Hybrids between new species are often sterile. To understand the causes of this reproductive barrier, Schwahn et al. examined testis histology in a large number of hybrids between two nascent species of house mice...
Keywords: speciation, hybrid sterility, testis histopathology, meiosis defect, stage VII
Abstract
Hybrid sterility is a common form of reproductive isolation between nascent species. Although hybrid sterility is routinely documented and genetically dissected in speciation studies, its developmental basis is rarely examined, especially in generations beyond the F1 generation. To identify phenotypic and genetic determinants of hybrid male sterility from a developmental perspective, we characterized testis histology in 312 F2 hybrids generated by intercrossing inbred strains of Mus musculus domesticus and M. m. musculus, two subspecies of house mice. Hybrids display a range of histologic abnormalities that indicate defective spermatogenesis. Among these abnormalities, we quantified decreased testis size, reductions in spermatocyte and spermatid number, increased apoptosis of meiosis I spermatocytes, and more multinucleated syncytia. Collectively, our phenotypic data point to defects in meiosis I as a primary barrier to reproduction. We identified seven quantitative trait loci (QTL) controlling five histologic traits. A region of chromosome 17 that contains Prdm9, a gene known to confer F1 hybrid male sterility, affects multinucleated syncytia and round spermatids, potentially extending the phenotypic outcomes of this incompatibility. The X chromosome also plays a key role, with loci affecting multinucleated syncytia, apoptosis of round spermatids, and round spermatid numbers. We detected an epistatic interaction between QTL on chromosomes 17 and X for multinucleated syncytia. Our results refine the developmental basis of a key reproductive barrier in a classic model system for speciation genetics.
THE reduced fertility of hybrids constitutes a major barrier to reproduction between species (Coyne and Orr 2004). When such “hybrid sterility” has a genetic component, this barrier can become permanent and is expected to increase in severity over time. Hybrid sterility tends to evolve faster than hybrid inviability (Coyne and Orr 2004), and in species with XY sex determination, males usually evolve sterility before females (Coyne and Orr 1989), suggesting that hybrid male sterility is often the first form of postzygotic isolation to appear. The genetics of hybrid male sterility between nascent species therefore provides a window into the mechanisms of a primary determinant of speciation in the early stages of the process.
The most direct way to evaluate hybrid male sterility is to measure the relative ability of hybrid males to sire offspring through mating experiments. Although this method focuses on the functional trait of interest, reproductive performance, it yields no insight into the phenotypic and developmental causes of sterility. A second strategy examines phenotypes known to be correlated with fertility. This approach has the potential to pinpoint the mechanisms of hybrid dysfunction, but it is rarely extended beyond assessment of a few outcomes of spermatogenesis (e.g., the count, shape, and/or motility of sperm) or gross reproductive morphology (e.g., testis weight). Because a variety of changes in the testis can produce similar phenotypic outcomes, measuring these traits also leaves the primary drivers of hybrid sterility unaddressed.
Ideally, the search for genetic determinants of hybrid male sterility would recognize the dynamic nature of spermatogenesis. By reproductive maturity in some species, a single testis contains mitotic stem cells (spermatogonia), two kinds of meiotic cells (primary and secondary spermatocytes), morphologically differentiating postmeiotic cells (spermatids), and maturing sperm, as well as Sertoli (“nurse”) cells and interstitial (Leydig) cells. Because spermatogenesis is continuous, these diverse cell populations can be surveyed simultaneously in individual males by histologic examination of the testis. The result is a developmental characterization of hybrid male sterility that specifies what stages of spermatogenesis are disrupted in hybrids (Oka et al. 2010). This increased phenotypic resolution has the potential to accelerate both the identification of the genes that determine this key reproductive barrier and the discovery of their functions.
The house mouse, Mus musculus, is a powerful model species for dissecting the genetics of hybrid male sterility. In the house mouse, the temporal and spatial progression of spermatogenesis in the testis is well understood, making it straightforward to identify specific histologic abnormalities in hybrids. Subspecies of house mice diverged from a common ancestor at least 350,000 years ago (Boursot et al. 1993; Duvaux et al. 2011; Geraldes et al. 2011) and display partial reproductive isolation (Forejt and Ivanyi 1974; Alibert et al. 1997; Britton-Davidian et al. 2005; Good et al. 2008b; Turner et al. 2012; White et al. 2012). Therefore, house mice provide insights into hybrid male sterility as it evolves (Wang et al. 2015).
Reproductive isolation between two subspecies of mice, Mus musculus domesticus (the Western European house mouse) and M. m. musculus (the Eastern European house mouse), has been intensively studied. These subspecies meet and mate in a natural hybrid zone that stretches across Europe. Allele frequency clines are narrow relative to subspecies ranges, suggesting that reproductive isolation limits gene flow (Tucker et al. 1992; Sage et al. 1993; Payseur et al. 2004; Teeter et al. 2010; Janoušek et al. 2012). Although there is evidence for assortative mating between M. m. musculus and M. m. domesticus (Smadja et al. 2004; Smadja and Ganem 2005) and fertility reductions in hybrid females (Good et al. 2008b; Suzuki and Nachman 2015), the most consistently documented reproductive barrier is F1 hybrid male sterility (sterility in this context refers to greatly reduced fertility). Crosses involving wild animals, wild-derived inbred strains, and classical inbred strains produce males with reduced fertility (Forejt and Ivanyi 1974; Chubb and Nolan 1987; Yoshiki et al. 1993; Alibert et al. 1997; Britton-Davidian et al. 2005; Vyskočilová et al. 2005; Good et al. 2008b; White et al. 2011). F1 hybrid male sterility is polymorphic within M. m. domesticus and within M. m. musculus (Britton-Davidian et al. 2005; Good et al. 2008b; Larson et al. 2018), an example of the intraspecific variation in intrinsic postzygotic isolation observed in a variety of species pairs [reviewed in Cutter (2012)]. In some crosses, a severe reduction in fertility is only found when the mother is M. m. musculus (Good et al. 2008b).
Much is understood about the genetic basis of F1 hybrid male sterility between M. m. domesticus and M. m. musculus. F1 males from crosses between some strains of M. m. musculus and C57BL/10 (a classical inbred strain of predominantly M. m. domesticus origin) are effectively sterile, whereas F1 males from similar crosses using classical strain C3H as the M. m. domesticus representative are fertile (Forejt 1996). Forejt and colleagues exploited this polymorphism to identify the first known hybrid sterility gene in vertebrates (Forejt and Ivanyi 1974; Forejt et al. 1991; Trachtulec et al. 2005; Mihola et al. 2009). Prdm9 is a histone H3 methyltransferase (Hayashi et al. 2005) that facilitates double-strand DNA break generation by locally modifying chromatin structure (Baudat et al. 2010; Parvanov et al. 2010; Paigen and Petkov 2018). In F1 hybrids between some strains of M. m. musculus and M. m. domesticus, Prdm9 alleles contribute to meiotic arrest in primary spermatocytes (Mihola et al. 2009). The arrest is caused by incompatibility between the Prdm9 heterozygous genotype, a M. m. musculus allele on the X chromosome (at the Hstx2 locus), and multiple additional autosomal loci (Dzur-Gejdošova et al. 2012). The overall role of the X chromosome in hybrid male sterility is complex, with contributions from multiple segments of the chromosome (Storchová et al. 2004; Good et al. 2008a), incompatibility with the Y chromosome in addition to the autosomes (Campbell and Nachman 2014), and large numbers of overexpressed genes in sterile F1 hybrids (Good et al. 2010; Bhattacharyya et al. 2013; Turner et al. 2014; Larson et al. 2017). Asynapsis of hetero-subspecific chromosomes modulated by Prdm9 and Hstx2 provides a potential mechanism for this hybrid incompatibility (Mihola et al. 2009; Bhattacharyya et al. 2013, 2014).
Disrupted epistatic interactions between alleles at different loci (such as Prdm9 and Hstx2), known as hybrid incompatibilities, play a major role in hybrid male sterility across a range of species pairs (Coyne and Orr 2004). These incompatibilities are expected to be enriched for recessive alleles (Muller 1942; Masly and Presgraves 2007), which are invisible in F1 hybrids at autosomal loci. Natural hybrids between M. m. domesticus and M. m. musculus are highly backcrossed (Teeter et al. 2010), suggesting that examination of hybrid male sterility in subsequent generations is crucial for understanding barriers to gene flow in the wild. Genome-wide association mapping in the hybrid zone revealed complex incompatibilities connected to reduced testis weight (Turner and Harr 2014), one of several signs of subfertility in natural hybrids (Turner et al. 2012). A suite of quantitative trait loci (QTL) was identified that confer decreased testis weight, reduced sperm count, or abnormal sperm head shape in F2 hybrids generated by controlled intercrosses (White et al. 2011). Detailed histological explanations for decreased testis weight and other phenotypes were not investigated in these studies of sterility in later-generation hybrids.
Multiple mechanisms for functional sterility in hybrid mice are possible, and we hypothesized that histological abnormalities could help define the pathogenesis of decreased fertility. Developmental defects could lead to fewer seminiferous tubules, resulting in smaller testes. Genetic incompatibilities in later-generation hybrids could lead to defective spermatogonial mitosis, meiosis I, meiosis II, or spermiogenesis. It is also possible that hybrid testes lack enough testosterone-secreting interstitial (Leydig) cells to support proper spermatogenesis and spermiogenesis. Hybrid incompatibilities could reduce the number or function of Sertoli cells. Finally, mechanisms leading to hybrid sterility could have minimal histological impact, affecting only spermiogenesis.
In this article, we go beyond the usual examination of endpoint phenotypes in mature sperm to evaluate these potential developmental causes of hybrid male sterility. We use detailed inspection of testis histopathology in a large number of F2 hybrids between M. m. domesticus and M. m. musculus to identify and characterize disruptions in key steps of spermatogenesis. We identify problems with completing meiosis I as a major cause of F2 hybrid male sterility. We proceed to identify QTL for novel indicators of hybrid male sterility, significantly refining the developmental roles of hybrid incompatibilities in the process.
Materials and Methods
Strains and crosses
We used the wild-derived inbred strains WSB/EiJ and PWD/PhJ as representatives of M. m. domesticus and M. m. musculus, respectively (subsequently denoted as domesticusWSB and musculusPWD). All testis histology phenotypes reported here were collected from mice generated by White et al. (2011) and examined at 70 (±5) days of age. Briefly, domesticusWSB and musculusPWD were crossed in both directions to produce F1 hybrids, and F1 hybrids were sib-mated to generate F2 hybrids. Testis histology phenotypes were collected from 312 F2 males. Because F1 males from musculusPWD × domesticusWSB crosses (following the convention of listing the mother first) were effectively sterile, 300 of the F2 males came from the domesticusWSB × musculusPWD cross direction. Detailed phenotypes were also collected from six musculusPWD × domesticusWSB F1 hybrids, six domesticusWSB × musculusPWD F1 hybrids, six musculusPWD, and six domesticusWSB.
Testis histology
Testes were dissected, weighed, fixed overnight in Bouin’s fixative, and washed in an ethanol series of 25, 50, and 75%. After standard tissue processing, right testes were bisected across the short axis, embedded in paraffin, sectioned at 5 mm, and stained with hematoxylin and eosin.
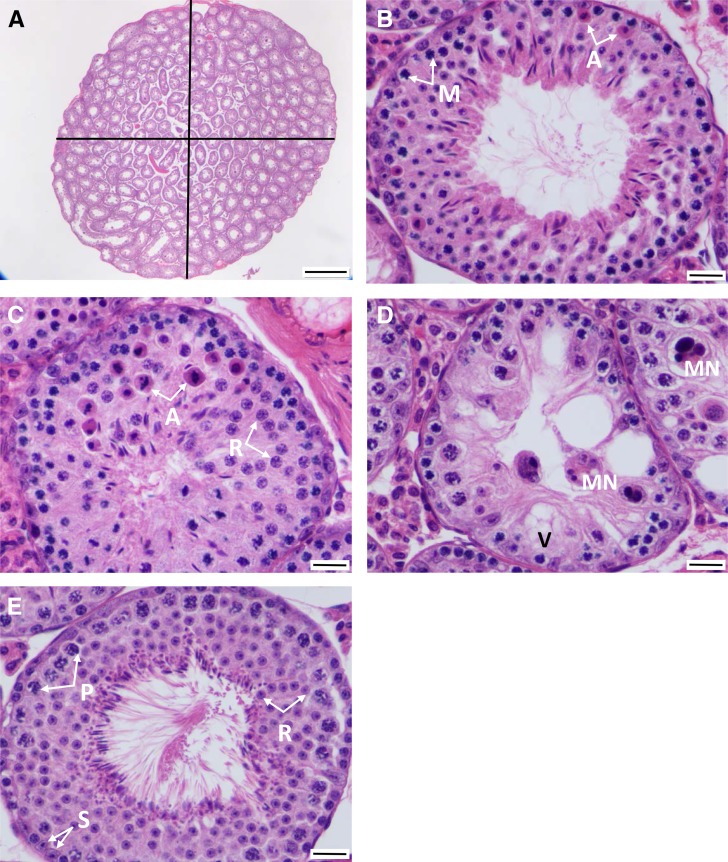
Slides were scanned using an Aperio scanner (Leica Biosystems, Buffalo Grove, IL). Digital measurements of testis width and height were taken, and testis area was estimated as an ellipse (Figure 1A). When at least 10 stage VII tubules were present (see below), similar measurements were captured for each of them (Figure 1A) (White et al. 2011).
Figure 1.
Schematic of testis histology measurements. (A) Entire testis. Testicular area was calculated using the area of an oval following measurements of its width and height. ×40 magnification was also used to count the number of tubules per testis. Bar, 500 μm. (B) Apoptotic meiosis I cells (A) showing hyper-eosinophilic cytoplasm and dense chromatin. Primary spermatocytes entering meiosis I (M). Bar, 20 μm. (C) Apoptosis of round spermatids (A) and round spermatocytes (R). Bar, 20 μm. (D) Multinucleated syncytia (MN; central and in adjacent tubules). Vacuolated Sertoli cell (V). Bar, 20 μm. (E) A healthy stage VII tubule used to count Sertoli cells (S), pachytene spermatocytes (P), and round spermatids (R). Bar, 20 μm.
Histology phenotypes
Testes were histologically examined for any phenotypic abnormalities. Once abnormalities were identified, they were scored for distribution and severity. Whenever possible, all testes were scored for a single trait on the same day or on consecutive days. As a threshold screen for further evaluation, we first asked whether each testis had at least 10 stage VII tubules. This approach focused our study on the least-affected testes, thereby decreasing the effects of nonspecific, late-stage testicular degeneration (Figure 1, A and E). Stage VII tubules were chosen for examination because they are readily identifiable, with pachytene spermatocytes and a full lining of elongating spermatids, which are released in stage VIII. Testes with <10 stage VII tubules were not considered further. The number of tubules with at least three apoptotic cells was recorded (Figure 1, B and C). Furthermore, apoptotic cells were identified and recorded as spermatogonia, meiotic cells (Figure 1B), round spermatids (Figure 1C), and/or elongating spermatids. We also recorded the number of multinucleated syncytia in each tubule section, a frequent abnormality in hybrid testes when spermatogonia fail to complete meiosis (Figure 1D). Sertoli cell vacuolation was identified (Figure 1D). Finally, the stage VII tubule in each testis with the thickest spermatogenic epithelium (considered the “healthiest”) was identified (Figure 1E) for detailed examination. The rationale for focusing on healthy stage VII tubules was that many of the lesions observed in less healthy tubules were consistent with end-stage disease rather than an active process. By focusing on early changes in the healthiest tubules, we enriched our data set for phenotypes mechanistically connected to hybrid male sterility rather than those common to degenerative changes in the testis. For each of these stage VII tubules, the number of Sertoli cells, spermatogonia, pachytene spermatocytes, and round spermatids were recorded (Figure 1E). These testis histology phenotypes were also compared to other fertility traits measured in the same mice, including testis weight, tubule area, epididymal sperm count, and epididymal sperm head morphology (White et al. 2011). Testis histology phenotypes and their expected relationships to fertility are listed in Table 1.
Table 1. Testis histology phenotypes and their expected relationships to fertility.
| Histology phenotype | Process/state measured | Expected pattern in subfertile testis |
|---|---|---|
| Testis area | Overall fertility | Lower |
| Stage VII tubule area | Overall fertility | Lower |
| Number of Sertoli cells in healthiest stage VII tubule | Support of meiosis | Fewer/same |
| Number of pachytene spermatocytes in healthiest stage VII tubule | Induction of meiosis | Fewer |
| Number of round spermatids in healthiest stage VII tubules | Meiotic progression to meiosis II | Fewer |
| Multinucleated syncytia per testis | Spermatocyte degeneration; failure of spermatogenesis or spermiogenesis | More |
| Number of tubules with ≥3 apoptotic primary (meiosis I) spermatocytes | Degeneration of developing spermatocytes | More |
| Number of tubules with ≥3 apoptotic round spermatids | Degeneration of developing spermatids | More |
QTL mapping
Genotypes at 198 informative single nucleotide polymorphisms (SNPs) taken from White et al. (2011) were used for QTL mapping. This set of genotypes included SNPs from across the autosomes and the X chromosome. SNPs on the Y chromosome and the mitochondrion were not considered because most F2 hybrids came from one direction of the intercross. Genotypes were assembled using stringent quality control filters described in Dumont et al. (2011) and White et al. (2011). Genotypes from a total of 553 F2 hybrids (males and females) were used to construct a genetic map with the est.map procedure and a Carter–Falconer mapping function in R/qtl (Broman et al. 2003; White et al. 2011). The average distance between SNPs on the genetic map was 7 cM. Physical positions between SNPs were interpolated using genetic and physical positions of flanking SNPs.
We conducted QTL analyses for the following phenotypes: testis area, number of multinucleated syncytia per testis, number of tubules with apoptotic spermatocytes, number of tubules with apoptotic round spermatids, number of tubules containing only Sertoli cells, and the numbers of Sertoli cells, pachytene spermatocytes, and round spermatids (and associated ratios) within the healthiest stage VII tubule. F2 phenotypic distributions (Supplemental Material, Figure S1) were inspected to determine how each trait should be treated in QTL analyses. The following phenotypes showed non-normal distributions skewed toward the zero-class: tubules with apoptotic meiotic cells, tubules with apoptotic round spermatids, and multinucleated syncytia. These traits were recoded and analyzed as binary presence/absence traits. Numbers of pachytene spermatocytes, round spermatids, and Sertoli cells, as well as testis area, were approximately normally distributed and were analyzed on the observed scale.
QTL were identified using standard interval mapping (Lander and Botstein 1989) or binary interval mapping, implemented using the scan.one function in R/qtl. Genome-wide significance thresholds were calculated from 1000 permutations for the autosomes and 15,868 permutations for the X chromosome (Broman et al. 2006). Models incorporating multiple QTL were fit using a stepwise forward/backward search algorithm (Manichaikul et al. 2009) implemented in the stepwiseqtl function in R/qtl, with genome-wide significance thresholds based on 1000 permutations. When multiple QTL were detected for a trait, we performed tests for potential epistatic interactions between detected QTL. Interaction terms for each pair of QTL were added to the multiple-QTL model one at a time, and a P-value for each interaction was calculated based on the improved fit as measured by the F-statistic. All QTL analyses assumed a genotyping error rate of 0.0001. The differential treatment of traits as binary or continuous made it difficult to jointly consider multiple phenotypes in QTL mapping. As a result, we analyzed each trait separately.
Data availability
Phenotypic and genotypic data are available at Data Dryad (http://datadryad.org; DOI: 10.5061/dryad.vd6k4b2). Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7161119.
Results
Testis histology in parental strains and F1 hybrids
musculusPWD testis sections all contain at least 10 stage VII tubules lined by a plush seminiferous epithelium consisting of Sertoli cells, meiotic spermatocytes, at least three layers of secondary spermatocytes and round spermatids (as these two cell types cannot be distinguished histologically, the term “round spermatids” is used throughout to include both), and elongating spermatids (Figure 2A). These animals appeared to be fully fertile in our laboratory (White et al. 2011).
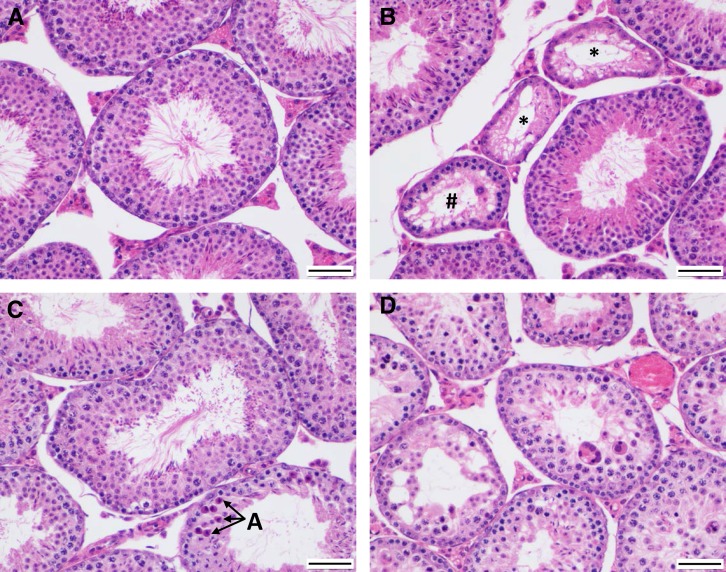
Figure 2.
Examples of testis histology phenotypes in parental strains and F1 hybrids (×200 magnification). (A) Representative musculusPWD testis showing multiple tubules containing a plush spermatogenic epithelium. Bar, 50 μm. (B) Representative domesticusWSB testis showing several Sertoli-only tubules (indicated by *), commensurate with decreased fertility in this strain. The tubule containing # is atrophic and exhibits a few meiotic cells. Bar, 50 μm. (C) Representative domesticusWSB × musculusPWD F1 testis showing mild to moderate degeneration with epithelial thinning and decreased numbers of round spermatids (atrophy) within the spermatogenic epithelium (center tubule) and marked apoptosis (A) of meiosis I cells and round spermatids in an adjacent (lower) tubule. Bar, 50 μm. (D) Representative musculusPWD × domesticusWSB F1 testis showing marked degeneration (multinucleated syncytia, apoptosis) and atrophy of the spermatogenic epithelium in multiple tubules. Bar, 50 μm.
domesticusWSB testis sections often contain multiple tubules exhibiting marked atrophy (Figure 2B, #) or complete absence of the spermatogenic epithelium (Figure 2B, *). Tubules that could not be staged because of their degeneration are shrunken and contain heavily or partially vacuolated Sertoli cells (Figure 2B, *) and contain a few meiotic cells (Figure 2B, #), indicating that spermatogonia are still present. These histologic changes likely contribute to this strain’s lower fertility (Odet et al. 2015), despite its normal sperm density (White et al. 2011).
domesticusWSB × musculusPWD F1 testis sections exhibit an intermediate phenotype, with mild atrophy (thinning) of the stage VII spermatogenic epithelium typified by a reduced number of developing round spermatids (generally fewer than three layers; Figure 2C). Apoptosis, as identified by shrunken cells with hyper-eosinophilic cytoplasm and pyknotic nuclei, is increased in these testes (Figure 2C, A in adjacent tubule; Table 2); this may be a mechanism of atrophy in this direction of the cross. domesticusWSB × musculusPWD F1 testis sections contain significantly fewer stage VII round spermatids than both parental strains (Table 2). Despite these histopathologic abnormalities, domesticusWSB × musculusPWD F1 hybrids bred well in our laboratory (White et al. 2011).
Table 2. Testis histology phenotypic means.
| Phenotype | domesticusWSB (n = 6) | musculusPWD (n = 6) | domesticusWSB × musculusPWD F1 (n = 6) | musculusPWD × domesticusWSB F1 (n = 6) | F2 (n = 312a) |
|---|---|---|---|---|---|
| Testis area (mm2) | 26.7 (5.1) | 28.2 (3.1) | 30.2 (4.0) | 25.6 (5.9) | 35.6 (8.1) |
| Stage VII tubule areab (µm2 × 103) | 44.0 (6.8)f | 46.3 (2.8)h,i | 36.4 (1.6)h,j | 28.2 (1.6)f,i,j | 42.6 (7.5) |
| Stage VII Sertoli cellsc | 14.5 (2.9) | 11.7 (2.3) | 13.5 (2.9) | 13.6 (3.2) | 15.1 (3.1) |
| Stage VII pachytene spermatocytesc | 56.8 (12.3)f | 57.5 (11.3)i | 43.7 (9.4) | 36.6 (4.7)f,i | 53.7 (14.8) |
| Stage VII round spermatidsc | 170.7 (41.3)f,g | 146.0 (20.8)h,i | 85.7 (23.0)g,h,j | 43.80 (24.0)f,i,j | 137.2 (36.0) |
| Multinucleated syncytiad | 2.8 (2.7)f,g | 0.3 (0.5)i | 0.0 (0.0)g,j | 29.5 (8.3)f,i,j | 3.5 (15.1) |
| Tubules with apoptosisg (meiotic) | 0.3 (0.5) | 0.0 (0.0) | 5.0 (2.6) | N/A | 0.5 (1.3) |
| Tubules with apoptosise (round spermatids) | 0.0 (0.0) | 0.0 (0.0) | 0.2 (0.4) | N/A | 0.4 (1.0) |
Values reported as mean (SD).
300 domesticusWSB × musculusPWD cross direction, 12 musculusPWD × domesticusWSB cross direction.
Calculated from the 10 healthiest stage VII tubules in each testis section.
Counts from healthiest tubule in each testis section.
Total count from each testis section.
Scored for tubules with three or more apoptotic cells of respective type.
P < 0.05 (Mann–Whitney U) in pairwise comparison between groups. F2s were not included in these comparisons.
Compared to testes from musculusPWD, domesticusWSB, and domesticusWSB × musculusPWD F1 mice, testes from musculusPWD × domesticusWSB F1 mice show severe degeneration (Figure 2D and Table 2). The spermatogenic epithelium is highly atrophied, Sertoli cell vacuolation is marked, and multiple multinucleated syncytia are visible (Figure 2D and Table 2). musculusPWD × domesticusWSB F1 testes have significantly less stage VII tubule area, fewer stage VII pachytene spermatocytes, and fewer stage VII round spermatids than both parental strains (Table 2). F1 hybrids from this direction of the cross exhibited very poor fertility in the laboratory setting (White et al. 2011).
Testis histology in F2 hybrids
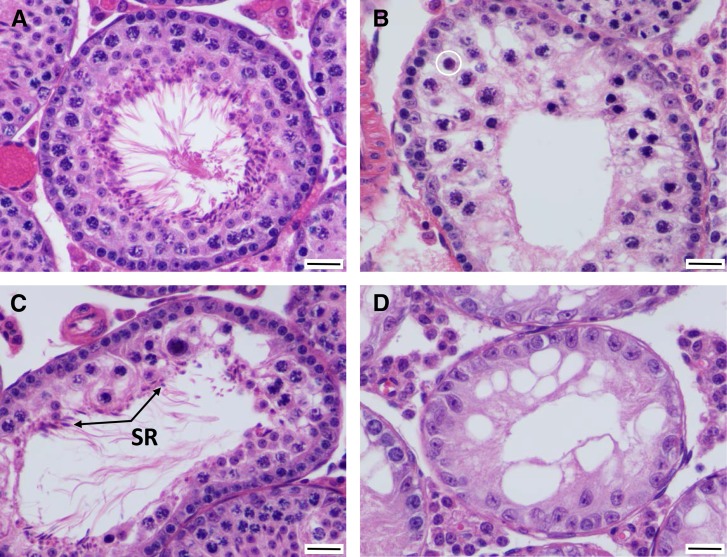
Testes from domesticusWSB × musculusPWD F2 mice present a range of phenotypes that indicate defective spermatogenesis (Figure 3 and Figure S1). The severity of each phenotype is typically milder than seen in either F1 cross or domesticusWSB testes (Table 2). Many domesticusWSB × musculusPWD F2 testes show substantial and progressive atrophy of the spermatogenic epithelium, including loss of elongating spermatids (Figure 3, B and C), round spermatids (Figure 3, A–C), meiotic spermatocytes (Figure 3, B and C), and/or spermatogonia (leaving only Sertoli cells; Figure 3D) (Table 2). Normal tubules should contain at least three layers of round spermatids (e.g., Figure 2A), but this is often not the case (e.g., Figure 3A). This result suggests an initial defect in meiosis I. Apoptosis is increased, both within pachytene spermatocytes (again suggesting a defect in meiosis I), and within the more basal layers of round spermatids. Fewer round spermatids are present (Figure S1), suggesting that fewer primary spermatocytes survive to meiosis II and spermiogenesis; this is an expected outcome from an increase in apoptosis within the meiotic I compartment. We observed no increase in apoptosis among spermatogonia.
Figure 3.
Examples of testis histology phenotypes in stage VII tubules in F2 hybrids from the musculusPWD × domesticusWSB cross direction (×400 magnification). (A) In this F2 tubule, there is a markedly attenuated/atrophic spermatogenic epithelium, consisting of only one or two (rather than three or four) layers of round spermatids. Bar, 20 μm. (B) In this F2 tubule, there is luminal displacement of primary (meiosis I) spermatocytes, many of which are undergoing apoptosis. There are no maturing round spermatids, and only meiosis I is identified. The circle indicates a meiotic cell entering apoptosis. Bar, 20 μm. (C) In this F2 tubule, there is marked disruption of spermatogenesis, with only a few meiotic spermatocytes, few round spermatids, formation of a multinucleated syncytium, and spermatid retention (SR), where elongated spermatids are still held by Sertoli cells. Bar, 20 μm. (D) In this (and adjacent) F2 tubule, there is complete loss of spermatogonia, meiotic spermatocytes, and round spermatids, leaving only Sertoli cells. Bar, 20 μm.
Some tubules contain abnormal meiotic cells at all levels of the tubule and completely lack round spermatids (Figure 3, B and C). These abnormal cells exhibit densely compacted and clumped chromatin but lack obvious cytoplasmic eosinophilia (Figure 3, B and C). Chromatin clumping is likely associated with early stages of apoptosis, and a few of these abnormal meiotic cells appear to be entering apoptosis (Figure 3B, circle). Pachytene spermatocytes do not accumulate in stage VII tubules, suggesting that meiotic arrest, if it occurs, is brief and apoptosis is rapidly triggered. Additionally, there is no change in the ratio of pachytene spermatocytes to either Sertoli cells or round spermatids that would be indicative of meiotic arrest. The observation that abnormal meiotic cells are found at all levels of the tubule (Figure 3, B and C) when they should be anatomically restricted to the suprabasilar compartment, completing meiosis II and attaining a rounded phenotype, again points to a defect in meiosis I.
Another common sign of degeneration in F2 testes is the formation of multinucleated syncytial cells from round spermatids, indicating a failure to complete meiosis. Small numbers of multinucleated syncytial cells (0–1 per testis) are expected in healthy testes. The average number of multinucleated syncytia for F2 testis sections from the domesticusWSB × musculusPWD cross is 3.5 (SD 15.1) (Table 2). Additionally, a few tubules exhibit a phenomenon known as spermatid retention (Figure 3C). Spermatid retention occurs when elongating spermatids are not released to the lumen of the tubule. It may be caused by the absence of the next wave of developing spermatocytes and loss of their maturation stimuli. Sertoli cell vacuolation is also present, indicating dysfunction of Sertoli cells (Figure 3, B–D). F2 distributions for testis histology phenotypes used for QTL mapping are shown in Figure S1.
Phenotypic correlations across F2 hybrids
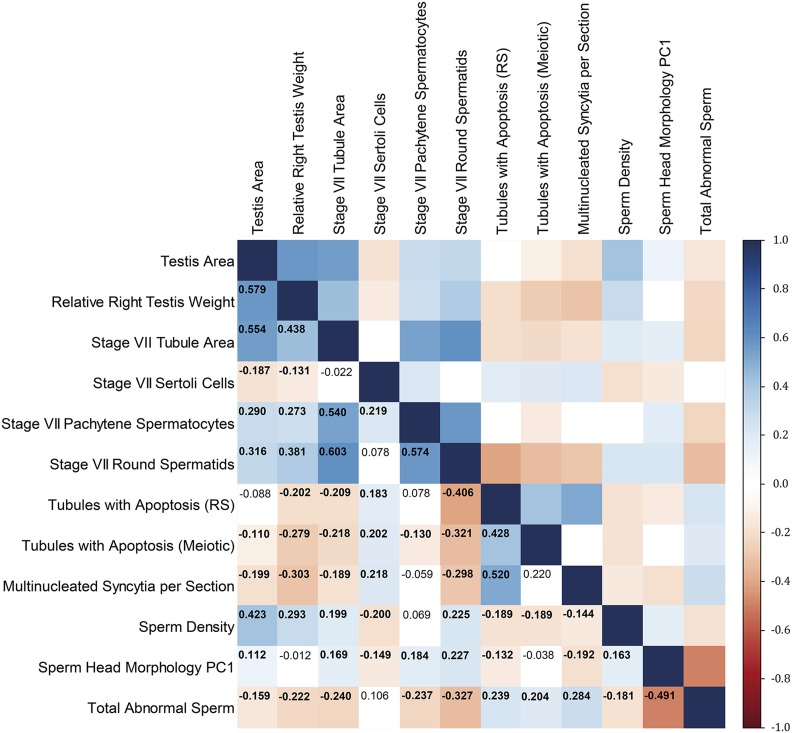
Our data set provided an unusual opportunity to characterize relationships between different histologic defects associated with hybrid male sterility during the processes of spermatogenesis and spermiogenesis. Across F2 hybrids, many pairs of traits are significantly correlated (Figure 4). Most correlations are weak in magnitude (Figure 4), suggesting that each trait contains distinct information about hybrid male sterility and motivating the independent treatment of phenotypes in subsequent genetic analyses. A set of stronger correlations highlight temporally and/or functionally connected processes. These relationships include positive correlations between numbers of pachytene spermatocytes and round spermatids, numbers of tubules with apoptotic meiotic cells and tubules with apoptotic round spermatids, numbers of tubules with apoptotic round spermatids and multinucleated syncytia, tubule area and number of pachytene spermatocytes, and tubule area and number of round spermatids (Figure 4).
Figure 4.
Pearson’s correlations among testis histology phenotypes across F2 mice (displayed as a heat map). Correlations with P < 0.05 are bolded. Data for relative right testis weight, stage VII tubule area, sperm density, sperm head morphology PC1, and total abnormal sperm were originally reported in White et al. (2011). PC1, principal component 1; RS, round spermatids.
QTL for testis histology defects
Single QTL mapping identified loci linked to five phenotypes (Table 3). Four of these traits are connected to round spermatids: the number of round spermatids (with the number of pachytene spermatocytes as covariate), the number of round spermatids per Sertoli cell, the presence/absence of tubules with apoptotic round spermatids, and the presence/absence of multinucleated syncytia. QTL for testis area also were found.
Table 3. QTL from single QTL analyses.
| Phenotype | Chromosome | Position (cM) | LOD | Position (Mb) | 1.5 LOD interval (Mb) | DDa | DMa | MMa |
|---|---|---|---|---|---|---|---|---|
| Testis area (cm2) | 10 | 18.6 | 5.76 | 65.1 | 40.4–121.1 | 0.34 (0.01) | 0.35 (0.01) | 0.40 (0.01) |
| X | 0.0 | 2.92 | 10.2 | 10.2–166.3 | 0.38 (0.01) | — | 0.34 (0.01) | |
| Multinucleated syncytiab | 17 | 13.3 | 4.26 | 30.0 | 3.1–65.2 | 2.02 (1.80) | 5.26 (1.21) | 1.14 (1.98) |
| X | 0.0 | 8.00 | 10.2 | 10.2–58.3 | 0.95 (1.33) | — | 5.78 (1.20) | |
| With tubule area as covariatec | 8 | 15.0 | 4.54 | 53.6 | 33.5–66.5 | 1.51 (1.97) | 3.17 (1.25) | 6.22 (1.81) |
| Tubules with apoptosis (round spermatids)d | X | 0.0 | 4.81 | 10.2 | 10.2–45.1 | 0.17 (0.09) | — | 0.68 (0.08) |
| Round spermatids per Sertoli cell | 17 | 13.3 | 4.27 | 30.0 | 3.1–65.2 | 10.1 (0.33) | 8.7 (0.22) | 10.3 (0.36) |
| X | 15.0 | 3.91 | 58.3 | 10.2–71.5 | 10.2 (0.24) | — | 8.8 (0.22) | |
| Round spermatidse | 17 | 13.3 | 4.99 | 30.0 | 23.3–65.2 | 140.0 (4.1) | 130.5 (2.8) | 150.9 (4.5) |
| With pachytene as covariate |
D, M. m. domesticusWSB; M, M. m. musculusPWD.
Phenotypic means for each genotype with SE in parenthesis.
Mapped as binary trait, presence of more than one multinucleated syncytium in testis section.
QTL identified when tubule area in testis section is used as a covariate.
Mapped as binary trait, presence or absence of apoptotic cells among round spermatids in testis section.
QTL identified when number of stage VII pachytene spermatocytes are used as a covariate.
QTL on chromosome 17 affect the number of round spermatids and the presence of multinucleated syncytia. Heterozygotes for these QTL show phenotypes consistent with reduced fertility, matching the previously reported underdominant effects (on other fertility traits) of this genomic region (Dzur-Gejdošova et al. 2012; Flachs et al. 2012; Turner et al. 2014).
QTL on the X chromosome determine the number of round spermatids per Sertoli cell, the presence of tubules with apoptotic round spermatids, the presence of multinucleated syncytia, and testis area. The estimated position of the QTL for the number of round spermatids per Sertoli cell is 48 Mb distal to the QTL for the other three traits, raising the possibility that the X chromosome contains multiple QTL (although the 1.5 LOD intervals overlap). For each of the four phenotypes, the musculusPWD X chromosome is associated with reduced fertility, consistent with previous reports (Storchová et al. 2004; White et al. 2011).
Some QTL were only identified once variation in other traits was considered. QTL on chromosome 17 for round spermatid number were only detected when this phenotype was divided by the number of Sertoli cells or when the number of pachytene spermatocytes was treated as a covariate. The X-linked QTL only appeared when the number of pachytene spermatocytes was used as a covariate. These conditional mapping results suggest that the relationship between round spermatids and meiotic cells and/or Sertoli cells is an important part of QTL activity on chromosomes 17 and X.
Multiple QTL mapping identified loci linked to the same five phenotypes as single QTL mapping (Table 4). Multiple QTL mapping revealed additional QTL that contribute to the presence of multinucleated syncytia (chromosome 18) and testis area (chromosome 3) (Table 4). For some QTL, 1.5 LOD intervals were narrowed compared to single QTL mapping.
Table 4. QTL from multiple QTL analyses.
| Phenotype | Chromosome | Position (cM) | LOD | Position (Mb) | 1.5 LOD interval (Mb) | % Variancea | Additive effectb | Dominance deviationc |
|---|---|---|---|---|---|---|---|---|
| Testis area (cm2) | 3 | 52.0 | 3.62 | 125.0 | 97.2–141.0 | 4.9 | −0.62 (0.2) | −0.88 (0.3) |
| 10 | 19.0 | 5.35 | 66.4 | 49.5–82.7 | 7.3 | 0.87 (0.2) | −0.80 (0.3) | |
| X | 0.0 | 3.47 | 10.2 | 10.2–56.4 | 4.7 | −0.57 (0.1) | — | |
| Multinucleated syncytiad | 17 | 12.0 | 4.46 | 26.9 | 8.9–44.0 | 5.7 | −0.52 (3.5) | 22 (4.8) |
| 18 | 36.0 | 3.80 | 71.9 | 61.5–82.8 | 4.8 | 4.8 (3.5) | 20 (4.9) | |
| X | 1.0 | 8.03 | 14.4 | 10.2–54.4 | 10.5 | 15 (2.4) | — | |
| Multinucleated syncytiad | 8 | 15.0 | 3.84 | 53.6 | 35.5–66.5 | 5.4 | 14 (3.4) | 1.8 (4.9) |
| With tubule area as covariate | 17 | 13.0 | 4.18 | 29.2 | 10.8–44.0 | 5.9 | −0.91 (3.3) | 20 (4.6) |
| X | 0.0 | 4.83 | 10.2 | 10.2–91.9 | 6.9 | 11 (2.3) | — | |
| Tubules with apoptosis (round spermatids)e | X | 0.0 | 4.67 | 10.2 | 10.2–44.2 | 7.2 | 12 (2.5) | — |
| Round spermatids per Sertoli cell | 17 | 12.0 | 4.20 | 26.9 | 6.9–60.1 | 6.1 | −4.4 (24) | −147 (33) |
| X | 11.0 | 4.00 | 50.5 | 10.2–70.1 | 5.8 | −73 (17) | — | |
| Round spermatidsf | 17 | 13.3 | 4.98 | 29.9 | 6.9–53.4 | 5.0 | 512 (244) | −1487 (330) |
| With pachytene as covariate |
Values in effect columns scaled by 102.
Percentage of phenotypic variance explained by QTL.
Additive effects calculated as half the difference between phenotypic average of homozygotes, estimated effect with SE in parentheses.
Dominance deviation calculated as difference between phenotypic average of heterozygotes and the midpoint between phenotypic averages of homozygotes, estimated deviation with SE in parenthesis.
Mapped as binary trait, presence of more than one multinucleated syncytium in testis section. Using tubule area as a covariate produces a best-fitting model with a differing set of QTL.
Mapped as binary trait, presence or absence of apoptotic cells among round spermatids in testis section.
QTL identified when number of stage VII pachytene spermatocytes is used as a covariate.
Tests for epistasis revealed a significant interaction between the QTL on chromosomes 17 and X for the presence of multinucleated syncytia (pointwise P < 0.005) that explains an additional 3% of the variance. There was no evidence for epistasis between any of the other detected QTL.
We compared QTL positions with those found previously in the same cross for additional traits connected to hybrid male sterility (White et al. 2011). The chromosome 17 positions of QTL for the number of round spermatids and the presence of multinucleated syncytia are the same or very similar to QTL for epididymal sperm density. The position of the X-linked QTL for the number of round spermatids is close to QTL for some measures of sperm head morphology. X-linked QTL for the presence of tubules with apoptotic round spermatids and multinucleated syncytia, as well as testis area, are located more proximally than X-linked QTL for sperm head morphology, although there is some overlap in 1.5 LOD intervals. QTL on chromosomes 8 and 18 for the presence of multinucleated syncytia overlap with QTL for seminiferous tubule area. Testis area QTL located on chromosomes 3 and 10 overlap with QTL for relative right testis weight.
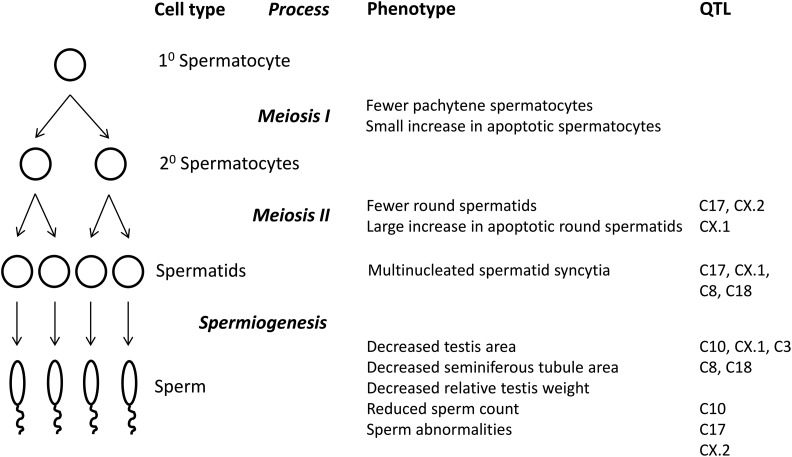
A schematic summarizing QTL activities in the temporal context of spermatogenesis and spermiogenesis is provided in Figure 5.
Figure 5.
Schematic illustrating phenotypic effects of F2 hybrid male sterility QTL.
Discussion
The continuous and spatially structured nature of spermatogenesis makes it possible to assign abnormalities to specific stages and processes through detailed histological examination of testes. Our application of this strategy to a large sample of hybrid mouse testes elucidated the phenotypic and genetic causes of a primary reproductive barrier—hybrid male sterility—between two subspecies of house mice.
Phenotypic determinants of F2 hybrid male sterility
Although we found significant phenotypic heterogeneity across F2 mice, a few overall patterns point to the developmental bases of F2 hybrid male sterility. Testes generally develop normally, as indicated by the presence of interstitial (Leydig) cells, Sertoli cells, seminiferous tubules, and spermatogonia in expected numbers. Active division and entry into meiosis of spermatogonia show that mitosis proceeds normally.
Increases in apoptotic pachytene meiotic cells and decreases in round spermatids suggest that the failure to complete meiosis I is a primary barrier. This failure leads to increased apoptosis within round spermatids and an increase in multinucleated syncytia (degenerating spermatids). Our results are consistent with cell death by activation of the pachytene checkpoint in meiosis I in hybrids. As secondary spermatocytes and round spermatids cannot be histologically distinguished, and an increase in apoptotic round spermatids was noted, the possibility exists of an additional downstream defect in meiosis II. Identification of multinucleated syncytia also suggests potential postmeiotic defects in spermiogenesis, although syncytia can result from aberrant meiosis as well.
Although F2 hybrids harbor normal numbers of Sertoli cells, Sertoli cell vacuolation in some tubules suggests dysfunction of these cells as well. The frequency of tubules with vacuolation is associated with infertility in extinct lines from the Collaborative Cross (Shorter et al. 2017), which are themselves hybrids of three subspecies of house mice (Threadgill and Churchill 2012). Sertoli cells form the blood–testis barrier, which prevents the immune system from attacking the genetically novel cells generated by meiosis. We attribute Sertoli cell vacuolation to a loss of trophic feedback from developing spermatocytes held within the Sertoli cells; these two cell types are cytoplasmically linked via gap junctions.
Our study is the first to comprehensively characterize testis histology in hundreds of hybrid mice. Comparisons to other studies of hybrids that reported testis histology in smaller numbers of mice reveal potential generalities. Wild hybrids from the European contact zone between M. m. domesticus and M. m. musculus exhibit some of the same testicular abnormalities we identified, including significant apoptosis of round spermatids, vacuolated Sertoli cells, multinucleated syncytia, and spermatid retention (Turner et al. 2012). The apoptosis we detected in laboratory-reared F2 hybrids was earlier (in pachytene spermatocytes) than that observed in natural hybrids (chiefly round spermatids) (Turner et al. 2012). Nevertheless, the overall histologic similarities suggest that F2 hybrid mice generated in the laboratory provide strong models for reductions in hybrid fertility in the wild.
The defects we observed in F1 hybrids were less severe than those previously reported for sterile F1 hybrids from crosses between two M. m. musculus strains and C57BL/6 (an inbred strain mostly descended from M. m. domesticus). Testes from NJL × C57BL/6 F1 hybrids contain spermatogonia and primary spermatocytes, but no secondary spermatocytes or spermatids (Kaku et al. 1995). Testes from these hybrids undergo primary spermatocyte apoptosis and harbor a reduced number of pachytene cells relative to zygotene cells (Oka et al. 2010). Testes from musculusPWD × C57BL/6 F1 hybrids completely lack postmeiotic cells, with apoptosis in meiosis I leading to a deficiency in the number of midpachytene cells and an absence of diplotene cells (Bhattacharyya et al. 2013). Testes from musculusPWD × domesticusWSB F1 hybrids show similarly drastic signs of reduced fertility. Like other traits associated with hybrid male sterility (Good et al. 2008b), testis histology in F1 hybrids between M. m. musculus and M. m. domesticus differs among strains. Another factor that complicates comparisons among studies is that tubule degeneration results in Sertoli-only tubules lacking primary and secondary spermatocytes. As a result, studies of older mice could miss the primary degenerative process, instead revealing the end-stage (infertile) testis consisting of chiefly Sertoli-only tubules.
Genetic determinants of F2 hybrid male sterility
We used substantial variation in testis histology phenotypes among F2 mice to identify QTL that contribute to a subset of these traits. Our study is the first to characterize the genetic architecture of these five measures of testis abnormalities.
Our results indicate important roles for loci on multiple chromosomes in the development of F2 hybrid male sterility. The effects of QTL on chromosomes 17 and X on testis histology mirror those previously documented for other sterility traits in F1 hybrids and in consomics/congenics (Forejt and Ivanyi 1974; Forejt et al. 1991; Storchová et al. 2004; Mihola et al. 2009; Dzur-Gejdošova et al. 2012). In particular, mice heterozygous for chromosome 17 QTL and/or carrying chromosome X QTL alleles from musculusPWD show histologic traits associated with reduced fertility.
The underdominant action and genomic positions of the QTL on chromosome 17 suggest the causative mutation(s) lie(s) in Prdm9 (Mihola et al. 2009). If this turns out to be the case, our results would extend the phenotypic effects of Prdm9-based incompatibilities to include multinucleated syncytia. Our findings would also confirm that these incompatibilities reduce the number of round spermatids in F2 hybrids. Furthermore, these patterns are consistent with the hypothesis that Prdm9-based incompatibilities cause F1 hybrid male sterility by triggering pachytene checkpoint activation in meiotic prophase I with reductions in synapsis and double-strand-break repair (Bhattacharyya et al. 2013; Gregorova et al. 2018). The estimated positions of QTL on chromosome 17 reported here and in White et al. (2011) (∼26–30 Mb) are far enough away from the location of Prdm9 (15.5 Mb) to raise the possibility that this gene is not the causative locus (although the confidence intervals include Prdm9). Reaching a definitive conclusion will require finer genetic mapping in this genomic region.
Testis area, the presence of tubules with apoptotic round spermatids, and the presence of multinucleated syncytia all map to the proximal part of the X chromosome. These results suggest that this X-linked region primarily affects apoptosis, leading to both decreased testis area (due to loss of spermatocytes) and subsequent formation of multinucleated syncytia (degenerate spermatocytes). Based on position, QTL in this region appear to be distinct from previously reported F1 hybrid male sterility QTL Hst1x (which disrupts spermiogenesis) (Storchová et al. 2004) and Hst2x (which arrests meiosis) (Bhattacharyya et al. 2014). Introgression of the proximal third of the X chromosome from the PWK strain of M. m. musculus on to the genomic background of the LEWES strain of M. m. domesticus also reduces sperm count and testis weight (Good et al. 2008a). Our results suggest that a second QTL on the X chromosome controls the number of round spermatids (although confidence intervals on position overlap). Overall, our findings support the idea that the X chromosome contributes to hybrid male sterility in multiple ways (Storchová et al. 2004; Good et al. 2008a, 2010; Bhattacharyya et al. 2014; Turner et al. 2014; Larson et al. 2017).
QTL for multinucleated syncytia on chromosomes 8 and 18 localize near QTL for seminiferous tubule area (White et al. 2011). Syncytia indicate failures in differentiation from round spermatids to spermatozoa, as the preexisting junctions between these cells fail to separate and the cells are not released from Sertoli cells. Because multinucleated syncytial cells represent spermatocyte degeneration, spermatocyte loss is also likely; this in turn could reduce tubule area. The overlap of QTL for testis area with QTL for testis weight (White et al. 2011) suggests that these loci control the overall size of the testis in hybrids.
The absence of QTL for other histologic traits could indicate that these phenotypes are not important contributors to hybrid male sterility in this cross. In this vein, most F2 hybrids could be characterized as displaying normal aspects of testis development (as revealed by the numbers of tubules and Sertoli cells) and normal initiation of meiosis I (as revealed by the number of pachytene spermatocytes). Alternatively, genetic incompatibilities could exist for these traits, but with effects too small to detect using our sample size. Finally, these phenotypes could feature higher measurement error, although we have no indication that this is the case.
Examination of testis abnormalities in intercrosses featuring other strains could identify different QTL or distinct phenotypic effects of the same QTL. Both M. m. domesticus and M. m. musculus subspecies harbor polymorphism for F1 hybrid male sterility involving chromosomes X (Good et al. 2008b) and 17 (Forejt and Ivanyi 1974; Vyskočilová et al. 2009), and other autosomes (Larson et al. 2018). Detailed examination of testis histology in crosses with other subspecies of house mice, including M. m. castaneus, would help resolve the evolutionary origins of the hybrid incompatibilities we identified (Moyle and Payseur 2009; White et al. 2012; Wang et al. 2015).
Acknowledgments
We gratefully acknowledge the expert technical assistance of Beth Gray with slide preparation. We thank Daniel Barbash and two anonymous reviewers for helpful feedback on the manuscript. R.J.W. and M.A.W. were both supported by a National Institutes of Health (NIH) training grant in Genetics (T32 GM007133) and an NIH training grant in Computation and Informatics in Biology and Medicine (T15 NLM2M007359). R.J.W. was also supported by a University of Wisconsin-Madison Science and Medicine Graduate Research Scholarship. This research was supported by National Science Foundation grants DEB1353737 and DEB0918000, and NIH grant R01 GM120051 to B.A.P.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7161119.
Communicating editor: D. Barbash
Literature Cited
- Alibert P., Fel-Clair F., Manolakou K., Britton-Davidian J., Auffray J. C., 1997. Developmental stability, fitness, and trait size in laboratory hybrids between European subspecies of the house mouse. Evolution 51: 1284–1295. 10.1111/j.1558-5646.1997.tb03975.x [DOI] [PubMed] [Google Scholar]
- Baudat F., Buard J., Grey C., Fledel-Alon A., Ober C., et al. , 2010. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science 327: 836–840. 10.1126/science.1183439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya T., Gregorova S., Mihola O., Anger M., Sebestova J., et al. , 2013. Mechanistic basis of infertility of mouse intersubspecific hybrids. Proc. Natl. Acad. Sci. USA 110: E468–E477. 10.1073/pnas.1219126110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya T., Reifova R., Gregorova S., Simecek P., Gergelits V., et al. , 2014. X chromosome control of meiotic chromosome synapsis in mouse inter-subspecific hybrids. PLoS Genet. 10: e1004088 10.1371/journal.pgen.1004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursot P., Auffray J. C., Britton-Davidian J., Bonhomme F., 1993. The evolution of house mice. Annu. Rev. Ecol. Syst. 24: 119–152. 10.1146/annurev.es.24.110193.001003 [DOI] [Google Scholar]
- Britton-Davidian J., Fel-Clair F., Lopez J., Alibert P., Boursot P., 2005. Postzygotic isolation between the two European subspecies of the house mouse: estimates from fertility patterns in wild and laboratory-bred hybrids. Biol. J. Linn. Soc. Lond. 84: 379–393. 10.1111/j.1095-8312.2005.00441.x [DOI] [Google Scholar]
- Broman K. W., Wu H., Sen S., Churchill G. A., 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19: 889–890. 10.1093/bioinformatics/btg112 [DOI] [PubMed] [Google Scholar]
- Broman K. W., Sen S., Owens S. E., Manichaikul A., Southard-Smith E. M., et al. , 2006. The X chromosome in quantitative trait locus mapping. Genetics 174: 2151–2158. 10.1534/genetics.106.061176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P., Nachman M. W., 2014. X-Y interactions underlie sperm head abnormality in hybrid male house mice. Genetics 196: 1231–1240. 10.1534/genetics.114.161703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb C., Nolan C., 1987. Mouse hybrid sterility and testicular function. Biol. Reprod. 36: 1343–1348. 10.1095/biolreprod36.5.1343 [DOI] [PubMed] [Google Scholar]
- Coyne J. A., Orr H. A., 1989. Patterns of speciation in Drosophila. Evolution 43: 362–381. 10.1111/j.1558-5646.1989.tb04233.x [DOI] [PubMed] [Google Scholar]
- Coyne J. A., Orr H. A., 2004. Speciation. Sinauer Associates, Sunderland, MA. [Google Scholar]
- Cutter A. D., 2012. The polymorphic prelude to Bateson-Dobzhansky-Muller incompatibilities. Trends Ecol. Evol. 27: 209–218. 10.1016/j.tree.2011.11.004 [DOI] [PubMed] [Google Scholar]
- Dumont B. L., White M. A., Steffy B., Wiltshire T., Payseur B. A., 2011. Extensive recombination rate variation in the house mouse species complex inferred from genetic linkage maps. Genome Res. 21: 114–125. 10.1101/gr.111252.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvaux L., Belkhir K., Boulesteix M., Boursot P., 2011. Isolation and gene flow: inferring the speciation history of European house mice. Mol. Ecol. 20: 5248–5264. 10.1111/j.1365-294X.2011.05343.x [DOI] [PubMed] [Google Scholar]
- Dzur-Gejdošova M., Simecek P., Gregorova S., Bhattacharyya T., Forejt J., 2012. Dissecting the genetic architecture of F1 hybrid sterility in house mice. Evolution. 66: 3321–3335. 10.1111/j.1558-5646.2012.01684.x [DOI] [PubMed] [Google Scholar]
- Flachs P., Mihola O., Šimeček P., Gregorová S., Schimenti J. C., et al. , 2012. Interallelic and intergenic incompatibilities of the Prdm9 (Hst1) gene in mouse hybrid sterility. PLoS Genet. 8: e1003044 10.1371/journal.pgen.1003044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forejt J., 1996. Hybrid sterility in the mouse. Trends Genet. 12: 412–417. 10.1016/0168-9525(96)10040-8 [DOI] [PubMed] [Google Scholar]
- Forejt J., Ivanyi P., 1974. Genetic studies on male sterility of hybrids between laboratory and wild mice (Mus musculus L.). Genet. Res. 24: 189–206. 10.1017/S0016672300015214 [DOI] [PubMed] [Google Scholar]
- Forejt J., Vincek V., Klein J., Lehrach H., Loudovamickova M., 1991. Genetic mapping of the T-complex region on mouse chromosome 17 including the hybrid sterility-1 gene. Mamm. Genome 1: 84–91. 10.1007/BF02443783 [DOI] [PubMed] [Google Scholar]
- Geraldes A., Basset P., Smith K. L., Nachman M. W., 2011. Higher differentiation among subspecies of the house mouse (Mus musculus) in genomic regions with low recombination. Mol. Ecol. 20: 4722–4736. 10.1111/j.1365-294X.2011.05285.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good J. M., Dean M. D., Nachman M. W., 2008a A complex genetic basis to X-linked hybrid male sterility between two species of house mice. Genetics 179: 2213–2228. 10.1534/genetics.107.085340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good J. M., Handel M. A., Nachman M. W., 2008b Asymmetry and polymorphism of hybrid male sterility during the early stages of speciation in house mice. Evolution. 62: 50–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good J. M., Giger T., Dean M. D., Nachman M. W., 2010. Widespread over-expression of the X chromosome in sterile F1 hybrid mice. PLoS Genet. 6: e1001148 10.1371/journal.pgen.1001148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorova S., Gergelits V., Chvatalova I., Bhattacharyya T., Valiskova B., et al. , 2018. Modulation of Prdm9-controlled meiotic chromosome asynapsis overrides hybrid sterility in mice. eLife 7: e34282 10.7554/eLife.34282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Yoshida K., Matsui Y., 2005. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature 438: 374–378. 10.1038/nature04112 [DOI] [PubMed] [Google Scholar]
- Janoušek V., Wang L., Luzynski K., Dufkova P., Vyskocilova M. M., et al. , 2012. Genome-wide architecture of reproductive isolation in a naturally occurring hybrid zone between Mus musculus musculus and M. m. domesticus. Mol. Ecol. 21: 3032–3047. 10.1111/j.1365-294X.2012.05583.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku Y., Kon Y., Takagi N., Yamashita T., Hayashi M., et al. , 1995. Histological analysis of male hybrid sterility induced by the Hst-1 gene in mice. J. Vet. Med. Sci. 57: 973–975. 10.1292/jvms.57.973 [DOI] [PubMed] [Google Scholar]
- Lander E. S., Botstein D., 1989. Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121: 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E. L., Keeble S., Vanderpool D., Dean M. D., Good J. M., 2017. The composite regulatory basis of the large X-effect in mouse speciation. Mol. Biol. Evol. 34: 282–295. 10.1093/molbev/msw243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E. L., Vanderpool D., Sarver B. A. J., Callahan C., Keeble S., et al. , 2018. The evolution of polymorphic hybrid incompatibilities in house mice. Genetics 209: 845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manichaikul A., Moon J. Y., Sen S., Yandell B. S., Broman K. W., 2009. A model selection approach for the identification of quantitative trait loci in experimental crosses, allowing epistasis. Genetics 181: 1077–1086. 10.1534/genetics.108.094565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masly J. P., Presgraves D. C., 2007. High-resolution genome-wide dissection of the two rules of speciation in Drosophila. PLoS Biol. 5: e243 10.1371/journal.pbio.0050243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihola O., Trachtulec Z., Vlcek C., Schimenti J. C., Forejt J., 2009. A mouse speciation gene encodes a meiotic histone H3 methyltransferase. Science 323: 373–375. 10.1126/science.1163601 [DOI] [PubMed] [Google Scholar]
- Moyle L. C., Payseur B. A., 2009. Reproductive isolation grows on trees. Trends Ecol. Evol. 24: 591–598. 10.1016/j.tree.2009.05.010 [DOI] [PubMed] [Google Scholar]
- Muller H. J., 1942. Isolating mechanisms, evolution, and temperature. Biol. Symp. 6: 71–125. [Google Scholar]
- Odet F., Pan W., Bell T. A., Goodson S. G., Stevans A. M., et al. , 2015. The founder strains of the collaborative cross express a complex combination of advantageous and deleterious traits for male reproduction. G3 (Bethesda) 5: 2671–2683. 10.1534/g3.115.020172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Mita A., Takada Y., Koseki H., Shiroishi T., 2010. Reproductive isolation in hybrid mice due to spermatogenesis defects at three meiotic stages. Genetics 186: 339–351. 10.1534/genetics.110.118976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen K., Petkov P. M., 2018. PRDM9 and its role in genetic recombination. Trends Genet. 34: 291–300. 10.1016/j.tig.2017.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvanov E. D., Petkov P. M., Paigen K., 2010. Prdm9 controls activation of mammalian recombination hotspots. Science 327: 835 10.1126/science.1181495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payseur B. A., Krenz J. G., Nachman M. W., 2004. Differential patterns of introgression across the X chromosome in a hybrid zone between two species of house mice. Evolution 58: 2064–2078. 10.1111/j.0014-3820.2004.tb00490.x [DOI] [PubMed] [Google Scholar]
- Sage R. D., Atchley W. R., Capanna E., 1993. House mice as models in systematic biology. Syst. Biol. 42: 523–561. 10.1093/sysbio/42.4.523 [DOI] [Google Scholar]
- Shorter J. R., Odet F., Aylor D. L., Pan W., Kao C. Y., et al. , 2017. Male infertility is responsible for nearly half of the extinction observed in the mouse collaborative cross. Genetics 206: 557–572. 10.1534/genetics.116.199596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smadja C., Ganem G., 2005. Asymmetrical reproductive character displacement in the house mouse. J. Evol. Biol. 18: 1485–1493. 10.1111/j.1420-9101.2005.00944.x [DOI] [PubMed] [Google Scholar]
- Smadja G., Catalan J., Ganem C., 2004. Strong premating divergence in a unimodal hybrid zone between two subspecies of the house mouse. J. Evol. Biol. 17: 165–176. 10.1046/j.1420-9101.2003.00647.x [DOI] [PubMed] [Google Scholar]
- Storchová R., Gregorová S., Buckiová D., Kyselová V., Divina P., et al. , 2004. Genetic analysis of X-linked hybrid sterility in the house mouse. Mamm. Genome 15: 515–524. 10.1007/s00335-004-2386-0 [DOI] [PubMed] [Google Scholar]
- Suzuki T. A., Nachman M. W., 2015. Speciation and reduced hybrid female fertility in house mice. Evolution. 69: 2468–2481. 10.1111/evo.12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeter K. C., Thibodeau L. M., Gompert Z., Buerkle C. A., Nachman M. W., et al. , 2010. The variable genomic architecture of isolation between hybridizing species of house mice. Evolution. 64: 472–485. 10.1111/j.1558-5646.2009.00846.x [DOI] [PubMed] [Google Scholar]
- Threadgill D. W., Churchill G. A., 2012. Ten years of the collaborative cross. Genetics 190: 291–294. 10.1534/genetics.111.138032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtulec Z., Mihola O., Vlcek C., Himmelbauer H., Paces V., et al. , 2005. Positional cloning of the Hybrid sterility 1 gene: fine genetic mapping and evaluation of two candidate genes. Biol. J. Linn. Soc. Lond. 84: 637–641. 10.1111/j.1095-8312.2005.00460.x [DOI] [Google Scholar]
- Tucker P. K., Sage R. D., Warner J., Wilson A. C., Eicher E. M., 1992. Abrupt cline for sex chromosomes in a hybrid zone between two species of mice. Evolution 46: 1146–1163. 10.1111/j.1558-5646.1992.tb00625.x [DOI] [PubMed] [Google Scholar]
- Turner L. M., Harr B., 2014. Genome-wide mapping in a house mouse hybrid zone reveals hybrid sterility loci and Dobzhansky-Muller interactions. eLife 3: e02504 10.7554/eLife.02504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L. M., Schwahn D. J., Harr B., 2012. Reduced male fertility is common but highly variable in form and severity in a natural house mouse hybrid zone. Evolution. 66: 443–458. 10.1111/j.1558-5646.2011.01445.x [DOI] [PubMed] [Google Scholar]
- Turner L. M., White M. A., Tautz D., Payseur B. A., 2014. Genomic networks of hybrid sterility. PLoS Genet. 10: e1004162 10.1371/journal.pgen.1004162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskočilová M., Trachtulec Z., Forejt J., Pialek J., 2005. Does geography matter in hybrid sterility in house mice? Biol. J. Linn. Soc. Lond. 84: 663–674. 10.1111/j.1095-8312.2005.00463.x [DOI] [Google Scholar]
- Vyskočilová M., Pražanová G., Piálek J., 2009. Polymorphism in hybrid male sterility in wild-derived Mus musculus musculus strains on proximal chromosome 17. Mamm. Genome 20: 83–91. 10.1007/s00335-008-9164-3 [DOI] [PubMed] [Google Scholar]
- Wang R. J., White M. A., Payseur B. A., 2015. The pace of hybrid incompatibility evolution in house mice. Genetics 201: 229–242. 10.1534/genetics.115.179499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. A., Steffy B., Wiltshire T., Payseur B. A., 2011. Genetic dissection of a key reproductive barrier between nascent species of house mice. Genetics 189: 289–304. 10.1534/genetics.111.129171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. A., Stubbings M., Dumont B. L., Payseur B. A., 2012. Genetics and evolution of hybrid male sterility in house mice. Genetics 191: 917–934. 10.1534/genetics.112.140251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiki A., Moriwaki K., Sakakura T., Kusakabe M., 1993. Histological studies on male sterility of hybrids between laboratory and wild mouse strains. Dev. Growth Differ. 35: 271–281. 10.1111/j.1440-169X.1993.00271.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Phenotypic and genotypic data are available at Data Dryad (http://datadryad.org; DOI: 10.5061/dryad.vd6k4b2). Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7161119.