Abstract
The ratio between the absolute number of neutrophils and the number of lymphocytes (NLR) has recently emerged as a potential new biomarker predicting worse clinical conditions ranging from infectious disease to cardiovascular disease. Prognostic significance of NLR in patients with ST-elevation myocardial infarction (STEMI) is not established. This study aimed to investigate prognostic impact of NLR in patients with STEMI.
We analyzed the data and clinical outcomes of 30-day survivors with STEMI who received successful coronary intervention from 2006 to 2010. NLR was computed from the absolute values of neutrophils and lymphocytes from the complete blood count at admission. Occurrence of major adverse cardiovascular events (MACEs; death, recurrent MI, target vessel revascularization (TVR)) at 5 years was evaluated.
We enrolled 326 patients and mean follow-up duration was 68 ± 36 months. The mean NLR was 4.7 ± 5.2. Among all patients, all-cause mortality occurred in 46 patients (14%). Initial NLR was higher in patients who experienced all-cause mortality (6.39 ± 8.9 vs 4.2 ± 3.1, P = .004). In a multivariate regression model, the higher NLR was independently associated with increased risk for all-cause mortality (Hazard ratio, 1.085; 95% confidence interval, 1.002–1.174, P = .044).
Increased NLR was associated increased rate of all-cause mortality in 30-day survivors after index STEMI, who received successful coronary intervention.
Keywords: myocardial infarction, neutrophil to lymphocyte, prognosis
1. Introduction
Myocardial infarction (MI) is associated with an inflammatory reaction, which is a prerequisite for healing and scar formation.[1–3] Our previous study demonstrated that epicardial adipose tissue, representing local inflammation, closely related to long-term clinical outcomes in patients with ST-elevation MI (STEMI).[4] Systemic inflammation also has a pivotal role in adverse outcomes in patients with STEMI.[5–7]
One of circulating inflammatory markers, the ratio between the absolute number of neutrophils and the number of lymphocytes (NLR) has been recently demonstrated as a predictor of worse clinical conditions ranging from infectious disease to cardiovascular disease.[8–10] Prognostic significance of NLR in patients with ST-elevation MI (STEMI) is not established. This study aimed to investigate prognostic impact of NLR in patients with STEMI.
2. Materials and methods
We consecutively enrolled 30-day survivors after STEMI, who underwent successful revascularization. Successful revascularization was defined as thrombolysis in myocardial infarction trial (TIMI) grade 3 flow and <30% residual stenosis in infarct-related artery after primary percutaneous coronary intervention (PCI). Medical records of all patients were retrospectively reviewed. This study was approved by the Ajou University Hospital Institutional Review Board (approval number: AJIRB-MED-MDB-17–193). We excluded patients from this study if they had any of the following: a history of prior revascularization, left ventricular (LV) dysfunction caused by predisposing cardiomyopathy or severe valvular heart disease including symptomatic aortic stenosis or more than moderate aortic and mitral regurgitation, active inflammation (such as infection or systemic autoimmune disease) or malignancy.
Venous blood was collected in all the patients at admission, preceding primary PCI. Total white blood cell count and neutrophil, lymphocyte, and monocyte counts were calculated using an automated blood cell counter. NLR, calculated as total neutrophil counts divided by total lymphocyte counts, was computed from the absolute values of neutrophils and lymphocytes from the same blood sample collected at admission.
Occurrence of major adverse cardiovascular events (MACEs), including all-cause mortality, recurrent myocardial infarction (MI), and target vessel revascularization (TVR), within 5 years was evaluated. Recurrent myocardial infarction was defined according to the universal definition of MI.[11] The TVR was defined as clinically indicated percutaneous or surgical revascularization of the index vessel during follow-up. At 5 years after index STEMI, follow-up data were obtained by reviewing medical records and/or telephone interview with patients.
SPSS 18.0 statistical software package (SPSS, Chicago, IL) was used for all calculations. Data are shown as the mean ± standard deviation for continuous variables and as numbers and percentages for categorical variables. Comparisons were conducted by unpaired Student t test for continuous variables and Pearson chi-square test for categorical variables. Multivariate regression analysis was performed to assess the effect of NLR on clinical outcomes. NLR was compared by MACEs controlling for additional well-known risk factors of MACEs. These predictors were age, the presence of hypertension, diabetes mellitus and dyslipidemia, family history, smoking, and LV ejection fraction (EF). By constructing a receiver operating characteristic curve, we derived a cut-off value of NLR for MACEs. Based this cut-off value, all patients were reclassified into a high NLR group and a low NLR group. Event-free survival analysis for patients in these groups was performed using the Kaplan–Meier method, and the differences between groups were assessed by the log-rank test. Null hypotheses of no difference were rejected if P values were <.05.
3. Results
From 2006 to 2010, a total of 326 patients (254 males, 58 ± 12-year-old) were enrolled. The mean follow-up interval was 68 ± 36 months. Mean value of NLR of these patients was 4.7 ± 5.2.
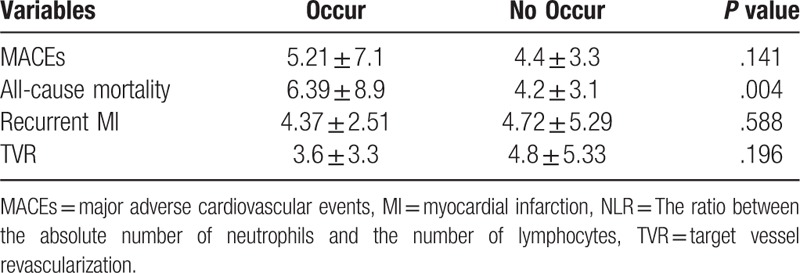
The MACEs was occurred in 90 patients (28%). Of 326 patients, 46 patients died (14%), 10 patients experienced recurrent MI (3%), and 53 patients needed TVR (16%). According to the occurrence of MACEs, all patients were regrouped and NLR level was compared by unpaired Student t test (Table 1). Initial NLR was significantly higher in patients who experienced all-cause mortality (6.39 ± 8.9 vs 4.2 ± 3.1, P = .004). There was no statistical difference according to the occurrence of MACEs, recurrent MI, and TVR.
Table 1.
Initial NLR according to occurrence of MACEs.
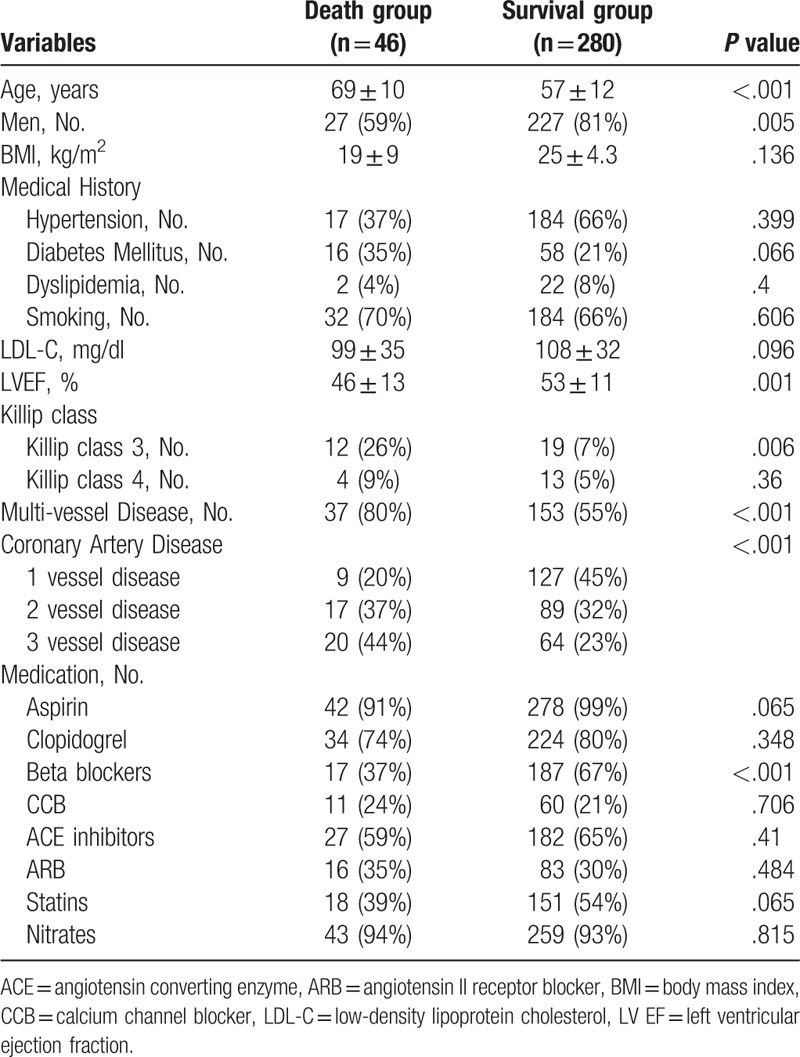
Baseline clinical characteristics by all-cause mortality are summarized in Table 2. Patients in the death group were older (69 ± 10 vs 57 ± 12-year-old, P <.001), had less males (59 vs 81%, P <.001) than patients in the survival group. In the medical history, there was no statistical difference between the groups. In the death group, LV dysfunction was more prevalent (EF: 46 ± 13 vs 53 ± 11%, P = .001 and killip class 3: 26 vs 7%, P = .006). Compared to the survival group, subjects in the death group had significantly higher rate of multi-vessel disease (80 vs 55%, P <.001). The death group had less beta-blocker use (37 vs 67%, P <.001).
Table 2.
Baseline characteristics by all-cause mortality.
In a multivariate regression model, old age, diabetes, dyslipidemia, smoking, LV EF, and NLR were strongly related to all-cause mortality (Table 3). The higher NLR was independently associated with increased with for all-cause mortality (Hazard ratio, 1.085; 95% confidence interval, 1.002–1.174, P = .044).
Table 3.
Multiple logistic regression analysis of the clinical predictors for all-cause mortality.

For predicting all-cause mortality, the cut-off value of NLR was 4.3 with a sensitivity and specificity of 57% and 61%, respectively (area under the curve = 0.566, 95% confidence interval = 0.48–0.652, P = .044). When the patients were reclassified based on the derived cut-off value of NLR for all-cause mortality, the high NLR group (NLR ≥4.3) consisted of 136 patients (98 males) with a mean age of 61 ± 13 years. The low NLR group (NLR <4.3 mm) consisted of 190 patients (156 males) with a mean age of 57 ± 12 years. The high NLR group was significantly older than the low NLR group (P <.001). There were more males in the high NLR group (P = .036). Kaplan–Meier analysis revealed that survival rate in the high NLR group was significantly lower than in the low NLR group (log-rank P = .024, Fig. 1). The event-free survival curves for freedom of MACEs and TVR were not significantly different between the groups (log-rank P = .307 and .063, respectively). In addition, the recurrent MI-free survival rate in the high NLR group tended to be lower than in the low NLR group (log-rank P = .052).
Figure 1.
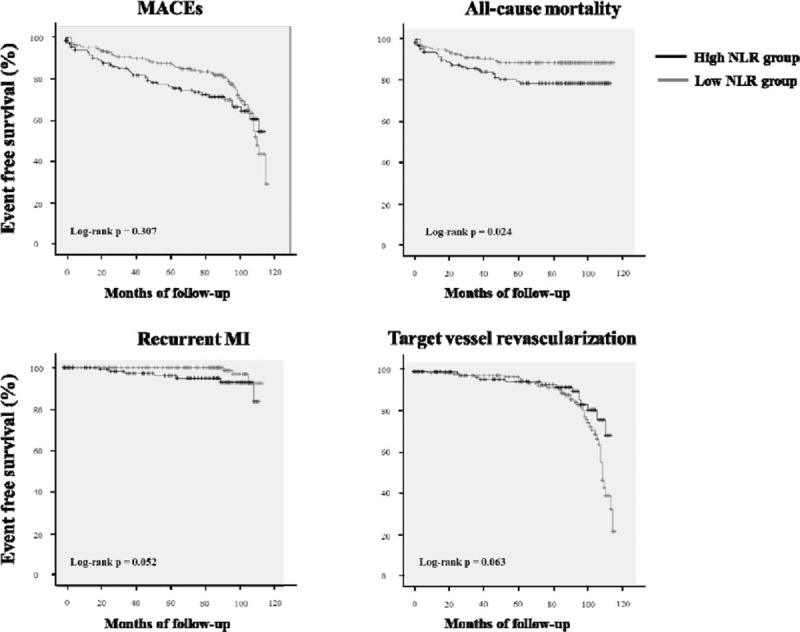
Kaplan–Meier survival curve in the high NLR group and the low NLR group. MACEs = major adverse cardiovascular events, MI = myocardial infarction, NLR = the ratio between the absolute number of neutrophils and the number of lymphocytes.
4. Discussion and conclusions
The present study demonstrated the close relationship between higher NLR and increased rate of all-cause mortality in patients with STEMI.
Patients with inflammatory or autoimmune disorders are well-known to have an increased risk of cardiovascular disease, which is most likely the consequence of inflammatory responses affecting plaque destabilization.[12] Most of studies found that systemic inflammation independently predicts MACEs.[5,6,7] Some emerging inflammatory markers, such as neopterin, uric acid, and circulation micro ribonucleic acid, have been suggested as markers of disease activity or inflammation in patients with cardiovascular disease.[13–15] Independent of lipid-lowering, anti-inflammatory therapy was demonstrated to significantly lower MACEs.[16] Necrotic cell death in response to infarction stimulates a host inflammatory response.[17] STEMI can result in obstructed blood flow, with myocardial necrosis and inflammation as a consequences.[9] In STEMI, inflammatory cells are also functionally activated.[18] After PCI, functionally active neutrophil infiltration is aggravated locally in the balloon-injured arteries.[19]
Neutrophils are mostly related to an acute inflammatory reaction on cardiac injury.[9] Acute inflammatory reaction, reflected by high C-reactive protein promoting tissue factor, integrin mediating the fast recruitment of neutrophils and cellular adhesion molecules mediating the transmigration of neutrophils, is related with proatherothombotic state.[20–22] Neutrophils mediate increased plaque rupture and thrombosis by secreting proteolytic enzymes causing vascular damage, activation of coagulation pathways, micro vascular plugging and myocyte necrosis mediated by secretion of pro-inflammatory cytokines.[10] By releasing oxygen free radicals, functionally active neutrophils modulate the electrical activity of the myocardium causing arrhythmogenesis.[23] Neutrophil count also associates with myocardial extension of the areas of infarction, development of heart failure and impaired epicardial and microvascular perfusion.[5,24] Activated neutrophils contribute to further cellular and intracellular edema and clogging of microvasculature resulting in no-reflow phenomenon and abnormal myocardial perfusion.[25] Although none of the patients have moderate or severe LV dysfunction in the present study, the myocardial structural and functional alteration beyond EF representing LV systolic function, which might be mediated by inflammatory reaction, might affect the adverse clinical outcomes after STEMI.
Lymphocytes are known to have protective effect on clinical outcomes in cardiovascular disease.[9] Critical inflammatory states induce lymphopenia due to increased lymphocyte apoptosis.[26] After STEMI, physiological stress, and the subsequent activation of the neurohormonal system, which lead to cortisol release, also result in lymphopenia related strongly to LV dysfunction and high myocardial mass destruction.[27] Cortisol is significantly raised in acute MI and correlates with the development of LV dysfunction, arrhythmogenesis, cardiogenic shock, and in-hospital mortality.[28] However, cortisol levels, itself, have limitations for clinical prognostic factor due to their variations associated with diurnal rhythm, psychological, or physical stress.
The NLR could act as a combined surrogate marker for both acute inflammatory reaction and activated neurohormonal system. As a consequence, NLR could be a more potent clinical predictor after STEMI than other inflammatory markers and markers representing activated neurohormonal system, such as cortisol, norepinephrine, and angiotensin II. The NLR might be related with serious post-MI complication, such as acute thrombosis, lethal arrhythmias, cardiogenic shock, LV remodeling, or LV dysfunction. The present study demonstrated that NLR was independent predictor for all-cause mortality in patients with STEMI. It suggests that NLR has incremental value for predicting adverse clinical outcomes after STEMI over traditional cardiac risk factors. As total white blood cell count including neutrophil, lymphocyte, and monocyte counts is routinely checked in patients with STEMI, NLR may be readily available at no extra cost.
There are several limitations to the present study. First, the present study logically implied that NLR might be related to LV remodeling or LV dysfunction after STEMI. Due to early intervention, most of patients had mild LV dysfunction or normal LV function. Myocardial structural and functional alterations beyond EF might affect adverse clinical outcomes. In our previous study, we demonstrated, using the speckle tracking imaging, that the myocardial fibrotic process may affect the early contractile dysfunction of LV even in patients with normal EF.[29] Considering well-known incidences of serious post-MI complications, NLR might also be related with acute thrombosis including stent thrombosis or stroke resulting in increased rate of all-cause mortality. As autopsy was not performed, the cause of death was not fully evaluated in the present study. Second, levels of other inflammatory markers or markers representing activated neurohormonal system, such as cortisol, norepinephrine, and angiotensin II were not checked in the present study. Although NLR, a combined surrogate marker for both acute inflammatory reaction and activated neurohormonal system, might be more potent than these other surrogate markers, there is lack of comparison studies with these markers and NLR. To prove this, further studies might be needed. Fourth, the results of the present study could not be applied to the general population. The derived cut-off value of NLR was 4.3 mm for predicting all-cause mortality. A normal upper-limit value for NLR has not been established. Also, it was a cross-sectional study, not a cohort study. Therefore, the derived cut-off value of the study could not be applied to the general population.
In conclusion, increased NLR was associated increased rate of all-cause mortality in 30-day survivors after index STEMI, who received successful coronary intervention. In STEMI, baseline NLR could help to assess the prognosis and a more intensive treatment might be needed for patients with increased NLR.
Author contributions
Conceptualization: Jin-Sun Park.
Data curation: Gyo-Seung Hwang.
Formal analysis: Byoung-Joo Choi.
Methodology: Kyoung-Woo Seo.
Resources: So-Yeon Choi.
Software: Myeong-Ho Yoon.
Supervision: Joon-Han Shin.
Validation: Seung-Jea Tahk.
Writing – original draft: Jin-Sun Park.
Writing – review & editing: Jin-Sun Park.
Footnotes
Abbreviations: EF= ejection fraction, LV = left ventricular, MACEs = major adverse cardiovascular events, NLR = the ratio between the absolute number of neutrophils and the number of lymphocytes, PCI = percutaneous coronary intervention, STEMI = ST-elevation myocardial infarction, TVR = target vessel revascularization.
This work was supported by the new faculty research fund of Ajou University School of Medicine.
The authors report no conflicts of interest.
References
- [1].Entman ML, Smith CW. Postreperfusion inflammation: a model for reaction to injury in cardiovascular disease. Cardiovasc Res 1994;28:1301–11. [DOI] [PubMed] [Google Scholar]
- [2].Frangogiannis NG, Youker KA, Rossen RD, et al. Cytokines and the microcirculation in ischemia and reperfusion. J Mol Cell Cardiol 1998;30:2567–76. [DOI] [PubMed] [Google Scholar]
- [3].Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 2002;53:31–47. [DOI] [PubMed] [Google Scholar]
- [4].Park JS, Lee YH, Seo KW, et al. Echocardiographic epicardial fat thickness is a predictor for target vessel revascularization in patients with ST-elevation myocardial infarction. Lipids Health Dis 2016;15:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Husser O, Bodi V, Sanchis J, et al. White blood cell subtypes after STEMI: temporal evolution, association with cardiovascular magnetic resonance—derived infarct size and impact on outcome. Inflammation 2011;34:73–84. [DOI] [PubMed] [Google Scholar]
- [6].Anzai T, Yoshikawa T, Takahashi T, et al. Early use of beta-blockers is associated with attenuation of serum C-reactive protein elevation and favorable short-term prognosis after acute myocardial infarction. Cardiology 2003;99:47–53. [DOI] [PubMed] [Google Scholar]
- [7].Carrick D, Haig C, Rauhalammi S, et al. Pathophysiology of LV remodeling in survivors of STEMI: inflammation, remote myocardium, and prognosis. JACC Cardiovasc Imaging 2015;8:779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Arbel Y, Shacham Y, Ziv-Baran T, et al. Higher neutrophil/lymphocyte ratio is related to lower ejection fraction and higher long-term all-cause mortality in ST-elevation myocardial infarction patients. Can J Cardiol 2014;30:1177–82. [DOI] [PubMed] [Google Scholar]
- [9].Meeuwsen JAL, Wesseling M, Hoefer IE, et al. Prognostic value of circulating inflammatory cells in patients with stable and acute coronary artery disease. Front Cardiovasc Med 2017;4:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sawant AC, Adhikari P, Narra SR, et al. Neutrophil to lymphocyte ratio predicts short- and long-term mortality following revascularization therapy for ST elevation myocardial infarction. Cardiol J 2014;21:500–8. [DOI] [PubMed] [Google Scholar]
- [11].Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation 2007;116:2634–53. [DOI] [PubMed] [Google Scholar]
- [12].Raffel OC, Tearney GJ, Gauthier DD, et al. Relationship between a systemic inflammatory marker, plaque inflammation, and plaque characteristics determined by intravascular optical coherence tomography. Arterioscler Thromb Vasc Biol 2007;27:1820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pacileo M, Cirillo P, De Rosa S, et al. The role of neopterin in cardiovascular disease. Monaldi Arch Chest Dis 2007;68:68–73. [DOI] [PubMed] [Google Scholar]
- [14].Prasad M, Matteson EL, Herrmann J, et al. Uric acid is associated with inflammation, coronary microvascular dysfunction, and adverse outcomes in postmenopausal women. Hypertension 2017;69:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].De Rosa S, Indolfi C. Circulating microRNAs as biomarkers in cardiovascular diseases. EXS 2015;106:139–49. [DOI] [PubMed] [Google Scholar]
- [16].Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- [17].Majno G, La Gattuta M, Thompson TE. Cellular death and necrosis: chemical, physical and morphologic changes in rat liver. Virchows Arch Pathol Anat Physiol Klin Med 1960;333:41–65. [DOI] [PubMed] [Google Scholar]
- [18].De Servi S, Mazzone A, Ricevuti G, et al. Clinical and angiographic correlates of leukocyte activation in unstable angina. J Am Coll Cardiol 1995;26:1146–50. [DOI] [PubMed] [Google Scholar]
- [19].Welt FG, Edelman ER, Simon DI, et al. Neutrophil, not macrophage, infiltration precedes neointimal thickening in balloon-injured arteries. Arterioscler Thromb Vasc Biol 2000;20:2553–8. [DOI] [PubMed] [Google Scholar]
- [20].Cirillo P1, Golino P, Calabrò P, et al. C-reactive protein induces tissue factor expression and promotes smooth muscle and endothelial cell proliferation. Cardiovasc Res 2005;68:47–55. [DOI] [PubMed] [Google Scholar]
- [21].Wolf D, Anto-Michel N, Blankenbach H, et al. A ligand-specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense. Nat Commun 2018;9:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cirillo P, Pacileo M, De Rosa S, et al. HMG-CoA reductase inhibitors reduce nicotine-induced expression of cellular adhesion molecules in cultured human coronary endothelial cells. J Vasc Res 2007;44:460–70. [DOI] [PubMed] [Google Scholar]
- [23].Lucchesi BR, Werns SW, Fantone JC. The role of the neutrophil and free radicals in ischemic myocardial injury. J Mol Cell Cardiol 1989;21:1241–51. [DOI] [PubMed] [Google Scholar]
- [24].Sezer M, Okcular I, Goren T, et al. Association of haematological indices with the degree of microvascular injury in patients with acute anterior wall myocardial infarction treated with primary percutaneous coronary intervention. Heart 2007;93:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sen N, Afsar B, Ozcan F, et al. The neutrophil to lymphocyte ratio was associated with impaired myocardial perfusion and long term adverse outcome in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Atherosclerosis 2013;228:203–10. [DOI] [PubMed] [Google Scholar]
- [26].Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–50. [DOI] [PubMed] [Google Scholar]
- [27].Blum A, Sclarovsky S, Rehavia E, et al. Levels of T-lymphocyte subpopulations, interleukin-1 beta, and soluble interleukin-2 receptor in acute myocardial infarction. Am Heart J 1994;127:1226–30. [DOI] [PubMed] [Google Scholar]
- [28].Jutla SK, Yuyun MF, Quinn PA, et al. Plasma cortisol and prognosis of patients with acute myocardial infarction. J Cardiovasc Med (Hagerstown) 2014;15:33–41. [DOI] [PubMed] [Google Scholar]
- [29].Kang SJ, Lim HS, Choi BJ, et al. Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase-1, a marker of myocardial fibrosis, in patients with hypertension. J Am Soc Echocardiogr 2008;21:907–11. [DOI] [PubMed] [Google Scholar]


