Abstract
Rationale:
Follicular dendritic cell (FDC) sarcoma is a rare tumor with FDC differentiation that typically arises within lymph nodes but can also occur extranodally. To date, the primary esophageal FDC sarcoma has not been reported in the English literature.
Patient concerns:
We described a 67-year-old female who foremostly presented with dysphagia, and the patient was readmitted due to a dry cough and pain of his right shoulder 2 years after initial treatment.
Diagnoses:
Primary esophageal FDC sarcoma with the right superior mediastinal lymph node metastasis.
Interventions:
The esophageal tumor was removed by endoscopic submucosal dissection at the first hospitalization. At the second hospitalization 2 years after the initial visit, the tracheal stent loaded with (125) iodine radioactive seeds was placed. The profiles of genetic variations and immunotherapeutic biomarkers were also explored by next-generation sequencing protocol from the patient's blood, esophageal primary, and mediastinal metastatic tumor samples.
Outcomes:
The patient's symptom transitorily relieved, but she gave up further treatment and died 2 months after the tracheal stent was placed. As for the genomic alterations, we found 9 gene mutations in all the samples, including checkpoint kinase 2(CHEK2), FAT atypical cadherin 1 (FAT1), tumor protein 53 (TP53), DPYD, ERBB2 interacting protein (ERBB2IP), FBXW7, KMT2D, PPP2R1A, TSC2, whereas amplification of MYC was only in the metastatic example. The analysis of clonal evolution and phylogenetic tree showed the propagation and replay of polyclonal esophageal FDC sarcoma. At the same time, the detection of biomarkers for immunotherapy revealed microsatellite stable and mismatch repair-proficient (pMMR), which predicted a relatively poor anti-programmed death (PD-1)/programmed death ligand (PD-L1) immunotherapy outcome. On the contrary, the tumor mutational burdens were 10 mutations per 1 million bases in both the primary and metastatic tumor sample, which ranked the top 23.3% in solid tumors mutational burdens database of Geneseeq and might be a good predictor of the efficacy of anti-PD-1/PD-L1 immunotherapy.
Lessons:
To the best of our knowledge, this case report announced the first case of extranodal primary esophageal FDC sarcoma in the world, and firstly revealed its unique genetic alterations profiles, which might contribute to further in-depth study of this rare disease.
Keywords: chemotherapy, esophagus, follicular dendritic cell sarcoma, immunotherapy, radiotherapy, sarcoma
1. Introduction
Follicular dendritic cell sarcoma (FDCS), a rare neoplasm with follicular dendritic cell (FDC) differentiation, was first reported in 1986 by Monda et al.[1] FDCS is a neoplastic proliferation of spindled to ovoid cells with morphologic and immunophenotypic features similar to those of ordinary FDCs and is classified under histiocytic and dendritic cell neoplasms by the World Health Organization Classification of Tumours.[2] Approximately 60% of FDCSs arise in lymph nodes, most commonly involving cervical and mediastinal lymph nodes. Meanwhile, one-third of FDCSs can also occur in extranodal sites.[3] The predominant extranodal site of FDCS was the head and neck region followed by the liver, spleen, gastrointestinal tract, and thoracic viscera.[3] Due to its rarity, FDCS was often initially misdiagnosed. Identification 1 or more of the following markers: CD21, CD23, CD35, podoplanin,[4] and CXCL-13[5] by immunohistochemistry (IHC) can help differentiate FDCS.
Here, in the world, we report the first case of extranodal FDCS arising in the esophagus. Because of the absolute rarity of esophageal FDCS, lack of benefit with conventional systemic agents, and absence of genomic characterization in the literature, we collected the patient's blood, esophageal primary, and mediastinal metastatic tumor samples to evaluate the status of 422 tumor-related genes and immunotherapy biomarkers using the next-generation sequencing (NGS) protocol (Geneseeq biotechnology corporation, Nanjing, Jiangsu, China).
2. Case report
A 67-year-old woman without family cancer history presenting with dysphagia for 1 month was admitted to Nanjing Drum Tower Hospital in August 2015. Endoscopic ultrasound revealed a 1.55cm × 0.65 cm hemispherical neoplasm with a smooth surface arising from muscularis mucosae of the middle thoracic esophagus (25 cm from the incisors, Fig. 1A, B). The initial clinical diagnosis was esophageal leiomyoma. Then the esophageal neoplasm was removed by endoscopic submucosal dissection (ESD) on August 12, 2015 (Fig. 2). The postoperative pathology report revealed that the patient actually had an esophageal FDCS. Under the microscope, the tumor cells had ovoid and spindle nuclei containing prominent nucleoli and vesicular chromatin, showing a high mitotic activity and a large number of pathological mitotic figures (Fig. 3A). The number of mitotic figures was up to 15 per 10 high-power fields. With the use of IHC, the tumor cells were strongly positive for clusters of differentiation (CD) 21 (Fig. 3B), positive for vimentin (Vim), weakly positive for CD35 and CD23, and negative for leukocyte common antigen (LCA), S100, CD3, CD20, TdT, CD30, ALK, pan-cytokeratins (pan-CK), EMA, CK7, CK19, CK5/6, CK8/18, P63, P40, HMB45, A103, and programmed death ligand (PD-L1) (VENTANA, SP142). Epstein-Barr virus-encoded small RNA (EBER) in situ hybridization was also negative. Ki-67 revealed a proliferation index of approximately 80%. Postoperative fluorodeoxyglucose (FDG)—positron emission tomography (PET)/computed tomography (CT) found a lymph node measuring 0.9cm × 0.9 cm located in the tracheoesophageal groove of the right upper mediastinum with slightly increased metabolism (standardized uptake value maximum [SUVmax], 2.6), and no significantly abnormal metabolism in the esophageal area on August 27, 2015 (Fig. 4A). Since the tracheoesophageal groove lymph node metastasis is one of the most common metastatic sites of the middle thoracic esophageal tumors, we concluded that the diagnosis of the patient is primary esophageal FDCS with mediastinal lymph node metastasis. We had recommended chemoradiotherapy for the patient, but she refused.
Figure 1.
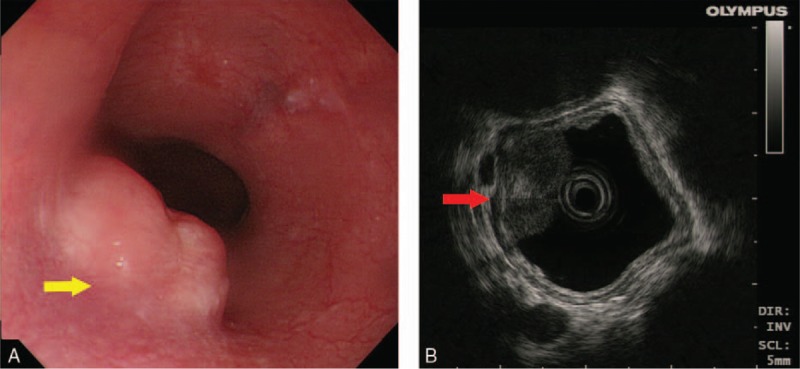
Endoscopy and endoscopic ultrasound image. (A) Endoscopy showed a 1.55cm × 0.65 cm hemispherical neoplasm with a smooth surface of the middle thoracic esophagus (25 cm from incisor, yellow arrow). (B) The tumor arose from muscularis mucosae under endoscopic ultrasound (red arrow).
Figure 2.
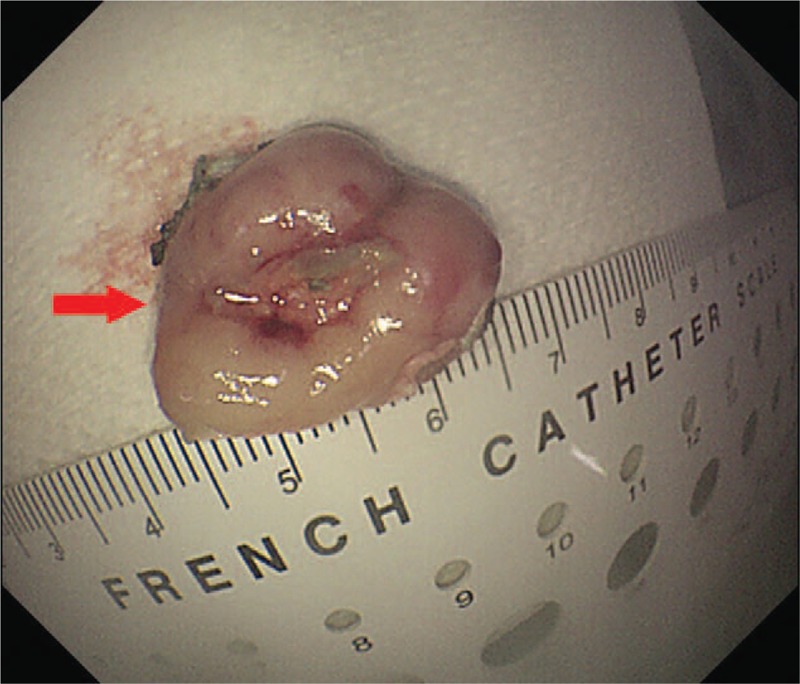
Specimens after ESD. The primary esophageal tumor specimens after ESD surgery (red arrow). ESD = endoscopic submucosal dissection.
Figure 3.
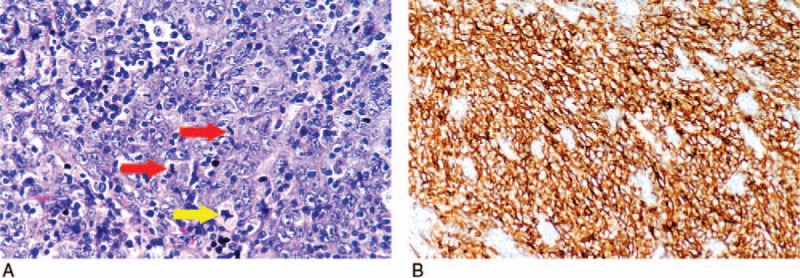
Pathological images of primary esophageal tumor. (A) The primary esophageal tumor cells had ovoid and spindle nuclei containing prominent nucleoli and vesicular chromatin, showing a high mitotic activity (red arrow × 2) and a large number of pathological mitotic figures (yellow arrow). The number of mitotic figures was up to 15 per 10 high-power fields (Hematoxylin-eosin, X400). (B) The tumor cells were strongly positive for CD21 (Envison 2-step method, X200).
Figure 4.
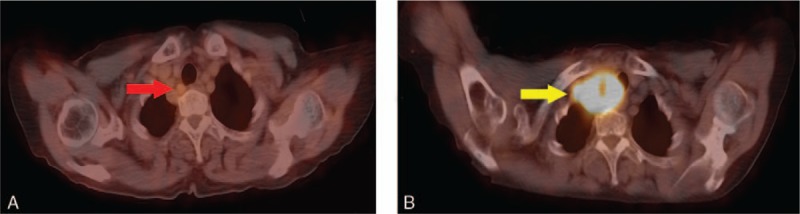
PET/CT image of mediastinal metastatic lymph node. (A) PET/CT (2015-08-27) found a lymph node measuring 0.9cm × 0.9 cm located in the tracheoesophageal groove of the right superior mediastinum, and the SUVmax was slightly increased to 2.6 (red arrow). (B) PET/CT (2017-11-09) showed a large metastatic lymph node size of 5.2cm × 4.0 cm of the upper right mediastinum, and the SUVmax was significantly increased to 12.3 (yellow arrow).CT = computed tomography, PET = positron emission tomography, SUVmax = standardized uptake value maximum.
The patient was admitted again due to a dry cough and pain in his right shoulder for 1 month in November 2017. PET/CT examination showed an enlarged lymph node size of 5.2cm × 4.0 cm in the upper right mediastinum, and the SUVmax was significantly increased to 12.3 on November 9, 2017 (Fig. 4B). The patient underwent a CT-guided biopsy of the lymph node on November 14, 2017, and the pathology report also revealed an FDCS. Immunostains for CD21, CD35, PD-L1 (VENTANA, SP142) were strongly positive, weakly positive, and negative, respectively. EBER in situ hybridization was negative. We inferred that the patient's right upper mediastinal metastatic lymph node had gradually enlarged in 2 years, and compressed the trachea and brachial plexus nerves, resulting in the clinical symptoms described above. At the same time, we collected the patient's samples and sent to the core facility of Geneseeq biotechnology corporation (Nanjing, China). The NGS test was performed by the steps of “DNA extraction”, “Library preparation and sequencing” and “sequence data processing and identification of clinically actionable mutations”. Because no clinically available targeted drugs were found, we designed a concurrent chemoradiation treatment for the patient. The chemotherapeutic regimen consisted of gemcitabine and paclitaxel liposomes. Unexpectedly, the patient started to have dyspnea before treatment. CT scan showed more severe compression of the trachea by the tumor. After 2 fractions of radiotherapy and 1 cycle of chemotherapy, the above symptoms aggravated, the treatment stopped and the tracheal stent loaded with (125)iodine radioactive seeds was urgently placed[6] (Fig. 5). Later, the patient's symptom temporarily relieved, but she gave up further treatment for economic reasons and died 2 months after the tracheal stent was placed.
Figure 5.
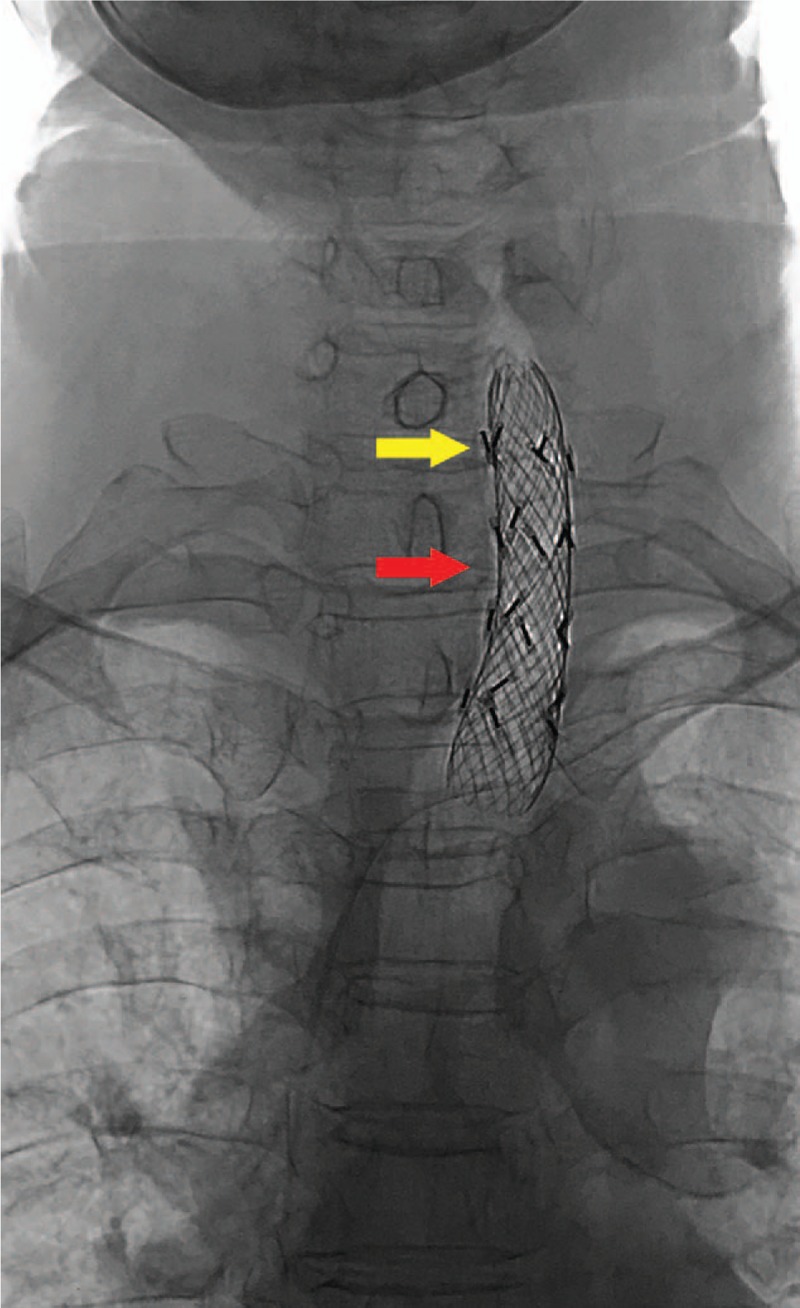
X-ray radiography after the tracheal stent placed. The X-ray radiography was taken after the tracheal stent (red arrow) loaded with (125) iodine radioactive seeds (yellow arrow) was urgently placed.
3. Discussion
Etiopathogenesis of FDCS remains unclear. Some studies have identified that Epstein-Barr virus has been associated with a specific subset of FDCS presenting in liver and spleen,[7] and there are reports of FDCS being associated with the hyaline-vascular type of Castleman's disease (HVCD).[8,9] In 3 published studies with a large number of samples (54, 97, and 142 patients), the median age of FDCS varied from 42.7 to 49 without gender predilection.[7,10,11] FDCS is often considered to be a low-to-intermediate grade sarcoma,[10] but due to the rarity of the disease, there have been only a few systematic evaluation to date on the outcomes and prognostic factors of FDCS. Pang et al[11] analyzed the results of FDCS of the head and neck with the largest sample size through a pooled analysis. They reported 2-year overall survival (OS), 5-year OS, 2-year disease-free survival (DFS) and 5-year DFS were 91%, 81%, 64%, and 34%, respectively. Further statistical analysis revealed that patients who received surgery plus radiotherapy compared to surgery alone, and patients who had a neck dissection, were significantly less likely to recur locoregionally. The most substantial review of extranodal FDCS by Wang et al[7] included 130 cases with follow-up data. The overall recurrence, metastasis, and mortality rates were 49.2%, 21.5%, and 13.8%, respectively (mean follow-up: 34.5 months). A series of 54 patients with FDCS described by Perkins et al[10] reported that the median survival did not reach, and the mortality rate was 22% (mean follow-up: 28 months). Prognostic factors indicating an unfortunate outcome of FDCS include an intra-abdominal location, a large tumor size, a high mitotic rate, coagulative necrosis, and nuclear pleomorphism.[3,7,12]
Radical resection is the primary therapy for FDCS, but the value of radiotherapy and chemotherapy in the treatment of this neoplasm remains uncertain. Jain et al[12] analyzed 66 patients with FDCS. They reported that patients who underwent an upfront gross total resection (GTR) experienced better prognosis than who not, and the pattern of relapse was predominantly locoregional. Consolidative radiotherapy, instead of adjuvant chemotherapy could improve the local control, PFS, and OS of patients who underwent a GTR. There are currently no standard chemotherapeutic regimens for FDCS. It is still controversial whether chemotherapy drugs of FDCS based on hematologic malignancies or sarcomas regimens.[11] Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) is the most commonly used chemotherapeutic regimen, but the response rate (RR) of patients treated with CHOP alone is inadequate.[13] Some studies have elucidated that FDCs have a vascular stromal rather than a hematopoietic origin, a finding that is consistent with their clinical behavior and prognosis being similar to low-intermediate grade soft tissue sarcomas.[14] Therefore, treatment with agents better suited to mesenchymal stromal tumors rather than hematopoietic malignancies may be more appropriate.[15] In another report of MD Anderson cancer center, gemcitabine with a taxane yielded an RR of 80% in patients with FDCS.[12] This finding corroborated another statement of favorable outcomes with this combination.[16] Our patient underwent a palliative ESD and did not receive chemoradiation after surgery. Two years later, the trachea stent was implanted due to the vast metastatic right superior mediastinal lymph node that had compressed the trachea and caused dyspnea. Radiotherapy and chemotherapy could not be tolerated by the patient. The patient's clinical course showed an intermediate-grade malignancy, which was associated with having poor prognostic factors of sizeable metastatic lymph node size, high mitosis rate, nuclear pleomorphism, and high Ki67 index.
It was reported that the profiles of genomic alterations in esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) differed widely.[17]KRAS and ERBB2 frequently altered far more in EAC compared with ESCC, whereas changes of PIK3CA, PTEN, and NOTCH1 were more predominant in ESCC compared with EAC.[18] But little is known about molecular driven mutation and targeted treatment of FDCS. To date, tumor protein 53 (TP53), PTEN mutations had been identified by NGS in 3 patients with FDCS in 2 studies.[12,19] Go et al[20] demonstrated that 18.5% of patients with FDCS harbored BRAFV600E mutation. Their result provided a theoretical basis for introducing BRAF mutation-targeting therapy (Vemurafenib and Dabrafenib) in patients with FDCS.
To explore the DNA potential mutations for our reported esophageal FDCS; we utilized NGS to detect all exons of 422 tumor-related genes and biomarkers for immunotherapy. Our analysis identified the following 9 gene mutations in all the 3 tested samples: Checkpoint kinase 2(CHEK2), FAT atypical cadherin 1 (FAT1), TP53, DPYD, ERBB2 interacting protein (ERBB2IP), FBXW7, KMT2D, PPP2R1A, TSC2 (Table 1). Whereas amplification of MYC was only found in the metastatic sample. CHEK2 emerges as an essential signaling transducer of cellular responses to DNA damage and a candidate tumor suppressor, defect of which contributes to the pathogenesis of diverse types of human malignancies, mainly family breast cancer.[21] FAT atypical cadherin 1 (FAT1) regulates cell adhesion, cell growth, cell migration, and actin dynamics as either oncogene or tumor suppressor in human cancers. FAT1 has been reported as one of the significant mutant genes in ESCC.[22]TP53 is the most frequently mutated gene in human cancers, mutation of which is linked to poor prognosis.[23]ERBB2IP is a tumor suppressor that binds to ERBB2 and attenuates downstream RAS/ERK signaling. Tran et al[24] have reported a successful immunotherapy based on ERBB2IP mutation-specific CD4+ T cells in a patient with widely metastatic cholangiocarcinoma. Meanwhile, MYC gene amplification predicts the poorest prognosis in patients with non-small cell lung cancers.[25]DPYD mutation has been reported to be associated with fluorouracil drug toxicity.[26] In addition to these, there are few studies on the genetic variations of FBXW7, KMT2D, PPP2R1A, and TSC2.
Table 1.
Summary of tumor genomic profiles of our case by Geneseeq assay.
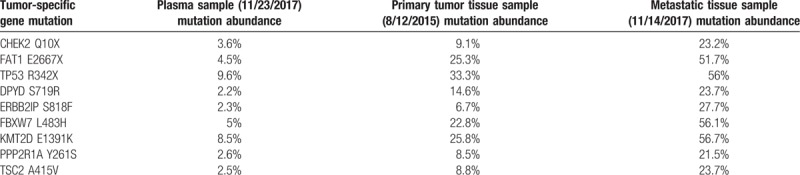
From Table 1, we could draw the following conclusions: First, the plasma circulating tumor DNA (ctDNA) and tissue mutation profiles were precisely the same, indicating that liquid biopsy could to some extent replace the biopsy. Second, the mutation abundance in metastatic tissue specimens was significantly higher than that in primary lesions, which might be related to tumor heterogeneity. Given the prevalence of heterogeneity of primary and metastatic tumors, it was clinically necessary to re-biopsy metastatic tumors. Third, it could be seen from Table 1 that the mutation spectrum of esophageal FDCS and esophageal epithelial tumors is not precisely the same,[17,18] which is unique. To in-depth discuss the heterogeneity of our case, we analyzed the mutation profiles of primary and metastatic tumor specimens and plasma ctDNA mutations and then performed mutation clonal clustering to aggregated 3 clones. For these 3 clones, we plotted the clonal evolution and generated the phylogenetic tree. It could be seen that mutated clone 1 first evolved then clone 1 branched clone 2 and clone 3. Three clones appeared in different tumor tissues and plasma simultaneously, showing the propagation and replay of polyclonal (Fig. 6).
Figure 6.
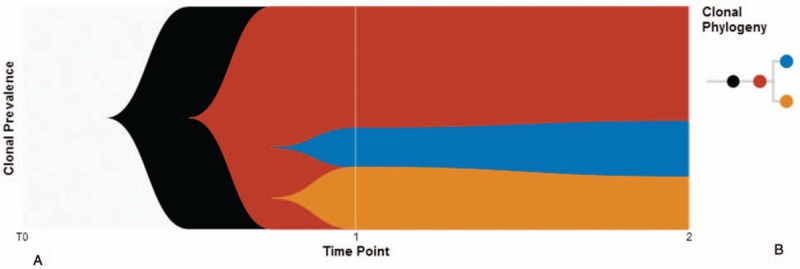
Clonal evolution and phylogenetic tree. (A) Clonal evolution. We performed mutation clonal clustering to aggregated 3 clones (red = clone 1, blue = clone 2, orange = clone 3). (B) Clonal phylogenetic tree. Clone 1 (red circle) first evolved, then cloned 1 branched clone 2 (blue circle) and cloned 3 (orange circle).
Up to now, there has been minimal clinical experience with targeted drug treatment in FDCS. Lemech et al[27] detected a case of lung and pleura FDCS with a breast cancer family history and confirmed a BRCA2 germline mutation. The patient was treated with carboplatin and veliparib based on BRCA2 mutation after a failure of frontline surgery and chemoradiotherapy and achieved a durable disease stabilization. However, whether we can use the genetic information to guide therapy in the patient with FDCS remains unknown as the functional significance of these mutations is not clear, and the lack of appropriately targeted drugs.
Laginestra et al[28] first investigated the immune landscape of FDCS. They provided evidence of a peculiar immunologic microenvironment associated with FDCS's proliferation and underscored the potential relevance of programmed death-1 (PD-1) and PD-L1/2 axes in the regulation of FDCS immune contexture. This finding provides a prospective basis for the use of PD-1/PD-L1 immune checkpoint inhibitors for FDCS. In our sample, the detection of biomarkers for immunotherapy revealed microsatellite stable (MSS) and mismatch repair-proficient (pMMR), which predicted a relatively poor anti-PD-1/PD-L1 immunotherapy outcome.[29] On the contrary, the tumor mutational burdens (TMB) were 10 mutations per 1 million bases in both the primary and metastatic tumor sample in our research, which ranked the top 23.3% in solid tumors TMB database of Geneseeq and belonged to high TMB. Carbone et al[30] had reported that high TMB might be a good predictor of the efficacy of anti-PD-1/PD-L1 immunotherapy. These above results may provide some references for the immunotherapy of FDCS.
4. Conclusions
We described a rare case of primary esophageal FDCS pursuing an aggressive clinical course, explored the landscape of its genetic mutations and immunotherapeutic biomarkers, and evaluated its clonal evolution and phylogenetic tree. Our case indicated that esophageal FDCS had a unique genetic mutation status that differed from ESCC or EAC. To our knowledge, this is the first report in the world to announce an extranodal FDCS involving esophagus and characterize its genomic alterations and immunotherapeutic biomarkers.
Author contributions
Conceptualization: Wei Ren.
Data curation: Wei Ren, Qi Sun, Pu-Yuan Wu.
Formal analysis: Wei Ren.
Investigation: Wei Ren, Qi Sun, Pu-Yuan Wu, Bin Huang, Ju Yang.
Methodology: Wei Ren, Qi Sun, Jing Yan, Bao-Rui Liu.
Software: Wei Ren, Ju Yang.
Supervision: Bao-Rui Liu.
Validation: Wei Ren, Pu-Yuan Wu.
Visualization: Wei Ren, Qi Sun.
Writing – original draft: Wei Ren.
Writing – review & editing: Wei Ren, Qi Sun, Bao-Rui Liu.
Footnotes
Abbreviations: CD = clusters of differentiation, CHEK2 = checkpoint kinase 2, CHOP = cyclophosphamide, doxorubicin, vincristine, and prednisone, CT = computed tomography, ctDNA = circulating tumor DNA, DFS = disease-free survival, EAC = esophageal adenocarcinoma, EBER = Epstein-Barr virus-encoded small RNA, ERBB2IP = ERBB2 interacting protein, ESCC = esophageal squamous cell carcinoma, ESD = endoscopic submucosal dissection, FAT1 = FAT atypical cadherin 1, FDC = follicular dendritic cell, FDCS = follicular dendritic cell sarcoma, GTR = gross total resection, IHC = immunohistochemistry, MSS = microsatellite stable, NGS = next-generation sequencing, OS = overall survival, PD-1 = programmed death-1, PD-L1 = programmed death ligand-1, PET = positron emission tomography, SUVmax = standardized uptake value maximum, TMB = tumor mutational burdens, TP53 = tumor protein 53.
WR and QS contributed equally to this study
The present study was approved by the Ethic Committee of the Affiliated Drum Tower Hospital of Medical School of Nanjing University. In accordance with Chinese regulations and due to the observational nature of this single patient retrospective study, no formal ethics approval is required. The authors obtained patient's consent to participate.
The authors obtained informed consent from the patient to publish information on her disease and clinical course.
This study was supported by Nanjing Medical Science and Technology Development Fund (YKK16103).
The authors report no conflicts of interest.
References
- [1].Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation: A report of 4 cases. Am J Pathol 1986;122:562–72. [PMC free article] [PubMed] [Google Scholar]
- [2].Kairouz S, Hashash J, Kabbara W, et al. Dendritic cell neoplasms: an overview. Am J Hematol 2007;82:924–8. [DOI] [PubMed] [Google Scholar]
- [3].Chen T, Gopal P. Follicular dendritic cell sarcoma. Arch Pathol Lab Med 2017;141:596–9. [DOI] [PubMed] [Google Scholar]
- [4].Yu H, Gibson JA, Pinkus GS, et al. Podoplanin (D2-40) is a novel marker for follicular dendritic cell tumors. Am J Clin Pathol 2007;128:776–82. [DOI] [PubMed] [Google Scholar]
- [5].Vermi W, Lonardi S, Bosisio D, et al. Identification of CXCL13 as a new marker for follicular dendritic cell sarcoma. J Pathol 2008;216:356–64. [DOI] [PubMed] [Google Scholar]
- [6].Zhu HD, Guo JH, Mao AW, et al. Conventional stents versus stents loaded with (125)iodine seeds for the treatment of unresectable oesophageal cancer: a multicentre, randomised phase 3 trial. Lancet Oncol 2014;15:612–9. [DOI] [PubMed] [Google Scholar]
- [7].Wang RF, Han W, Qi L, et al. Extranodal follicular dendritic cell sarcoma: a clinicopathological report of four cases and a literature review. Oncol Lett 2015;9:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chan AC, Chan KW, Chan JK, et al. Development of follicular dendritic cell sarcoma in hyaline-vascular Castleman's disease of the nasopharynx: tracing its evolution by sequential biopsies. Histopathology 2001;38:510–8. [DOI] [PubMed] [Google Scholar]
- [9].Chan JK, Tsang WY, Ng CS. Follicular dendritic cell tumor and vascular neoplasm complicating hyaline-vascular Castleman's disease. Am J Surg Pathol 1994;18:517–25. [DOI] [PubMed] [Google Scholar]
- [10].Perkins SM, Shinohara ET. Interdigitating and follicular dendritic cell sarcomas: a SEER analysis. Am J Clin Oncol 2013;36:395–8. [DOI] [PubMed] [Google Scholar]
- [11].Pang J, Mydlarz WK, Gooi Z, et al. Follicular dendritic cell sarcoma of the head and neck: case report, literature review, and pooled analysis of 97 cases. Head Neck 2016;38suppl 1:E2241–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jain P, Milgrom SA, Patel KP, et al. Characteristics, management, and outcomes of patients with follicular dendritic cell sarcoma. Br J Haematol 2017;178:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Soriano AO, Thompson MA, Admirand JH, et al. Follicular dendritic cell sarcoma: a report of 14 cases and a review of the literature. Am J Hematol 2007;82:725–8. [DOI] [PubMed] [Google Scholar]
- [14].Aguzzi A, Kranich J, Krautler NJ. Follicular dendritic cells: origin, phenotype, and function in health and disease. Trends Immunol 2014;35:105–13. [DOI] [PubMed] [Google Scholar]
- [15].Hartmann S, Doring C, Agostinelli C, et al. miRNA expression profiling divides follicular dendritic cell sarcomas into two groups, related to fibroblasts and myopericytomas or Castleman's disease. Eur J Cancer 2016;64:159–66. [DOI] [PubMed] [Google Scholar]
- [16].Conry RM. Response of follicular dendritic cell sarcoma to gemcitabine and docetaxel: report of two cases and literature review. Clin Sarcoma Res 2014;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cancer Genome Atlas Research Network, Analysis Working Group: Asan University, BC Cancer Agency, et al Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang K, Johnson A, Ali SM, et al. Comprehensive genomic profiling of advanced esophageal squamous cell carcinomas and esophageal adenocarcinomas reveals similarities and differences. Oncologist 2015;20:1132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Starr JS, Attia S, Joseph RW, et al. Follicular dendritic cell sarcoma presenting as a thyroid mass. J Clin Oncol 2015;33:e74–6. [DOI] [PubMed] [Google Scholar]
- [20].Go H, Jeon YK, Huh J, et al. Frequent detection of BRAF (V600E) mutations in histiocytic and dendritic cell neoplasms. Histopathology 2014;65:261–72. [DOI] [PubMed] [Google Scholar]
- [21].Nevanlinna H, Bartek J. The CHEK2 gene and inherited breast cancer susceptibility. Oncogene 2006;25:5912–9. [DOI] [PubMed] [Google Scholar]
- [22].Hu X, Zhai Y, Kong P, et al. FAT1 prevents epithelial mesenchymal transition (EMT) via MAPK/ERK signaling pathway in esophageal squamous cell cancer. Cancer Lett 2017;397:83–93. [DOI] [PubMed] [Google Scholar]
- [23].Kastenhuber ER, Lowe SW. Putting p53 in context. Cell 2017;170:1062–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014;344:641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kortlever RM, Sodir NM, Wilson CH, et al. Myc cooperates with ras by programming inflammation and immune suppression. Cell 2017;171:1301–15.e1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Thomas F, Hennebelle I, Delmas C, et al. Genotyping of a family with a novel deleterious DPYD mutation supports the pretherapeutic screening of DPD deficiency with dihydrouracil/uracil ratio. Clin Pharmacol Ther 2016;99:235–42. [DOI] [PubMed] [Google Scholar]
- [27].Lemech CR, Williams R, Thompson SR, et al. Treatment of breast cancer 2 (BRCA2)-mutant follicular dendritic cell sarcoma with a poly ADP-ribose polymerase (PARP) inhibitor: a case report. BMC Res Notes 2016;9:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Laginestra MA, Tripodo C, Agostinelli C, et al. Distinctive histogenesis and immunological microenvironment based on transcriptional profiles of follicular dendritic cell sarcomas. Mol Cancer Res 2017;15:541–52. [DOI] [PubMed] [Google Scholar]
- [29].Le DT, Durham JN. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017;376:2415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


