Abstract
STUDY QUESTION
Is junctional adhesion molecule A (JAM-A), a sperm protein essential for normal motility, expressed in the murine post-testicular pathway and involved in sperm maturation?
SUMMARY ANSWER
JAM-A is present in the prostate and seminal vesicles and in all three regions of the epididymis where it is secreted in epididymosomes in the luminal fluid and can be delivered to sperm in vitro.
WHAT IS KNOWN ALREADY
JAM-A shares with the plasma membrane Ca2+ATPase 4 (PMCA4, the major Ca2+ efflux pump in murine sperm) a common interacting partner, CASK (Ca2+/CaM-dependent serine kinase). JAM-A, like PMCA4, plays a role in Ca2+ regulation, since deletion of Jam-A results in significantly elevated intracellular Ca2+ levels and reduced sperm motility. Recently, PMCA4 was reported to be expressed in the epididymis and along with CASK was shown to be in a complex on epididymosomes where it was transferred to sperm. Because of the association of JAM-A with CASK in sperm and because of the presence of PMCA4 and CASK in the epididymis, the present study was performed to determine whether JAM-A is expressed in the epididymis and delivered to sperm during their maturation.
STUDY DESIGN, SIZE, DURATION
The epididymides, prostate and seminal vesicles were collected from sexually mature C57BL/6J and Institute for Cancer Research mice and antibodies specific for JAM-A and Ser285 -phosphorylated JAM-A (pJAM-A) were used for the analysis. Tissues, sperm and epididymal luminal fluid (ELF) were studied. Epididymosomes were also isolated for study. Caput and caudal sperm were co-incubated with ELF individually to determine their abilities to acquire JAM-A in vitro.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Sections of all three regions of the epididymis were subjected to indirect immunofluorescence analysis. Epididymal tissues, fluid, sperm, prostate and seminal vesicle tissues were analyzed for JAM-A and/or pJAM-A via western blotting analysis. The relative amounts of JAM-A and pJAM-A among epididymal tissues, ELF and sperm were detected by western blot via quantification of band intensities. Epididymosomes were isolated by ultracentrifugation of the ELF after it was clarified to remove cells and tissue fragments, and the proteins western blotted for JAM-A and pJAM-A, and exosomal biochemical markers. FACS analysis was used to quantify the amount of JAM-A present on caput and caudal sperm, as well as the amount of JAM-A acquired in vitro after their co-incubation with ELF.
MAIN RESULTS AND THE ROLE OF CHANCE
Western blots revealed that JAM-A is expressed in all three regions of the epididymis, the prostate and seminal vesicles. As confirmed by indirect immunofluorescence, a western blot showed that JAM-A has a higher expression in the corpus and caudal regions, where it is significantly (P < 0.01) more abundant than in the caput. Both JAM-A and Ser285-phosphorylated JAM-A (pJAM-A) are secreted into the ELF where it is highest in the distal regions. In the ELF, both JAM-A and pJAM-A were detected in epididymosomes. Western blotting of sperm proteins showed a significant (P < 0.01) increase of JAM-A and pJAM-A in caudal, compared with caput, sperm. Flow-cytometric analysis confirmed the increase in JAM-A in caudal sperm where it was 1.4-fold higher than in caput ones. Co-incubation of caput and caudal sperm with ELF demonstrated ~2.3- and ~1.3-fold increases, respectively, in JAM-A levels indicating that epididymosomes transfer more JAM-A to caput sperm that are less saturated with the protein than caudal ones.
LARGE SCALE DATA
Not applicable.
LIMITATIONS, REASONS FOR CAUTION
First, although the ELF was clarified prior to ultracentrifugation for epididymosome isolation, we cannot rule out contamination of the epididymosomal proteins by those from epididymal epithelial cells. Second, the JAM-A detected in the prostate and seminal vesicles might not necessarily be secreted from those organs and may only be present within the tissues, where it would be unable to impact sperm in the ejaculate.
WIDER IMPLICATIONS OF THE FINDINGS
Although performed in the mouse the study has implications for humans, as the highly conserved JAM-A is a signaling protein in human sperm. There is physiological significance to the finding that JAM-A, which regulates sperm motility and intracellular Ca2+, exists in elevated levels in the cauda where sperm gain motility and fertilizing ability. The study suggests that the acquisition of JAM-A in the epididymal tract is involved in the mechanism by which sperm gain their motility during epididymal maturation. This increased understanding of sperm physiology is important for aspects of ART.
STUDY FUNDING AND COMPETING INTEREST(S)
The work was supported by NIH-RO3HD073523 and NIH-5P20RR015588 grants to P.A.M.-D. The authors declare there are no conflicts of interests.
Keywords: epididymosomes, epididymal luminal fluid, sperm maturation, post-testicular maturation, infertility, junctional adhesion molecule A, phosphorylation
Introduction
Sperm that leave the testis are morphologically mature but functionally incompetent, as they lack synthetic machinery. As they enter and traverse the epididymis, an extended convoluted duct, their exposure to epididymal luminal fluid (ELF) contributes to their maturation (Cosentino and Cockett, 1986). During this process, which lasts ~5–10 days in mice (Robaire et al., 2006), sperm acquire many molecular components from the ELF on their plasma membrane to promote their maturation (Young and Goodman, 1982; Cooper, 1998; Jones, 1998). Recent work has shown that transmembrane proteins, similar to glycosyl phosphatidylinositol (GPI)-linked proteins, are secreted into the luminal fluid and are carried on epididymosomes, membranous vesicles (Frenette and Sullivan, 2001; Saez et al., 2003; Martin-DeLeon, 2006, 2016), which are thought to transfer proteins to the sperm surface (Sullivan, 2016; Sullivan and Mieusset, 2016).
Recently it was shown that plasma membrane Ca2+ATPase 4 (PMCA4), a transmembrane protein and the major Ca2+ efflux protein in murine sperm (Wennemuth, 2003), is carried on epididymosomes and can be delivered to sperm during their epididymal maturation (Patel et al., 2013). PMCA4 is an essential sperm protein whose absence leads to loss of motility (Okunade et al., 2004; Schuh et al., 2004), similar to the loss of junctional adhesion molecule A (JAM-A) (Shao et al., 2008), which is an essential transmembrane sperm protein involved in the regulation of intracellular Ca2+. A key regulator of sperm function, intracellular Ca2+ is significantly elevated in Jam-A null sperm (Aravindan et al., 2012). Interestingly, these sperm have significantly reduced PMCA4 activity (Aravindan et al., 2012) and electron dense mitochondria, due to calcium overload (Shao et al., 2008). This histopathology in Jam-A null sperm is shared with Pmca4 null sperm (Okunade et al., 2004) where significant losses of progressive and hyperactivated motility lead to infertility (Okunade et al., 2004; Schuh et al., 2004).
Furthermore, in sperm JAM-A and PMCA4 share a common interacting partner, Ca2+/CaM-dependent serine kinase (CASK), which they bind sequentially via a PDZ (PSD-95/D1g/ZO-1)-ligand interaction, to respectively promote or inhibit Ca2+ efflux and maintain Ca2+ homeostasis (Aravindan et al., 2012). It is noteworthy that CASK is also an epididymal secretory protein (Burkin et al., 2004; Patel et al., 2013), and interestingly has been shown to co-immunoprecipitate with PMCA4 from epididymosomes in murine ELF (Patel et al., 2013). Based on these observations, we hypothesized that JAM-A may also be expressed in the epididymis and secreted in the luminal fluid where it can be acquired by sperm transiting the epididymal tract during their maturation.
Like PMCA4, JAM-A was shown to be expressed in testicular germ cells and is present in elongated spermatids (Shao et al., 2008; Patel et al., 2013). However, in somatic cells JAM-A is expressed at tight junctions in endothelial and epithelial cells, and is known to be present in the Sertoli-Sertoli tight junctions in the seminiferous tubules of the testis (Shao et al., 2008). Thus, it is possible that JAM-A could be expressed in the epididymal epithelium. Consequently, we also hypothesized that it is present in the prostatic and seminal vesicle epithelium/secretions where other epididymal proteins are expressed (Zhang et al., 2004; Andrews et al., 2015), to enhance maturation of ejaculated sperm.
Materials and Methods
Animal and reagent use
The studies, conducted in mice, are in agreement with the Guide for the Care and Use of Laboratory Animals published by the National Research Council of the National Academies (2011) and animal care followed guidelines outlined by the Animal Care and Use Committee at the University of Delaware. All mice used throughout the investigation were of the C57BL/6J and Institute for Cancer Research strains and were sexually mature (at least 3 months of age). All enzymes and chemicals were from Fisher Scientific Co. (Malvern, PA, USA), Sigma (St. Louis, MO, USA) or Invitrogen (Carlsbad, CA, USA), unless otherwise specified.
Antibodies and fluorescent dyes
A rabbit polyclonal anti-JAM-A antibody (ab180821) from Abcam (Cambridge, MA, USA) was used for the detection of JAM-A protein in western blotting and a rat monoclonal anti-JAM-A (BV12) antibody (ab16896) was used for immunofluorescence and flow-cytometric studies. Previously used to detect JAM-A in sperm and testis (Shao et al., 2008), the BV12 antibody used for immunofluorescence and flow cytometry was also used for western blots and revealed a band with the characteristic MW (~34–40 KDa), in the absence of non-specific bands (Supplementary Fig. 1).
Rat monoclonal anti-CD9 antibody (sc-18869), mouse monoclonal anti-HSC70 antibody (sc-7298) and a rabbit polyclonal anti-Ser285-phosphorylated JAM-A (sc-17430) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA) and used for westerns. Alexa Fluor 647 goat anti-rat (ab150159) was obtained from Abcam (Cambridge, MA, USA) and Alexa Fluor 488 goat anti-rat was obtained from Invitrogen. Fluoro-Gel II with DAPI (17985-50) was purchased from Electron Microscopy Sciences (Hatfield, PA, USA).
Collection of tissues, ELF and sperm
The procedure for the collection of tissues, fluid and sperm was similar to that previously described (Deng et al., 2000; Zhang et al., 2004; Patel et al., 2013). Groups of 4–5 males were used for pooled samples for each experiment, which was performed at least three times. Epididymal tissues were dissected into the three regions (caput, corpus and cauda) and along with the prostate and the seminal vesicles were minced in 1× phosphate-buffered saline (PBS). The fluid and tissues of each epididymal region were transferred to separate Eppendorf tubes and allowed to gravity settle. The supernatant, without tissue fragments, was centrifuged at 500g for 15 min at room temperature to pellet sperm and recover the raw ELF. Following this, the sperm pellet and tissues were washed twice with 1 ml of PBS and frozen separately at −80°C until ready for processing. The supernatant from the original sperm pellet or the raw ELF was centrifuged at 13 000g for 20 min at 4°C to remove cellular debris, and the clarified supernatant, designated as ELF, was stored at −80°C until processing.
Fractionation of ELF for epididymosome collection
Clarified ELF, obtained as described above, was subjected to ultracentrifugation at 120 000g for 2 h at 4°C using an Optima L-70 centrifuge with a Ti60 rotor (Beckman), as previously described (Griffiths et al., 2008), to isolate the soluble from the insoluble fraction (the vesicles) (Griffiths et al., 2008; Patel et al., 2013).
Sodium dodecyl sulfate polyacrylamide gel electrophoresis and western blot analysis
Preparation of protein extracts from the epididymal tissues, luminal fluid, sperm, prostate and seminal vesicles was performed as outlined previously (Zhang et al., 2004), using testis tissue as a positive control. The total protein concentration was analyzed using the bicinchoninic acid protein assay kit (Pierce, Thermo Scientific, Rockford, IL, USA), according to manufacturer's protocol. Samples for electrophoresis were prepared in 2× Laemmli sample buffer with dithiothreitol (100 mM) and urea (125 mg/ml) and heated for 5 min at 100°C. Sixty micrograms of protein from tissues, fluid, sperm and epididymosomes were loaded per lane on 10% polyacrylamide gels and transferred onto nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ, USA). Western blotting was performed with the WesternBreeze Chemiluminescent Immunodetection Kit (Invitrogen) accrding to manufacturer's protocol. Membranes were incubated with 2% blocking solution for 2 h at 4°C and then incubated in primary antibodies: rabbit polyclonal anti-JAM-A (1:500), rat monoclonal (BV12) anti-JAM-A antibody (1:500) or rabbit polyclonal phospho-JAM-A antibodies (1:500) overnight at 4°C. Non-specific primary antibody binding was removed with 3× washes of 1× TBST solution (20 mM Tris, pH 8.0, 150 mM NaCl and 0.5% Tween 20) and proteins were visualized using alkaline phosphatase (AP)-conjugated secondary antibodies and reagents from the Western blotting detection kit (Invitrogen). The membrane was washed six times more for 15 min with 1× TBST before using the ECL kit (Bio-Rad, Hercules, CA, USA) to detect chemiluminescence. The actual amounts of protein loaded on the gels were assessed using heat shock cognate 70 (HSC70), which is a biochemical marker of epididymosomes, as an internal loading control for normalization. Thus, the membranes were re-probed with the anti-HSC70 antibody (1:1000) immediately after probing with the anti-JAM-A/anti-pJAM-A antibodies (Supplementary Fig. 2) or after stripping (Supplementary Fig. 1). This was followed by using the appropriate AP-conjugated secondary antibody. The normalization approach was as described (http://bitesizebio.com/23411/the-4-important-steps-for-western-blot-quantification/).
To quantify the intensity of the bands, images from shorter film exposures were selected and the Image J software (Rasband, 1997) was used to subtract the background from the JAM-A/pJAM-A band and that of the HSC70 loading control. The HSC70 band with the highest intensity was selected and all HSC70 values were divided by it to obtain their relative intensities. JAM-A values were divided by the relative HSC70 values in their respective lanes to obtained normalized values. The presence of JAM-A in its phosphorylated state (pJAM-A) was similarly assessed.
Statistical analysis
Experiments were performed at least three times and the mean ± SEM of the normalized data for band density analyzed using a Student's t-test or ANOVA with SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). The assumption of a parametric test was made by conducting the test of homogeneity of variances. When there was significance, data were analyzed by the Dunnett's T3 test; otherwise, the least significant difference (LSD) test was used. P < 0.05 was considered statistically significant.
Indirect immunofluorescence
Mouse tissues from the three epididymal regions and from the testis were collected after euthanasia and imbedded in OCT (Tissue Tek, Torrance, CA, USA) and frozen at −80°C. Cryostat sections of 20 μm were kept at −80°C until use. A 1:1 ratio of acetone:methanol solution was used to fix the slides for 20 min at −20°C before air drying and being placed in protein blocker, 2% bovine serum albumin (BSA) in PBS for 1–2 h at room temperature. The slides were then incubated at 4°C overnight with a 1:50 rat anti-JAM-A primary antibody and washed twice with 1× PBS for 20 min. Sections were then incubated for 1 h at room temperature, in the dark, with 1:200 Alexa Fluor 647-conjugated goat anti-rat (ab150159). The slides were washed twice with 1× PBS for 20 min and then mounted with Fluoro-Gel II with DAPI (Electron Microscopy Sciences) and cover-slipped. The sections were imaged using a Zeiss LSM 780 (Carl Zeiss Inc., Gottingen, Germany) confocal microscope. Controls were prepared by replacing primary antibody with rat IgG.
Analysis of sperm uptake of JAM-A from ELF in vitro
Caput and caudal sperm collected from 2 to 3 sexually mature mice, as previously outlined (Griffiths et al., 2008; Patel et al., 2013), were incubated in ELF recovered in PBS with 2 μM zinc acetate and protease inhibitor (#P2714, Sigma) at a pH of 5.5 or the PBS vehicle (control). Half of the sperm suspension was incubated in 200 μl ELF and the other half in 200 μl PBS control. All samples were incubated for 2 h at 37°C then washed 3× with PBS and centrifuged at 500g for 15 min at room temperature. Cells were fixed with 1.5% paraformaldehyde for 1 h at room temperature, washed 3× for 15 min each with 1× PBS and then permeabilized with 0.1% Triton X-100 for 10 min at room temperature. Permeabilized cells were processed for indirect immunofluorescence by incubation in 2% BSA blocking solution for 30 min and then in 1:200 rat anti-JAM-A primary antibody for 1 h at room temperature. Sperm were washed 3× with PBS and incubated in 1:200 Alexa Fluor 488 goat anti-rat for 30 min in the dark. Cells were again washed 3× for 15 min with PBS and re-suspended in 1 ml PBS for fluorescence analysis.
Quantification of JAM-A on sperm by flow cytometry
Flow cytometry was used to quantify JAM-A in caput and caudal sperm soon after they were collected from the animals, as well as after they were co-incubated with ELF. Sperm samples collected from the animals were immediately washed in PBS, fixed and processed for indirect immunofluorescence as described above. A population of ~50 000 cells from each group was analyzed for the amount of JAM-A present using a FACSCalibur (Becton Dickinson, San Diego, CA, USA) flow cytometer equipped with an argon laser at 488 nm excitation. Flow cytometry was also used to quantify the amount of JAM-A acquired on sperm following co-incubation in ELF.
Results
Detection of JAM-A in the epididymal tract and the accessory organs
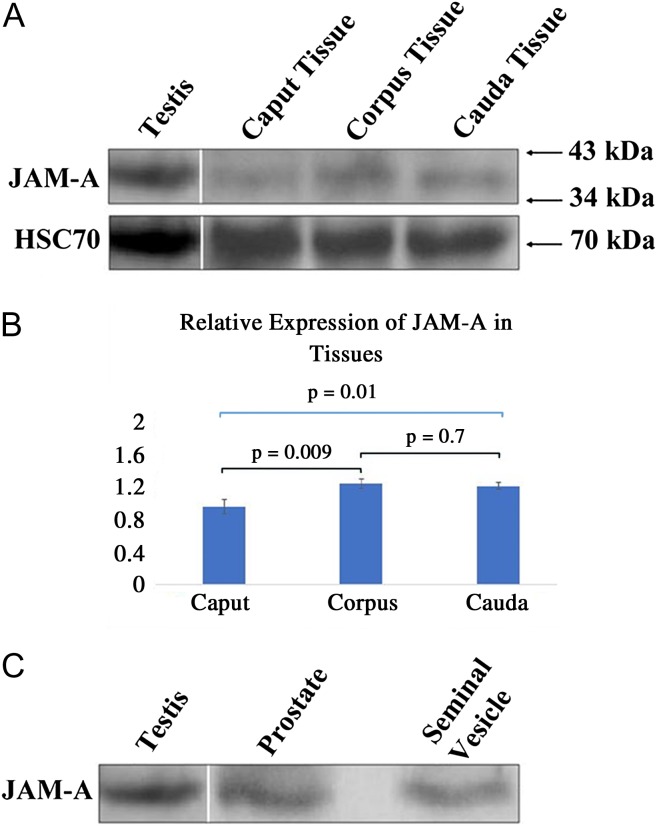
The presence of JAM-A in the epididymal tract was analyzed using western blots. Figure 1A shows the presence of the protein in the tissues of all three epididymal regions at ~40 kDa. Statistical analysis shows differential expression among the three regions with the caput containing the lowest levels of the protein, being significantly lower than the corpus (P < 0.01) and the cauda (P = 0.01) (Fig. 1B). For the accessory organs, the prostate and seminal vesicle, western blots also showed the presence of JAM-A (Fig. 1C).
Figure 1.
Western blot analysis reveals that junctional adhesion molecule A (JAM-A) is expressed in murine epididymal tissues, prostate and seminal vesicles. Sixty micrograms of proteins were loaded in (A) and (C). JAM-A was detected using a rabbit polyclonal anti-JAM-A antibody (1:500 dilution). An ~40 kDa JAM-A band is present in the testis (positive control) and all three epididymal regions. Blots were stripped and re-probed for heat shock cognate 70 (HSC70) as a loading control to analyze the intensities of the bands via densitometry, using Image J software. The relative expression of JAM-A is seen in (B) where values are expressed as mean ± SEM for three independent experiments. Significant differences are seen between caput and corpus (P < 0.01) and caput and cauda (P = 0.01), using ANOVA. (C) Protein extracts from tissue of the whole prostate and seminal vesicles revealed the typical JAM-A band seen in testis (positive control).
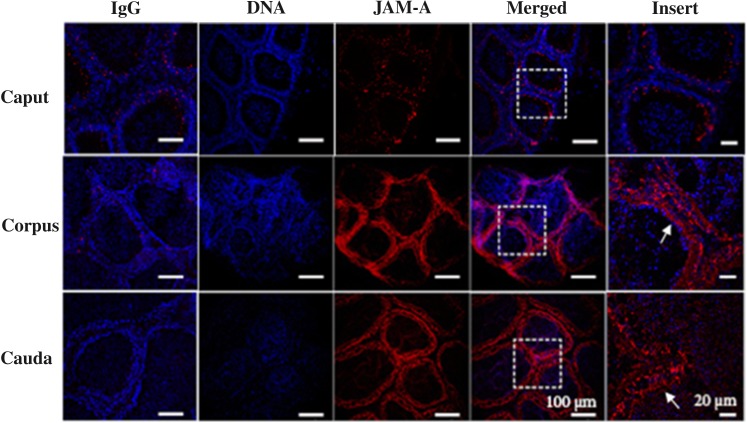
Immunofluorescence analysis was used to confirm JAM-A's expression in the epididymis. Sections of the caput, corpus and cauda were stained with the rat monoclonal anti-JAM-A (BV12) antibody (ab16896). Expression was seen in all three regions and was lowest in the caput (Fig. 2), corroborating the western blot data. In the corpus and caudal regions, JAM-A was distributed throughout the epithelial cells in the duct, with staining seen in both the basal and the luminal lining (Fig. 2), from which proteins are secreted into the lumen. Although the immunofluorescence assay is not quantitative, the corpus appeared to be more intensely fluorescent than the cauda.
Figure 2.
Indirect immunofluorescence of frozen sections of the murine epididymis reveals different regional distribution of JAM-A. The IgG control, in the first column for each of the three regions, when stained with the secondary antibody and the DAPI staining shows only the blue DNA staining of the nuclei, with no signal and low background. On the other hand, the rat monoclonal anti-JAM-A antibody showed a signal (red) in ducts of the three epididymal regions, in addition to the blue DAPI staining of the nuclei. When the DNA- and JAM-A-staining images were merged and the inset enlarged, the JAM-A signal could be seen throughout the walls of the ducts (arrows) of the corpus and cauda and was least abundant in the caput.
Presence of JAM-A in the ELF
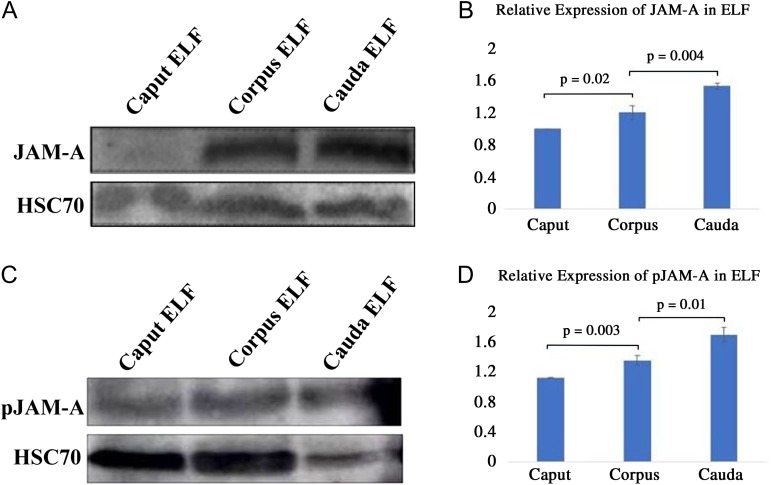
Using either of the anti-JAM-A antibodies, ab180821 or ab16896 (BV12), western blot analysis detected the presence of JAM-A in the ELF of all three regions of the epididymis (Fig. 3A). Statistical analysis shows differential expression in the ELF with the caudal fluid having significantly (P < 0.01) higher levels of JAM-A than the corpus which had significantly (P = 0.02) higher levels than the caput (Fig. 3B). When anti-Ser285-phosphorylated JAM-A antibody was used to probe the ELF, the amounts of pJAM-A were similar to that of JAM-A, with the cauda showing the highest levels (Fig. 3C and D). The HSC70 band for the caudal ELF in Fig. 3C is less dense than those for the corpus and caput, indicating that it accounts for proportionately less of the same amounts of proteins loaded for all three regions. For each sample, we loaded 60 μg proteins containing HSC70 produced in a fairly constant amount. However, because many other proteins are being released over time into the ELF, the relative amount of HSC70 in the total protein output decreases with time. It should be noted that the relative levels of HSC70 in the total proteins, like that for other extracellular vesicular proteins such as α-tubulin, typically vary with the sperm storage period in the cauda. After prolonged storage, a large variety of proteins are released in the ELF via epididymosomes. Thus in Fig. 3C, HSC70 constitutes a smaller proportion of the total proteins than that in Fig. 3A where mating or spontaneous seminal emission might have occurred just before sacrifice of the animal(s). This difference for caudal constituents, using α-tubulin as a loading control, can also be seen in a recent paper where sperm from the three regions were purified on a percoll gradient (Nixon et al., 2015).
Figure 3.
Western blot analysis shows that the murine luminal fluids contain JAM-A and Ser285-phosphorylated JAM-A (pJAM-A). Sixty micrograms of proteins were loaded in each lane and JAM-A was detected (A) and can be seen to be differentially distributed (B) with the levels being significantly higher in the cauda than the caput (P = 0.02) and in the corpus than the cauda (P = 0.004), using ANOVA. (C) Anti-pJAM-A antibodies revealed pJAM-A bands, which also varied in intensity (D) and were significantly greater (P = 0.003) for corpus, compared with caput, and cauda compared with corpus (P = 0.01), using ANOVA. These results are from four independent experiments, using 12 animals, N = 4, mean ± SEM.
Immunodetection of JAM-A and pJAM-A in caput and caudal sperm
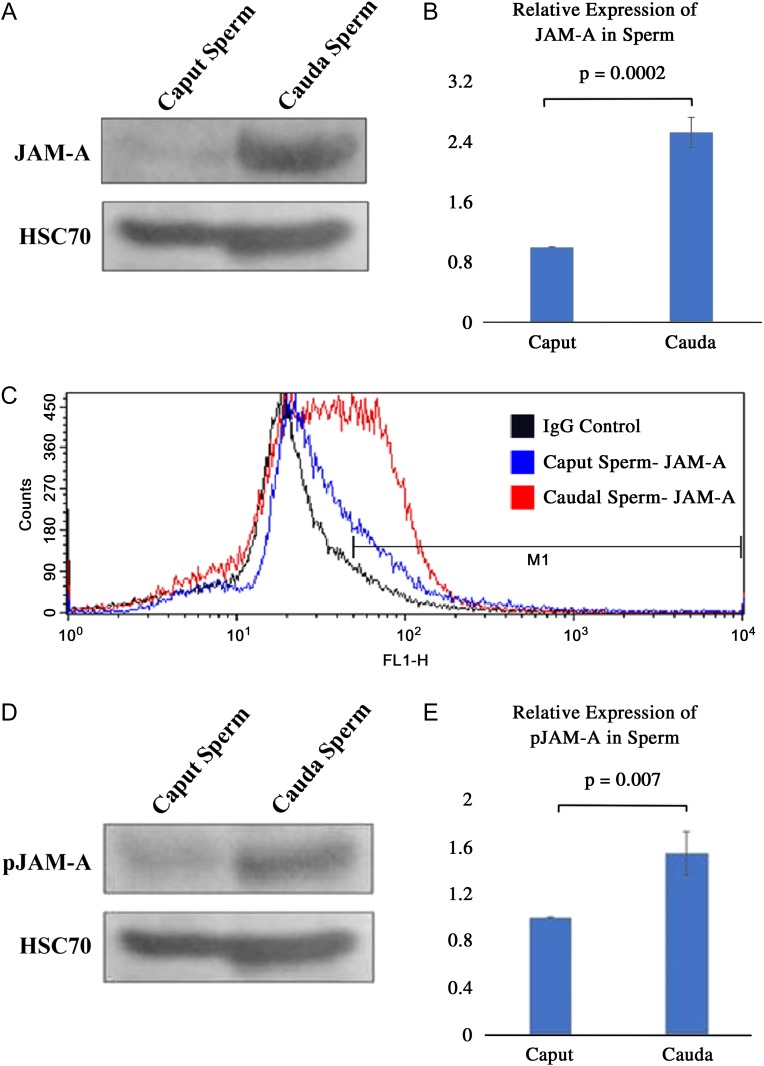
To detect the level of JAM-A protein in maturing sperm, western blot analysis was performed and revealed significantly (P < 0.01) higher levels of JAM-A in caudal, compared with caput, sperm (Fig. 4A and B). The increase was confirmed by flow-cytometric analysis, which showed a 1.4-fold increase in the percentage of cells with the highest fluorescence intensity (M1 region) in the cauda, compared with the caput (Fig. 4C). In line with these findings, Western blots showed that pJAM-A levels were significantly (P < 0.01) higher in caudal sperm where there was ~1.5-fold increase in the mean band density (Fig. 4D and E).
Figure 4.
Western and flow-cytometric analyses indicate that JAM-A and pJAM-A exist in different amounts in caput and caudal sperm. (A) The characteristic JAM-A band is seen for caput and caudal sperm with a greater intensity in the latter (60 µg proteins loaded in both lanes). (B) Band intensities were significantly different (P < 0.01), for N = 3, mean ± SEM. (C) The difference in the expression of JAM-A in caput and caudal sperm was confirmed by flow-cytometric analysis. The IgG control (black), which consisted of a 1:1 mixture of caput and caudal sperm is shown, to compare with the specificity of the anti-JAM-A antibody used for caput (blue) and caudal (red) sperm. With ~50 000 cells analyzed for each group, the graph shows a right shift for caudal sperm, indicating a 1.4-fold increase in the percentage of cells with the highest level of fluorescence, compared with caput sperm. (D) pJAM-A expression is seen in both sperm types (with 60 µg proteins loaded), and the relative amounts displayed in the bar graph (E), which reveals a significant (P < 0.01) increase in caudal (~1.5-fold), compared with caput sperm. N = 3, mean ± SEM, using the Student's t-test.
Sperm uptake of JAM-A from ELF in vitro
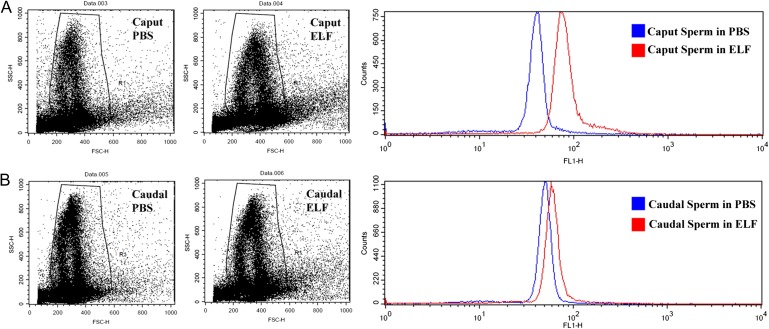
Both caput and caudal sperm were able to acquire JAM-A following co-incubation with ELF combined from all three regions of the epididymis. Flow cytometry detected an ~2.3-fold increase of JAM-A (as detected by the mean fluorescence intensity) in caput sperm in ELF, compared with control sperm co-incubated in PBS, while the increase for caudal sperm was ~1.3-fold (Fig. 5A and B).
Figure 5.
Flow-cytometric analysis reveals that caput and caudal sperm have different capacities for JAM-A uptake from epididymal luminal fluid (ELF) in vitro. The acquisition of JAM-A is seen in caput (A) and caudal (B) sperm, respectively, following co-incubation in ELF combined from all three epididymal regions or phosphate-buffered saline (PBS) (control) for 90 min at 37°C. Analysis of ~50 000 cells/group revealed uptake in both caput and caudal cells, as reflected in a right shift of the graph for ELF compared with the control. The resulting increase in JAM-A is ~2.3-and ~1.3-fold for caput and caudal sperm, respectively, compared with the control.
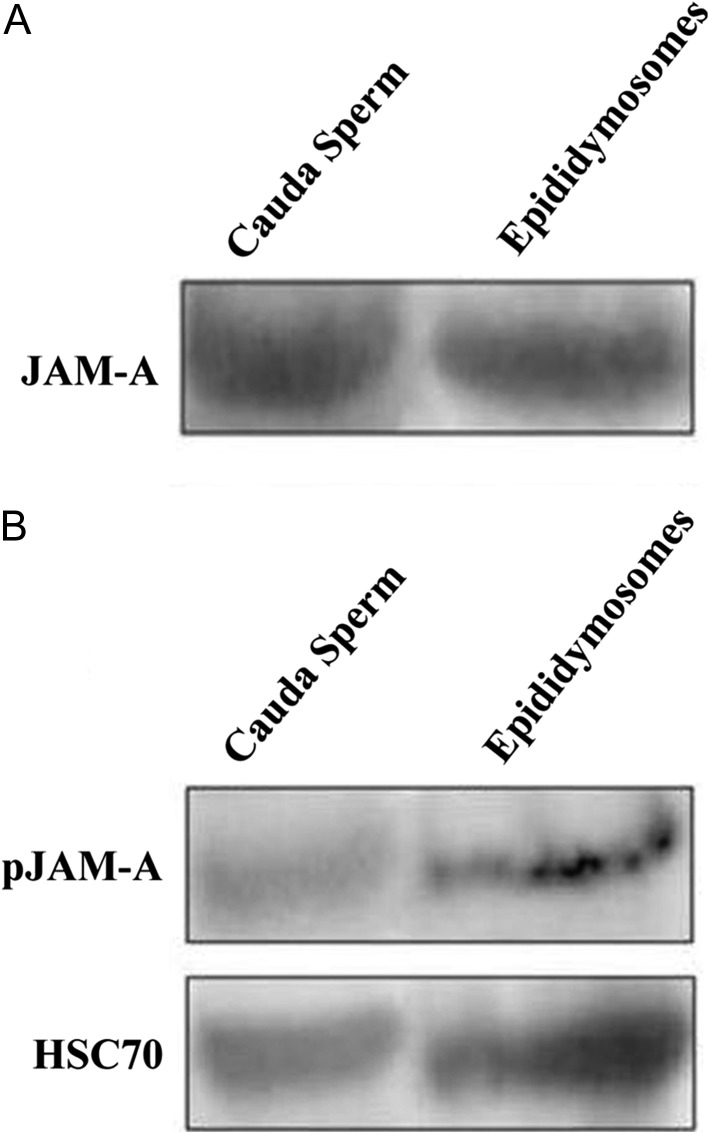
Detection of JAM-A in epididymosomes
When isolated epididymosomes were probed in western analysis, JAM-A (Fig. 6A), as well as pJAM-A (Fig. 6B), was found to be present. Detection of HSC70 exosomal biochemical marker is consistent with the finding that the collected vesicles were exosomes/microvesicles (Fig. 6A and B). Western analysis also showed that CD9 tetraspanin biochemical exosomal marker was also present in the epididymosomes (data not shown).
Figure 6.
Immunoblot of epididymosomal proteins shows the presence of JAM-A and pJAM-A. Following ultracentrifugation of the clarified ELF to isolate epididymosomes, 60 µg of proteins from the pellet were loaded and the blots revealed (A) JAM-A and (B) pJAM-A. Sperm proteins were used as a positive control. When the membrane was re-probed with HSC70, a biochemical marker for exosomes, a positive band was detected, consistent with the presence of epididymosomes. Only one set of HSC70 bands is shown since the same aliquots of epididymosomal proteins were used for both the JAM-A and pJAM-A membranes.
Discussion
Our research has shown that JAM-A functions in conjunction with PMCA4 and CASK to regulate calcium levels within spermatozoa (Aravindan et al., 2012). Both PMCA4 and CASK have been detected in epididymal tissues and have been shown to be associated with epididymal sperm maturation (Burkin et al., 2004; Patel et al., 2013). In the present study, both indirect immunofluorescence and western analysis revealed the presence of JAM-A in all three regions of the murine epididymis and showed that it is more abundant in the distal region than in the caput. Immunofluorescence localized it within the epithelial lining, on both the apical and basal membranes, while western analysis showed that it is also present in the accessory organs, the seminal vesicles and the prostate. Similar to SPAM1, a sperm protein expressed in the epididymis and the accessory organs and released in the secretions (Zhang et al., 2004), JAM-A is likely to appear in the seminal plasma where it is able to interact with sperm. It should be noted that PMCA4 and CASK, which interacts with JAM-A, are present in human seminal plasma and prostasomes (Andrews et al., 2015).
Western blotting showed that JAM-A is present in the Ser285-phosphorylated state, as well as the unphosphorylated state in the ELF. With a protein of 300 amino acids, phosphorylation of JAM-A at Ser285 occurs near the C-terminal PDZ-binding ligand (Naik and Eckfeld, 2003) and this is likely to result from its interaction with CASK at its PDZ domain (Mukherjee et al., 2008). It should be noted that as post-translational modification of JAM-A is associated with its activation and signaling activity (Naik et al., 2003), we hypothesize that the presence of JAM-A in its phosphorylated state (pJAM-A) in the luminal fluids may be associated with the existence of JAM-A in a JAM-A-CASK complex. This would be similar to the PMCA4-CASK complex seen in the ELF (Patel et al., 2013).
In the luminal fluids, there were regional differences in the levels of both JAM-A and pJAM-A: levels were significantly higher in the cauda than in the corpus, where they were significantly higher than in the caput. It should be noted that the difference in JAM-A and pJAM-A levels in ELF between the cauda and the other regions is likely to be exaggerated because HSC70 accounts for relatively less of the total proteins after prolonged sperm storage. As mentioned earlier, the differences seen in the fluid might be more reflective of sperm storage time rather than protein expression levels. Therefore, conservatively, it can be stated that JAM-A and pJAM-A are significantly higher in the distal, compared with the proximal, ELF.
The presence of JAM-A and pJAM-A in all three regions indicates that this protein is involved in both the maturation and storage of sperm, which occur in the proximal and distal epididymal regions, respectively (Anakwe et al., 1991). Sullivan and Mieusset (2016) have reported that in both mice and human (where JAM-A is also present in sperm, Shao et al. 2008), sperm gain motility and fertilizing ability in the cauda epididymis. It is of physiological relevance that the levels of JAM-A, which is known to regulate sperm motility (Shao et al., 2008), are highest in ELF in the cauda where sperm motility is attained. Thus, it appears that the acquisition of JAM-A by sperm in the cauda, similar to that of PMCA4 (Patel et al., 2013), is involved in the mechanism by which sperm motility and fertilizing ability are acquired by sperm during epididymal maturation. It might also be argued that the lower levels of JAM-A in the caput might play a physiological role in preventing maturation during the early stages of sperm transit, to avoid premature aging.
When JAM-A and pJAM-A were analyzed in caput and caudal sperm via western analysis, the levels were significantly higher in the latter (Fig. 4); corresponding to the regional abundance of the protein in ELF. Flow-cytometric analysis corroborated this finding for JAM-A. This observation signifies that as sperm traverse the epididymal tract they acquire JAM-A. To date, testicular sperm that enter the epididymis have not been analyzed for the presence of JAM-A. However, the finding that JAM-A is expressed in elongated spermatids (Shao et al., 2008) suggests that it is present on the plasma membrane of testicular sperm entering the epididymis, although the relative amount compared with epididymal sperm is unknown. It should also be noted that since the level of pJAM-A was assessed based on the HSC70 level rather than the total proteins, the higher level detected in caudal sperm does not necessarily reflect a greater level of phosphorylation, but merely the presence of more JAM-A.
To determine whether JAM-A can be acquired by sperm from the ELF in vitro, caput and caudal sperm were separately co-incubated with ELF combined from all three regions. The results demonstrate that sperm can acquire JAM-A from the luminal fluids with caput sperm having an ~2.3-fold increase in JAM-A after co-incubation, compared with the control. For caudal sperm, there was only an ~1.3-fold increase in JAM-A. These findings suggest that caudal sperm are more saturated with JAM-A than caput sperm, reflecting in vivo acquisition of JAM-A as sperm transit the epididymis and arrive in the cauda where they are stored.
The vehicles for delivery of epididymal secretory proteins from the luminal fluid to the sperm surface are thought to be exosomes/microvesicles, known as epididymosomes (Saez et al., 2003; Griffiths et al., 2008; Sullivan, 2016; Sullivan and Mieusset, 2016). These are released into the ELF from the epithelial lining via the apocrine pathway (Hermo and Jacks, 2002). In our study, epididymosomes that were isolated from the ELF showed the presence of both JAM-A and pJAM-A (Fig. 6) in their cargo. The presence of pJAM-A in epididymosomes indicates that they carry JAM-A in a form that is activated (Naik et al., 2003) and likely complexed with CASK (Mukherjee et al., 2008). The findings therefore suggest that JAM-A is carried and delivered to sperm in both the inactive unphosphorylated and the activated phosphorylated states.
The mechanism(s) by which epididymosomes and other extracellular vesicles deliver transmembrane proteins to sperm are unlikely to include endocytosis (Martin-DeLeon, 2016), as occurs in somatic cells (Raposa and Stoorvogel, 2013). Schwarz et al. (2013) have suggested a fusogenic mechanism and Al-dossary et al. (2015) used Super-resolution Structured Illumination Microscopy to provide strong supporting evidence for this pathway, one that is likely to be responsible for the delivery of JAM-A and pJAM-A to sperm.
Conclusion
We have shown that JAM-A, which is an essential protein for normal sperm motility, is also an epididymal secretory protein that can be acquired by sperm during epididymal maturation. This testicular protein was also detected in the accessory organs and is likely to contribute to the molecular components of the murine seminal plasma of the ejaculate. The highest levels of JAM-A and pJAM-A are present in the caudal ELF and caudal sperm where motility and fertilizing ability are gained. This implies a role for JAM-A in the mechanism underlying the attainment of these characteristics during sperm maturation. Detected in both its unphosphorylated and its Ser285-phosphorylated states in epididymosomes, JAM-A is likely to be acquired by sperm in the inactivated as well as the activated state. As a highly conserved signaling protein (Naik and Eckfeld, 2003), JAM-A is expected to play a role in the post-testicular maturation of human sperm, as suggested by the presence of its interacting partner, CASK, in human prostasomes (Andrews et al., 2015).
Supplementary Material
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Authors’ roles
K.Z.W. and P.A.M.-D. designed the experiments and analyzed the data, K.Z.W. performed most of the experiments, K.L. performed some of the westerns, D.S.G. carried out the FACS analysis, and K.Z.W. and P.A.M.-D. wrote the paper.
Funding
National Institutes of Health (RO3HD073523 and 5P20RR015588 to P.A.M.-D.).
Conflict of interest
The authors declare there are no conflicts of interests.
References
- Al-Dossary AA, Bathala P, Caplan J, Martin-DeLeon PA. Oviductosome-sperm membrane interaction in cargo delivery: detection of fusion and underlying molecular players using 3D Super-Resolution Structured Illumination Microscopy (SR-SIM). J Biol Chem 2015;290:17710–17723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anakwe OO, Sharma S, Hoff HB, Hardy DM, Gerton GL. Maturation of guinea pig sperm in the epididymis involves the modification of proacrosin oligosaccharide side chains. Mol Reprod Dev 1991;29:294–301. [DOI] [PubMed] [Google Scholar]
- Andrews RE, Galileo DS, Martin-DeLeon PA. Plasma membrane Ca2+- ATPase 4: interaction with constitutive nitric oxide synthases in human sperm and prostasomes which carry Ca2+/CaM-dependent serine kinase. Mol Hum Reprod 2015;21:832–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravindan GR, Fomin VR, Naik UP, Modelski MJ, Naik MU, Galileo DS, Duncan RL, Martin-DeLeon PA. CASK interacts with PMCA4b and JAM-A on the mouse sperm flagellum to regulate motility and Ca2+ homeostasis. J Cell Physiol 2012;227:3138–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkin HR, Zhao L, Miller DJ. CASK is in the mammalian sperm head and is processed during epididymal maturation. Mol Reprod Dev 2004;68:500–506. [DOI] [PubMed] [Google Scholar]
- Cooper TG. Interactions between epididymal secretions and spermatozoa. J Reprod Fertil Suppl 1998;53:119–136. [PubMed] [Google Scholar]
- Cosentino MJ, Cockett ATK. Review article: Structure and function of the epididymis. Urol Res 1986;14:229–240. [DOI] [PubMed] [Google Scholar]
- Deng X, He Y, Martin-DeLeon PA. Mouse Spam1 (PH-20): evidence for its expression in the epididymis and for a new category of spermatogenic-expressed genes. J Androl 2000;21:822–832. [PubMed] [Google Scholar]
- Frenette G, Sullivan R. Prostasome-like particles are involved in the transfer of P25b from the bovine epididymal fluid to the sperm surface. Mol Reprod Dev 2001;59:115–121. [DOI] [PubMed] [Google Scholar]
- Griffiths GS, Reese KL, Galileo DS, Martin-DeLeon PA. Investigating the role of murine epididymosomes and uterosomes in GPI-linked protein transfer to sperm using SPAM1 as a model. Mol Reprod Dev 2008;75:1627–1636. [DOI] [PubMed] [Google Scholar]
- Hermo L, Jacks D. Nature's ingenuity: bypassing the classical secretory route via apocrine secretion. Mol Reprod Dev 2002;63:394–410. [DOI] [PubMed] [Google Scholar]
- Jones RC. Plasma membrane structure and remodeling during sperm maturation in the epididymis. J Reprod Fertil Suppl 1998;53:73–84. [PubMed] [Google Scholar]
- Martin-DeLeon PA. Epididymal Spam1 and its impact on sperm function. Mol Cell Endocrinol 2006;250:114–121. [DOI] [PubMed] [Google Scholar]
- Martin-DeLeon PA. Uterosomes: exosomal cargo during the estrus cycle and interaction with sperm. Front Biosci 2016;8:115–122. [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Sharma M, Urlaub H, Bourenkov GP, Jahn R, Sudhof TC, Wahl MC. CASK functions as a Mg2+-independent neurexin kinase. Cell 2008;133:328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik MU, Mousa SA, Parkos CA, Naik UP. Signaling through JAM-1 and αVβ3 is required for the angiogenic action of bFGF: dissociation of the JAM1 and αVβ3 complex. Blood 2003;102:2108–2114. [DOI] [PubMed] [Google Scholar]
- Naik UP, Eckfeld K. Junctional adhesion molecule 1 (JAM-1). J Biol Regul Homeost Agents 2003;17:341–347. [PubMed] [Google Scholar]
- Nixon B, Stanger SJ, Mihalas BP, Reilly JN, Anderson AL, Tyagi S, Holt JE, McLaughlin EA. The MicroRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biol Reprod 2015;93:91, 1–20. [DOI] [PubMed] [Google Scholar]
- Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O'Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T et al. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem 2004;279:33742–33750. [DOI] [PubMed] [Google Scholar]
- Patel R, Al-Dossary A, Stabley DL, Barone C, Galileo DS, Strehler E, Martin-DeLeon PA. Plasma membrane Ca2+-ATPase4 in murine epididymis: secretion of splice variants in the luminal fluid and a role in sperm maturation. Biol Reprod 2013;89:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposa G, Stoorvogel W. Extracellular vesicles, exosomes, microvesicles and friends. J Cell Biol 2013;200:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997. –2016.
- Robaire B, Hinton BT, Orgebin-Crist M. The epididymis In: Neill J, Plant T, Pfaff D, Challis J, Kretser D (eds). . Knobil and Neill's Physiology of Reproduction; 3rd edn Vol. 1. New York: Raven Press, 2006,1071–1148. [Google Scholar]
- Saez FJ, Frenette GP, Sullivan RM. Epididymosomes and prostasomes: their roles in post-testicular maturation of the sperm cells. J Androl 2003;24:149–154. [DOI] [PubMed] [Google Scholar]
- Schuh K, Cartwright EJ, Jankevics E, Bundschu K, Liebermann J, Williams JC, Armesilla AL, Emerson M, Oceandy D, Knobeloch KP et al. Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J Biol Chem 2004;279:28220–28226. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Wennemuth G, Post H, Brandenburger T, Aumuller G, Wilhelm B. Vesicular transfer of membrane components to bovine epididymal spermatozoa. Cell Tissue Res 2013;353:549–561. [DOI] [PubMed] [Google Scholar]
- Shao M, Ghosh A, Cooke VG, Naik UP, Martin-DeLeon PA. JAM-A is present in mammalian spermatozoa where it is essential for normal motility. Dev Biol 2008;313:246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R. Epididymosomes: Role of extracellular microvesicles in sperm maturation. Front Biosci 2016;8:106–114. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Mieusset R. The human epididymis: its function in sperm maturation. Hum Reprod Update 2016;22:574–587. [DOI] [PubMed] [Google Scholar]
- Wennemuth G. Calcium clearance mechanisms of mouse sperm. J Gen Physiol 2003;122:115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LG, Goodman SA. Analysis of lipid and protein components of ejaculated bull sperm surface and seminal plasma. Gamete Res 1982;6:281–291. [Google Scholar]
- Zhang H, Carlos R, Morales CR, Badran H, El-Falfy M, Martin-DeLeon PA. Expression of Spam1 (PH-20) in the extratesticular duct and accessory organs of the mouse: a possible role in sperm fluid reabsorption. Biol Reprod 2004;71:1101–1107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.