation, attention should be paid to hemostasis to avoid the bleeding affecting the surgical field and causing interference to the operation.
-
(4)
during the operation, structures such as the yellow ligament and vertebral plate should be retained as much as possible to prevent postoperative scar formation and cause tension and compression on the dural and nerve roots.
The transforaminal approach is recommended in patients with:
-
(1)
central paramedian-type lumbar disc herniation or lesions located in the upper lumbar segment;
-
(2)
prolapse or drift of lumbar intervertebral disc accompanied by a higher iliac crest.
We also found that lumbar disc herniation can be divided into 2 types: stress type and tension type. Numbness is a typical symptom of the stress type, while pain is characteristic of the tension type. Local anesthetic may cause pain, leading to shifts in the lumbar disc in patients with the tension type of disc herniation, which could result in nerve root damage if the needle enters into the epidural space over the ligamentum of the intervertebral foramen. Therefore, the selection of approach should depend on the characteristics of the patient. The transforaminal approach should be selected when pain is obvious.
Several study limitations should be addressed. First, this was a retrospective cohort study, which means the strength of evidence was slightly inferior to the randomized controlled trial. Second, this study is a single center, retrospective analysis that was based on an existing database. It remains unknown if residual confounders may have affected the outcomes despite the use of multivariable analyses. Third, the present study just evaluated the short-term efficacy. Finally, the sample size of poor curative group was relatively small and long-term follow-up was needed.
Percutaneous endoscopic surgery for lumbar disc herniation has the advantages of being less traumatic than traditional surgery and producing a reliable curative effect. The choice of a transforaminal or interlaminar approach does not appear to affect the treatment efficacy of percutaneous endoscopic discectomy for lumbar disc herniation. The selection of surgical approach depends on anatomical structure and physiological characteristics. It should be noted that surgery is likely to be less successful in patients aged 45 years or older, with a disease course lasting over 12 months, symptoms including both lumbocrural pain and lumbar disc herniation of Pfirrmann grade IV, and/or a history of trauma.
Acknowledgments
We thank to the colleagues working in the Department of Orthopedics, The Affiliated Changzhou No. 2 Hospital of Nanjing Medical University.
Author contributions
Conceptualization: Xindie Zhou.
Data curation: Nanwei Xu.
Formal analysis: Xijia Jiang.
Funding acquisition: Xindie Zhou.
Investigation: Xijia Jiang, Nanwei Xu.
Methodology: Nanwei Xu.
Supervision: Xindie Zhou.
Validation: Nanwei Xu.
Writing – original draft: Xijia Jiang.
Writing – review & editing: Xindie Zhou.
Footnotes
Abbreviations: CI = confidence interval, JOA = Japanese Orthopedic Association, ODI = Oswestry Disability Index, OR = odd risk, VAS = Visual Analog Scale.
The authors have no conflicts of interest to disclose.
References
- [1].Zatevakhin II, Pasechnik IN, Gubaidullin RR, et al. Accelerated postoperative rehabilitation: multidisciplinary issue (Part 1). Khirurgiia (Mosk) 2015;4–8. [DOI] [PubMed] [Google Scholar]
- [2].Previnaire JG, De Bont N, Bordi H, et al. Open surgery for haemorrhoids in persons with spinal cord injury. Spinal Cord Ser Cases 2018;4:35doi:10.1038/s41394-018-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sammour T, Kahokehr A, Srinivasa S, et al. Laparoscopic colorectal surgery is associated with a higher intraoperative complication rate than open surgery. Ann Surg 2011;253:35–43. [DOI] [PubMed] [Google Scholar]
- [4].Schwartz C. What is minimally invasive surgery. Eur J Orthop Surg Traumatol 2018;28:759–60. [DOI] [PubMed] [Google Scholar]
- [5].Walker CT, Xu DS, Godzik J, et al. Minimally invasive surgery for thoracolumbar spinal trauma. Ann Transl Med 2018;6:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rushfeldt C, Pham KD, Aabakken L. Endoscopic surgery. Tidsskr Nor Laegeforen 2016;136:827–30. [DOI] [PubMed] [Google Scholar]
- [7].Liao Z, Wang CH, Cui WL. Comparison of allograft and autograft in lumbar fusion for lumbar degenerative diseases: a systematic review. J Invest Surg 2016;29:373–82. [DOI] [PubMed] [Google Scholar]
- [8].Hlubek RJ, Mundis GJ. Treatment for recurrent lumbar disc herniation. Curr Rev Musculoskelet Med 2017;10:517–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Alvi MA, Kerezoudis P, Wahood W, et al. Operative approaches for lumbar disc herniation: a systematic review and multiple treatment meta-analysis of conventional and minimally invasive surgeries. World Neurosurg 2018;114:391–407. [DOI] [PubMed] [Google Scholar]
- [10].van Hooff ML, Spruit M, Fairbank JC, et al. The oswestry disability index (version 2.1a): validation of a Dutch language version. Spine (Phila Pa 1976) 2015;40:E83–90. [DOI] [PubMed] [Google Scholar]
- [11].Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am 1971;53:891–903. [PubMed] [Google Scholar]
- [12].Wang F, Cai F, Shi R, et al. Aging and age-related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthritis Cartilage 2016;24:398–408. [DOI] [PubMed] [Google Scholar]
- [13].Urrutia J, Besa P, Lobos D, et al. Lumbar paraspinal muscle fat infiltration is independently associated with sex, age, and inter-vertebral disc degeneration in symptomatic patients. Skeletal Radiol 2018;47:955–61. [DOI] [PubMed] [Google Scholar]
- [14].Xu H, Mei Q, Xu B, et al. Expression of matrix metalloproteinases is positively related to the severity of disc degeneration and growing age in the East Asian lumbar disc herniation patients. Cell Biochem Biophys 2014;70:1219–25. [DOI] [PubMed] [Google Scholar]
- [15].Briseno MR, Phukan RD, Leonard DA, et al. The influence of adjacent level disc disease on discectomy outcomes. Eur Spine J 2016;25:230–4. [DOI] [PubMed] [Google Scholar]
- [16].Lee SM, Lee GW. The impact of generalized joint laxity on the clinical and radiological outcomes of single-level posterior lumbar interbody fusion. Spine J 2015;15:809–16. [DOI] [PubMed] [Google Scholar]
- [17].Manchikanti L, Singh V, Falco FJ, et al. An updated review of automated percutaneous mechanical lumbar discectomy for the contained herniated lumbar disc. Pain Physician 2013;16:E151–84. [PubMed] [Google Scholar]
- [18].Daureeawoo R, Baliga S, Mohahmed W, et al. A case report of an unusual cause of postoperative leg pain after posterior lumbar fusion. Ann Med Surg (Lond) 2017;19:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Abbott AD, Tyni-Lenne R, Hedlund R. Leg pain and psychological variables predict outcome 2-3 years after lumbar fusion surgery. Eur Spine J 2011;20:1626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Orita S, Inage K, Eguchi Y, et al. Lumbar foraminal stenosis, the hidden stenosis including at L5/S1. Eur J Orthop Surg Traumatol 2016;26:685–93. [DOI] [PubMed] [Google Scholar]
- [21].Wang B, Lu G, Patel AA, et al. An evaluation of the learning curve for a complex surgical technique: the full endoscopic interlaminar approach for lumbar disc herniations. Spine J 2011;11:122–30. [DOI] [PubMed] [Google Scholar]
- [22].Wang B, Lu GH, Li J, et al. Contrast study of full-endoscopic interlaminar approach for the surgical treatment of lumbar disc herniation. Zhonghua Wai Ke Za Zhi 2011;49:74–8. [PubMed] [Google Scholar]


