Abstract
The indication for autotransplantation of parathyroid glands is still controversial. A new classification of parathyroid glands based on the positional relationship among parathyroid glands, thyroid gland and thymus was created to decide in situ preservation or autotransplantation during thyroid surgery.
A retrospective study included patients with papillary thyroid cancer who underwent total thyroidectomy with bilateral central lymph node dissection between November 2014 and November 2016. According to the application of the new classification (December 2015–November 2016) or traditional method (preservation of all functional parathyroid glands in situ, November 2014–November 2015), the patients were divided into new classification and traditional groups.
The traditional method was utilized in 288 patients who underwent surgery during the first half of the study, while the new classification was applied to 249 patients during the latter half of the study. The incidence of transient hypoparathyroidism was 43.0% (107/249) in new classification group and 35.8% (103/288) in the traditional group, respectively (P = .093). The corresponding incidence of permanent hypoparathyroidism was 0.4% (1/249) and 4.5% (13/288) (P = .002).
The new classification of parathyroid glands potentially reflects the difficulty of preservation and helps to make a reasonable decision on preservation or autotransplantation of a parathyroid gland, which may minimize the incidence of permanent hypoparathyroidism.
Keywords: classification, papillary thyroid cancer, parathyroid gland, permanent hypoparathyroidism
1. Introduction
Hypoparathyroidism is one of the most common complications after thyroidectomy. Theoretically, the best method, which is also the general recommendation, to prevent hypoparathyroidism is to preserve all parathyroid glands and their blood supply in situ. However, the blood supply is frequently injured in spite of meticulous dissection.[1–4] Selective autotransplantation is performed as a remedial measure when a parathyroid gland is devascularized or resected inadvertently.[4] However, the blood supply of parathyroid glands often varies due to the unique embryonic development. Color judgment for devascularization of parathyroid glands is extremely subjective.[3] Different surgeons may have different subjectivity on discoloration and adopt different methods of in situ preservation or autotransplantation. Controversy surrounds the method because permanent hypoparathyroidism still often occurs.
Autotransplantation is an effective strategy for restoration of parathyroid function.[1,5,6] Therefore, some surgeons advocate routine parathyroid autotransplantation to prevent permanent hypoparathyroidism because they believe the function of the autotransplanted glands is more predictable than that of the glands left in situ presumably with adequate blood supply.[4] However, it increases the incidence of transient hypoparathyroidism.[7,8] In addition, several studies reported increased risk of permanent hypoparathyroidism after parathyroid autotransplantation.[9,10] The primary causes of parathyroid deficiency include mechanical or thermal trauma, accidental resection, devascularization with secondary necrosis of parathyroid glands preserved in situ, and dysfunction of parathyroid glands before and/or after autotransplantation. Therefore, identification of an appropriate occasion to make a decision on preservation or autotransplantation of parathyroid glands is crucial to preserve their function and prevent postoperative hypoparathyroidism.
No existing noninvasive method can accurately decide when to autotransplant parathyroid glands. The indication for autotransplantation is still controversial. Based on the positional relationship among parathyroid glands, thyroid gland and thymus, a new classification of parathyroid glands was created to reflect the difficulty of preservation and help to decide in situ preservation or autotransplantation. The aim of the study was to investigate whether the new classification of parathyroid glands could effectively decrease the incidence of permanent hypoparathyroidism.
2. Patients and methods
2.1. Patients
Consecutive papillary thyroid cancer (PTC) patients who underwent total thyroidectomy with bilateral central lymph node dissection in the Department of Thyroid Surgery, West China Hospital of Sichuan University between November 2014 and November 2016 were included retrospectively from a prospective database. PTC was diagnosed by histopathological examination on ultrasound-guided fine needle aspiration biopsy and finally confirmed by paraffin section examination. Exclusion criteria included previous thyroid or parathyroid surgery, preoperative parathyroid glands dysfunction, pregnancy, lobectomy, ipsilateral central lymph node dissection, and lateral lymph node dissection. According to the application of the new method (the new classification of parathyroid glands) or traditional method (selective autotransplantation) to determine in situ preservation or autotransplantation of parathyroid glands, patients were divided into the new group and the traditional group. The traditional method was applied until November 30, 2015, and the new method was used after December 1, 2015. All patients were informed and agreed to follow-up meetings at least 6 months after surgery. The study was approved by the medical ethics committee of West China Hospital, Sichuan University.
2.2. Indications of total thyroidectomy with bilateral central lymph node dissection
The indications for total thyroidectomy in our department are as followed:
-
1)
high risk (family history and radiation exposure);
-
2)
bilateral or multifocal PTC;
-
3)
unilateral PTC with contralateral nodule(s);
-
4)
isthmus PTC;
-
5)
PTC>4 cm (stage T3);
-
6)
extrathyroidal invasion (stages T3 and T4);
-
7)
pathologic variables including tall cell variant, diffuse sclerosis variant, solid variant, and follicular variant;
-
8)
bilateral central lymph node or lateral lymph node metastases;
-
9)
distant metastases; and
-
10)
TERT (+) (by analysis of preoperative fine needle aspiration).
The indications for bilateral central lymph node dissection include:
-
1)
bilateral PTC;
-
2)
isthmus PTC;
-
3)
PTC with stage T3 and T4;
-
4)
prelaryngeal and/or pretracheal lymph node metastases;
-
5)
bilateral central lymph node or lateral lymph node metastases; and
-
6)
TERT (+) (by analysis of preoperative fine needle aspiration).
2.3. Classification of parathyroid glands
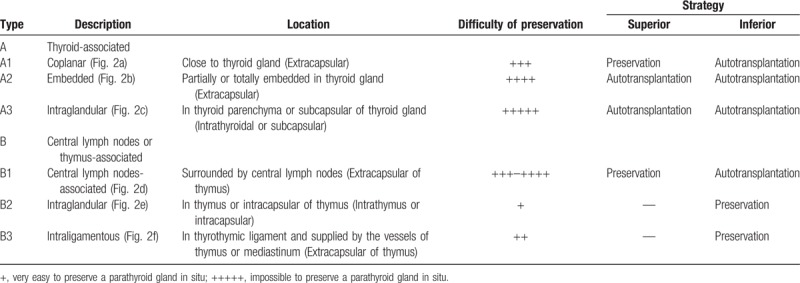
According to the positional relationship among parathyroid glands, thyroid gland, and thymus, parathyroid glands are classified as type A and type B in our department (Fig. 1). There are 3 subtypes in each type of parathyroid glands. The details of the 6 subtypes of parathyroid glands are shown in Figure 2. The type A PG has close relationship with thyroid gland, which contains 3 subtypes (types A1, A2, and A3). The type A1 PG clings to thyroid gland and locates outside of thyroid capsule (extracapsular), which is not easy to separate from thyroid gland (Fig. 2A). The type A2 PG is partly or totally embedded in thyroid gland, but also locates outside of thyroid capsule (extracapsular) (Fig. 2B). It is very difficult to separate type A2 PG from thyroid gland. The type A3 PG locates in thyroid parenchyma (intrathyroidal) or thyroid subcapsular (intracapsular), which is only found in resected thyroid specimen (Fig. 2C). The type B PG has no close relationship with thyroid gland, but some have certain relationship with thymus. There are also 3 subtypes in the type B PG, including types B1, B2, and B3. The type B1 PG locates around thyroid gland including all the type B PG except for types B2 and B3 PG (Fig. 2D). The type B2 PG locates in thymus parenchyma (intrathymus), which is easy to preserve in situ (Fig. 2E). The type B3 PG locates around thymus, which is mainly supplied by the vessels of thymus or mediastinum and also easy to preserve in situ (Fig. 2F). The strategies of the new classification for in situ preservation and autotransplantation during total thyroidectomy with bilateral central lymph node dissection include preservation of types B2 and B3 parathyroid glands and superior types A1 and B1 parathyroid glands in situ except for PTC invasion or accidental resection; autotransplantation of types A2 and A3 parathyroid glands; and autotransplantation of inferior types A1 and B1 parathyroid glands (Table 1).
Figure 1.
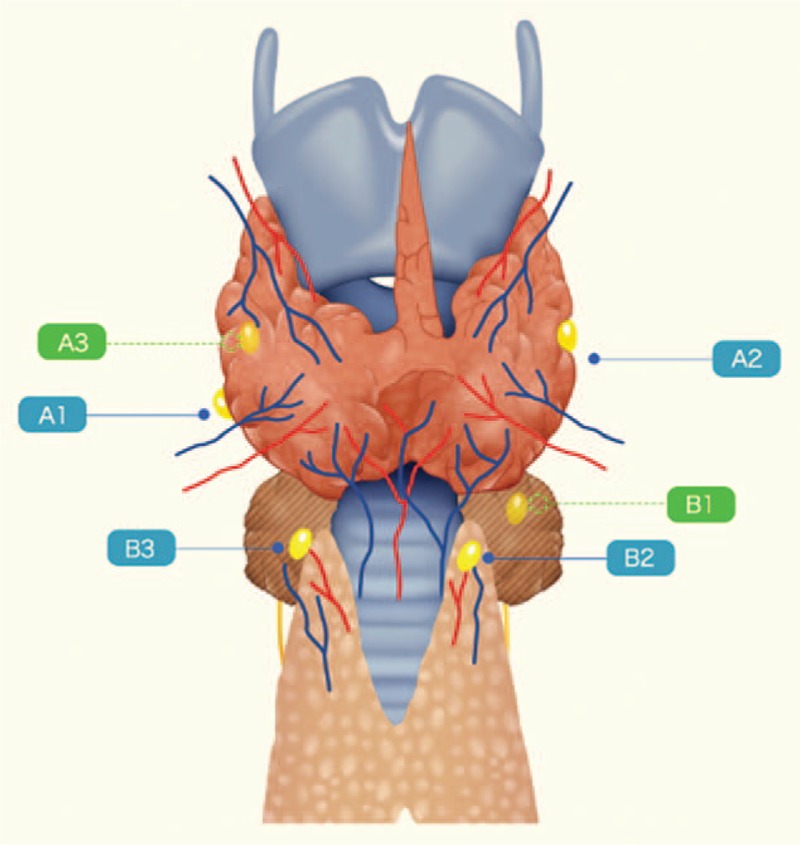
Schematic illustration of the types of parathyroid gland.
Figure 2.
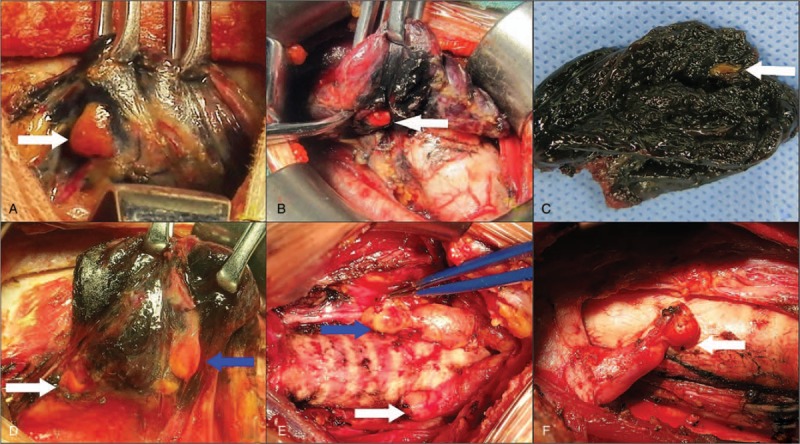
Intraoperative figures description of types of parathyroid gland. (A) Type A1 parathyroid gland locates next to thyroid gland and outside of thyroid capsule (white arrow). (B) Type A2 parathyroid gland is partially or totally embedded in thyroid gland and locates outside of thyroid capsule (white arrow). (C) Type A3 parathyroid gland locates in the resected thyroid gland (white arrow). (D) Type B1 parathyroid gland locates in the central compartment and around thyroid gland, but had no close relationship with thyroid gland (white arrow indicates type B1 parathyroid gland and blue arrow indicates type A1 parathyroid gland). (E) Type B2 parathyroid gland locates in thymus parenchyma (white arrow indicates type B2 parathyroid gland and blue arrow indicates type B3 parathyroid gland). (F) Type B3 parathyroid gland locates within thyrothymic ligament, which is mainly supplied by the vessels of thymus or mediastinum (white arrow).
Table 1.
The details of the new classification of parathyroid glands.
2.4. Surgical procedures
All surgeries were conducted by a same thyroid surgeon (Su A). Surgical procedures were as followed: a curved incision was made at the 2-finger distance above the suprasternal notch. The platysma was separated transversely and the gap between thyroid gland and cervical anterior muscles was opened, fully exposing the surgical site. Then carbon nanoparticles suspension (Lai Mei Pharmaceutical Co, Chongqing, China) was slowly injected into the upper, middle, and lower poles (≤ 0.1 mL per point) of thyroid gland to facilitate bilateral central lymph node dissection and parathyroid glands identification.[11] Surgical resection was started 10 minutes later. Total thyroidectomy was performed and the latter was associated with bilateral central lymph node dissection. Thymus was preserved except for PTC invasion. Parathyroid glands were classified by a same surgeon and the classifications were recorded in both groups. In the new group, when a parathyroid gland was found during surgery, classification was determined instantly and then a decision was made on in situ preservation or autotransplantation based on the strategies shown in Table 1. In the traditional group, attempt was made firstly to preserve all parathyroid glands in situ. Selective autotransplantation was performed as a remedial measure when a parathyroid gland was devascularized or resected inadvertently. If a parathyroid gland could not be preserved in situ, a small part was sent to intraoperative frozen biopsy and the remaining was wrapped in gauze which was soaked with normal saline solution. When the parathyroid gland was confirmed, it was immediately chopped into 1 mm3 fragments and buried into several pockets in the sternocleidomastoid muscle. Intraoperative neuromonitoring (Medtronic NIM-Response 2.0) was used to identify and protect recurrent laryngeal nerve. The same group of pathologists analyzed all surgical specimens and the presence of parathyroid glands in the surgical specimens (accidental resection) was recorded.
2.5. Perioperative management
Perioperative management was standardized. Preoperative evaluation in each patient included serum calcium, parathyroid hormone (PTH), thyroid function, thyroid ultrasound, and laryngoscopy. PTH was routinely tested 1 day, 30 days, and 6 months after surgery. Oral and/or intravenous calcium supplementation was conducted to the patients with symptomatic hypocalcemia. Postoperative hypoparathyroidism was defined as a PTH measurement <1.6 pmol/L (normal range, 1.6–6.9 pmol/L) after surgery. If serum PTH level did not return to normal at 6 months postoperatively, hypoparathyroidism was defined as permanent. If serum PTH level returned to normal within 6 months postoperatively, hypoparathyroidism was classified as transient.[12]
2.6. Data collection
All data were collected retrospectively, including general characteristics, preoperative examination, intraoperative factors, pathologic examination, postoperative examination, and complications. American Joint Committee on Cancer (AJCC) staging (the seventh edition) was applied for all PTC patients.[13] The primary endpoints were the number of parathyroid glands identified and autotransplanted, the details of each type of parathyroid glands and the incidence of permanent hypoparathyroidism.
2.7. Statistical analysis
Continuous variables were presented as mean ± standard deviation. Statistical analysis was conducted with IBM SPSS Statistics 19.0. Statistical comparison was carried out between the new group and traditional group by using the χ2 test or Student t test. A multivariate analysis with logistic regression was performed to identify the perioperative factors, which were considered to affect permanent hypoparathyroidism. The results of the multivariate analysis were expressed as odds ratio, 95% confidence interval (CI) and P value. Statistical significance was set at P <.05.
3. Results
3.1. Patient characteristics
Of the 537 patients included in the study, 249 (46.4%) used the new method and 288 (53.6%) accepted the traditional method. Table 2 shows a comparison of baseline characteristics of the patients in the 2 groups. The age of the patients ranged from 17 to 72 years, with a median age of 41 years. The ratio between male and female was nearly 1:3 (148:389). No significant differences were found between the 2 groups in age, sex, body mass index (BMI), comorbidities (hypertension, diabetes, thyroiditis, Graves’ disease, hypothyroidism, and nodular goiter), preoperative levels of serum calcium and PTH and vocal cord paralysis.
Table 2.
The clinical characteristics of the patients in the 2 groups.
3.2. Characteristics of tumors and central lymph nodes
The 2 groups were comparable in the largest tumor size, multifocality, primary tumor location, and AJCC stage. The mean number of harvested central lymph nodes was 11.5 ± 5.9 in the new group and 10.9 ± 5.3 in the traditional group, respectively (P = .177). Of these lymph nodes, 3.1 ± 3.9 in the new group and 3.0 ± 3.7 in the traditional group had metastases (P = .717). Rate of lymph node metastases for the entire cohort was 68.7% (369/537). It occurred in 177 (71.1%) and 192 (66.7%) patients in both groups (P = .305). No significant difference in terms of the largest size of lymph nodes (7.3 ± 4.2 vs 8.3 ± 4.5 mm, P = .621) was also found between the 2 groups.
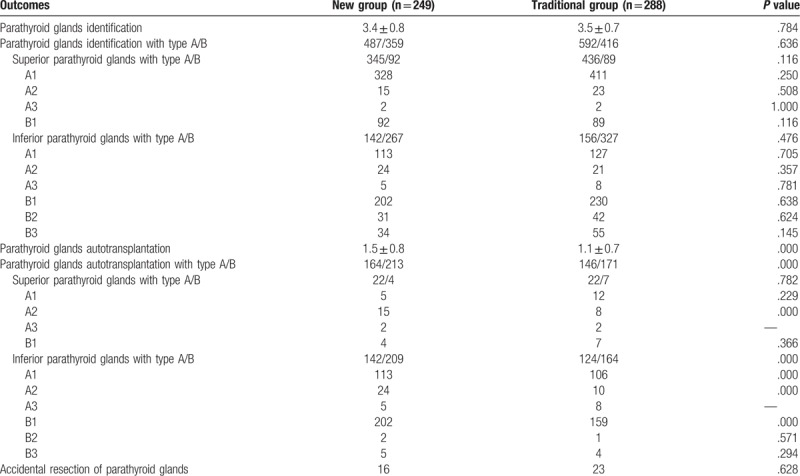
3.3. Parathyroid glands identification, classification, autotransplantation, and accidental resection
The details of parathyroid glands identification, classification, autotransplantation and accidental resection in the 2 groups are presented and compared in Table 3. In the new group, 487 type A and 359 type B parathyroid glands were identified, with an average of 3.4 ± 0.8 per patient. In the traditional group, 592 type A and 416 type B parathyroid glands were identified, with an average of 3.5 ± 0.7 per patient. No statistically significant differences aforementioned were observed between the 2 groups (all P >.05). The most common type of parathyroid glands was type A1 (979/1854, 52.8%), followed by type B1 (613/1854, 33.1%), B3 (89/1854, 4.8%), A2 (83/1854, 4.5%), B2 (73/1854, 3.9%), and A3 (17/1854, 0.9%). Two type A3 parathyroid glands were found in 1 patient. There was also no significant difference in each type of parathyroid glands between the 2 groups (all P >.05). The most common types of superior and inferior parathyroid glands were type A1 (739/962, 76.8%) and type B1 (432/892, 48.4%), respectively. Types B2 and B3 parathyroid glands were only found in the inferior ones. A significant difference in terms of the mean number of parathyroid glands for autotransplantation was observed between the new and traditional groups (1.5 ± 0.8 vs 1.1 ± 0.7, P = .000). The rate of parathyroid glands autotransplantation was significantly higher in the new group compared with the traditional group (377/846, 44.6% vs 317/1008, 31.4%, P = .028). The preservation rates of types A1, A2, A3, B1, B2, and B3 PGs were 73.2% (323/441), 0% (0/39), 0% (0/7), 29.9% (88/294), 93.5% (29/31), and 85.3% (29/34) in the new group, while they were 78.1% (420/538), 59.1% (26/44), 0% (0/10), 48.0% (153/319), 97.6% (41/42), and 92.7% (51/55) in the traditional group. More types A1 and B1 inferior parathyroid glands were autotransplanted in the new group (P = .000 and .000, respectively). The autotransplantation rates of type A2 superior and inferior parathyroid glands were significantly higher in the new group than in the traditional group (P = .000 and .000, respectively). The 2 groups had comparable autotransplantation rates for other types of parathyroid glands. The final pathological examination presented 16 cases (6.4%) with accidental resection of parathyroid glands in the new group and 23 (8.0%) in the control group (P = .628).
Table 3.
Parathyroid glands identification, classification, autotransplantation and accidental resection in the 2 groups.
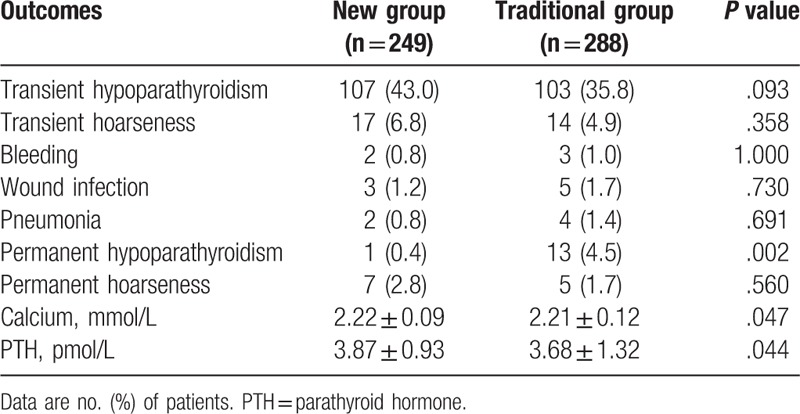
3.4. Postoperative complications and results of follow-up
Table 4 summarizes the postoperative complications and the results of follow-up in the 2 groups. Transient hypoparathyroidism was documented in 107 of the 249 patients (43.0%) in the new group and 103 of the 288 patients (35.8%) in the traditional group (P = .093). No significant difference was found between the 2 groups in terms of the occurrence of transient hoarseness, bleeding, wound infection and pneumonia. All the patients completed a 6-month follow-up period. The incidence of permanent hypoparathyroidism for the entire cohort was 2.6% (14/537). The incidence of permanent hypoparathyroidism in the new and traditional groups was 0.4% (1/249) and 4.5% (13/288), respectively (P = 0.002). Only 1 parathyroid gland was found intraoperatively and preserved in situ in the permanent hypoparathyroidism patient in the new group. However, 1 parathyroid gland was found in the surgical specimens by pathologists. Of the 13 patients with permanent hypoparathyroidism in the traditional group, 3, 8, and 2 had 0, 1, and 2 parathyroid glands autotransplanted, respectively. Numbers of parathyroid glands preserved in situ were as followed: 0 in 1 patient, 1 in 5 patients, 2 in 5 patients, and 3 in 2 patients. The level of serum PTH in the patients with permanent hypoparathyroidism was 1.21 ± 0.26 pmol/L. Permanent hoarseness due to vocal cord paralysis (confirmed by laryngoscopy) occurred in 7 patients (2.8%) in the new group and 5 (1.7%) in the traditional group (P = .560). The levels of serum calcium and PTH were significantly higher in the new group than in the traditional group at 6 months postoperatively (2.22 ± 0.09 vs 2.21 ± 0.12 mmol/L, P = .047 and 3.87 ± 0.93 vs 3.68 ± 1.32 pmol/L, P = .044, respectively).
Table 4.
The postoperative complications and the results of follow-up in the 2 groups.
3.5. Risk factors of permanent hypoparathyroidism
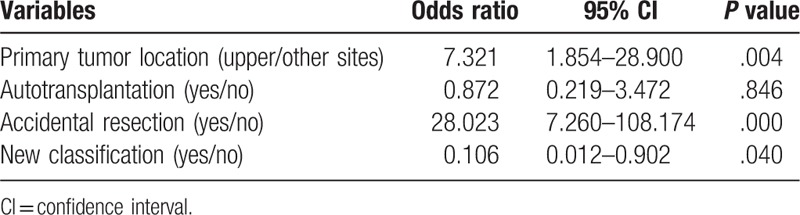
Perioperative factors, which might influence the incidence of permanent hypoparathyroidism, were sought by univariate and multivariate analyses. Primary tumor location, autotransplantation, accidental resection and application of new parathyroid glands classification had significant effects on the occurrence of permanent hypoparathyroidism on univariate analysis (Table 5). Multivariate analysis of the 4 factors demonstrated that primary tumor location, accidental resection, and application of new parathyroid glands classification were the independent risk factors of permanent hypoparathyroidism (Table 6).
Table 5.
Univariate analysis of risk factors for permanent hypoparathyroidism.
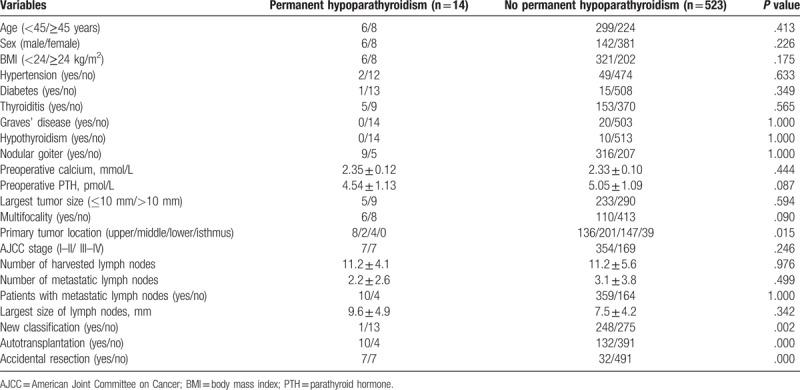
Table 6.
Multivariate analysis of risk factors for permanent hypoparathyroidism.
4. Discussion
The reported incidence of permanent hypoparathyroidism after total thyroidectomy with bilateral central lymph node dissection ranges from 1.5% to 16.2%.[14,15] It was 2.6% for the entire cohort in the present study. However, it was only 0.4% in the new classification patients. Preservation of at least 1 parathyroid gland with intact blood supply or routine autotransplantation of at least 1 parathyroid gland with good function can effectively reduce the incidence of permanent hypoparathyroidism.[5,12] It still occurred in 10 patients with autotransplantation of at least 1 parathyroid gland and 12 with preservation of at least 1 parathyroid gland in the traditional group, which indicated that parathyroid glands might be dysfunctional before autotransplantation and/or after preservation due to the prolonged devascularization. Compared to routine autotransplantation,[5] the new classification can more reasonably guide in situ preservation or autotransplantation of parathyroid glands, which may lead to lower incidence of transient hypoparathyroidism.[7,8] Significantly higher levels of PTH and calcium were also found in the patients with new parathyroid glands classification at 6 months after surgery. These results indicate that the new classification has potential advantage to preserve the function of parathyroid glands and decrease the incidence of permanent hypoparathyroidism.
The intraoperative PTH assay during thyroidectomy in monitoring of parathyroid function turned out to be of added-value for the surgical decision making with respect to the selective parathyroid autotransplantation.[16] However, it is not widely used in China due to the lack of relevant technology and equipment. Although a knife test or biopsy to confirm devascularization by observation of capsular bleeding is advocated, it may cause iatrogenic injury to the precarious blood supply of parathyroid glands.[2,3] The most important characteristic of the new classification is that parathyroid glands are classified based on their position instead of blood supply.[17] It reduces the assessment of the viability of parathyroid glands and makes the surgeries easier. Both the position and the type of parathyroid glands in the new classification are not altered with the change of surgeons. It objectively reflects the relationship among parathyroid glands, thyroid gland, and thymus. Therefore, it can be easily applied by thyroid surgeons.
In the present study, the most common types of parathyroid glands were type A1 (52.8%) and type B1 (33.1%). The most common types of superior and inferior parathyroid glands were type A1 (76.8%) and type B1 (48.4%), respectively. Due to relatively fixed position and blood supply, 97.9% of superior ones were preserved in situ regardless of types A1 or B1. Therefore, we strongly recommend preservation of superior parathyroid glands with types A1 and B1 during total thyroidectomy with bilateral central lymph node dissection. However, inferior ones with types A1 and B1 are always difficult to preserve in situ because their blood supply is easily damaged. Type A2 parathyroid glands accounted for 4.5% of all glands in the study. It is more difficult to preserve them in situ because of the complicated blood supply. In addition, mechanical or thermal trauma was not avoided during the separation of types A1 and A2 parathyroid glands from thyroid gland. Parathyroid deficiency is closely related with dysfunction or necrosis of the devascularized parathyroid glands preserved in situ. Although lower incidence of transient hypoparathyroidism was noted in the traditional group, preservation of more types A1, A2, and B1 parathyroid glands did not reduce the incidence of permanent hypoparathyroidism. Furthermore, autotransplantation of inferior parathyroid glands may decrease central lymph node recurrence in PTC patients.[18] Therefore, direct autotransplation of type A2 parathyroid glands and inferior types A1 and B1 ones can be an applicable first-line intervention. Type A3 parathyroid glands, accounting for 0.9% of all glands, play a key role in the prevention of permanent hypoparathyroidism as well. Since they only locate in thyroid gland and cannot be preserved in situ, careful examination of thyroid specimen intraoperatively decreases the incidence of accidental resection of type A3 parathyroid glands.[19] Some inferior parathyroid glands have a certain relationship with thymus because of similar embryonic development. Types B2 and B3 ones accounted for 3.9% and 4.8% of all glands, respectively. They are easily preserved in situ due to the gap between thymus and central lymph nodes. Thymus should be routinely preserved except for PTC invasion. Therefore, the new classification objectively reflects the difficulty to preserve parathyroid glands in situ. Combination preservation with autotransplantation of parathyroid glands is an effective strategy for minimizing the incidence of permanent hypoparathyroidism during thyroid surgery.[7,9] Under the guidance of the new classification, in situ preservation or autotransplantation can be reasonably determined to further preserve parathyroid function.
In the present study, primary tumor location, application of the new classification and accidental resection were the independent risk factors of permanent hypoparathyroidism. PTC in the upper pole of thyroid gland was first identified as a risk factor of permanent hypoparathyroidism. It can probably be explained by the facts that more direct invasion and less preservation of superior parathyroid glands are found when a primary tumor locates in the upper pole of thyroid gland. The incidence of accidental resection of parathyroid glands in the current study was 7.3%, which is in accord with recent reports (5%–29%).[12,20] The careful inspection of all surgical specimen (thyroid gland and fat lymphoid tissue) intraoperatively allows autotransplantation of inadvertently removed parathyroid glands during total thyroidectomy with central lymph node dissection. Multivariate analysis further confirmed the effectiveness of the new classification of parathyroid glands on the prevention of permanent hypoparathyroidism.
The present study has limitations in that it is retrospective and without randomization of application of the new classification. Surgeon's experience which is one of the most important factors in the prevention of permanent hypoparathyroidism may change during the 2 periods. In addition, the follow-up period was only 6 months. A prospective randomized trial with long-term follow-up period is needed to definitely confirm the effect of the new parathyroid glands classification on permanent hypoparathyroidism.
5. Conclusions
In conclusion, the new classification of parathyroid glands potentially reflects the difficulty of preservation and helps to make a reasonable decision on preservation or autotransplantation of a parathyroid gland, which may minimize the incidence of permanent hypoparathyroidism.
Author contributions
Conceptualization: Anping Su, Tao Wei.
Formal analysis: Zhihui Li.
Investigation: Rixiang Gong.
Methodology: Yanping Gong.
Supervision: Jingqiang Zhu.
Writing – original draft: Anping Su.
Writing – review & editing: Anping Su.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, PTC = papillary thyroid cancer, PTH = parathyroid hormone.
The study was supported by the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University.
The authors have no conflicts of interest to disclose.
References
- [1].Testini M, Rosato L, Avenia N, et al. The impact of single parathyroid gland autotransplantation during thyroid surgery on postoperative hypoparathyroidism: a multicenter study. Transplant Proc 2007;39:225–30. [DOI] [PubMed] [Google Scholar]
- [2].Lo CY. Parathyroid autotransplantation during thyroidectomy. ANZ J Surg 2002;72:902–7. [DOI] [PubMed] [Google Scholar]
- [3].Kuhel WI, Carew JF. Parathyroid biopsy to facilitate the preservation of functional parathyroid tissue during thyroidectomy. Head Neck 1999;21:442–6. [DOI] [PubMed] [Google Scholar]
- [4].Lorente-Poch L, Sancho JJ, Ruiz S, et al. Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 2015;102:359–67. [DOI] [PubMed] [Google Scholar]
- [5].Zedenius J, Wadstrom C, Delbridge L. Routine autotransplantation of at least one parathyroid gland during total thyroidectomy may reduce permanent hypoparathyroidism to zero. Aust N Z J Surg 1999;69:794–7. [DOI] [PubMed] [Google Scholar]
- [6].Ahmed N, Aurangzeb M, Muslim M, et al. Routine parathyroid autotransplantation during total thyroidectomy: a procedure with predictable outcome. J Pak Med Assoc 2013;63:190–3. [PubMed] [Google Scholar]
- [7].Palazzo FF, Sywak MS, Sidhu SB, et al. Parathyroid autotransplantation during total thyroidectomy--does the number of glands transplanted affect outcome? World J Surg 2005;29:629–31. [DOI] [PubMed] [Google Scholar]
- [8].Lo CY, Lam KY. Postoperative hypocalcemia in patients who did or did not undergo parathyroid autotransplantation during thyroidectomy: a comparative study. Surgery 1998;124:1081–6. [DOI] [PubMed] [Google Scholar]
- [9].Kihara M, Miyauchi A, Kontani K, et al. Recovery of parathyroid function after total thyroidectomy: long-term follow-up study. ANZ J Surg 2005;75:532–6. [DOI] [PubMed] [Google Scholar]
- [10].Kirdak T, Dundar HZ, Uysal E, et al. Outcomes of parathyroid autotransplantation during total thyroidectomy: a comparison with age- and sex-matched controls. J Invest Surg 2017;30:201–9. [DOI] [PubMed] [Google Scholar]
- [11].Li J, Li X, Wang Z. Negative developing of parathyroid using carbon nanoparticles during thyroid surgery. Gland Surg 2013;2:100–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Song CM, Jung JH, Ji YB, et al. Relationship between hypoparathyroidism and the number of parathyroid glands preserved during thyroidectomy. World J Surg Oncol 2014;12:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].NCCN Clinical Practice Guidelines in Oncology. NCCN Guidelines: Thyroid Carcinoma. Version 3, 2011. [Google Scholar]
- [14].Hall CM, Snyder SK, Maldonado YM, et al. Routine central lymph node dissection with total thyroidectomy for papillary thyroid cancer potentially minimizes level VI recurrence. Surgery 2016;160:1049–58. [DOI] [PubMed] [Google Scholar]
- [15].Giordano D, Valcavi R, Thompson GB, et al. Complications of central neck dissection in patients with papillary thyroid carcinoma: results of a study on 1087 patients and review of the literature. Thyroid 2012;22:911–7. [DOI] [PubMed] [Google Scholar]
- [16].Barczynski M, Cichon S, Konturek A, et al. Applicability of intraoperative parathyroid hormone assay during total thyroidectomy as a guide for the surgeon to selective parathyroid tissue autotransplantation. World J Surg 2008;32:822–8. [DOI] [PubMed] [Google Scholar]
- [17].Cui Q, Li Z, Kong D, et al. A prospective cohort study of novel functional types of parathyroid glands in thyroidectomy: in situ preservation or auto-transplantation. Medicine (Baltimore) 2016;95:e5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wei T, Li Z, Jin J, et al. Autotransplantation of inferior parathyroid glands during central neck dissection for papillary thyroid carcinoma: a retrospective cohort study. Int J Surg 2014;12:1286–90. [DOI] [PubMed] [Google Scholar]
- [19].Abboud B, Sleilaty G, Braidy C, et al. Careful examination of thyroid specimen intraoperatively to reduce incidence of inadvertent parathyroidectomy during thyroid surgery. Arch Otolaryngol Head Neck Surg 2007;133:1105–10. [DOI] [PubMed] [Google Scholar]
- [20].Paek SH, Lee YM, Min SY, et al. Risk factors of hypoparathyroidism following total thyroidectomy for thyroid cancer. World J Surg 2013;37:94–101. [DOI] [PubMed] [Google Scholar]


