Abstract
Motor dysfunction is a common and severe complication of stroke that affects the quality of life of these patients. Currently, motor function rehabilitation predominantly focuses on active movement training; nevertheless, the role of sensory input is usually overlooked. Sensory input is very important to motor function. Voluntary functional movement necessitates preparation, execution, and monitoring functions of the central nervous system, while the monitoring needs the participation of the sensory system. Sensory signals affect motor functions by inputting external environment information and intrinsic physiological status as well as by guiding initiation of the motor system. Recent studies focusing on sensory input-based rehabilitation training for post-stroke dyskinesia have demonstrated that sensory function has significant effects on voluntary functional movements. In conclusion, sensory input plays a crucial role in motor function rehabilitation, and the combined sensorimotor training modality is more effective than conventional motor-oriented approaches.
Keywords: motor rehabilitation, sensorimotor integration, stroke
1. Introduction
Stroke, whether ischemic or hemorrhagic, is a common cerebrovascular event with high disability and mortality rates. It is the leading contributor to secondary movement disorders in elderly patients.[1] Post-stroke dyskinesia is a common and severe complication that affects the quality of life of these patients. Currently, motor function rehabilitation predominantly focuses on active movement training, such as improving muscle strength, controlling convulsions, and adjusting movement patterns.[2,3] However, rehabilitation training based on sensory input has yet to be highlighted.[4]
Voluntary functional movement necessitates preparation, execution, and monitoring functions of the central nervous system; the preparation and execution require involvement of the motor system, while the monitoring needs the participation of the sensory system.[5] In higher-order motor behaviors, the brain must integrate sensory inputs to evaluate the surrounding environment accurately and to produce the corresponding motor outputs.[6] Movement adaptability refers to the ability to adjust constantly to the motor strategy in order to adapt to changes in the environment, which should be based on the feedback of sensory input.[7] Sensory signals affect motor functions in the following 2 ways: inputting external environment information and intrinsic physiological status, and guiding initiation of the motor system.[8]
In this review, we summarize the anatomical basis, relevant experimental studies, and clinical applications of sensory input training as well as discuss the therapeutic effects of sensory input training on motor function rehabilitation after stroke. This review highlights the importance of the sensory component of motor function and illuminates the application value of sensory input training for motor function rehabilitation.
2. Anatomical basis of sensory input training in motor rehabilitation
2.1. Basal ganglia circuit
Sensory afferent nerves directly or indirectly project to the brain stem, cerebellum, subcortex, and cortex. Basal ganglia connect with the frontal lobe, limbic system, and sensory system via the neural circuit; and this circuit participates in the motor control and the integration of cognitive, emotional, and sensorimotor information. Although basal ganglia have no sensory projection fibers, they can govern motor function by processing the sensory information indirectly. Numerous studies have shown that basal ganglia participate in the generation and maintenance of actions in 2 ways: by simultaneously activating the agonistic and antagonistic muscles and maintaining balance, or by sequentially activating the agonistic and antagonistic muscles and generating fast motion.[9] Additionally, basal ganglia can selectively inhibit certain active motions, assisting the body to complete a specific action.[10] Neurophysiological studies have confirmed that basal ganglia are the control center of multi-level sensory input and that abnormal sensorimotor integration is the pathological basis of motor dysfunctions.[11] Among the motor circuit components of the basal ganglia, the substantia nigra, hypothalamus, globus pallidus, and caudate nucleus are the main focus. Moreover, the basal ganglia circuit can be regulated by special dopamine receptors. Sensation-induced phase-related release of dopamine is deemed to be a crucial factor affecting the generation and reinforcement of involuntary movements.[12]
2.2. Cerebellum circuit
The cerebellum directly receives abundant sensory afferent fibers, which play an important role in guiding motion and regulating motor coordination.[13] The cortex-cerebellum circuit connects the frontal lobe, pons, cerebellar cortex, deep cerebellar nucleus, locus ruber, ventrolateral nucleus of the thalamus, and motor cortex, which provide an anatomical basis for the regulation of motor coordination. Moreover, the virus tracing technique has shown that dual fiber connections exist among the basal ganglia, sensorimotor cortex, and cerebellum (Fig. 1).[14,15] The cortex–basal ganglia–cerebellum circuit has an essential role in the motor, cognitive, emotional, and sensory functions in patients with dyskinesia.
Figure 1.
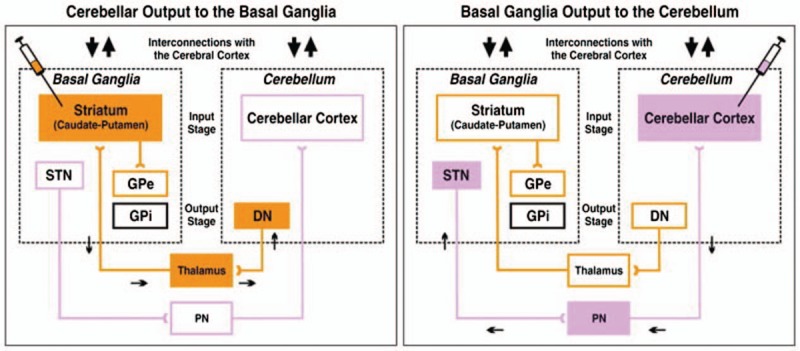
Experimental paradigms and circuits interconnecting the cerebellum and basal ganglia (reference [4]). DN = dentate nucleus, GPe = external segment of the globus pallidus, GPi = internal segment of the globus pallidus, PN = pontine nuclei, STN = subthalamic nucleus.
2.3. Sensorimotor center
Previous studies have shown reciprocal fiber projection between the primary motor area (M1) and the primary sensory area (S1).[16–19] The posterior parietal cortex (PPC) is located rostral to the primary and secondary visual cortex, and caudal to the somatosensory cortex; injury in the PPC can cause cognitive, sensory, or motor dysfunction.[20] The PPC receives afferent fibers from 20 cortical areas and 25 thalamic nuclei, and it projects to 25 cortical areas, based on which the PPC participates in the complicated sensorimotor network. Additionally, the PPC is the sensorimotor integration center for active tactile exploratory motions.
3. Experimental studies of sensory input training in motor rehabilitation
3.1. Animal experiments
A study on mammals has found that sensory input signals by stimulating the skin, muscles, and joints can activate M1 neurons.[21] In addition, Tanji et al have studied the sensorimotor cortex in an unanesthetized monkey; they found that the noncutaneous input activated the caudal part of the M1 and that the cutaneous input primarily activated the caudal part of the M1.[22] Moreover, Xerri et al have demonstrated that the motion control function is impaired in monkeys after neuronal damage in the S1.[23] Damage to the somatosensory cortex usually causes loss of voluntary motor functions and sensation to somatic stimulation. In adult rhesus monkeys, removal of the S1 cortex dominating the distal forearm has been shown to result in severe motor dysfunction and decreased sensation to a tactile stimulus.[24]
Experimental studies on rats have revealed that peripheral nerve injury can cause reorganization of the motor cortex.[25] Additionally, Petersen et al have found that whisker muscles are innervated by cholinergic motor neurons located in S1. Stimulation of M1 drives exploratory rhythmic whisking, while stimulation of S1 drives whisker retraction.[26]
3.2. Experimental studies in humans
Clinical evidence has confirmed the close relationship between sensory function and motor function. Some scholars have noted that a partial or complete loss of sensation impacts the accuracy and coordination of directional movements.[27,28] In a haptically deafferented patient, the loss of sensory input caused a lack of conscious recognition of her own actions.[29] In addition, Kiemel et al have found that light touch can improve postural stability; and they speculated that this may be due to the reinforced consciousness to active movements.[30] Hermsdörfer et al also have noted that the dynamic activation of tactile receptors in the thumb and forefinger guaranteed the stability and accuracy of gripping motions.[31] Furthermore, speech motor outputs are closely correlated with the auditory sensory input.[32]
Functional neuroimaging has demonstrated distinct anatomical structures in the M1 area and cerebellum between musicians and nonmusicians.[33] Some studies have proposed that musical training can reinforce the neural connectivity in certain brain areas;[34–36] furthermore, musical activities, such as playing a musical instrument, can improve the neural plasticity, especially in the frontal and temporal regions.[37,38] These findings indicate that the sensory input can help with motor function rehabilitation. Schneider et al have found that music-supported training can improve the motor functions of the upper extremities in post-stroke patients, via strengthening the cortical functional connections and increasing activation of the motor cortex.[39] In addition, Choi et al have used high-frequency repetitive transcranial magnetic stimulation (rTMS) to stimulate the somatosensory cortex, which resulted in improved sensory discrimination ability, muscular synchronized contraction, as well as motor coordination; these findings suggest that rTMS can enhance sensorimotor integration and promote motor rehabilitation.[40]
The study design was approved by the Ethics Committee of The First Hospital of Jilin University and written informed consent was obtained from each patient.
4. Clinical rehabilitation technologies based on sensory input
4.1. Bobath technique
The Bobath concept considers that post-stroke dyskinesia is due to the loss of control of the superior cerebral center to low-level centers and that the inhibition of primitive reflexes is reduced; thus, the Bobath technique advocates the use of a multi-channel sensory input to prevent motor compensation and to remodel the normal motor status.[41] The Bobath technique also emphasizes the role of sensorimotor integration in motor modulation, suggesting that sensory input training is beneficial for motor rehabilitation in post-stroke patients.[42]
4.2. Proprioceptive neuromuscular facilitation (PNF) approach
PNF refers to a recently advanced form of rehabilitation training involving both the stretching and contraction of targeted muscle groups.[43] This technique is based on human auxology, neurophysiology, and kinesiology. PNF training mobilizes multiple joints and muscle groups, comprehensively using kinesthetics and postural sense to motivate the neuromuscular reaction. This approach modulates the muscular contraction via the proprioceptive sensory system and facilitates motor rehabilitation.[44]
4.3. Rood technique
The Rood technique, also known as multisensory stimulation therapy, is suitable for all subtypes of motor control deficits. This treatment uses sensory stimulation, such as a fast brush or light touch on skin and tapping on the muscle tendon or belly, to motivate or inhibit the neuromuscular reaction. Additionally, this approach uses squeezing, stretching, or light touch to relieve muscular spasms.[45]
4.4. Cognitive-motor training
Recently, cognitive-motor training has been extensively used in post-stroke rehabilitation. Relevant studies have found that short-term cognitive-motor training can improve the gait and equilibrium functions in post-stroke patients; however, determining the long-term efficacy still requires further research.[46] Additionally, cognitive-motor training can be employed to predict the risk of falling in elderly patients.[47]
4.5. Virtual reality (VR) technology
VR rehabilitation is based on the theory that the central processing of postural stability and spatial direction sense rely on multi-sensory input and the requirement for specific motions.[48] VR rehabilitation can provide standardized or individualized intervention on patients’ motor functions in a circumstance with a multi-dimensional sensory input.
4.6. Music-based intervention
In recent years, music-based intervention has been widely used in neurorehabilitation, and it has shown remarkable efficacy in improving motor functions.[49] During gait training, rhythmic sound stimulation can significantly improve a patient's walking function, especially in terms of posture control, balance, walking velocity, stride length, standing time, walking rhythm, and symmetry.[50,51] Another study also has observed that music-based rehabilitation significantly improves the motor function of hemiplegic upper limbs.[39] Of note, “mute” musical instruments did not provide an obvious benefit, indicating that the functional improvement was associated with the music sensory input.[52] In addition, Altenmüller et al have administered a music-based intervention including self-paced movements of the index finger (MIDI-piano) and of the whole arm (drum pads), and they found that the music-supported therapy yielded significant improvement in both gross and fine motor functions of the hands; they speculated that the efficacy may be related to the external auditory feedback and neural reorganization induced by the melody and rhythm of music.[53]
4.7. Sensory-motor training in Parkinson's disease
Sensory input-based training is also a hot area of research in the rehabilitation of Parkinson's disease patients. For example, Taghizadeh et al have found that sensory-motor training for 2 weeks could improve both sensory performance (such as tactile acuity, wrist proprioception, and weight and texture discrimination) and upper extremity motor function in patients with Parkinson's disease; while these efficacies were limited to patients who had a score of 1 to 3 according to the Hoehn and Yahr Scale.[54] Recently, nondrug treatments, especially music-based motor training, have been found to be effective for the motor functional rehabilitation of Parkinson's disease patients.[47] Music can stimulate interactions between the sensory and motor systems, which may be helpful for evoking voluntary movements.[55]
4.8. Sensory input training on motor function recovery by interactions with neuroinflammation
Inflammation plays an important role in the pathogenesis of ischemic stroke and some metabolic diseases, and stroke represents an important central nervous system complication.[56,57] Pretreatment with anti-inflammatory drugs for acute ischemic stroke may help patients achieve a favorable outcome.[58] The sensory input training strategy may enhance motor rehabilitation through anti-apoptotic, neuroprotective, and anti-inflammatory effects.[59]
5. Conclusion
In conclusion, sensory input plays a crucial role in motor rehabilitation (Fig. 2), and impairment of the sensory system can impact the motor functions. Therefore, sensory input should be highlighted in post-stroke rehabilitation. Our analysis indicates that a combined sensorimotor training modality is more effective than conventional motor-oriented approaches.
Figure 2.
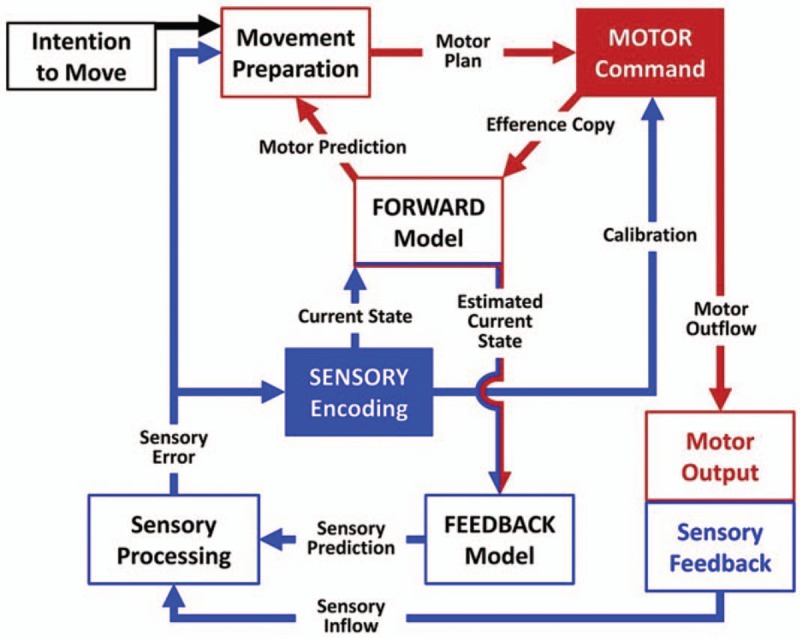
Sensory-motor integration circuits (reference [33]).
Author contributions
Data curation: Xiaowei Chen, Zhaohong Yan, Xunchan Liu.
Formal analysis: Fuqian Liu, Shihuan Cheng, He Li.
Funding acquisition: Zhenlan Li.
Investigation: Shihuan Cheng, Xunchan Liu.
Methodology: Fuqian Liu.
Resources: Fuqian Liu.
Validation: Zhaohong Yan, He Li.
Writing – original draft: Xiaowei Chen.
Writing – review & editing: Zhenlan Li.
Footnotes
Abbreviations: M1 = primary motor area, PNF = proprioceptive neuromuscular facilitation, PPC = posterior parietal cortex, rTMS = repetitive transcranial magnetic stimulation, S1 = primary sensory area, VR = virtual reality.
This work was supported by Changchun Municipal Science and Technology Bureau Major Medical and Health Industry Science and Technology Projects.
The authors have no conflicts of interest to disclose.
References
- [1].Brunner IC, Skouen JS, Strand LI. Recovery of upper extremity motor function post stroke with regard to eligibility for constraint-induced movement therapy. Top Stroke Rehabil 2011;18:248–57. [DOI] [PubMed] [Google Scholar]
- [2].Koh CL, Pan SL, Jeng JS, et al. Predicting recovery of voluntary upper extremity movement in subacute stroke patients with severe upper extremity paresis. PLoS One 2015;10:e0126857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mouawad MR, Doust CG, Max MD, et al. Wii-based movement therapy to promote improved upper extremity function post-stroke: a pilot study. J Rehabil Med 2011;43:527–33. [DOI] [PubMed] [Google Scholar]
- [4].Richards LG, Stewart KC, Woodbury ML, et al. Movement-dependent stroke recovery: a systematic review and meta-analysis of TMS and fMRI evidence. Neuropsychologia 2008;46:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Perruchoud D, Murray MM, Lefebvre J, et al. Focal dystonia and the sensory-motor integrative loop for enacting (SMILE). Front Hum Neurosci 2014;8:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].O'Sullivan SB, Schmitz TJ, Fulk GD. Physical Rehabilitation, 6th edition, F A Davis Co. 2014:p. 87. [Google Scholar]
- [7].Papale AE, Hooks BM. Circuit changes in motor cortex during motor skill learning. Neuroscience 2018;368:283–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Patel N, Jankovic J, Hallett M. Sensory aspects of movement disorders. Lancet Neurol 2014;13:100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hemami H, Moussavi Z. A model of the basal ganglia in voluntary movement and postural reactions. Comput Methods Biomech Biomed Engin 2014;17:1432–46. [DOI] [PubMed] [Google Scholar]
- [10].Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol 2003;60:1365–8. [DOI] [PubMed] [Google Scholar]
- [11].Rajagopal S, Seri, Cavanna AE. Premonitory urges and sensorimotor processing in Tourette syndrome. Behav Neurol 2013;27:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Redgrave P, Vautrelle N, Reynolds JN. Functional properties of the basal ganglia's re-entrant loop architecture: selection and reinforcement. Neuroscience 2011;198:138–51. [DOI] [PubMed] [Google Scholar]
- [13].Koziol LF, Budding DE, Chidekel D. From movement to thought: executive function, embodied cognition, and the cerebellum. Cerebellum 2012;11:505–25. [DOI] [PubMed] [Google Scholar]
- [14].Hoshi E, Tremblay L, Féger J, et al. The cerebellum communicates with the basal ganglia. Nat Neurosci 2005;8:1491–3. [DOI] [PubMed] [Google Scholar]
- [15].Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev 2010;20:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen JL, Carta S, Soldado-Magraner J, et al. Behaviour-dependent recruitment of long-range projection neurons in somatosensory cortex. Nature 2013;499:336–40. [DOI] [PubMed] [Google Scholar]
- [17].Kastner S, Chen Q, Jeong SK, et al. A brief comparative review of primate posterior parietal cortex: a novel hypothesis on the human toolmaker. Neuropsychologia 2017;105:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee S, Kruglikov I, Huang ZJ, et al. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci 2013;16:1662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mao T, Kusefoglu D, Hooks B, et al. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron 2011;72:111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mohan H, de Haan R, Mansvelder HD, et al. The posterior parietal cortex as integrative hub for whisker sensorimotor information. Neuroscience 2018;368:240–5. [DOI] [PubMed] [Google Scholar]
- [21].Strick PL, Preston JB. Two representations of the hand in area 4 of a primate. II. Somatosensory input organization. J Neurophysiol 1982;48:150–9. [DOI] [PubMed] [Google Scholar]
- [22].Tanji J, Wise SP. Submodality distribution in sensorimotor cortex of the unanesthetized monkey. J Neurophysiol 1981;45:467–81. [DOI] [PubMed] [Google Scholar]
- [23].Xerri C, Merzenich MM, Peterson BE, et al. Plasticity of primary somatosensory cortex paralleling sensorimotor skill recovery from stroke in adult monkeys. J Neurophysiol 1998;79:2119–48. [DOI] [PubMed] [Google Scholar]
- [24].Lamotte RH, Mountcastle VB. Disorders in somesthesis following lesions of parietal lobe. J Neurophysiol 1979;42:400. [DOI] [PubMed] [Google Scholar]
- [25].Sanes JN, Suner S, Donoghue JP. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Exp Brain Res 1990;79:479–91. [DOI] [PubMed] [Google Scholar]
- [26].Petersen CCH. Cortical control of whisker movement. Neuroscience 2013;37:183–203. [DOI] [PubMed] [Google Scholar]
- [27].Stenneken P, Prinz W, Cole J, et al. The effect of sensory feedback on the timing of movements: evidence from deafferented patients. Brain Res 2006;1084:123–31. [DOI] [PubMed] [Google Scholar]
- [28].Levin MF, Lamarre Y, Feldman AG. Control variables and proprioceptive feedback in fast single-joint movement. Can J Physiol Pharmacol 1995;73:316–30. [DOI] [PubMed] [Google Scholar]
- [29].Fourneret P, Paillard J, Lamarre Y, et al. Lack of conscious recognition of one's own actions in a haptically deafferented patient. Neuroreport 2002;13:541–7. [DOI] [PubMed] [Google Scholar]
- [30].Kiemel T, Oie KS, Jeka JJ. Multisensory fusion and the stochastic structure of postural sway. Biol Cybern 2002;87:262–77. [DOI] [PubMed] [Google Scholar]
- [31].Hermsdörfer J, Elias Z, Cole JD, et al. Preserved and impaired aspects of feed-forward grip force control after chronic somatosensory deafferentation. Neurorehabil Neural Repair 2008;22:374–84. [DOI] [PubMed] [Google Scholar]
- [32].Cogan GB, Thesen T, Carlson C, et al. Sensory-motor transformations for speech occur bilaterally. Nature 2014;507:94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Herholz Sibylle C, Zatorre Robert J. Musical training as a framework for brain plasticity: behavior, function, and structure. Neuron 2012;76:486–502. [DOI] [PubMed] [Google Scholar]
- [34].SK Brain correlates of music-evoked emotions. Nat Rev Neurosci 2014;15:170–80. [DOI] [PubMed] [Google Scholar]
- [35].Särkämö T, Tervaniemi M, Huotilainen M. Music perception and cognition: development, neural basis, and rehabilitative use of music. Wiley Interdiscip Rev Cogn Sci 2013;4:441–51. [DOI] [PubMed] [Google Scholar]
- [36].Alluri V, Toiviainen P, Jääskeläinen IP, et al. Large-scale brain networks emerge from dynamic processing of musical timbre, key and rhythm. Neuroimage 2012;59:3677–89. [DOI] [PubMed] [Google Scholar]
- [37].Schlaug G. Musicians and music making as a model for the study of brain plasticity. Progress in. Brain Res 2015;217:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vaquero L, Hartmann K, Ripollés P, et al. Structural neuroplasticity in expert pianists depends on the age of musical training onset. Neuroimage 2016;126:106–19. [DOI] [PubMed] [Google Scholar]
- [39].Schneider S, Schönle PW, Altenmüller E, et al. Using musical instruments to improve motor skill recovery following a stroke. J Neurol 2007;254:1339–46. [DOI] [PubMed] [Google Scholar]
- [40].Choi EH, Yoo WK, Ohn SH, et al. Enhancement of motor coordination by applying high frequency repetitive TMS on the sensory cortex. J Electromyogr Kinesiol 2016;28:17–22. [DOI] [PubMed] [Google Scholar]
- [41].Levin MF, Panturin E. Sensorimotor integration for functional recovery and the Bobath approach. Motor Control 2011;15:285–301. [DOI] [PubMed] [Google Scholar]
- [42].Gjelsvik BEB. The Bobath concept in adult neurology: Stuttgart Georg Thieme Verlag; 2008. [Google Scholar]
- [43].Adler SS, Beckers D, Buck M. PNF in Practice. 3rd edition. Springer, 2008; 94:355–6. [Google Scholar]
- [44].Guiutula FX, Cabanasvaldés R, Sitjàrabert M, et al. The efficacy of the proprioceptive neuromuscular facilitation (PNF) approach in stroke rehabilitation to improve basic activities of daily living and quality of life: a systematic review and meta-analysis protocol. BMJ Open 2017;7:e016739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stockmeyer SA. An interpretation of the approach of rood to the treatment of neuromuscular dysfunction. Am J Phys Med 1967;46:900–61. [PubMed] [Google Scholar]
- [46].Wang XQ, Pi YL, Chen BL, et al. Cognitive motor interference for gait and balance in stroke: a systematic review and meta-analysis. Eur J Neurol 2015;22:555-e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhang S, Liu D, Ye D, et al. Can music-based movement therapy improve motor dysfunction in patients with Parkinson's disease? Systematic review and meta-analysis. Neurol Sci 2017;38:1–8. [DOI] [PubMed] [Google Scholar]
- [48].Shumway-Cook A, Woollacott M. Attentional demands and postural control: the effect of sensory context. J Gerontol 2000;55:M10-6. [DOI] [PubMed] [Google Scholar]
- [49].Sihvonen AJ, Särkämö T, Leo V, et al. Music-based interventions in neurological rehabilitation. Lancet Neurol 2017;16:648–60. [DOI] [PubMed] [Google Scholar]
- [50].Cha Y, Kim Y, Hwang S, et al. Intensive gait training with rhythmic auditory stimulation in individuals with chronic hemiparetic stroke: a pilot randomized controlled study. Neurorehabilitation 2014;35:681–8. [DOI] [PubMed] [Google Scholar]
- [51].Thaut MH, Leins AK, Rice RR, et al. Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: a single-blind, randomized trial. Neurorehabil Neural Repair 2007;21:455–9. [DOI] [PubMed] [Google Scholar]
- [52].Tong Y, Forreider B, Sun X, et al. Music-supported therapy (MST) in improving post-stroke patients’ upper-limb motor function: a randomised controlled pilot study. Neurol Res 2015;37:434–40. [DOI] [PubMed] [Google Scholar]
- [53].Altenmüller E, Marco-Pallares J, Münte TF, et al. Neural reorganization underlies improvement in stroke-induced motor dysfunction by music-supported therapy. Ann New York Acad Sci 2009;1169:395–405. [DOI] [PubMed] [Google Scholar]
- [54].Taghizadeh G, Azad A, Kashefi S, et al. The effect of sensory-motor training on hand and upper extremity sensory and motor function in patients with idiopathic Parkinson disease. J Hand Ther 2017;pii: S0894-1130(17)30004-2. [DOI] [PubMed] [Google Scholar]
- [55].Trombetti A, Hars M, Herrmann FR, et al. Effect of music-based multitask training on gait, balance, and fall risk in elderly people: a randomized controlled trial. Arch Intern Med 2011;171:525–33. [DOI] [PubMed] [Google Scholar]
- [56].Tuttolomondo A, Pecoraro R, Simonetta I, et al. Neurological complications of Anderson-Fabry disease. Curr Pharm Des 2013;19:6014–30. [DOI] [PubMed] [Google Scholar]
- [57].Tuttolomondo A, Pecoraro R, Simonetta I, et al. Anderson-Fabry disease: a multiorgan disease. Curr Pharm Des 2013;19:5974–96. [DOI] [PubMed] [Google Scholar]
- [58].Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: a retrospective chart review from the GIFA study. Int J Cardiol 2011;151:318–22. [DOI] [PubMed] [Google Scholar]
- [59].Chan HH, Wathen CA, Mathews ND, et al. Lateral cerebellar nucleus stimulation promotes motor recovery and suppresses neuroinflammation in a fluid percussion injury rodent model. Brain Stimul 2018;11:1356–67. [DOI] [PubMed] [Google Scholar]


