Supplemental Digital Content is available in the text
Keywords: dinoprostone, Foley catheter balloon, induction, meta-analysis
Abstract
Background:
Successful labor induction depends on the cervical status at the time of induction. Currently, both a Foley catheter and a dinoprostone insert are used for effective cervical ripening. This study compared the efficacy and safety of the intracervical Foley catheter and dinoprostone insert for cervical ripening to achieve successful labor induction.
Methods:
PubMed, Embase, and the Cochrane Library were searched from January 2000 to February 2017 for relevant articles. Only published randomized, controlled trials comparing the dinoprostone insert with the Foley catheter were included.
Results:
Eight trials including 1191 women who received the intracervical Foley catheter balloon and 1199 who received the dinoprostone insert were used for this study. There was no significant difference between the 2 groups regarding the induction-to-delivery (I-D) interval in a random effect model (mean difference, 0.71 hours; 95% confidence interval [CI], −2.50 to 3.91; P = .67). The highly significant heterogeneity (I2 = 97%) could be explained by the subgroup analysis of the type of Foley catheter and balloon volume. There was no significant difference between the 2 methods regarding the cesarean delivery rate (relative risk, 0.91; 95% CI, 0.78–1.07; P = .24), Apgar score, or side effects, including maternal infection rate, postpartum hemorrhage, and hyperstimulation. No obvious publication bias was found.
Conclusions:
According to the cesarean delivery rate, the intracervical Foley catheter balloon was as efficient as the dinoprostone insert. A moderate balloon volume (30 mL) and higher dose of dinoprostone (≥6 mg) were related to shorter I-D intervals. Additionally, there was no significant difference between the two methods regarding maternal or neonatal safety.
1. Introduction
Labor induction has become more widespread in most countries during the past decade,[1] with 20% to 30% of all deliveries worldwide involving induced labor.[2–4] Successful labor induction depends on the cervical status at the time of induction. It is predicted that patients with a poor Bishop score will have an unacceptably high rate of induction failure.[5] However, a variety of methods, including mechanical and pharmacologic methods, are available for cervical ripening.
Mechanical methods (laminaria tent, various types of balloon catheters, and extra-amniotic saline infusion) that were initially developed to ripen the cervix are thought to work by physically dilating the cervix and stimulating the release of endogenous prostaglandins. Pharmacologic ripening agents include oxytocin administration and various forms of exogenous prostaglandin administered orally or vaginally. Currently, prostaglandin E2 preparations (Prostin1, Cervidil1, Propess1) are the preferred choices in many countries.[6–10] However, the best method of labor induction is unknown.
To determine the optimal method of labor induction, investigators have conducted many clinical trials that have compared the efficacy and safety of the Foley catheter balloon with the dinoprostone insert. However, the results have not led to a consensus. Therefore, we performed a meta-analysis to compare the efficacy and safety of the intracervical Foley catheter and the dinoprostone insert for cervical ripening for successful labor induction.
2. Methods
This study was developed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions. All statistical analyses were performed using data reported in previously published studies. Ethical approval and informed consent were not required.
2.1. Search strategy
We performed a systematic literature search of PubMed, Embase, and the Cochrane Library from 2000 to February 2017. The following MeSH terms and text words were used: “Delivery,” “Deliveries,” “Obstetric,” “Obstetric,” “Parturition,” “Birth,” “Births,” “Childbirth,” “Childbirths,” “labor,” “Induced,” “Induction,” “Abortion,” “Cervical,” “Ripenings,” “Prostaglandin,” “Prostaglandins,” “Prostanoids,” “Prostanoid,” “Endoperoxides,” “Analogues,” “PGE,” “ProstaglandinsE,” “Alprostadil,” “PGE1,” “Dinoprostone,” “PGE2,” “PGE2?alpha,” “Prostenon,” “Misoprostol,” “Novo?Misoprostol,” “misoprostol?acid,” “propess,” “water?bag,” “water?balloon,” “bladder,” “Foley,” “Foley?Catheter,” “seaweed?stick,” “laminaria,” “laminaria?tent,” “RCT,” “randomised,” “Clinical,” “Controlled,” and “Trial∗.” The “AND” or “OR” operator was used to combine these terms in varying combinations. The article language was limited to English. The latest search was conducted on February 28, 2017. Two authors (JHX and JQL) independently reviewed the titles and abstracts identified during the search. Any disagreement was resolved by discussion or by involving a 3rd assessor (FC). No protocol was developed for this review.
2.2. Study selection
Inclusion criteria were as follows: primigravida; ≥37 and <42 weeks of gestation; singleton pregnancy; cephalic presentation; Bishop score ≤3; intact membranes; intracervical Foley catheter balloon for cervical ripening and labor induction; dinoprostone insert for cervical ripening and labor induction; study reported both primary outcomes (cesarean delivery rate and induction-to-delivery [I-D] interval); and study reported a randomized, controlled trial. Exclusion criteria were as follows: literature published as letters, editorials, abstracts, reviews, case reports, or expert opinions; experiment was in vitro or in vivo but not based on patients; no cesarean delivery rate and I-D interval mentioned; and repeated and similar studies. Cohen kappa statistic was used to assess the chance-corrected agreement between reviewers (SPSS version 18.0; SPSS Inc, Chicago, IL).[11]
2.3. Data extraction
We designed a form to extract data (Appendix 1 of the supplementary data). For eligible studies, 2 review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, a 3rd review author (FC) was consulted. The following clinical data were extracted for each trial: the first author's last name; publication year; country; study design; study interval; and criteria for participant inclusion. The primary outcomes of this meta-analysis were cesarean delivery rate and I-D interval. The secondary outcomes included other maternal parameters and neonatal outcomes. When an article only included the median and interquartile range (IQR), we used a formula (when data distribution was close to normal: IQR = 1.35 × standard deviation) to calculate the estimated mean and corresponding 95% confidence interval (CI).
2.4. Assessment of risk of bias
Two review authors (LZ and CZ) independently assessed the risk of bias for each study using the following information outlined in the Cochrane Handbook for Systematic Reviews of Interventions[12]: whether blinding was implemented for participants, staff, and outcome assessments; what proportion of subjects completed follow-up; and whether there was evidence of selective reporting of outcomes. Any disagreement was resolved by discussion or by involving a third assessor (FC).
2.5. Data synthesis and statistical analysis
We performed a statistical analysis using Review Manager software (RevMan 5.3). The primary outcomes were cesarean delivery rate and I-D interval of the intracervical Foley catheter balloon compared with the dinoprostone insert. Relative risk (RR) and 95% CI were used to measure the cesarean delivery rate. Cases of maternal or neonatal adverse events were also recorded. Heterogeneity among studies was measured by the I2 test. The latent publication bias was assessed by a funnel plot and Egger linear regression test. All statistical tests were 2-tailed, and P < .05 was considered statistically significant. All analyses were conducted using Review Manager software version 5.3 (The Cochrane Collaboration) and STATA statistical software package version 12.0 (Stata Corporation, College Station, TX).
3. Results
3.1. Search results
A total of 552 potential articles were identified from the literature searches. After selection, 8 studies matched the inclusion criteria and were suitable for our meta-analysis.[13–20] The flow diagram in Figure 1 shows the selection process. A total of 2390 cases were analyzed; 1191 (49.8%) patients received a Foley catheter balloon and 1199 (50.2%) received a dinoprostone insert. A review of the study selection and data extraction indicated excellent agreement between reviewers (k = 0.821).
Figure 1.
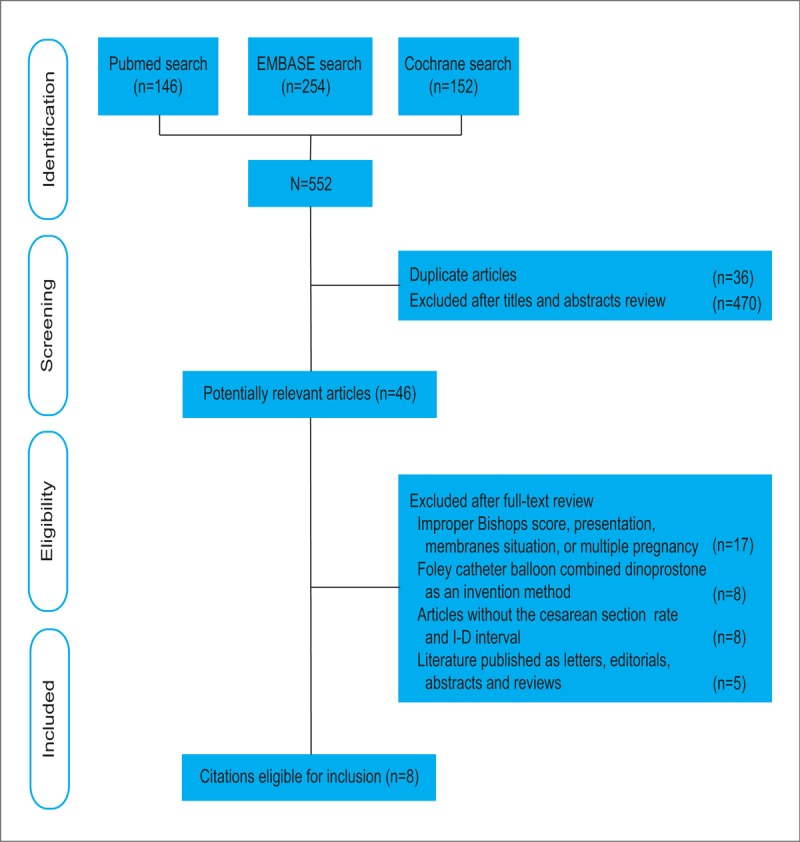
Flow chart for the selection of records. The search strategy identified 552 citations. Titles and abstracts were reviewed. Forty-six potential articles were retrieved and evaluated. Finally, 8 randomized, controlled trials appeared to be eligible.
3.2. Study characteristics and quality assessment
Study characteristics are summarized in Table 1. There were no significant differences between the 2 groups regarding maternal age, body mass index, gestational age, and baseline Bishop score. Study sample sizes ranged from 70 to 824. Two studies involved white populations.[16,17] The remaining studies involved Asian populations.[13–15,18–20] The type of Foley catheter used in each study ranged from 14F to 22F. The methodologic quality assessment based on the Cochrane risk of bias is presented in Figures 2 and 3. Overall, the studies included in our analysis were of moderate quality.
Table 1.
Main characteristics of all the studies included in the meta-analysis.
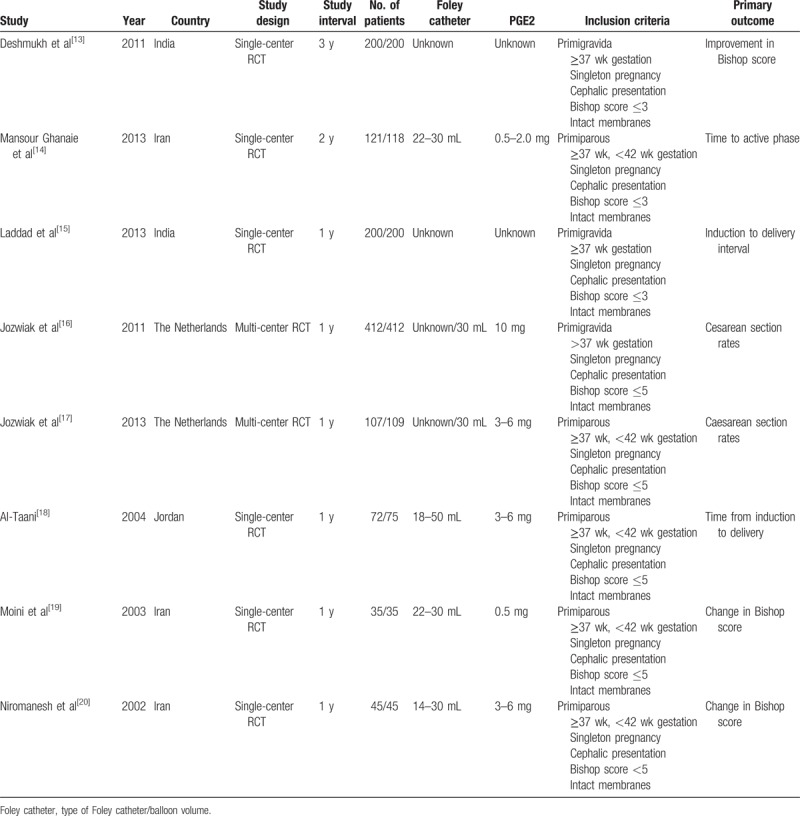
Figure 2.
Risk of bias graph. The review authors’ judgments of each risk of bias item are presented as percentages.
Figure 3.
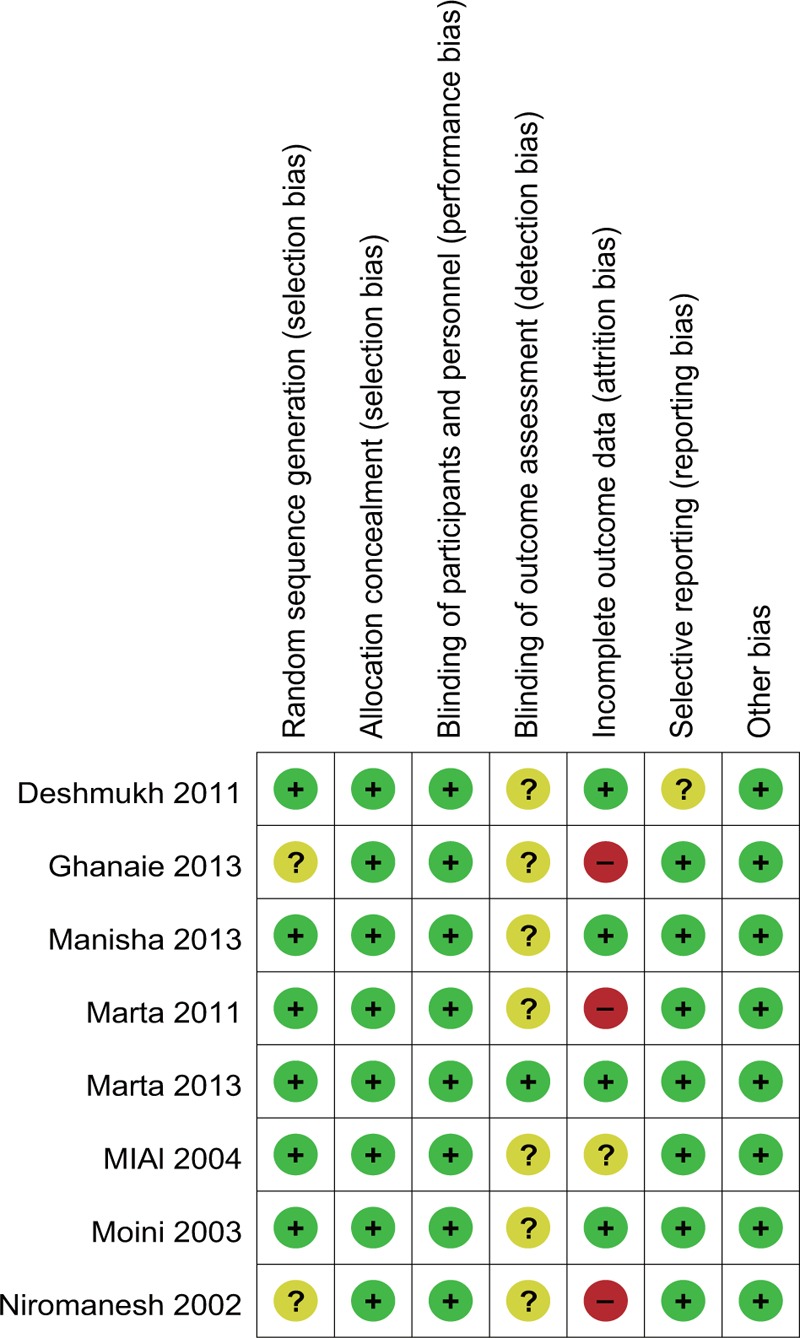
Risk of bias summary. The review authors’ judgments of each risk of bias item for each included study.
3.2.1. Cesarean delivery
All studies in our analysis reported data regarding cesarean delivery. Two hundred thirty-two (19.5%) patients in the intracervical Foley catheter balloon group and 256 (21.4%) in the dinoprostone insert group had a cesarean delivery. Due to the low heterogeneity across studies (I2 = 15%), a fixed-effect model was used. No significant differences were found between groups regarding the cesarean delivery rate (RR, 0.91; 95% CI, .78–1.07; P = .24) (Fig. 4).
Figure 4.
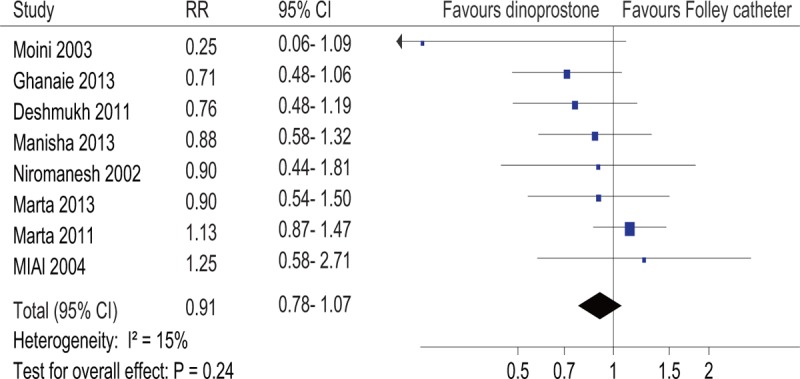
Forest plot of the cesarean delivery rate. RR, relative risk.
3.3. Time from the start of intervention to birth
Data regarding the I-D interval were described in eight studies.[13–20] Due to the significant heterogeneity across studies (I2 = 97%), a random-effect model was used. The pooled mean difference of the studies had no statistical significance (P = .67) (Fig. 5).
Figure 5.
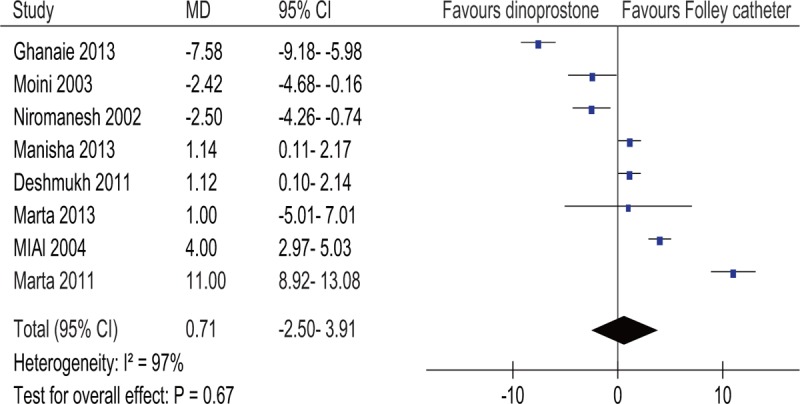
Forest plot of the induction-to-delivery interval. MD, mean difference.
3.4. Subgroup analysis
Based on the high heterogeneity of the I-D interval analysis, we conducted a subgroup analysis by stratifying the pooled data according to year (2010 or after vs before 2010), study region (Asian vs white), sample size (≥200 vs <200), type of Foley catheter (14F vs 18F vs 22F), balloon volume (30 mL vs 50 mL), and dinoprostone dose (<3 mg vs ≥3 mg and <6 mg vs ≥6 mg) (Table 2).
Table 2.
Subgroup analyses on I-D interval∗.
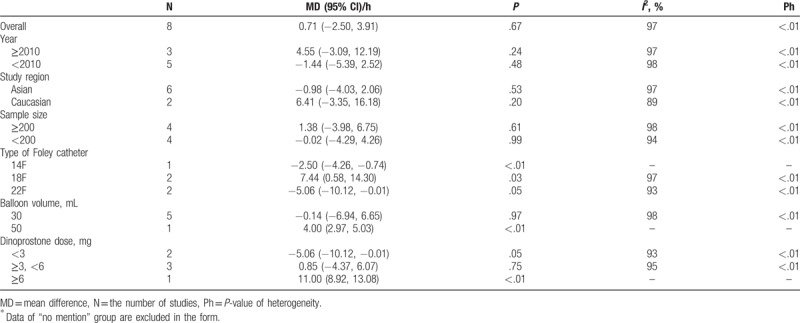
According to Table 2, heterogeneity of each subgroup decreased only during the dinoprostone dose analysis (I2 = 93%; I2 = 95%). In addition, the results of the subgroups were different but logical. When a low dose of dinoprostone (<3 mg) was used for comparison, the Foley catheter resulted in a shorter I-D interval (mean difference, −5.06 hours; 95% CI, −10.12 to −0.01; P < .05). Conversely, a high dose of dinoprostone (>6 mg) resulted in a shorter I-D interval (mean difference, 11.00 hours; 95% CI, 8.92–13.08; P < .00001). When the dose of dinoprostone was between 3 and 6 mg, there was no significant difference between the 2 methods regarding the I-D interval (mean difference, 0.85 hours; 95% CI, −4.37 to 6.07; P = .75). This indicated that the dose of dinoprostone was negatively related to the I-D interval, and that the individual study[21] had a great impact on total heterogeneity due to its high dose of dinoprostone (10 mg). However, the heterogeneities were still high, suggesting other confounding factors.
Therefore, subgroup analyses were repeated without including the study by Marta, and a further decrease in heterogeneity was found for the Foley catheter type (I2 = 93%) (Table 3) and balloon volume analyses (I2 = 88%) (Table 3). Although the heterogeneity was not largely reduced, the results for each subgroup varied widely. Interestingly, the Foley catheter balloon with a small diameter (14F) or large diameter (22F) had a shorter I-D interval than the dinoprostone insert (14F: mean difference −2.50 hours; 95% CI, −4.26 to −0.74; P < .005; 22F: mean difference, −5.06 hours; 95% CI, −10.12 to −0.01; P < .05) (Table 3). However, the Foley catheter balloon with a moderate diameter (18F) had a longer I-D interval (mean difference, 4.00 hours; 95% CI, 2.97–5.03; P < .00001) (Table 3). This illogic result was believed to be due to the small sample effects (only 1 study was included in the 2 subgroups), and the mechanical compression of the Foley catheter balloon on the cervix was mainly dependent on balloon volume. Moderate balloon volume (30 mL) was more efficient than large balloon volume (50 mL) regarding the I-D interval (30 mL: mean difference, −3.40 hours; 95% CI, −6.76 to −0.04; P < .05; 50 mL: mean difference, 4.00 hours; 95% CI, 2.97–5.03; P < .00001) (Table 3). It was suggested that the dose of dinoprostone and balloon volume were the main causes of heterogeneity.
Table 3.
Subgroup analyses on I-D interval excluded 1 outlier study∗.
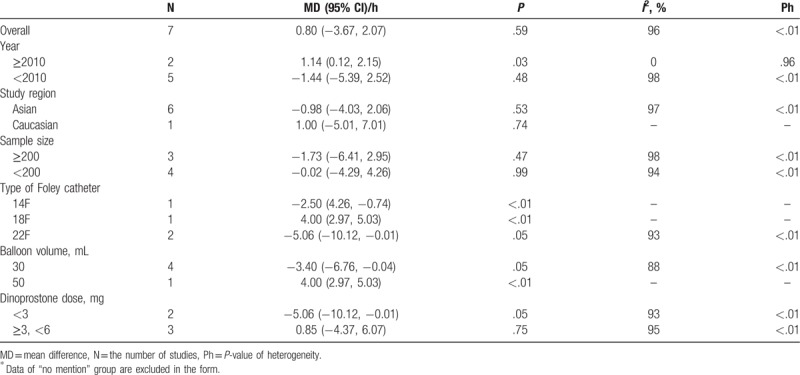
3.5. Secondary outcomes
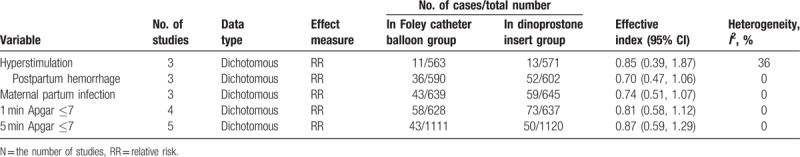
Cases of maternal or neonatal adverse events were recorded, and no significant difference was found between 2 groups (Table 4). There was no significant difference between the 2 methods regarding Apgar score and side effects such as maternal infection rate, postpartum hemorrhage, and hyperstimulation.
Table 4.
Cases of maternal or neonatal adverse events in Foley catheter balloon group and dinoprostone insert group.
3.6. Sensitivity analysis
To determine the stability of our results, a sensitivity analysis was performed. We conducted a metatrim analysis of the cesarean delivery rate. The metatrim analysis is a rank-based data augmentation technique that estimates the number and outcomes of missing studies and adjusts the meta-analysis to incorporate the missing theoretical studies. No data point was filled, which meant that the result was stable. According to the multivariate analysis and the univariate analysis, no significant changes were detected between the previous RR and new RR for the cesarean delivery rate. The new RR was pooled using the remaining studies after we deleted 1 individual study at a time. However, regarding the I-D interval, a new pooled significant mean difference (0.40; 95% CI, 0.31−0.49) indicated that the I-D interval was longer for the dinoprostone insert group when the study by Mansour Ghanaie et al was not included.[14] Therefore, this study might have affected the stability of this meta-analysis.
3.7. Publication bias
The funnel plot (Fig. 6) and Harbord modified test for small study effects (P = .140) on the cesarean delivery rate indicated no publication bias. According to the funnel plot (Fig. 7) and Egger test for small study effects (P = .221), no publication bias was found for the I-D interval.
Figure 6.
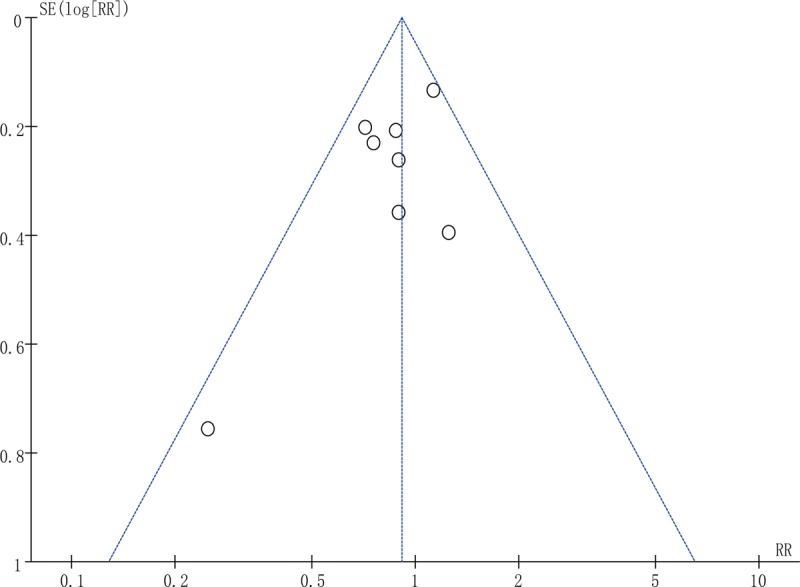
Funnel plot of the cesarean delivery rate.
Figure 7.
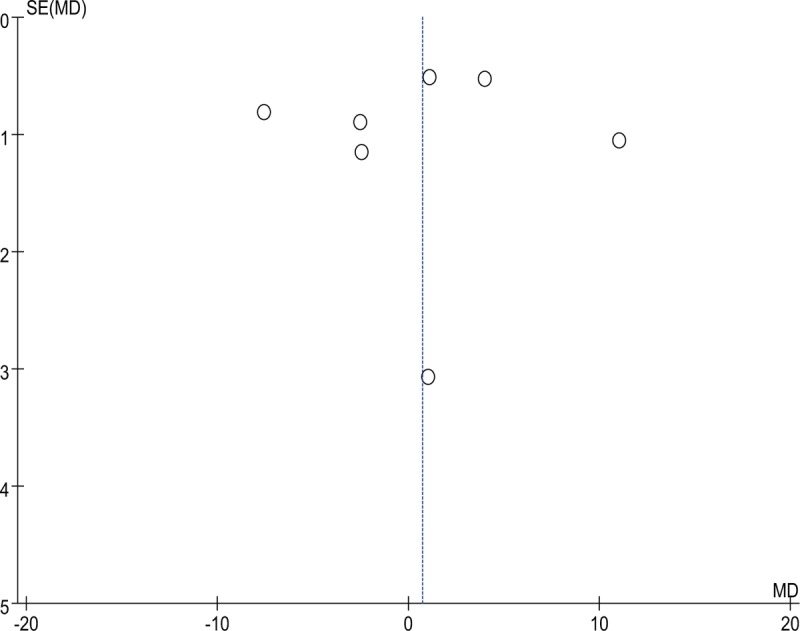
Funnel plot of the induction-to-delivery interval.
4. Discussion
Labor induction is one of the most common interventions in clinical obstetrics.[22] Although numerous studies have been conducted to compare ripening methods, no agreement has been universally achieved. The cesarean delivery rate, I-D interval, maternal complications, and neonatal outcomes need to be considered for ideal ripening. Many outcomes can be used to assess the effectiveness of induction methods. Cesarean delivery, which is surgery, is not acceptable to many pregnant women. Therefore, we chose the cesarean delivery rate as the primary outcome. As was expected, the cesarean delivery rate was similar for both groups, indicating further similarities between the 2 methods. Furthermore, low heterogeneity suggested credibility of the results (I2 = 15%; P = .24).
When pregnant women were interviewed about their expectations regarding childbirth, one of the main hopes was a short duration of labor.[23] Therefore, the time required for cervical ripening should be taken into consideration when selecting a method of labor induction. Three of the studies included (Mansour Ghanaie et al,[14] Moini et al,[19] and Niromanesh et al[20]) in this analysis claimed that the dinoprostone insert was associated with shorter time to delivery than the intracervical Foley catheter balloon. However, four of the studies included (Deshmukh et al,[13] Laddad et al,[15] Jozwiak et al,[16] and Al-Taani[18]) in this analysis reported that a shorter time to delivery was found with the use of an intracervical Foley catheter balloon. Jozwiak et al[17] showed no difference between the 2 groups regarding the I-D interval. Our pooled results showed high heterogeneity (I2 = 97%), and the subgroup analysis suggested that a moderate balloon volume (30 mL) and higher dose of dinoprostone (≥6 mg) were related to shorter I-D intervals.
Efficacy and safety are equally important factors when evaluating a cervical ripening method. Therefore, an ideal ripening agent should offer the best balance of these 2 factors and minimal side effects. Our meta-analysis also pooled outcomes of maternal complications, including hyperstimulation, postpartum hemorrhage, and maternal partum infection. No significant difference was found between the 2 groups. However, compared to prostaglandin E2, Foley catheters have additional advantages such as wide availability, easy storage, and low cost. A cost-effectiveness analysis of ripening methods was not performed because of limited data. However, the lower costs associated with oxytocin use, required personnel, and ancillary expenses might counterbalance the high cost of the dinoprostone insert method.[24] No significant differences were observed between the 2 groups regarding neonatal outcomes, including Apgar scores at 1 and 5 minutes.
4.1. Comparisons with similar systematic reviews
Researchers were able to reach a consensus regarding efficacy. They concluded that both methods have comparable efficacy. Jozwiak et al conducted a meta-analysis that included 3 individual studies that suggested comparable cesarean delivery rates (RR, 0.94; 95% CI, 0.73–1.21).[17] Wang et al conducted a meta-analysis that included 6 individual studies and reached the same conclusion (RR, 0.94; 95% CI, 0.80–1.21).[24] However, regarding the I-D interval, the results were inconsistent. A recent meta-analysis conducted by Vaknin showed no difference between I-D intervals for those using the Foley catheter balloon and those using the prostaglandin insert for cervical ripening.[25] In contrast, the results of the study by Wang et al indicated that the I-D interval was significantly shorter for the dinoprostone insert group compared to Foley catheter group (mean difference, 5.73 hours; 95% CI, 1.26–10.20),[24] which indicated that the dinoprostone insert was more effective than the Foley catheter for labor induction. Our research indicated that this could not be generalized and that time depended on the balloon volume and dose of dinoprostone.
Regarding maternal and neonatal complications, no significant difference was found between the 2 groups in our study. In the present analysis, McMaster et al observed similar results regarding maternal and neonatal adverse events, including maternal infection, chorioamnionitis, endometritis, and neonatal infection.[26] Wang et al observed no significant differences between the two groups regarding epidural analgesia, meconium staining, and neonatal complications (low birth weight, 5-minute Apgar score <7, umbilical artery blood pH < 7, and admission to the neonatal intensive care unit); however, a significantly increased risk of excessive uterine activity in the dinoprostone insert group was reported (RR, 0.07; 95% CI, 0.03–0.19; P < .01).[24]
4.2. Limitations
There were some limitations to our analysis that deserve discussion. First, we observed considerable heterogeneity between the studies when analyzing the I-D interval. Although we performed a subgroup analysis and analyzed the dose of dinoprostone and balloon volume, heterogeneity did not decrease to a reasonable range. There was evidence that a Foley catheter balloon with a higher balloon volume (60 or 80 mL) was more effective than a volume of 30 mL,[27,28] which was inconsistent with the results of our subgroup analysis. However, no randomized, controlled trials proved this theory. Second, during this analysis, we selected eligible studies only from those published in English, which might have caused publication bias. However, this bias was not supported by the funnel plot.
5. Conclusion
According to the cesarean delivery rate, an intracervical Foley catheter balloon was as efficient as a dinoprostone insert for inducing cervical ripening. A moderate balloon volume (30 mL) and higher dose of dinoprostone (≥6 mg) were related to shorter I-D intervals. In addition, there was no significant difference between the 2 methods regarding maternal or neonatal safety.
Author contributions
Conceptualization: Lixia Zhu, Qin Liu.
Data curation: Lixia Zhu, Qin Liu.
Formal analysis: Cong Zhang, Xing Gu.
Investigation: Cong Zhang, Xing Gu, Qin Liu, Jianqing Li.
Methodology: Cong Zhang, Fang Cao, Qin Liu.
Project administration: Fang Cao.
Resources: Fang Cao, Xing Gu.
Software: Cong Zhang, Xing Gu.
Supervision: Jianqing Li, Jianhao Xu.
Visualization: Cong Zhang, Jianhao Xu.
Writing – original draft: Lixia Zhu.
Writing – review & editing: Jianqing Li, Jianhao Xu.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CS% = cesarean section rate, I-D interval = induction to delivery interval, IQR = interquartile range, MD = mean difference, N = number of studies, Ph = P-value of heterogeneity, RR = relative risk.
LZ and CZ authors contributed equally to this work.
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Supplemental Digital Content is available for this article.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Dögl M, Vanky E, Heimstad R. Changes in induction methods have not influenced cesarean section rates among women with induced labour. Acta Obstet Gynecol Scand 2016;95:112–5. [DOI] [PubMed] [Google Scholar]
- [2].Zeitlin J, Mohangoo A, Cuttini M. The European Perinatal Health Report: comparing the health and care of pregnant women and newborn babies in Europe. J Epidemiol Commun H 2009;63:681–2. [DOI] [PubMed] [Google Scholar]
- [3].Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2009. Natl Vital Stat Rep 2011;60:1–70. [PubMed] [Google Scholar]
- [4].Werkmeister G, Party MCW. Normal childbirth. Birth 2010;37:178–178. [DOI] [PubMed] [Google Scholar]
- [5].St Onge RD, Conners GT. Preinduction cervical ripening: a comparison of intracervical PGE2 gel vs. the Foley catheter. Am J Obstet Gynecol 1995;172:687–90. [DOI] [PubMed] [Google Scholar]
- [6].Turnbull AC. Induction of labour. Bjog Int J Obstet Gy 2010;75:24–31. [DOI] [PubMed] [Google Scholar]
- [7].ACOG Committee on Practice Bulletins - Obstetrics ACOG practice bulletin no. 107: induction of labour. Obstet Gynecol 2009;114:386–97. [DOI] [PubMed] [Google Scholar]
- [8].Seloojeme D, Pisal P, Barigye O, et al. Are we complying with NICE guidelines on the use of prostaglandin E2 for induction of labour? A survey of obstetric units in the UK. J Obstet Gynaecol Re 2007;27:144–7. [DOI] [PubMed] [Google Scholar]
- [9].Rayburn WF. Preinduction cervical ripening: basis and methods of current practice. Obstet Gynecol Surv 2002;57:683–92. [DOI] [PubMed] [Google Scholar]
- [10].Grol RP, Gaj WM, Marcus MA, et al. Haemorrhagia post partum; an implementation study on the evidence-based guideline of the Dutch Society of Obstetrics and Gynaecology (NVOG) and the MOET (Managing Obstetric Emergencies and Trauma-course) instructions; the Fluxim study. Bmc Pregnancy Childb 2010;10:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull 1968;70:213–20. [DOI] [PubMed] [Google Scholar]
- [12].Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. Available at: www.cochrane-handbook.org Accessed March 30, 2015 [Google Scholar]
- [13].Deshmukh VL, Yelikar KA, Deshmukh AB. Study of intra-cervical Foley's catheter and PGE2 gel. J Obstet Gynecol India 2011;61:418–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mansour Ghanaie M, Jafarabadi M, Milani F, et al. A randomised controlled trial of Foley catheter, extra-amniotic saline infusion and prostaglandin E2 suppository for labour induction. J Fam Reprod Health 2013;7:49–55. [PMC free article] [PubMed] [Google Scholar]
- [15].Laddad MM, Kshirsagar NS, Karale AV. A prospective randomised comparative study of intra-cervical Foley's catheter insertion versus PGE2 gel for pre-induction cervical ripening. Int J Reprod Contracept Obstet Gynecol 2013;2:217–20. [Google Scholar]
- [16].Jozwiak M, Oude Rengerink K, Benthem M, et al. Foley catheter versus vaginal prostaglandin E2 gel for induction of labour at term (PROBAAT Trial): An open-label, randomised controlled trial. Lancet 2011;378:2095–103. [DOI] [PubMed] [Google Scholar]
- [17].Jozwiak M, Oude Rengerink K, Ten Eikelder MLG, et al. Foley catheter or prostaglandin E2 inserts for induction of labour at term: an open-label randomised controlled trial (PROBAAT-P trial) and systematic review of literature. Eur J Obstet Gynecol Reprod Biol 2013;170:137–45. [DOI] [PubMed] [Google Scholar]
- [18].Al-Taani MI. Comparison of prostaglandin E2 tablets or Foley catheter for labour induction in grand multiparas. East Med Health J 2004;10:547–53. [PubMed] [Google Scholar]
- [19].Moini A, Riazi K, Honar H, et al. Preinduction cervical ripening with the Foley catheter and saline infusion vs. cervical dinoprostone. Int J Gynecol Obstet 2003;83:211–3. [DOI] [PubMed] [Google Scholar]
- [20].Niromanesh S, Mosavi-Jarrahi A, Samkhaniani F. Intracervical Foley catheter balloon vs. prostaglandin in preinduction cervical ripening. Int J Gynecol Obstet 2003;81:23–7. [DOI] [PubMed] [Google Scholar]
- [21].Jozwiak M, Oude RK, Benthem M, et al. Foley catheter versus vaginal prostaglandin E2 gel for induction of labour at term (PROBAAT trial): an open-label, randomised controlled trial. Lancet 2011;378:2095–103. [DOI] [PubMed] [Google Scholar]
- [22].Londero AP, Schmitz R, Bertozzi S, et al. Diagnostic accuracy of cervical elastography in predicting labour induction success: a systematic review and meta-analysis. J Perinat Med 2016;44:167–78. [DOI] [PubMed] [Google Scholar]
- [23].Gibbins J, Thomson AM. Women's expectations and experiences of childbirth. Midwifery 2001;17:302–13. [DOI] [PubMed] [Google Scholar]
- [24].Wang H, Hong S, Liu Y, et al. Controlled-release dinoprostone insert versus Foley catheter for labour induction: a meta-analysis. J Matern Fetal Neonatal Med 2016;29:2382–8. [DOI] [PubMed] [Google Scholar]
- [25].Vaknin Z, Kurzweil Y, Sherman D. Foley catheter balloon vs locally applied prostaglandins for cervical ripening and labour induction: a systematic review and meta-analysis. Am J Obstet Gynecol 2010;203:418–29. [DOI] [PubMed] [Google Scholar]
- [26].McMaster K, Sanchez-Ramos L, Kaunitz AM. Evaluation of a transcervical Foley catheter as a source of infection: a systematic review and meta-analysis. Obstet Gynecol 2015;126:539–51. [DOI] [PubMed] [Google Scholar]
- [27].Levy R, Kanengiser B, Furman B, et al. A randomised trial comparing a 30-mL and an 80-mL Foley catheter balloon for preinduction cervical ripening. Am J Obstet Gynecol 2004;191:1632–6. [DOI] [PubMed] [Google Scholar]
- [28].Delaney S, Shaffer BL, Cheng YW, et al. Labour induction with a Foley balloon inflated to 30 mL compared with 60 mL: a randomised controlled trial. Obstet Gynecol 2010;115:1239–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


