Abstract
We previously reported satisfactory results with the Karakoca resector balloon in 10 patients with stage IV chronic obstructive pulmonary disease (COPD) who did not respond to medical treatment. In this article, we present the outcomes of the Karakoca resector balloon dilatation and curettage technique in a larger case series (n = 188).
A total of 188 COPD patients [mean age (SD): 69.2 (8.0) years; 46 females] classified as stage III to IV by the Global Initiative for Obstructive Lung Disease criteria underwent balloon desobstruction for segmental and subsegmental bronchi by therapeutic bronchoscopy. None of the patients could have achieved symptom relief even under high-dose inhaled bronchodilators and corticosteroids, oral corticosteroids, or oxygen and noninvasive mechanical ventilation therapy before the intervention. Forced expiratory volume in 1 s (FEV1) and oxygen saturation (SpO2) were measured, and modified Borg dyspnea scale (MBS) scores were determined before and 1 week and 1 month after the intervention.
All patients were active smokers and 80% had concomitant chronic diseases. After the intervention, there was a notable reduction in the oxygen need of the patients. Comparison of lung function tests 1 week after the procedure with results before the procedure showed significant improvements in FEV1, MBS, and SpO2 levels (P < 0.001 for each), and the improvements were maintained for the entire postprocedural month (P < 0.001 for each). Except for 4 males, all patients were free of symptoms.
These results confirmed our early observations that balloon dilatation and curettage is a safe and successful technique for medical treatment-resistant COPD.
Keywords: chronic obstructive pulmonary disease, Karakoca resector balloon desobstruction, stage III to IV
1. Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow limitations associated with a chronic inflammatory process in the airways and lung parenchyma. In many COPD patients, the pathological hallmark is inflammation of the small airways (bronchiolitis). Increased volume of tissue in the small airway walls, epithelial proliferation, squamous metaplasia, goblet cell hyperplasia, and the accumulation of mucous exudates in the lumen contribute to the narrowing of the lumen of the airways with a 3 to 8 mm diameter by increasing wall thickness.[1–4] The functional consequence of these abnormalities is airflow limitation. Despite optimal pharmacological and rehabilitation therapies, a significant percentage of COPD patients suffer from symptoms of airflow limitations, but interventional therapies are limited.[5–7]
Recently, we reported pathological and functional improvement with the Karakoca resector balloon equipped with a specific curettage/resection function that enabled the removal of the goblet cell layer in 10 severe COPD patients resistant to medical treatments.[8] Herein, we present the results of Karakoca resector balloon dilatation and curettage (DC) in a larger series (n = 188) of stage III to IV COPD cases.
2. Methods
2.1. Patients
Of the 4450 patients with COPD admitted to our clinic between 2012 and 2017, 188 patients who underwent therapeutic DC with Karakoca resector balloon desobstruction based on their diagnosis with Stage III to IV COPD according to the Global Initiative for Obstructive Lung Disease (GOLD) criteria and with predominantly chronic bronchitis findings on respiratory function tests, high resolution thorax CT and quantitative ventilation and perfusion scintigraphy were included in this study (Fig. 1). The patients have been followed up since 2012. Written informed consent from each subject for publishing their medical findings and approval of the institutional ethics committee was obtained.
Figure 1.
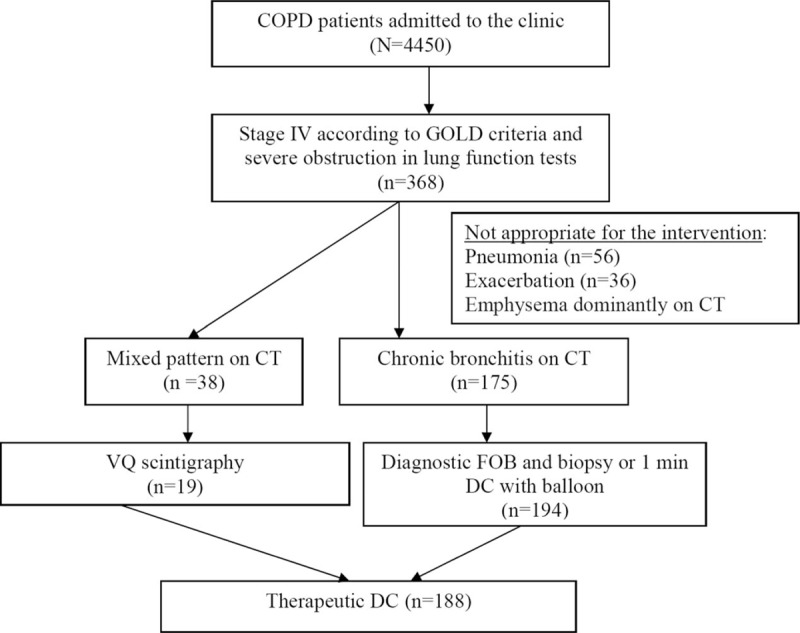
Evaluation of the appropriateness for the Karakoca resector balloon therapeutic dilatation and curettage intervention. COPD = chronic obstructive pulmonary disease, DC = dilatation and curettage, VQ = ventilation/perfusion lung scan.
2.2. Appropriateness of the balloon desobstruction intervention
Patients were considered appropriate for the balloon desobstruction intervention based on baseline characteristics as described previously in our first 10-case series.[8] Briefly, except for one, all patients were diagnosed with stage IV COPD based on the GOLD classification. One patient was considered as having stage III COPD, but his cough and sputum symptoms were not relieved with conventional therapy; he underwent this procedure for the relief of symptoms. All patients had chronic bronchitis findings on high resolution thorax CT. Quantitative ventilation and perfusion scintigraphy were performed for the patient's mixed pattern, and those with emphysema were excluded. They were considered to be appropriate for balloon DC upon preintervention diagnostic fiber-optic bronchoscopy and therapeutic aspiration examination: 166 patients were evaluated with 1 min balloon DC, while in 22 patients bronchoscopic biopsy for goblet cell hyperplasia was performed.
2.3. Intervention
The patients were examined by a cardiologist and an anesthesiologist before the intervention. The intervention was performed under general anesthesia using a flexible therapeutic bronchoscope (Pentax model BF-XT, Tokyo, Japan, channel diameter was 3.2 mm and Olympus ultrathin bronchoscope, Tokyo, Japan, channel diameter was 2.0 mm).
2.4. Karakoca resector balloon DC
Karakoca resector balloon equipment developed for cancer patients by Y.K. was first introduced to the medical community in the 16th World Bronchology Congress in Tokyo, Japan. It is manufactured in Turkey. It is a single-use, sterilized product available in 6 different dimensions (40∼25, 50∼30, 10∼10, 10∼15, 15∼20, and 15∼30 mm). The design of the balloon enables an intervention at the tracheal level and the small bronchi as considered by the expert performing bronchoscopy. For a single treatment session, an average of 2 to 3 balloons is needed. The Karakoca resection balloon equipment consists of a nonlatex balloon mounted onto the distal end of a double lumen polyethylene tube with a length of 120 cm and outer diameter of 2.6 or 1.6 mm. The balloon length varied between 10 and 50 mm with a maximum inflated diameter of 10 to 25 mm. A mesh structure of 0.2 mm thick polyurethane/Lycra (ENBIO MEDICAL, Istanbul, Turkey) fibers covered the balloon. The minimum diameter of the balloon was 1.7 mm together with the mesh structure when deflated.
During the intervention, the first step was deep therapeutic aspiration with 3.2 and 2.0 mm channel bronchoscopes. The DC was then started with fiber-optic bronchoscopy with the 3.2 mm channel from the 1/2 distal of the trachea toward the segmental and subsegmental bronchi. The balloon is inserted into the bronchial lumen so that the mucosal obstruction covers it, and it is repeatedly inflated and deflated until the lumen patency is established by an electronic pump in a regular pulse mode. The force applied directly to the bronchial mucosa via the balloon is approximately 2.5 to 2.0 bar, compressing the hyperplasic goblet cells.
The larger balloon used at the trachea and main and lobar bronchi provides 2.5 to 2.0 bar pressure, and a medium sized balloon used in segmental and subsegmental bronchi provides 2 to 1.5 bar pressure. For the 4 to 5 to 6 subsegmental bronchi, a 2.0-mm channel bronchoscope and the smallest balloon providing 1.5 to 1.0 bar pressure were used.
Although the balloon pressure that thins the mucosal hyperplasia does not cause any lacerations or bleeding, the egested mucous material flowing into the bronchial lumen is aspirated to provide complete patency of the obstructed airways. Therapeutic aspiration is repeated after DC with 3.2 and 2.0 mm channel bronchoscopes.
Based on the recommended operating time decided by the preoperative cardiac evaluation, the number of treated segmental bronchi that could be accessed over 60 min ranged from 100 to 300 during the desobstruction in our cases, while it was 60 to 90 in our first 10 cases.[8] The procedure was performed using 3.2 and 2.0-mm inner channel bronchoscopes that provided access to 8 to 3-mm bronchi, those with most extensive goblet cell accumulation, along with the use of resector balloon maneuvers in 3-mm bronchi, which could be reached by a 4.1 mm outer diameter bronchoscope. Segmental and subsegmental bronchi were viewed during fiberoptic bronchoscopy, whereas therapeutic aspiration applied to patients with a mucous layer lining the entire mucosa and bronchoscopic biopsies were taken from the right inferior posterior segment to compare preoperative and postoperative pathologic findings based on the goblet cells and mucus accumulation.
2.5. Follow-up measurements
Lung function tests and clinical findings were recorded before and 1 week and 1 month after the intervention. Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and forced expiratory flow at 25% to 75% (FEF 25–75) were measured by a spirometer (Zan GPI 3.00, nSpire Health, Longmont, CO) and oxygen saturation (SpO2) was measured by a pulse oximeter (Mindray, China). Difficulty in breathing is evaluated by a modified Borg dyspnea scale, which starts at 0, where breathing causes the patient no difficulty at all, and progresses through to 10, where breathing difficulty is maximal. Aerobic capacity/endurance of the patients were tested by 6-min walking test (6MWT).
2.6. Statistical analysis
Statistical analyses were performed using the IBM Statistical Package of Social Sciences for Windows, Version 20.0 (IBM Corp., Armonk, NY). Numeric variables are presented as the mean ± SD or median (minimum–maximum). The Wilcoxon signed-rank test was used to compare pre and postoperative parameters. P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Baseline characteristics
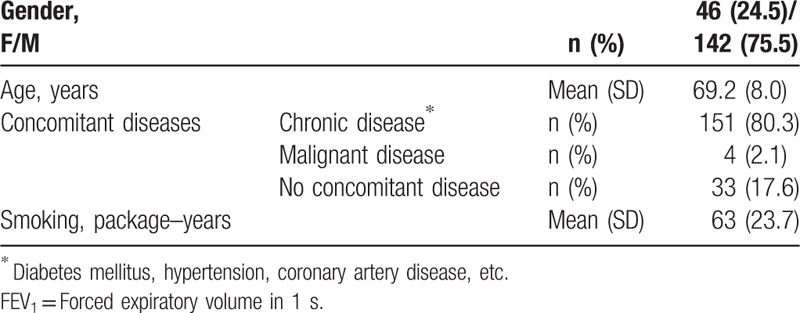
The mean (±SD) age of the 188 patients presented in this report was 69.2 (±8.0) years; 75.5% were males; all were active smokers and 80% had concomitant chronic diseases (Table 1).
Table 1.
Baseline characteristics of the patients considered appropriate for Karakoca resector balloon desobstruction (n = 188).
3.2. Follow-up outcomes
Medical therapy in all patients included high-dose bronchodilator, regular oxygen, and oral corticosteroid therapy because of frequent exacerbation history. One hundred five patients were on noninvasive mechanical ventilation therapy, 4 patients were tracheotomized and under home ventilator support, and 4 patients were under mechanical ventilator support for exacerbations.
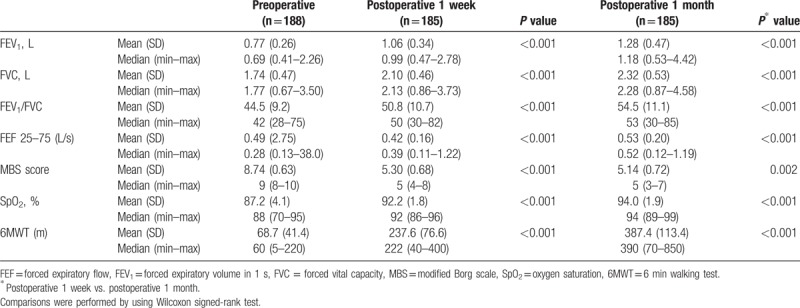
After the intervention, there was a notable reduction in the oxygen need of the patients, while significant improvements were observed in 6MWT results (Table 2). Comparison of lung function tests 1 week after the procedure with results before the procedure showed significant improvements in FEV1, FVC, FEV1/FVC, FEF 25–75 modified Borg dyspnea scale scores, and SpO2 levels (P < 0.001 for each), and the improvements were maintained for the entire postprocedural month (P < 0.001 for each) (Table 2).
Table 2.
Respiratory parameters prior to and 1 week and 1 month after the operation (n = 188).
None of the patients developed intraoperative, perioperative, and postoperative complications since 2012.
Tracheostomy was closed in 2 of the 4 patients; the other 2 tracheotomized patients died due to septicemia in 1 month. Routine inhaler and nebulizer treatments were maintained along with no need for oral corticosteroid treatment.
In 4 patients who were in the ICU due to respiratory failure before the operation, invasive and noninvasive mechanical ventilation support was discontinued and 3 of them were stabilized with no history of exacerbations in the past year; and 1 patient died of heart attack at 1 month after the operation. In another patient with a mixed-emphysema pattern on the ventilation/perfusion lung scan (VQ scintigraphy), symptom relief could not be achieved and the FEV1 level did not increase. Except for 4 males, all patients were free of symptoms.
Exacerbation frequency was also markedly improved in the early postoperative period. No exacerbations were observed during the postoperative 3 months of follow-up. For the 5-year follow-up, 1 patient had an acute COPD attack in 2 years, and 2 patients had an acute attack in 1 year.
3.3. Survival
Of the 188 patients who underwent the procedure, 5 were intubated at the ICU. Three of them could not be discharged of the ICU, and died after 2 months. A total of 4 treated patients (2 males, 2 females), died due to acute exacerbation at the ICU (6, 12, 22, and 35 months after the treatment). All remaining 185 patients survived and are under routine follow-up. Of the 165 patients admitted to our clinic, but had not undergone this procedure, 86 died after 1 year, 122 in 2 years, and 137 in 5 years (unpublished observations).
3.4. Hospital and ICU stay
The median hospitalization time of the 183 outpatient patients was 2 (1–4) days.
Two out of 5 patients who were already at the ICU were extubated immediately after the operation and transferred to the normal ward, and discharged from the hospital in 2 days. The other 3 ICU patients died of sepsis and multiorgan failure after 2 months of ICU stay.
4. Discussion
Despite optimal pharmacological therapy and pulmonary rehabilitation, patients with COPD remain significantly disabled. Although various interventional endoscopic techniques of lung volume reduction have been developed for COPD, proper patient selection is required.[5–7] A significant percentage of COPD patients have airway inflammation. Bronchitis and bronchiolitis prominently contribute to airflow limitation not only by narrowing and obliterating the airway lumen but also by actively constricting the airways.[9,10] Small airway disease and parenchymal destruction are the main structural abnormalities; low FEV1 values have been associated with an enlargement of bronchial smooth muscle and the accumulation of mucous exudates in the lumen of the small airways.[10–13]
In a recent pilot study, we reported that DC with the Karakoca resector balloon provided specific curettage/resection of the goblet cell layer, thus increasing SpO2 and FEV1 in 10 patients.[8] In the present study, we focused on lung functions, quality of life, and exercise capacity. We treated 188 stage III/IV COPD patients with chronic bronchitis and mixed COPD. Our results show improvements not only in SpO2 levels and lung function tests but also in effort capacity accompanied by the rapid relief of symptoms. The only unsuccessful result was that 3 of the ICU patients died in 2 months due to septicemia and heart attack. Except for these patients, we achieved symptom relief and improved lung function in all patients. Increase in FEV1 was found to be significant at 1 week and 1 month after the operation; thus, exercise capacity and quality of life was significantly improved. Two of the tracheotomized patients and 3 of the ICU patients were relieved of symptoms, and mechanical ventilation support was discontinued in the postoperative period. None of the patients developed complications within the postoperative 1 to 3 months of follow-up. We believe that appropriate selection of patients (patients with bronchitis and mixed COPD) for this treatment yielded our successful outcomes.[8] The only patient who did not achieve symptom relief had a concomitant condition (i.e., emphysema pattern).
Exacerbation frequency was also markedly improved in the early postoperative period.
Given the recommended operating time determined by the preoperative cardiac evaluation, 100 to 300 segmental and subsegmental bronchi could be reached during the desobstruction in our case series. Nonetheless, it should be noted that the number of accessed segments within the operating time will increase with experience.
In conclusion, we achieved successful results in our 188 patients with stage III/IV chronic bronchitis and mixed COPD treated with Karakoca balloon desobstruction, which is a safe and repeatable method. However, multicenter, larger scale, controlled studies are needed to provide further evidence of the efficacy and safety of the intervention.
Author contributions
Conceptualization: Yalcin Karakoca, Guler Gogus, Seha Akduman, Baykal Erturk.
Data curation: Yalcin Karakoca, Guler Gogus, Seha Akduman, Baykal Erturk.
Investigation: Yalcin Karakoca, Guler Gogus, Seha Akduman, Baykal Erturk.
Methodology: Yalcin Karakoca.
Supervision: Yalcin Karakoca.
Writing – original draft: Yalcin Karakoca.
Writing – review & editing: Yalcin Karakoca, Guler Gogus, Seha Akduman, Baykal Erturk.
Footnotes
Abbreviations: COPD = chronic obstructive pulmonary disease, DC = dilatation and curettage, FEF = forced expiratory flow, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity, GOLD = Global Initiative for Obstructive Lung Disease, MBS = modified Borg scale, SpO2 = oxygen saturation, VQ = ventilation/perfusion lung scan.
The authors have no conflicts of interest to disclose.
The authors have no funding.
References
- [1].Piqueras MG, Cosio MG. Disease of the airways in chronic obstructive pulmonary disease. Eur Respir J Suppl 2001;34:S41–9. [DOI] [PubMed] [Google Scholar]
- [2].Niewoeher DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med 1974;291:755–8. [DOI] [PubMed] [Google Scholar]
- [3].Berend N, Wright JL, Thurlberck WM, et al. Small airways disease: reproducibility of measurements and correlation with lung function. Chest 1981;79:263–8. [DOI] [PubMed] [Google Scholar]
- [4].McDonough JE, Yuan R, Suzuki M, et al. Small airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011;365:1567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Spruit MA, Chavannes NH, Herth FJ, et al. Clinical highlights from the 2011 ERS congress in Amsterdam. Eur Respir J 2012;39:1501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hopkinson NS, Kemp SV, Toma TP, et al. Atelectasis and survival after bronchoscopic lung volume reduction for COPD. Eur Respir J 2011;37:1346–51. [DOI] [PubMed] [Google Scholar]
- [7].Gasparini S, Zuccatosta L, Bonifazi M, et al. Bronchoscopic treatment of emphysema: state of the art. Respiration 2012;84:250–63. [DOI] [PubMed] [Google Scholar]
- [8].Karakoca Y, Gogus Karaagac G, Yapicier O. Use of resector balloon desobstruction a patient with chronic bronchitis: a feasibility study on a novel desobstruction technique. J Bronchol Intervent Pulmonol 2015;22:209–14. [DOI] [PubMed] [Google Scholar]
- [9].Hogg C, Chu F, Utokaparch S, et al. The nature of small airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–53. [DOI] [PubMed] [Google Scholar]
- [10].Caramori G, Di Gregorio C, Carlstedt I, et al. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology 2004;45:477–84. [DOI] [PubMed] [Google Scholar]
- [11].Saetta M, Turato G, Baraldo S, et al. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med 2000;161(Pt 1):1016–21. [DOI] [PubMed] [Google Scholar]
- [12].Araya J, Cambier S, Markovics JA, et al. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest 2007;117:3551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Martin C, Frija J, Burgel PR. Dysfunctional lung anatomy and small airways degeneration in COPD. Int J Chron Obstruct Pulmon Dis 2013;8:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


