Abstract
Silent cerebrovascular diseases, including silent brain infarcts (SBI), white matter hyperintensity (WMH), and cerebral microbleed, are closely correlated with stroke progression. The purpose of this study was to investigate the prevalence and potential risk factors of SBI and WMH in young patients with first-ever stroke.
A total of 400 young patients with first-ever stroke were included in this study and received magnetic resonance imaging test. The distributions of stroke subtypes were analyzed based on patients’ age and gender. The prevalence of SBI and WMH was evaluated in different age groups and stroke subtypes. Independent risk factors for SBI and WMH were identified using logistic regression analysis.
The distribution of stroke subtypes was not correlated with patients’ age or gender in our study. The incidence of SBI and WMH among all of the young stroke patients was 14.50% and 8.75%, respectively, which showed an upward tendency with age. The percentages of both SBI and WMH were significantly higher in small-vessel disease patients than in cases with other subtype diseases (all P < .05). Hypertension (odds ratio [OR] = 2.645, 95% confidence interval [CI] = 1.429–4.896, P = .002 for SBI; OR = 5.474, 95% CI = 2.319–12.921, P = .000 for WMH; OR = 39.988, 95% CI = 3.988–400.949, P = .002 for SBI and WMH) and homocysteine (OR = 4.033, 95% CI = 2.191–7.425, P = .000 for SBI; OR = 5.989, 95% CI = 2.637–13.602, P = .000 for WMH; OR = 4.068, 95% CI = 1.207–13.715, P = .024 for SBI and WMH) might be potential risk factors for SBI and WMH.
The prevalence of silent cerebrovascular disease was elevated with age. Hypertension and elevated homocysteine levels were 2 risk factors for silent cerebrovascular disease in young stroke patients.
Keywords: risk factors, silent brain infarcts, silent cerebrovascular disease, stroke, white matter hyperintensity, young adult
1. Introduction
Stroke is directly caused by cell deaths in brain due to poor blood supply, representing one of the leading causes for disability and deaths worldwide.[1] In recent decades, the incidence of stroke in young adults is increasing, accounting for about 10% to 15% of all stroke cases.[2] This disorder poses great economic and mental burdens on the patients and their family. Currently, various medical cares and strategies have already been developed to improve the treatment and rehabilitation for stroke patients. For example, telestroke system is an emerging tool to provide stroke care and treatment, functioning especially aiming at regions lacking neurologic services. A meta-analysis constructed by Baratloo et al demonstrated that telestroke significantly shortened onset-to-door duration and hospital stay time without increasing the risk of mortality or symptomatic intracranial hemorrhage.[3] Main emerging rehabilitation strategies include noninvasive brain stimulation, brain–computer interface, biotherapeutics, and pharmacologic agents, etc.[4] However, clinical outcomes of stroke patients are still unsatisfactory. Even worse, stroke may make young patients disabled in their most productive age.[5] According to published studies, the prevalence of and risk factors for stroke are significantly different between young and older patients.[6–8] However, clinical outcome is poor both in young and in older patients. So far, prevention is the primary way against this disease.[9] Silent cerebrovascular disease shows no outward symptoms, and can damage brain tissues, leading to high susceptibility to stroke. Therefore, more attentions should be paid on silent cerebrovascular diseases, thus reducing the morbidity and mortality of stroke.
Cerebrovascular disease is a group of vascular disorders in cerebral circulation. Silent cerebrovascular disease is reported to be highly prevalent among older individuals,[10] with 3 main manifestations: silent brain infarcts (SBI), white matter lesions of presumed vascular origin, and cerebral microbleed (CMB).[11] The incidence rate of silent cerebrovascular disease is dramatically higher than that of symptomatic stroke, imposing a great threat to human health around the world.[12] SBI shows gliosis and atrophy in subcortical cavity or cortical areas, which is attributed to previous infarction. White matter lesions of presumed vascular origin represent gliosis, demyelination, microinfarction, and arteriosclerosis that may result from ischemia. Meanwhile, CMB refers to silent hemorrhages shown as silent demarcated, hypointense, rounded lesions.[13,14] Although silent cerebrovascular disease plays an important role in stroke, its potential relationship with stroke among Chinese young adults remains unclear. Compared to recurrent stroke, first-ever stroke in young adults are more likely to be caused by cervical artery dissection and hematologic disorders, and less likely attributed to cardioembolic source.[15] So young patients with first-ever stroke were selected as the subjects in our study.
In the present study, we aimed to investigate the prevalence and risk factors of SBI and WHM in young adults with first-ever stroke.
2. Materials and methods
2.1. Study population
Four hundred consecutive patients who were diagnosed with first-ever stroke were recruited from the Beijing Chaoyang Hospital Affiliated to Capital Medical University between 2011 and 2016. These patients consisted of 230 (57.5%) males and 170 (42.5%) females, with a mean age of 39.5 ± 8.7 years old (ranging from 18 to 49 years). The diagnosis of stoke was implemented based on computer tomography (CT), brain magnetic resonance imaging (MRI), and echocardiography according to the World Health Organization definition: focal neurological deficit persisting for 24 hours with no apparent other causes than vascular ones.[16] The patients with first-ever stroke were included in our study. Exclusion criteria for the participants were as follows: with the history of symptomatic stroke; with acute infarction; having at least 1 silent lesion with a diameter more than 2 cm; and presenting malignancy or cerebral hemorrhage.
The protocols of this study were in accordance with the guidelines of the Ethics Committee of Beijing Chaoyang Hospital Affiliated to Capital Medical University. Signed informed consents were obtained from all the participants or their family members.
2.2. Risk factor collection
Demographic features, vascular risk factors, blood test data, and the severity of brain infarcts for the young stroke patients are all recorded and summarized in Table 1. Demographic features included age, gender, body mass index (BMI), smoking, and drinking status. Vascular risk factors contained migraine, diabetes mellitus (DM), hyperlipidemia, hypertension, cardiovascular disease, and atrial fibrillation. Total cholesterol, triglyceride, fasting blood glucose, and homocysteine were detected as blood test data for the patients. The affirmation of DM, hyperlipidemia, and hypertension were formed based on standard diagnosis. Cardiovascular disease was confirmed if any one of the following conditions appeared: coronary heart disease, myocardial infarction, peripheral arterial disease, and heart failure. The definitions of migraine were according to the International Headache Society criteria,[17] while the diagnosis of atrial fibrillation was conducted based on the ESC Guidelines for the management of atrial fibrillation proposed in 2012.[18]
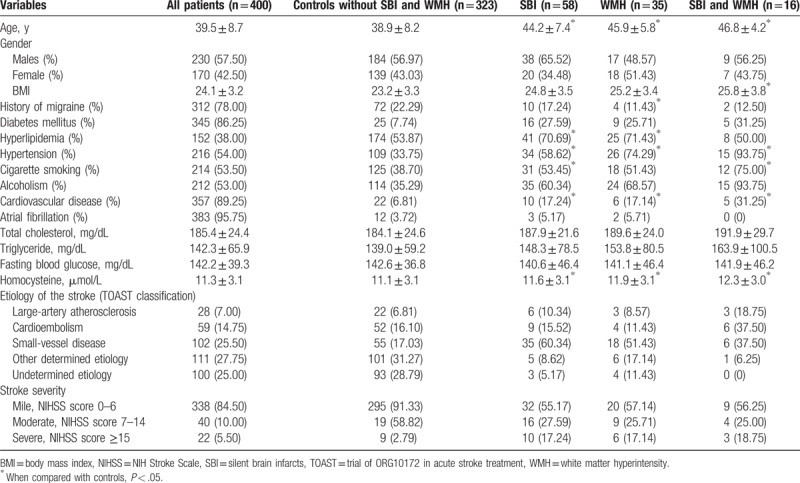
Table 1.
Demographic data, risk factors, subtypes, and severity of the stroke in the study population.
2.3. Diagnosis of SBI and WHM
MRIs were acquired using the same 3.0 T Siemens scanner (Erlangen,Germany). The parameters of MRI examination were as follows: axial T2-weighted (T2W; repetition time 4,500 ms; echo time 93 ms), axial T1-weighted imaging(T1W; repetition time 2,000 ms; echo time 9.2 ms), axialdiffusion-weighted imaging (repetition time 3,300 ms; echo time 91 ms), and coronal fluid-attenuated inversion recovery sequences(repetition time 8,000 ms; echo time 86 ms). The obtained images were reviewed by 2 experienced neurology specialists, and SBI was defined based on the established definition: hypointense lesions in T1WI are surrounded by hyperintense rim in FLAIR.
WHM was detected by the same neuroradiologists using FLAIR images. Based on previous studies, the severity of WHM was evaluated using the Fazekas scoring system.[19] Four grades for lesions were adopted in this system: grade 0 (absent), grade 1 (punctate), grade 2 (early confluent), and grade 3 (confluent). Grades 2 and 3 represented advanced WHM lesions.
2.4. Classification of stroke in young adults
According to clinical characteristics and neuroimaging data, stroke was divided into 5 etiologic subtypes by the 2 neuroradiologists according to the Trial of ORG10172 in Acute Stroke Treatment (TOAST) classification,[20] namely large-artery atherosclerosis (LAA), cardioembolism (CE), small-vessel disease (SVD), other determined etiology, and undetermined etiology. In addition, the severity of stroke was assessed using NIH Stroke Scale (NIHSS) score, thus classified into mild (0–6), moderate (7–14), and severe (≥15).[21]
2.5. Statistical analysis
All statistical analyses were performed using SPSS 18.0 software (SPSS, Chicago, IL). Continuous variables were expressed as mean ± standard deviation, and their comparisons between 2 groups were performed using Student t test. Chi-squared test was adopted to compare categorical variables between the groups. Potential risk factors for silent cerebrovascular disease were identified using multiple logistic regression analysis, and the results were estimated using odds ratios (ORs) and 95% confidence intervals (CIs). P < .05 was considered as statistical significance.
3. Results
3.1. Baseline characteristics of the study population
In the current study, demographic data, vascular risk factors, blood test data, and the severity of the stroke were collected from the medical records on the included patients, and analyzed between the young stroke patients with and without silent cerebrovascular disease. When compared with the patients without silent cerebrovascular disease, patients with SBI, white matter hyperintensity (WMH), and both of SBI and WMH had higher age distribution, more prevalent hypertension and cardiovascular disease, and higher concentration of homocysteine. All of the differences had statistical significance (P < .05). In SBI patients, hyperlipidemia and cigarette smoking were more frequent than in controls (P < .05). Migraine and hyperlipidemia were more common among WMH cases than in control group (P < .05) (Table 1). According to the NIHSS score system, the young stroke patients were divided into mile (338, 84.50%), moderate (40, 10.00%), and severe (22, 5.50%) groups.
3.2. The distribution of stroke subtypes in the young patients with first-ever stroke
A total of 400 young patients with first-ever stroke were recruited in our study. According to TOSAT classification, LAA was observed in 28 (7.00%) cases, CE presented in 59 (14.75%) cases, SVD was found in 102 (25.50%) cases, 111 (27.75%) cases were attributed to other determined etiology, while 100 (25.00%) patients had undetermined etiology. We estimated the distribution of stroke subtypes according to patients’ age and gender. As shown in Fig. 1, the prevalence of stroke caused by undetermined etiology was highest in patients <25 years, and decreased with age. Among patients >30 years old, stroke was frequently caused by other SVD, determined etiology and undetermined etiology.
Figure 1.
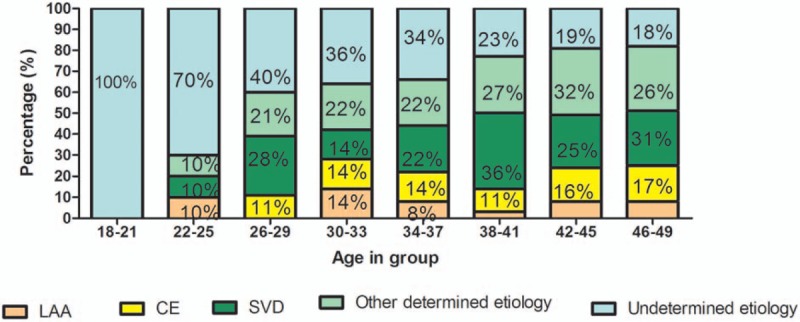
The distribution of stroke subtypes stratified by patients’ age.
In this study, we also investigated the distribution of stroke subtypes according to patients’ gender. The distribution of stroke subtypes in males was similar to that in females (P = .198). Detailed percentage of each subtype in males and females is presented in Fig. 2.
Figure 2.
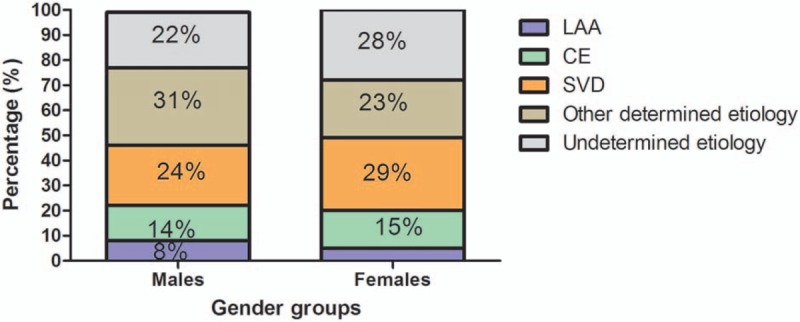
The frequency of stroke subtypes in males and females. The frequency of stroke subtypes in males was similar to that in females (P = .198).
3.3. Incidence of SBI and WMH in the young stroke patients
In order to assess the incidence rate of silent cerebrovascular disease in the young stroke patients, the prevalence of SBI and WMH in different age groups was estimated. Of these 400 young stroke patients, 58 (14.50%) were confirmed with SBI, 35 (8.75%) with WMH, and 16 (4.00%) with both SBI and WMH, while the rest of the patients (323, 80.75%) without silent cerebrovascular disease were employed as the control group. The minimal age of SBI detection in this study was 27 years old, while such figure for WMH was 36. The percentage of patients without SBI and WMH was significantly decreased with age (Fig. 3).
Figure 3.
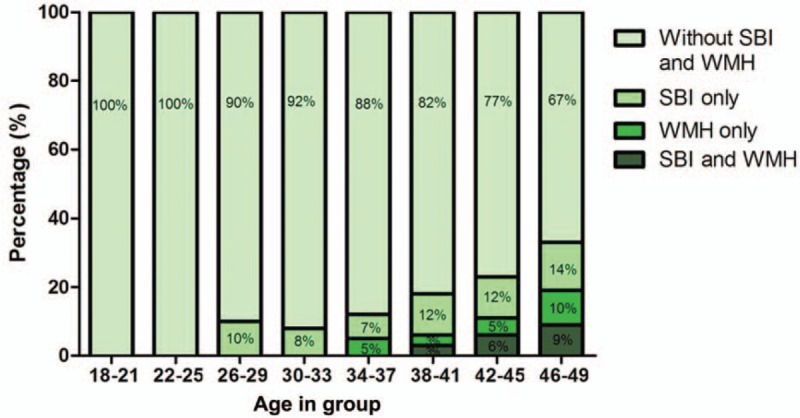
Incidence of silent cerebrovascular disease (SBI and WMH) in different age groups of young stroke patient cohort (n = 400). Total incidence rate of SBI and WMH was increased with age. SBI = silent brain infarcts, WMH = white matter hyperintensity.
In addition, the incidences of SBI and WMH in patients with different subtypes of stroke were also evaluated in the present study. The examination detected 28 (7.00%) LAA patients, 59 (14.75%) CE cases, 102 (25.50%) SVD sufferers, 111 (27.75%) cases caused by other determined etiology, and 100 (25.00%) patients caused by undermined etiology (Table 1). The proportion of SBI was higher in SVD (35/102, 34.31%) patients than in LAA (6/28, 21.43%) and CE (9/59, 15.52%) patients (P < .05). Similarly, the prevalence of WMH was increased in SVD (18/102, 17.65%) patients compared with LAA (3/28, 8.57%) and CE (4/59, 11.43%) patients (P < .05) (Fig. 4).
Figure 4.
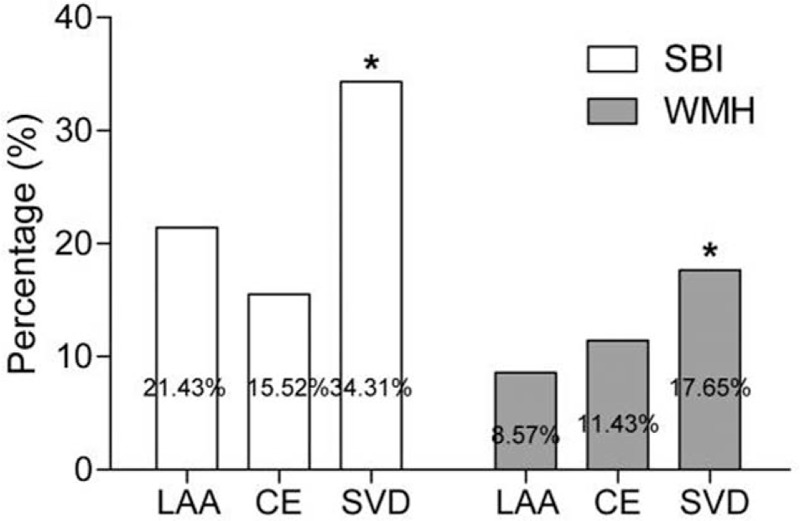
Prevalence of SBI and WMH in different stroke subtypes. SBI and WMH were more frequent in SVD patients. CE = cardioembolism, LAA = large-artery atherosclerosis, SBI = silent brain infarcts, SVD = small-vessel disease, WMH = white matter hyperintensity. ∗P < .05.
3.4. Risk factors associated with SBI and WMH
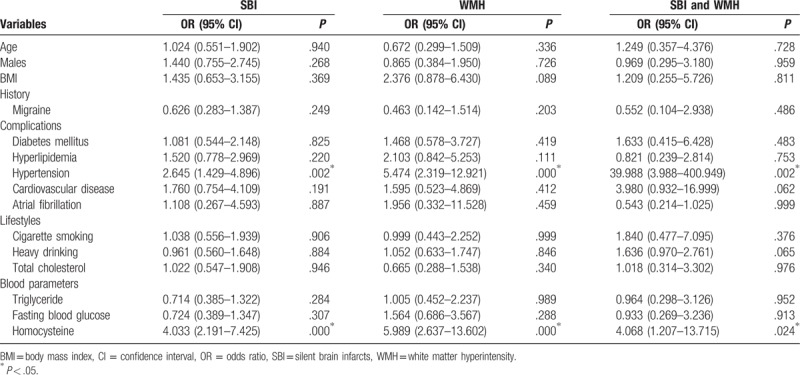
Multiple logistic regression analysis was performed to identify risk factors for SBI and WMH, and the following parameters entered into the model: age, gender, the history of migraine, hyperlipidemia, hypertension, cardiovascular disease and/or atrial fibrillation, smoking, drinking, and blood parameters. The results indicated that hypertension (OR = 2.645, 95% CI = 1.429–4.896, P = .002 for SBI; OR = 5.474, 95% CI = 2.319–12.921, P = .000 for WMH; OR = 39.988, 95% CI = 3.988–400.949, P = .002 for SBI and WMH) and homocysteine (OR = 4.033, 95% CI = 2.191–7.425, P < .001 for SBI; OR = 5.989, 95% CI = 2.637–13.602, P < .001 for WMH; OR = 4.068, 95% CI = 1.207–13.715, P = .024 for SBI and WMH) were independently correlated with the risk of SBI, WHM, and both of them (Table 2). However, age, gender, BMI, the history of migraine, DM, hyperlipidemia, cardiovascular disease or atrial fibrillation, cigarette smoking, heavy drinking, total cholesterol, blood triglyceride, or fasting blood glucose had no significant association with SBI, WHM, or both of them (P > .05 for all) (Table 2).
Table 2.
Multivariate logistic regression analysis applied to the SBI, WMH, and SBI and WMH groups.
4. Discussion
Stroke is the second-leading cause of disease-related deaths around the world, and one of the most common reasons for disability in human beings.[22] Stroke rarely attacks young adults between 18 and 45 years old, and stoke patients in this age-bracket only account for about <5% of all the cases.[23] According to relevant statistic data, the incidence rate of stroke in young adults is increasing worldwide.[24] Given the great threats of stroke on young adults, the prevention and treatment for this disease have received increasing attentions, especially in some developing countries where individuals in this age group are more important for economic development.[25]
Silent cerebrovascular disease, mainly including SBI, white matter lesions of presumed vascular origin and CMB, is closely related to stroke.[26] Although SBI is asymptomatic, data from previous researches revealed that it was correlated with increased risk of stroke.[27] Similarly, in stroke patients, WMH was associated with poor outcomes and elevated risk of relapse.[28] In addition, SBI and WMH have also been reported to be involved in cognitive impairment and dementia.[29,30] Since identifying clinical risk factors could contribute to the disease prevention, we aimed to investigate potential risk factors for SBI and WMH in young adults with first-ever stroke. In the present study, the prevalence of SBI and WMH as well as their potential risk factors were assessed.
In this study, 400 young patients with stroke were recruited, and the presence of silent cerebrovascular disease (SBI and WMH) was examined using MRI. SBI and WMH were relatively more common in young patients with stroke. Besides, the incidences of SBI and WMH were both increased with age. The incidence rate of SBI and WMH in different age groups revealed that SBI could be detected in early adulthood while WMH began at early midlife. SBI and WMH prevalence in stroke patients has also been explored in previous studies. For example, Putaala and colleagues assessed the features and risk factors of SBI and leukoaraiosis in patients with first ischemic stroke, and found increased proportion of SBI and leukoaraiosis with age.[31] In addition, Vermeer et al also revealed an increased prevalence of SBI in stroke patients in their study.[32] Our results about the prevalence of SBI and WMH were in accordance with these data, indicating close association of silent cerebrovascular disease with stroke in young patients.
Subtypes of stroke in young adults were also analyzed in the current study according to the TOAST classification. Three major subtypes, LAA, CE, and SVD, were all detected in the stroke cases. From the analysis results, we found that age and gender did not show significant association with stroke subtypes. However, the proportion of SBI was significantly higher in SVD patients than in LAA and CE patients. Similar results were also found for WMH. These data were consistent with earlier published studies, in which elevated prevalence of SBI was detected in SVD patients with a percentage between 41% and 81%.[33,34] Reportedly, diffuse white-matter lesions represents a surrogate marker for SVD, while SBI and WMH are considered to be involved in the progression of SVD.[35] Our findings combined with previous data suggested an important role of SBI and WMH in SVD progression.
This study also detected potential risk factors for SBI and WMH. Normal clinical characteristics and blood test data were all collected to analyze their association with the risk of SBI and WMH using logistic regression analysis. Accordingly, we found that both hypertension and homocysteine were independently correlated with the risk of SBI, WHM, and both of them. Thus, we concluded that hypertension and homocysteine were risk factors for silent cerebrovascular disease in young patients with stroke.
Although earlier researches have explored the role of SBI and WMH in stroke, our study is the first one to investigate the prevalence and risk factor of SBI and WMH in young stroke patients in China. The results suggested that silent cerebrovascular diseases might be correlated with the initiation of stroke, especially in young SVD adults. However, some limitations still existed in this study. First, the sample size in this research was relatively small, which might recede the precision of final results. Second, the causes of stroke in the study subjects were classified only based on the first evaluation for the patients. Due to the complexity in stroke etiology among young adult patients, more clinical estimations, like angiographic examinations, should be useful to improve the accuracy of our results. In addition, genetic factors also play an important role in stroke among young adults.[15] In further investigations, they should be taken into consideration as well. Consequently, future studies with larger sample size are required to verify and improve our conclusion.
In conclusion, the prevalence of silent cerebrovascular disease was increased with age. Hypertension and homocysteine were 2 independent risk factors for silent cerebrovascular disease in young patients with first-ever stroke.
Author contributions
Conceptualization: Huimin Fan, Xuezeng Hao, Shuna Yang, Yue Li, Junliang Yuan.
Data curation: Huimin Fan, Xuezeng Hao, Shuna Yang, Yue Li, Junliang Yuan, Wenli Hu.
Formal analysis: Huimin Fan, Xuezeng Hao, Shuna Yang, Yue Li, Wei Qin, Junliang Yuan, Wenli Hu.
Funding acquisition: Junliang Yuan, Wenli Hu.
Investigation: Lei Yang.
Writing – original draft: Huimin Fan, Xuezeng Hao, Wei Qin, Lei Yang.
Writing – review & editing: Huimin Fan, Xuezeng Hao, Wei Qin, Lei Yang.
Footnotes
Abbreviations: BMI = body mass index, CE = cardioembolism, CI = confidence interval, CMB = cerebral microbleed, DM = diabetes mellitus, FLAIR = fluid-attenuated inversion recovery, LAA = large-artery atherosclerosis, MRI = magnetic resonance imaging, NIHSS = NIH Stroke Scale, OR = odds ratio, SBI = silent brain infarcts, SVD = small-vessel disease, TOAST = trial of ORG10172 in acute stroke treatment, WMH = white matter hyperintensity.
This study was supported by the National Natural Science Foundation of China (Grant No. 81271309 and 81301016) and Beijing Municipal Administration of Hospitals’ Youth Programme (QML20150303).
The authors have no conflicts of interest to disclose.
References
- [1].Khaku AS, Dulebohn SC. Stroke. Treasure Island, FL: StatPearls; 2018. [Google Scholar]
- [2].Smajlovic D. Strokes in young adults: epidemiology and prevention. Vasc Health Risk Manag 2015;11:157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baratloo A, Rahimpour L, Abushouk AI, et al. Effects of telestroke on thrombolysis times and outcomes: a meta-analysis. Prehosp Emerg Care 2018;22:472–84. [DOI] [PubMed] [Google Scholar]
- [4].Feng W, Belagaje SR. Recent advances in stroke recovery and rehabilitation. Semin Neurol 2013;33:498–506. [DOI] [PubMed] [Google Scholar]
- [5].Maaijwee NA, Rutten-Jacobs LC, Schaapsmeerders P, et al. Ischaemic stroke in young adults: risk factors and long-term consequences. Nat Rev Neurol 2014;10:315–25. [DOI] [PubMed] [Google Scholar]
- [6].Renna R, Pilato F, Profice P, et al. Risk factor and etiology analysis of ischemic stroke in young adult patients. J Stroke Cerebrovasc Dis 2014;23:e221–7. [DOI] [PubMed] [Google Scholar]
- [7].Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke 2009;40:1195–203. [DOI] [PubMed] [Google Scholar]
- [8].Goeggel Simonetti B, Mono ML, Huynh-Do U, et al. Risk factors, aetiology and outcome of ischaemic stroke in young adults: the Swiss Young Stroke Study (SYSS). J Neurol 2015;262:2025–32. [DOI] [PubMed] [Google Scholar]
- [9].Singhal AB, Biller J, Elkind MS, et al. Recognition and management of stroke in young adults and adolescents. Neurology 2013;81:1089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nakanishi K, Jin Z, Homma S, et al. Left ventricular mass-geometry and silent cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) study. Am Heart J 2017;185:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Russo C, Jin Z, Homma S, et al. Subclinical left ventricular dysfunction and silent cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) study. Circulation 2013;128:1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Leary MC, Saver JL. Annual incidence of first silent stroke in the United States: a preliminary estimate. Cerebrovasc Dis 2003;16:280–5. [DOI] [PubMed] [Google Scholar]
- [13].Charidimou A, Werring DJ. Cerebral microbleeds and cognition in cerebrovascular disease: an update. J Neurol Sci 2012;322:50–5. [DOI] [PubMed] [Google Scholar]
- [14].Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lutski M, Zucker I, Shohat T, et al. Characteristics and outcomes of young patients with first-ever ischemic stroke compared to older patients: the National Acute Stroke ISraeli Registry. Front Neurol 2017;8:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Aho K, Harmsen P, Hatano S, et al. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ 1980;58:113–30. [PMC free article] [PubMed] [Google Scholar]
- [17].Olesen J, Goadsby P, Steiner T. The international classification of headache disorders. 2nd edition. Cephalalgia 2004;2:9–160. [DOI] [PubMed] [Google Scholar]
- [18].Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation-developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385–413. [DOI] [PubMed] [Google Scholar]
- [19].Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–6. [DOI] [PubMed] [Google Scholar]
- [20].Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of ORG10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- [21].Lyden P, Brott T, Tilley B, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 1994;25:2220–6. [DOI] [PubMed] [Google Scholar]
- [22].Balami JS, Chen RL, Buchan AM. Stroke syndromes and clinical management. QJM 2013;106:607–15. [DOI] [PubMed] [Google Scholar]
- [23].Ji R, Schwamm LH, Pervez MA, et al. Ischemic stroke and transient ischemic attack in young adults: risk factors, diagnostic yield, neuroimaging, and thrombolysis. JAMA Neurol 2013;70:51–7. [DOI] [PubMed] [Google Scholar]
- [24].Chraa M, Louhab N, Kissani N. Stroke in young adults: about 128 cases. Pan Afr Med J 2014;17:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gioia LC, Tollard E, Dubuc V, et al. Silent ischemic lesions in young adults with first stroke are associated with recurrent stroke. Neurology 2012;79:1208–14. [DOI] [PubMed] [Google Scholar]
- [26].Marfella R, Rizzo MR, Capoluongo MC, et al. Cryptogenic stroke and diabetes: a probable link between silent atrial fibrillation episodes and cerebrovascular disease. Exp Rev Cardiovasc Ther 2014;12:323–9. [DOI] [PubMed] [Google Scholar]
- [27].Vermeer SE, Hollander M, van Dijk EJ, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke 2003;34:1126–9. [DOI] [PubMed] [Google Scholar]
- [28].Putaala J, Haapaniemi E, Kurkinen M, et al. Silent brain infarcts, leukoaraiosis, and long-term prognosis in young ischemic stroke patients. Neurology 2011;76:1742–9. [DOI] [PubMed] [Google Scholar]
- [29].Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–22. [DOI] [PubMed] [Google Scholar]
- [30].Delgado P, Riba-Llena I, Tovar JL, et al. Prevalence and associated factors of silent brain infarcts in a Mediterranean cohort of hypertensives. Hypertension 2014;64:658–63. [DOI] [PubMed] [Google Scholar]
- [31].Putaala J, Kurkinen M, Tarvos V, et al. Silent brain infarcts and leukoaraiosis in young adults with first-ever ischemic stroke. Neurology 2009;72:1823–9. [DOI] [PubMed] [Google Scholar]
- [32].Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007;6:611–9. [DOI] [PubMed] [Google Scholar]
- [33].Adachi T, Kobayashi S, Yamaguchi S. Frequency and pathogenesis of silent subcortical brain infarction in acute first-ever ischemic stroke. Intern Med 2002;41:103–8. [DOI] [PubMed] [Google Scholar]
- [34].van Zagten M, Boiten J, Kessels F, et al. Significant progression of white matter lesions and small deep (lacunar) infarcts in patients with stroke. Arch Neurol 1996;53:650–5. [DOI] [PubMed] [Google Scholar]
- [35].Chen X, Wen W, Anstey KJ, et al. Prevalence, incidence, and risk factors of lacunar infarcts in a community sample. Neurology 2009;73:266–72. [DOI] [PubMed] [Google Scholar]


