Sixty years ago, Mogens Westergaard published a review analyzing genetic studies from a range of flowering plants. Westergaard provided strong genetic evidence that two separate factors are involved in sex...
Abstract
A long-standing question in biology concerns the genetic mechanisms by which two sexes can evolve (botanists call this the dioecious condition and zoologists call it gonochory) from a functionally ancestral hermaphroditic state (without separate sexes). In 1932, H. J. Muller, one of the great 20th century geneticists but also a fine evolutionary biologist, pointed out that two mutations were necessary. It was therefore puzzling that sex determination often involves a single genetic locus. Muller believed that the evolution of a single-gene system was possible, because maize geneticists had synthesized a single-gene system with separate sexes. However, this system is highly artificial, requiring geneticists to actively eliminate the wild-type allele at one of the two genes involved. This genetic system cannot therefore explain the natural evolution of dioecy. In 1958, Westergaard reviewed studies from a diversity of flowering plants, and showed that the genetics of natural sex determination in plants does not support the maize system. Instead, the genetic results pointed to a model involving two separate factors, with close linkage creating a single genetic locus. Moreover, Westergaard also pointed out that a two-gene model offers a natural explanation for the evolution of suppressed recombination between sex chromosome pairs. Studying plants allowed genetic analyses of the early steps in the evolution of dioecy, using dioecious species that evolved recently from species without separate sexes, whereas Muller failed to fully understand such evolutionary changes because he focused on animals, where later changes have often happened and obscured the early stages.
THIS year is the 60th anniversary of a paper by the Danish geneticist Mogens Westergaard that greatly contributed to understanding the genetics of sex determination in flowering plants, and thus to understanding the evolution of separate sexes more generally (Westergaard 1958). Although this paper was not published in GENETICS and appears to have been cited only four times in GENETICS papers, it is worth recalling in this journal. Perhaps its relative neglect in GENETICS papers reflects its focus on plants, which are often overlooked in papers about sex determination, or perhaps its publication as a review paper, in Advances In Genetics, obscured its important contributions. Westergaard’s review established a new hypothesis that is still important, as explained below.
Now that genome sequencing is opening up the possibility of studying sex determination in nonmodel species, more “cross-fertilization” between studies in animals and plants can be expected, and this somewhat neglected paper deserves to be better known. According to the Web of Science on May 2nd 2018, it has been cited 445 times, but accessed only 41 times since 2013. Yet the paper wonderfully illustrates how much can be understood using classical genetics reasoning, especially combined with cytological data. It also illustrates the breadth of interests at the Carlsberg Laboratory in Copenhagen, which produced several important figures in the history of genetics, and in which Westergaard trained. The development of the lab’s genetical work was greatly advanced with the appointment as director of Øjvind Winge, the “father of yeast genetics” (Szybalski 2001), who also had much broader interests. When the first International Congress on Human Genetics was held in Copenhagen in 1956, Winge served on the organizing committee.
Westergaard was a student with Winge, and obtained his Master’s degree in genetics in 1936. World War 2 interrupted his career, and he was active in the resistance against the Nazi German occupation and interned in a Danish-administered Polizeigefangenenlager, or police prison camp (Zickler 1977). He then worked with Herschel Mitchell, during a Rockefeller Fellowship at CalTech, and developed a widely used medium for Neurospora (Westergaard and Mitchell 1947). However, he was later refused a visa to attend the 1951 Cold Spring Harbor meeting on mutation as an invited speaker (see http://www.nytimes.com/1951/03/06/archives/danish-scientist-barred-as-exred-westergaard-says-us-will-not-let.html). As the Danish Communist party was a leading group resisting the occupation (while other major parties cooperated with the Germans), it was presumably these activities that led to this refusal, although by 1951 Westergaard had denounced Soviet communist promotion of Michurin’s genetical ideas. Protest against this visa refusal was led by Professor L. C. Dunn (who also actively worked to expose the perversion of genetics known as Lysenkoism, which advocated an important role for the inheritance of acquired characters); Dunn was supported by the bacterial geneticist M. Demerec (who had also worked on maize and Drosophila) and by the US secretary of state, and Westergaard did attend the meeting. He was coauthor of a paper about chemically induced mutations in Neurospora, which suggested a distinction between mutagens that cause mainly chromosome mutations, and ones that cause mutations within genes and can potentially be reversed by back mutation (Jensen et al. 1951). He appears among the many very distinguished participants photographed at the meeting, alongside Charlotte Auerbach; this was 6 years after she discovered the mutagenic effects of mustard gas. Westergaard returned to the US for the 1958 meeting on recombination, and he was elected a foreign associate of the National Academy of Sciences in 1972.
He was the University of Copenhagen’s first professor of genetics, in the Faculty of Science where Johannsen, then Professor of Plant Physiology, had performed his famous work showing that characters acquired during an organism’s life were not passed on to its progeny (Johannsen 1909). Westergaard built up the University’s Genetics Institute, continuing research on Neurospora genetics alongside his work on plants. In 1953, he wrote a textbook highlighting the importance of genetics for many areas of biology. He also worked to reform teaching in Danish universities, resulting in an 88-page book with the delightful English title On slaughter of sacred cows (Westergaard 1965), and popular articles emphasizing the duty of society to support and promote basic research.
Discovery of a Male-Determining Factor in the Plant Genus Silene, and Other Y-Linked Genes Affecting Gender Development
Westergaard’s work on plants with separate sexes (dioecious plants) began with experiments to determine the genetic control of gender in two plant species, the white and red campion, in the genus Silene (then called Melandrium). Like Drosophila, these plants have a cytologically visible male-specific chromosome, a Y chromosome. In 1916, Bridges had shown that the Drosophila melanogaster Y chromosome has no sex-determining function and carries only genes essential for male fertility: XO males appear normal, though they are sterile, while XXY individuals are morphologically normal females [reviewed by Ganetzky and Hawley (2016)]. Westergaard followed Bridges’ approach of experimentally changing chromosome numbers and showed that, unlike Drosophila, the Y chromosome of the plants he studied carries a strong male-determining factor or factors, and that even XXXY individuals develop as males (Westergaard 1940). This discovery in a plant came long before the human Y was known to carry a male-determining factor, in 1959 (Goodfellow and Darling 1988).
In three later cytogenetic papers (Westergaard 1946, 1948a,b), he studied deletion mutants, which showed that these plants’ Y carries a factor suppressing female development, whose loss leads to hermaphrodite flowers, and also a factor or factors promoting male functions (whose deletion leads to sterile flowers). It was probably these results that led Westergaard to doubt the prevailing single-gene model and review other work on plant sex determination. That survey, in turn, led him to conclude that, in flowering plants where genetic results were available, at least two genes are involved in the evolution of separate sexes, but that the genes are closely linked and behave as a single sex-determining locus. This two-gene hypothesis was first explicitly proposed in 1953 by both Westergaard (1953) and by the papaya geneticist William Benson Storey, who observed that hermaphroditism in papaya segregates as a third allele at the locus that controls male vs. female gender (Storey 1953). Storey concluded “that sex in papaya is determined not by a single gene but rather by a complex of genes,” and even suggested that a whole set of sexually dimorphic traits might be controlled by the same nonrecombining region.
The 1958 Review Paper
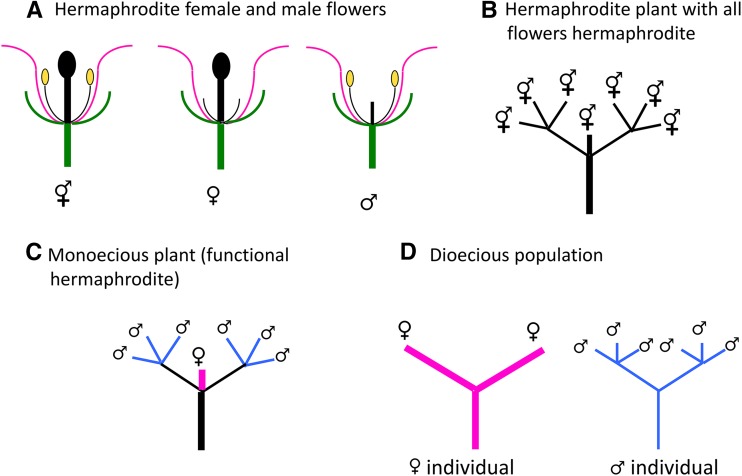
Flowering plants are particularly suitable for studying how separate sexes first evolve, because many species with separate, and genetically controlled, unisexual male and female individuals (the dioecious state) have close relatives that have no such genetic gender polymorphism. Separate sexes have evolved from hermaphroditism in other taxa, such as the Schistosomes within the Trematoda (Grossman et al. 1981; Platt and Brooks 1997), but relationships are mostly much less close. The relatives of dioecious plants either have hermaphrodite flowers (Figure 1, A and B) or are monoecious (a botanical term for hermaphrodite individuals that have separate male and female flowers distributed according to specific developmental patterns, such as maize; see Figure 1C). Among other evidence about the genetics of gender in plants, Westergaard analyzed data on the segregation of sex morphs in families derived from intercrosses where only one parent is unisexual.
Figure 1.
Hermaphrodite, female, and male flowers (A), and sex determination in hermaphrodite (B), monoecious (C), and dioecious (D) plants. In hermaphrodite plants, the plant body (B) has no gender (colored black), and structures with male and female functions develop in each flower (A). In monoecious plants (C), sex is also determined late in development, when individual flowers develop as male (thin blue stems) or female (thick pink stems) on a plant body that has no gender (black lines). In a diploid dioecious plant (D), sex is determined by genotype at fertilization, and expressed during the development of the inflorescences, under control by a genetic sexual polymorphism, sometimes involving sex chromosomes. Individual plants have different genders, and sometimes morphologically distinct sex chromosomes have evolved.
A surprising quantity of informative genetic data were available by 1958, partly because several important crops are dioecious and therefore of interest to geneticists. Westergaard’s review includes results from several crops in which female plants are the valued sex, including hops (Humulus lupulus and H. japonica) and Cannabis sativa, both with sex chromosome heteromorphism, and from species with no such heteromorphism, including asparagus, where male shoots are the crop, and spinach and Populus species where both sexes have value; other important crop plants without chromosomal heteromorphism included strawberries (Fragaria elatior), grapes (Vitis vinifera), Actinidia species (now known as kiwi fruit), mulberries, and papaya (Carica papaya). He also reviewed data from noncrop plants, including species in the genera Rumex and Silene (which both include some species with pronounced XY chromosome heteromorphism), as well as many species where no sex chromosome heteromorphism was detectable (including stinging nettles, Urtica dioica).
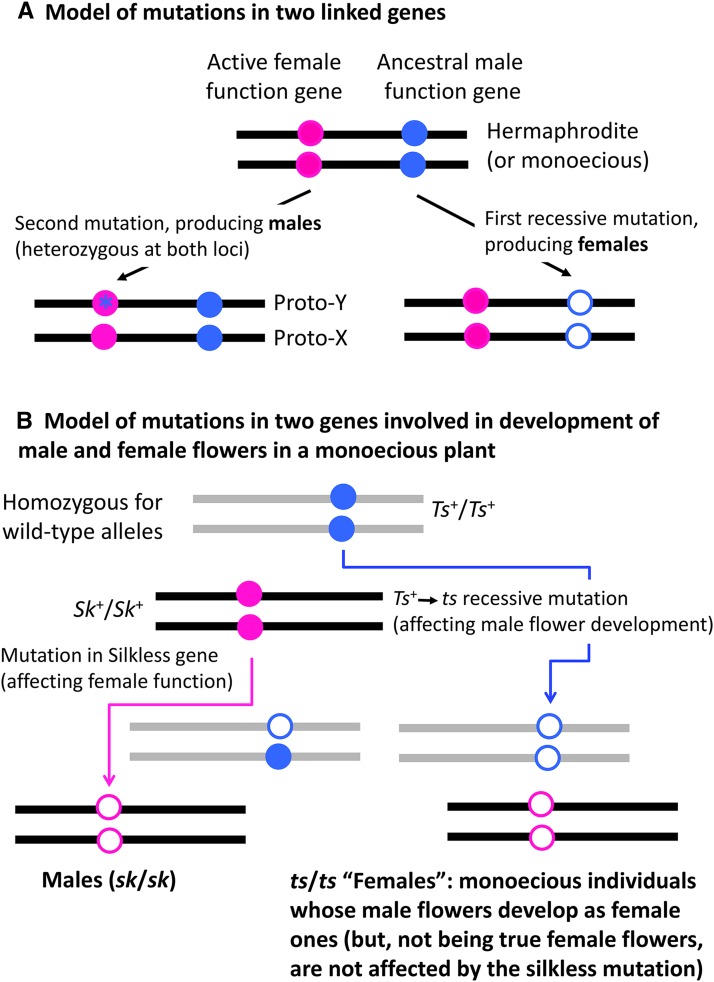
Lack of chromosomal heteromorphism might suggest single-gene control of gender in recently evolved and other nonheteromorphic systems (because heteromorphism suggests the presence of a nonrecombining region, but, with just a single gene, there is no selection against recombination). However, single-gene systems are puzzling, because, as Hermann Muller understood in his important review of the evolution of sex and sex determination (Muller 1932), two mutations must be involved in the initial evolution of two separate sexes (although, unusually for this geneticist, his ideas were not entirely clear, and he discussed both “sex-deciding” or “trigger” primary sex determination and the genes controlling the development of secondary sex differences, particularly a then current single-gene sex-determining model proposed by Richard Goldschmidt for the moth Lymantria, with factors of different strengths affecting gender development). Figure 2A illustrates the requirement for at least two mutations; the figure shows a situation where two separate, but linked, genes are involved and can recombine. Muller emphasized a different two-mutation model (Figure 2B) that had been used to create artificially dioecious strains of maize with single-gene control of gender, by combining two flower mutants (Emerson 1932; Jones 1932, 1934; Dellaporta and Urrea 1994). Females are produced by the recessive ts allele of the Tassel-seed gene, which changes male flowers of this monoecious plant into female ones, allowing some female function. Heterozygotes for the wild-type allele (Ts+ in Figure 2B) remain monoecious, but can be converted into males by making the plants homozygous for a second mutation, a recessive mutation (sk) of the silkless gene, and eliminating the wild-type allele (Sk+ in Figure 2B). In a homozygous sk/sk population, all plants are developmentally male because the sk allele sterilizes female flowers of monoecious individuals carry the Ts+ allele, while the ts/ts homozygotes are males whose female flowers are actually modified male ones; sk can be made homozygous because it affects only developmentally female flowers, and not these modified male flowers. Thus, a synthetic dioecious population was produced in which gender is controlled only by segregation of the Ts+ vs. ts alleles (a situation resembling female heterogamety can also be synthesized using different mutations). Muller suggested that flower gender-specific action like that of the sk allele is essential for the evolution of a sex-determining system, producing a single-gene system like those he knew about, despite the involvement of two mutations (Muller 1932).
Figure 2.
Two models in which two sex-determining mutations produce males and females (open circles indicate loss-of-function mutations). (A) Mutations in two linked genes on a single chromosome (indicated by the black horizontal line). A putative functionally hermaphrodite (hermaphrodite or monoecious) ancestor is shown at the top, with dots indicating functional genes involved in flower development. Females arise by a loss-of-function mutation in the right-hand gene, and the disc with a star in the left-hand gene indicates a dominant mutation that enhances male functions at the expense of female ones. Genetic polymorphism for the two sexes in the resulting dioecious population is controlled by segregation of the alleles at both the genes. If the genes are linked, they define a proto-sex chromosome pair, though the genes may still recombine. (B) The maize trigger with two mutations (on nonhomologous chromosomes denoted by black and gray lines). The first mutation changes male flowers into female ones, creating female individuals, and the second mutation is expressed only in individuals not homozygous for that mutation, and is not polymorphic in the derived dioecious population; gender is therefore controlled by a single segregating gene.
Westergaard calls this the “maize trigger.” It predicts that dioecious plants should differ from their nondioecious ancestors by only a single sex-linked gene, plus a fixed unlinked recessive factor (sk in Figure 2B). However, Westergaard emphasized that several plants, including papaya (as mentioned above) and Ecballium (see next section) display three segregating allelic types at their sex-determining locus, directly implying two genetic differences, even though they cosegregate. Figure 2A shows Westergaard’s own model, with at least two mutations involved in the evolution from hermaphroditism to separate sexes. After dioecy has evolved, two haplotypes control female and male genders, and the ancestral state is a third allelic type (at the top of the diagram) similar to that determining maleness, but lacking the male-promoting mutation; this model also predicts that maleness will be dominant to hermaphroditism, and femaleness recessive. The two models should be distinguishable using genetic data.
Genetic Data and Deletion Phenotypes Support the Two-Gene Model
Westergaard reviewed multiple kinds of genetic studies, showing that the sex ratios were as expected under his hypothesis. The most important observations were segregation data obtained by Fernando Galán, despite his work being impeded by the Spanish civil war (Pinar 2002). He crossed plants from dioecious and monoecious “types” of the genus Ecballium (Galán 1950, 1951); no sex chromosome heteromorphism has been detected in the dioecious type. Westergaard noted that when males of the dioecious type are the pollen parent, some males are always produced, and that the same was also true in interspecies hermaphrodite × male crosses from other taxa. These findings show that males must carry a dominant Y-linked maleness factor, as he had previously shown in his work with Silene. However, the remaining progeny are not always female; depending on the species studied, they can be male or hermaphrodite. Different possible explanations for such findings cannot be distinguished in the F1, because they might simply represent “incompatibilities” between the two species’ developmental systems.
In Ecballium, however, the F1 hybrids are fertile, allowing backcrosses and other informative crosses. These showed that the system involves three alleles (which we would now call haplotypes). However, Galán did not understand this implication of his results. J. B. S. Haldane, who visited Galán in Spain (Haldane 1937), reports that he interpreted them according to a form of Goldschmidt’s single-locus model (see above). Westergaard’s interpretation (which he called the “Melandrium model”) was simpler, as shown in Figure 2A. He also argued that, although in principle three alleles could arise through two different mutations in the same gene, this is unlikely to be the true situation. Instead, he suggested that two distinct genes will generally be involved, which cosegregate because they are closely linked. The Y would thus carry a dominant factor suppressing female functions, as well as a separate dominant male function gene that is absent or nonfunctional in females. Westergaard also showed that, unlike this simple model, the maize trigger (Figure 2B) cannot explain the sex segregations observed in crosses between dioecious and nondioecious populations, as neither sex has a mutation that is dominant to the ancestral state (Figure 2B).
A further test is possible: if two or more separate genes are indeed involved in sex determination they should be separable. As mentioned above, Westergaard’s own cytogenetic work in Silene had shown that deletions can indeed cause loss of either a Y-linked female-suppressing factor (leading to plants with functional hermaphrodite flowers) or of factor(s) that promote male functions. Importantly, his results from deletions showed that different regions of the Y chromosome carry factors with these differing functions. Distinct factors must therefore be involved. This has been confirmed in the white campion, S. latifolia, by studying flower phenotypes in deletions defined using molecular markers rather than cytologically [reviewed in Kazama et al. (2016)].
From Two-Gene Sex Determination to Sex Chromosomes
Westergaard’s hypothesis is particularly interesting because it proposes a stage with a two-locus polymorphism during evolution from hermaphroditism to a separate sexed state, with males differing from females by two distinct mutations (Figure 2A). Moreover, the two mutations can each spread in natural populations under plausible conditions (Charlesworth and Charlesworth 1978), and linkage between them is predicted if the mutation promoting male functions impairs female functions (making it advantageous to reduce the frequency with which females inherit the allele).
The first mutation generates females by a loss-of-function mutation leading to male sterility. A second mutation, generating males (indicated in Figure 2A by an asterisk in a gene affecting female functions), must be good for males (otherwise it would be eliminated from the population), and an obvious advantage is that it gains by abolishing or reducing the ancestral hermaphrodite’s female function. However, this would be detrimental for females (such mutations are termed “sexually antagonistic”), which could inherit it through crossing over between the evolving “proto-Y” and “proto-X” chromosomes (Figure 2A). This situation generates selection against recombinants (some of which would be female genotypes with the dominant male-promoting/femaleness-suppressing allele; recombinant males carrying the male-sterility allele could also arise, reducing male fitness unless this mutation is fully recessive). Westergaard’s model therefore predicts the evolution of suppressed recombination between evolving sex chromosomes (Charlesworth and Charlesworth 1978; Bull 1983), which can readily account for sex chromosome heteromorphism if separate sexes evolved long enough ago for recombination to have changed.
Species with no heteromorphism might then generally be younger systems. Westergaard analyzed published genetic ratios and showed that homozygotes for the Y-linked regions are viable in some plants, consistent with their sex-linked regions having evolved too recently to have undergone the major genetic degeneration that is known for several animal sex chromosome systems [reviewed in Bachtrog (2012)]. In other plants, including the white campion and papaya, the ratios indicate that this genotype is inviable. In the white campion, some X-linked genes are either missing from the Y-linked region or represented by Y-linked copies that appear to be nonfunctional, as expected if degeneration is ongoing in this plant (e.g., Papadopulos et al. 2015).
Different Ages of Different Systems?
Westergaard’s review of the plant data suggested that the difference between plants, often with two-gene sex-determination systems, and animals with single-gene systems simply represents different ages of their sex-determining systems. Plants may often have evolved separate sexes recently, with later changes masking the initial system. Muller was already aware in 1932 that changes may occur after the initial establishment of a sex-determining system, and that sex determination might work differently in different organisms. An astonishing variety of sex-determining systems has now been uncovered (Beukeboom and Perrin 2014). Westergaard reviewed the evidence for changes even among flowering plants. For example, one species of sorrel, Rumex acetosa, has an X-autosome balance system (Smith 1955) resembling the initially controversial hypothesis for Drosophila (Muller 1932).
Modern tools can allow the times of evolutionary transitions to be estimated, including the evolution of separate sexes within taxonomic groups and the times when sex-linked regions first evolved. It should therefore soon become clear whether Westergaard was right in suspecting that the X-autosome balance systems evolved from earlier-established XY systems (the genus Rumex also includes XY species). His suggestion that the viability of Y chromosome homozygotes in species with nonheteromorphic XY chromosome pairs could be because they often evolved recently and have not had enough time to degenerate is also potentially testable.
In animals, new genes have repeatedly taken control of sex determination (Wilkins 1995). Single-gene control could therefore probably evolve from ancestrally two-gene systems though similar “take-overs.” The persimmon, in which a single-gene system was recently discovered (Akagi et al. 2014), may represent such cases. Alternatively, genetic interactions may have been involved, such that one of the mutations involved became fixed in the species, so that it is no longer detectable by genetic approaches; an evolutionary model without the problems explained above for the maize trigger can account for the persimmon system (Charlesworth 2018).
Plant and Animal Systems and the Value of a Broad Perspective
Westergaard’s review illustrates how, even today, classical genetic results may yield the clearest understanding. The conclusion that separate genes are involved in controlling male and female functions could not have been inferred by genome sequencing, because the nonrecombining sex-determining regions are inherited as a block. If such a block includes a large number of genes, as in the white campion (e.g., Papadopulos et al. 2015), it becomes very difficult to identify the functionally relevant genes. Genetic studies in species with very recently evolved sex-determining regions, which have not yet evolved complete linkage between the different factors involved in determining gender (see Figure 2A), can be separable by recombination, and this has been found in a strawberry species (Spigler et al. 2008). However, in this situation it is again difficult to identify the sex-determining genome region, though this is becoming possible using DNA sequencing to examine large numbers of molecular markers [e.g., the single-amino acid variant that appears to be responsible in the tiger pufferfish, fugu, see Kamiya et al. (2012)]. When a small nonrecombining block exists, it is now becoming possible, using mutations, to infer regions with different functions within the block, as has recently been achieved in asparagus (Harkess et al. 2017).
The remarkably enlightened attitude of the Carlsberg Institute in allowing studies of organisms of no economic value, such as the campions, and fungi other than yeast, illustrates the valuable integration that genetic studies potentially offer. The Institute allowed work on distinctly nonmodel species, including a fish, the guppy (Poecilia reticulata, the genus was then called Lebistes), another system important for research on sex chromosomes, and species other than fungi [which do not have separate sexes and consequently do not have sex chromosomes, though mating-type loci in some fungi have suppressed recombination, and understanding sex chromosome evolution has contributed to understanding these systems, see, for example, Badouin et al. (2015) and Fontanillas et al. (2015)]. Work on different organisms was often done by the same scientist. Winge’s mentor was Johannes Schmidt, a previous director of the Carlsberg Institute, who was a botanist by training but also described the first case of Y-linked inheritance in the guppy. A paper in 1922 (Winge 1922) initiated Winge’s independent work on guppies, which outshone Schmidt’s contributions, and continued until 1947 alongside his studies of sex chromosomes in two plant genera, Humulus (hops) and Silene (Winge 1927). Westergaard’s work on sex determination in dioecious plants continued Winge’s research and, as described above, was inspired by work on Drosophila, and doubtless on the guppy. The genetic understanding that flowed from Winge’s and Westergaard’s studies in plants has continuing value, and current work is continuing to integrate concepts that apply in both plant and animal systems.
Footnotes
Communicating editor: A. S. Wilkins
Literature Cited
- Akagi T., Henry I. M., Tao R., Comai L., 2014. A Y-chromosome–encoded small RNA acts as a sex determinant in persimmons. Science 346: 646–650. 10.1126/science.1257225 [DOI] [PubMed] [Google Scholar]
- Bachtrog D., 2012. Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration. Nat. Rev. Genet. 14: 113–124. 10.1038/nrg3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badouin H., Hood M. E., Gouzy J., Aguileta G., Siguenza S., et al. , 2015. Chaos of rearrangements in the mating-type chromosomes of the anther-smut fungus Microbotryum lychnidis-dioicae. Genetics 200: 1275–1284. 10.1534/genetics.115.177709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukeboom L. W., Perrin N., 2014. The Evolution of Sex Determination. Oxford University Press, Oxford: 10.1093/acprof:oso/9780199657148.001.0001 [DOI] [Google Scholar]
- Bull J. J., 1983. Evolution of Sex Determining Mechanisms. Benjamin/Cummings, Menlo Park, CA. [Google Scholar]
- Charlesworth B., Charlesworth D., 1978. A model for the evolution of dioecy and gynodioecy. Am. Nat. 112: 975–997. 10.1086/283342 [DOI] [Google Scholar]
- Charlesworth D., 2018. Does sexual dimorphism in plants promote sex chromosome evolution? Environ. Exp. Bot. 146: 5–12. 10.1016/j.envexpbot.2017.11.005 [DOI] [Google Scholar]
- Dellaporta S. L., Urrea A. C., 1994. The sex determination process in maize. Science 266: 1501–1505. 10.1126/science.7985019 [DOI] [PubMed] [Google Scholar]
- Emerson, S., 1932 The present status of maize genetics, pp. 141–152 in Proceedings of the 6th International Congress of Genetics, edited by D. F. Jones. Genetics Society of America, Austin. [Google Scholar]
- Fontanillas E., Hood M. E., Badouin H., Petit E., Barbe V., et al. , 2015. Degeneration of the nonrecombining regions in the mating-type chromosomes of the anther-smut fungi. Mol. Biol. Evol. 32: 928–943. 10.1093/molbev/msu396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán F., 1950. Analyse génétique de la monoecie et de la dioecie zygotiques et de leur difference dans Ecballium elaterium, pp. 340 in 7th International Botanical Congress. Stockholm [Google Scholar]
- Galán F., 1951. Analyse génétique de la monoecie et de la dioecie zygotiques et de leur difference dans Ecballium elaterium. Acta Salamancticensia, Ciensias. Seccion Biologica 1: 7–15. [Google Scholar]
- Ganetzky B., Hawley R. S., 2016. The centenary of GENETICS: bridges to the future. Genetics 202: 15–23. 10.1534/genetics.115.180182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P., Darling S., 1988. Genetics of sex determination in man and mouse. Development 102: 251–258. [DOI] [PubMed] [Google Scholar]
- Grossman A., Short R., Cain G., 1981. Karyotype evolution and sex-chromosome differentiation in Schistosomes (Trematoda, Schistosomatidae). Chromosoma 84: 413–430. 10.1007/BF00286030 [DOI] [PubMed] [Google Scholar]
- Haldane J. B. S., 1937. Genetics in Madrid. Nature 139: 331 10.1038/139331a0 [DOI] [Google Scholar]
- Harkess A., Zhou J., Xu C., Bowers J. E., Hulst R. V., et al. , 2017. The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat. Commun. 8: 1279 10.1038/s41467-017-01064-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K., Kirk I., Kølmark G., Westergaard M., 1951. Chemically induced mutations in Neurospora. Cold Spring Harb. Symp. Quant. Biol. 16: 245–261. 10.1101/SQB.1951.016.01.020 [DOI] [PubMed] [Google Scholar]
- Johannsen W., 1909. Elemente der exakten Erblichkeitslehre. Fischer, Jena, Germany. [Google Scholar]
- Jones, D. F., 1932 The interaction of specific genes in doecious maize, pp. 104–107 in Proceedings of the 6th International Congress of Genetics, edited by D. F. Jones. Genetics Society of America, Austin. [Google Scholar]
- Jones D. F., 1934. Unisexual maize plants and their bearing on sex differentiation in pother plants and animals. Genetics 19: 552–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T., Kai W., Tasumi S., Oka A., Matsunaga T., et al. , 2012. A Trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (Fugu). PLoS Genet. 8: e1002798 10.1371/journal.pgen.1002798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama Y., Ishii K., Aonuma W., Ikeda T., Kawamoto H., et al. , 2016. A new physical mapping approach refines the sex-determining gene positions on the Silene latifolia Y-chromosome. Sci. Rep. 6: 18917 10.1038/srep18917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H. J., 1918. Genetic variability, twin hybrids and constant hybrids, in a case of balanced lethal factors. Genetics 3: 422–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H. J., 1932. Some genetic aspects of sex. Am. Nat. 66: 118–138. 10.1086/280418 [DOI] [Google Scholar]
- Papadopulos A. S. T., Chester M., Ridout K., Filatov D. A., 2015. Rapid Y degeneration and dosage compensation in plant sex chromosomes. Proc. Natl. Acad. Sci. USA 112: 13021–13026. 10.1073/pnas.1508454112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinar S., 2002. The emergence of modern genetics in Spain and the effects of the Spanish civil war (1936–1939) on Its development. J. Hist. Biol. 35: 111–148. 10.1023/A:1014544520340 [DOI] [PubMed] [Google Scholar]
- Platt T. R., Brooks D. R., 1997. Evolution of the Schistosomes (Digenea: Schistosomatoidea): the origin of dioecy and colonization of the venous system. J. Parasitol. 83: 1035–1044. 10.2307/3284358 [DOI] [PubMed] [Google Scholar]
- Smith B. W., 1955. Sex chromosomes and natural polyploidy in dioecious Rumex. J. Hered. 46: 226–232. 10.1093/oxfordjournals.jhered.a106563 [DOI] [Google Scholar]
- Spigler R., Lewers K., Main D., Ashman T.-L., 2008. Genetic mapping of sex determination in a wild strawberry, Fragaria virginiana, reveals earliest form of sex chromosome. Heredity 101: 507–517. 10.1038/hdy.2008.100 [DOI] [PubMed] [Google Scholar]
- Storey W. B., 1953. Genetics of the papaya. J. Hered. 44: 70–78. 10.1093/oxfordjournals.jhered.a106358 [DOI] [Google Scholar]
- Szybalski W., 2001. My road to Øjvind Winge, the father of yeast genetics. Genetics 158: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard M., 1940. Studies on the cytology and sex determination in polyploid forms of Melandrium album. Dansk Botan. Arkiv. 10: 1–13. [Google Scholar]
- Westergaard M., 1946. Structural changes of the Y-chromosomes in the offspring of polyploid Melandrium. Hereditas 32: 60–64. 10.1111/j.1601-5223.1946.tb02771.x [DOI] [PubMed] [Google Scholar]
- Westergaard M., 1948a Aberrant Y-chromosomes and sex expression in Melandrium album. Hereditas 32: 419–443. 10.1111/j.1601-5223.1946.tb02784.x [DOI] [PubMed] [Google Scholar]
- Westergaard M., 1948b The relation between chromosome constitution and sex in the offspring of triploid Melandrium. Hereditas 34: 257–279. 10.1111/j.1601-5223.1948.tb02841.x [DOI] [Google Scholar]
- Westergaard M., 1953. Uber den mechanismus der geschlechtsbestimmung bei Melandrium album. Naturwissenschaften 40: 253–260. 10.1007/BF00590417 [DOI] [Google Scholar]
- Westergaard M., 1958. The mechanism of sex determination in dioecious plants. Adv. Genet. 9: 217–281. [DOI] [PubMed] [Google Scholar]
- Westergaard M., 1965. Om slagtning af hellige køer. Hans Reitzels Forlag, Copenhagen. [Google Scholar]
- Westergaard M., Mitchell H., 1947. Neurospora-V - a synthetic medium favoring sexual reproduction. Am. J. Bot. 34: 573–577. 10.1002/j.1537-2197.1947.tb13032.x [DOI] [Google Scholar]
- Wilkins A., 1995. Moving up the hierarchy: a hypothesis on the evolution of a genetic sex-determination pathway. BioEssays 17: 71–77. 10.1002/bies.950170113 [DOI] [PubMed] [Google Scholar]
- Winge O., 1922. A peculiar mode of inheritance and its cytological explanation. J. Genet. 12: 137–144. 10.1007/BF02983077 [DOI] [Google Scholar]
- Winge O., 1927. On a Y-linked gene in Melandrium. Hereditas 9: 274–284. 10.1111/j.1601-5223.1927.tb03528.x [DOI] [Google Scholar]
- Zickler D., 1977. Mogens Westergaard: 1912–1975. Mycologia 69: 871–874. [Google Scholar]