Abstract
Primary biliary cholangitis (PBC) is a chronic disease that is increasingly being recognized in recent years. In this study, we sought to identify noninvasive markers of the severity of cirrhosis in patients with PBC based on routinely investigated laboratory parameters.
Ninety-four patients with histologically-confirmed PBC based on liver biopsy performed between January 2013 and December 2017 at the First Hospital of Jilin University were divided into 2 groups: early-stage cirrhosis (fibrosis stage F1 and F2; n = 74) and advanced-stage cirrhosis (fibrosis stage F3 and F4; n = 20).
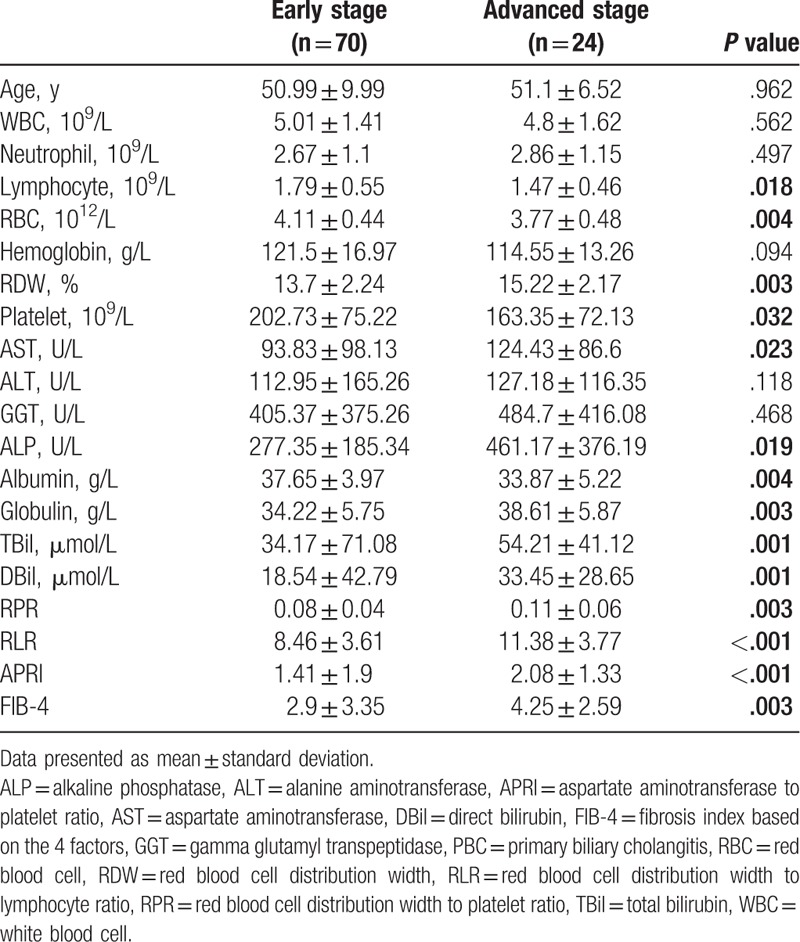
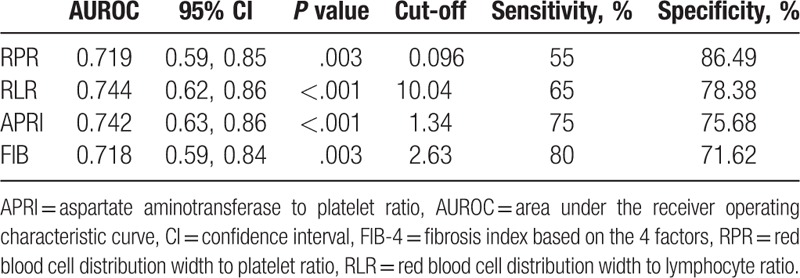
Patients with advanced-stage had significantly higher red blood cell distribution width (RDW) (15.2 vs 13.7; P = .003) and significantly lower platelet (163.35 vs 202.73; P = .032) and lymphocyte counts (1.47 vs 1.79; P = .018) as compared with patients with early-stage cirrhosis. Advanced-stage cirrhosis was associated with significantly higher RDW to platelet ratio (RPR), RDW to lymphocyte ratio (RLR), aspartate aminotransferase to platelet ratio index (APRI), and fibrosis index based on the 4 factors (FIB-4) as compared with early-stage cirrhosis. RLR showed the highest area under receiver operating characteristic curve (AUROC) (0.744). The sensitivity and specificity of RLR were 65% and 78.3%, respectively. RLR had higher AUROC than the other 3 noninvasive markers.
The noninvasive markers RPR, RLR, APRI, and FIB-4 showed good diagnostic accuracy for advanced-stage cirrhosis. These markers are easily acquired by routine laboratory tests and are reproducible predictors of the severity of PBC. RLR is a novel marker that may serve as a valuable supplement to APRI and FIB-4 for predicting the severity of cirrhosis.
Keywords: primary biliary cholangitis, noninvasive, histology
1. Introduction
Primary biliary cholangitis is a chronic disease caused by auto-immune destruction of small intrahepatic bile ducts, which results in fibrosis and cirrhosis with various life-threatening complications.[1] Primary sclerosing cholangitis (PSC) is also a chronic, progressive cholestatic disorder mostly seen in young men while primary biliary cholangitis (PBC) mainly affects middle-aged women with a wide variability in incidence rates across the world.[2–4] The diagnostic standard for PBC is the presence of 2 of the following 3 criteria: increased serum alkaline phosphatase (ALP) level as a biochemical evidence of cholestasis; presence of anti-mitochondrial antibody (AMA); and histological evidence of destructive and suppurative changes in small-sized and medium-sized bile ducts.[5] Liver biopsy provides valuable information pertaining to the development of the disease and is useful for monitoring of therapeutic response and prognostic assessment.[6] Although liver biopsy is the gold standard for diagnosis and staging of PBC, it is an invasive and painful procedure and its use for staging of PBC has been widely debated.[7] Therefore, it is not commonly performed for conventional diagnosis of PBC except in intractable cases. Several noninvasive serum markers for diagnosis of fibrosis and cirrhosis have been developed, such as aspartate transaminase-to-platelet ratio index (APRI) and fibrosis index based on 4 factors (FIB-4).[8,9] APRI has been shown to be useful as a predictor of histological severity as well as for follow-up of liver cirrhosis.[10] In a study conducted in United Kingdom, elevated APRI was shown to be associated with future risk of adverse events, independently and additively of the response to ursodeoxycholic acid (UDCA) therapy.[11] To the best of our knowledge, few studies have investigated noninvasive markers specifically for PBC. The need for reliable noninvasive markers of the severity of PBC is compelling.
Red blood cell distribution width reflects the range of variation in red blood cell volume in circulation; it is a commonly used index in routine blood tests.[12] The value of RDW for assessment of disease severity and prognostic assessment has been evaluated in various cardiovascular diseases, renal diseases, and some malignant diseases.[13–15] Some studies have indicated a correlation between RDW and liver disease in the context of nonalcoholic fatty liver disease and hepatitis B virus infection.[16,17] Other studies have shown an association of neutrophil to lymphocyte ratio with hepatitis B cirrhosis and hepatocellular carcinoma.[18,19]
In this study, we aimed to explore the relationship between the severity of histological stage and some noninvasive markers such as APRI, FIB-4, RDW to neutrophil ratio, and RDW to platelet ratio in patients with PBC.
2. Materials and methods
2.1. Study population
This was a retrospective study of patients with PBC treated at the First Hospital of Jilin University. Patients diagnosed with PBC between January 2013 and December 2017 were eligible for inclusion. The inclusion criterion was patients with liver biopsy-proven PBC. The exclusion criteria were hepatitis-related liver disease, overlap syndrome with autoimmune hepatitis/primary sclerosing cholangitis, and malignant diseases. Ninety-four patients with biopsy-proven PBC who were treatment-naive at the time of liver biopsy were included in the analysis. The study was conducted in accordance with the Declaration of Helsinki and was approved by the First Hospital of Jilin University Ethics Committee.
2.2. Clinical and laboratory data
The following data were obtained retrospectively from the electronic medical registry database: sex, age, white blood cell count (WBC), neutrophil count, lymphocyte count, red blood cell count (RBC), hemoglobin, red cell distribution width, platelet count, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transferase (GGT), ALP, albumin, globulin, total bilirubin (TBil), and direct bilirubin (DBil). The laboratory tests were conducted within the 1-week period immediately preceding the date of liver biopsy. The parameters investigated were calculated as follows: APRI = (AST/ULN)∗100/PLT; FIB-4 = (Age∗AST)/[PLT∗(ALT)1/2]; RPR = RDW(%)/PLT; RLR = RDW(%)/Lymphocyte. PLT and lymphocyte counts were expressed as 109/L.
2.3. Histological assessment
Liver needle biopsy was performed under color Doppler ultrasound guidance. A liver tissue of 1.5 cm requires a minimum of 5 portal tracts for diagnosis. Histological evaluation was performed according to the classification system defined by Ludwig and Scheuer. It was performed by 2 specialists at the Department of Pathology, First Hospital of Jilin University in a double-blind manner. Disease stage was classified into 4 stages according to the histologic staging system (stage 1, florid duct lesions; stage 2, proliferating ductular structures; stage 3, evidence of scarring; and stage 4, cirrhosis). The 94 patients were classified into 2 groups, i.e., early-stage PBC (Scheuer stage 1 and 2) and advanced-stage PBC (Scheuer stage 3 and 4).
2.4. Statistical analysis
Variables are presented as mean ± standard deviation. Categorical variables were analyzed using the chi-squared test. Student t test was used for normally distributed continuous variables. The Mann–Whitney U test was used for comparing differences between 2 independent samples, which were not normally distributed. Receiver operating characteristic curve analysis was used to determine the optimal cutoff value and the associated sensitivity and specificity for differentiating early and advanced stage of cirrhosis. P < .05 was defined as statistically significant. All statistical analyses were performed using SPSS version 18.0 (Chicago, IL).
3. Results
Out of the 94 patients, 74 (78.72%) patients were defined as early-stage PBC and the remaining 20 (21.28%) patients were defined as advanced-stage PBC. Women accounted for 86.4% and 95% of patients with early-stage and advanced-stage disease, respectively; no significant between-group difference was observed in this respect (P > .05). The mean age of patients with early-stage and advanced-stage PBC was 50.99 ± 9.99 years and 51.1 ± 6.52 years, respectively; the between-group difference in this respect was not statistically significant (P > .05).
No significant between-group differences were observed with respect to WBC count, neutrophil count, hemoglobin, or serum ALT (P > .05). Patients with advanced-stage PBC had significantly lower lymphocyte count, RBC count, and platelet count as compared with patients with early-stage PBC (P = .018 and P = .032). Advanced-stage PBC was associated with significantly higher RDW as compared with early-stage PBC (P = .003). Serum GGT, ALP, globulin, and bilirubin (total and direct) levels in advanced stage were higher than those in early stage. Only serum albumin levels were decreased in patients with advanced-stage PBC (P = .004). Patients with advanced-stage disease exhibited significantly higher RPR (P = .003), RLR (P = .001), APRI (P = .001), and FIB-4 (P = .003) levels as compared with patients with early-stage PBC (Table 1).
Table 1.
Comparison of clinical characteristics of patients with early stage and advanced stage PBC.
Receiver operating characteristic (ROC) curve analysis was performed for RPR, RLR, APRI, and FIB-4. The area under the ROC curve (AUROC) for RLR (0.744) was greater than the AUROC for the other 3 parameters (RPR: 0.719; APRI: 0.742; FIB-4: 0.718). The optimal cutoff value of RLR for distinguishing advanced cirrhosis from early stage (10.04) was associated with 65% sensitivity and 78.3% specificity (Fig. 1 and Table 2).
Figure 1.
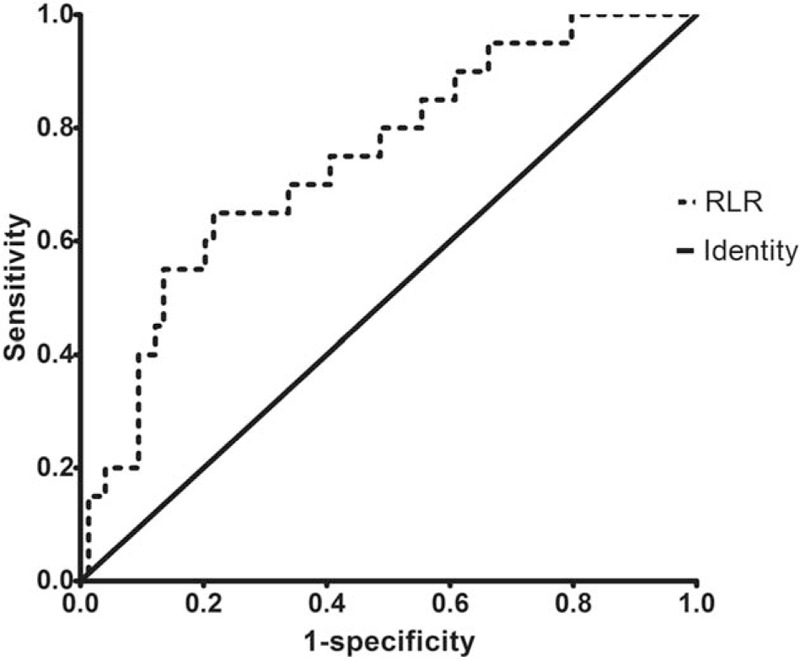
Receiver operating characteristic curve of RLR for differentiation of PBC patients with advanced stage cirrhosis. PBC = primary biliary cholangitis, RLR = red blood cell width distribution to lymphocyte ratio.
Table 2.
Diagnostic accuracy of different noninvasive markers for prediction of histological severity.
4. Discussion
To the best of our knowledge, this is the first study that evaluates the clinical relevance of RLR in patients with PBC. The results showed that RLR, an easily-acquired parameter through blood routine tests, can effectively differentiate advanced cirrhosis from early cirrhosis. RLR may also serve as a valuable marker for estimating the therapeutic response and to predict prognosis.
PBC is a chronic disease that is increasingly being recognized in recent years and its prevalence is growing worldwide.[20] The disease onset is typically insidious and is associated with non-specific symptoms. Pruritus and fatigue were reported in 80% and 20% to 70% patients, respectively.[1] Symptoms of decompensated cirrhosis may be seen in advanced stage. The presence of AMA is an important diagnostic marker of PBC that has a sensitivity and specificity of 90% to 95%.[21]
Liver biopsy is still the gold standard for diagnosis and staging of PBC and is useful for monitoring of therapeutic response.[22] Moreover, it can also predict prognosis of PBC.[23] Liver biopsy is essential when PBC-specific antibodies are absent, or when coexisting autoimmune hepatitis (AIH) or non-alcoholic steatohepatitis is suspected.[24] However, it is not essential and, indeed, not accepted by many patients owing to its invasive nature and the associated risk of complications.[25,26] The risk associated with liver biopsy in patients with advanced stage of cirrhosis is much higher than that in patients with early stage. Patients with early-stage disease generally show good clinical and biochemical response to ursodeoxycholic acid (UDCA) therapy.[22] Patients with advanced-stage cirrhosis tend to easily develop hepatic decompensation and show poor response to UDCA.[27] In addition, UDCA treatment in advanced stage does not decrease the risk of mortality or hepatocellular carcinoma in patients with PBC.[28] The prognosis of patients with early stage of cirrhosis is better than that of patients with advanced stage. Thus, figuring out the stage of PBC with noninvasive method is a key imperative.
Noninvasive methods were shown to have a high accuracy for detection of fibrosis.[29,30] An ideal noninvasive marker for predicting the severity of cirrhosis should be easy to acquire, cheap, and have a relatively high specificity. Previous studies had assessed the serum markers of histological severity of chronic hepatitis B, chronic hepatitis C, and non-alcoholic fatty liver disease.[31–33] However, not many studies have investigated noninvasive markers of PBC.
Parameters like RDW, RPR, AST/ALT ratio (AAR), and neutrophil to lymphocyte ratio (NLR) were shown to predict the degree of cirrhosis. In a study by Lou et al,[34] RDW value was shown to directly correlate with the severity of cirrhosis. In addition, RDW was shown to predict 3-month mortality in patients with hepatitis B. In a study by Chen et al,[35] RPR was shown to accurately predict the degree of cirrhosis. Use of this index may reduce the need for liver biopsy. Similar results pertaining to the ability of RPR to predict the degree of cirrhosis were reported by Taefi et al.[36] Another study from Sweden concluded that the AST/ALT ratio may help identify cirrhosis in patients with PBC.[37] Alkhouri et al[38] showed a positive correlation between NLR and the severity of cirrhosis.
RDW reflects the variation in the distribution of RBC volume in circulation. High values imply greater variation that may relate to anemia. Also, RDW is a marker of chronic inflammation.[39] RDW has been shown to be related with various diseases, such as cardiovascular diseases, renal diseases, and some malignant diseases.[13,15,40] In this study, RDW increased with increase in the severity of disease. Thrombocytopenia is a well-known complication of cirrhosis; therefore, platelet count decreases with the severity of cirrhosis. PBC is an autoimmune disease. Lymphocytes play a crucial role in immune surveillance. A decrease in lymphocyte count may relate with apoptosis and dysfunction of immune cells. Development of advanced cirrhosis is associated with a progressive decrease in lymphocyte count.
In this study, we observed significant difference between patients with early and advanced cirrhosis with respect to RPR, RLR, APRI, and FIB-4. In addition to APRI and FIB-4, which are commonly used noninvasive markers of disease severity, we observed a particularly good ability of RPR and RLR to distinguish between early and advanced cirrhosis. The diagnostic specificity of both RPR (86.4%) and RLR (78.3%) was higher than that of APRI and FIB-4. However, the sensitivity was lower than that of APRI and FIB-4. Therefore, RLR may serve as a valuable supplement to APRI and FIB-4 owing to its high specificity. RLR showed the highest AUROC among all the noninvasive markers (0.744). The results pertaining to APRI in this study contradict those reported from a study conducted in Turkey,[41] in which APRI value decreased with increase in the severity of PBC. Of note, there were no significant differences pertaining to baseline data between patients with early-stage and late-stage disease in the Turkish study; this may have contributed to the contradictory results. Nonetheless, our findings pertaining to APRI and FIB-4 are consistent with those of other studies.[42,43]
There were some limitations of this study. First, the sample size of this study was limited. Most patients in our hospital tend to refuse biopsy owing to the invasive nature and pain associated with this procedure. In addition, patients with decompensated cirrhosis are not suitable for invasive procedure due to the high risk of hemorrhage. In our further study, more patients will be enrolled to minimize the bias caused by sample size. Second, this was a single-center retrospective study, which lacked data from other areas. The results need to be validated internally and externally. We will enroll patients from other areas prospectively to validate these results.
In conclusion, this study provides insights into the relationship between noninvasive markers and histological severity of PBC. PRP, APRI, and FIB-4 showed their predictive value for estimation of the severity of PBC. To the best of our knowledge, this is the first study that investigated the clinical relevance of RLR as a new and easily acquired noninvasive marker of the severity of cirrhosis. Owing to its high diagnostic specificity, RLR may serve as a valuable supplement to APRI and FIB-4 for determining the severity of PBC.
Author contributions
Conceptualization: Jing Meng, Junqi Niu.
Data curation: Jing Meng, Hongqin Xu, Xu Liu, Ruihong Wu.
Formal analysis: Jing Meng, Hongqin Xu, Xu Liu, Ruihong Wu.
Methodology: Jing Meng, Hongqin Xu, Junqi Niu.
Software: Jing Meng, Xu Liu, Ruihong Wu.
Supervision: Hongqin Xu, Junqi Niu.
Writing – original draft: Jing Meng.
Writing – review & editing: Junqi Niu.
Footnotes
Abbreviations: AAR = AST/ALT ratio, AIH = autoimmune hepatitis, ALP = alkaline phosphatase levels, ALT = alanine aminotransferase, AMA = anti-mitochondrial antibody, APRI = aspartate aminotransferase-to-platelet ratio index, AST = aspartate aminotransferase, AUROC = area under receiver operating characteristic curve, DBil = direct bilirubin, FIB-4 = fibrosis index based on the 4 factors, GGT = gamma glutamyl transferase, PBC = primary biliary cholangitis, PSC = primary sclerosing cholangitis, RBC = red blood cell count, RDW = red cell width distribution, RLR = red blood cell width distribution to lymphocyte ratio, RPR = red cell width distribution to platelet ratio, TBil = total bilirubin, UDCA = ursodeoxycholic acid, WBC = white blood cell count.
Data availability: Data can be obtained by contacting correspondence author.
The authors declared no conflicts of interest.
References
- [1].Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet 2015;386:1565–75. [DOI] [PubMed] [Google Scholar]
- [2].Imam MH, Lindor KD. The natural history of primary biliary cirrhosis. Semin Liver Dis 2014;34:329–33. [DOI] [PubMed] [Google Scholar]
- [3].Yimam KK, Bowlus CL. Diagnosis and classification of primary sclerosing cholangitis. Autoimmun Rev 2014;13:445–50. [DOI] [PubMed] [Google Scholar]
- [4].Taghavi SA, Majd SK, Sianati M, et al. Prevalence of IgG-4-associated cholangiopathy based on serum IgG-4 levels in patients with primary sclerosing cholangitis and its relationship with inflammatory bowel disease. Turk J Gastroenterol 2016;27:547–52. [DOI] [PubMed] [Google Scholar]
- [5].Lindor KD, Gershwin ME, Poupon R, et al. Primary biliary cirrhosis. Hepatology 2009;50:291–308. [DOI] [PubMed] [Google Scholar]
- [6].Locke GR, Therneau TM, Ludwig J, et al. Time course of histological progression in primary biliary cirrhosis. Hepatology 1996;23:52–6. [DOI] [PubMed] [Google Scholar]
- [7].Pokorska-Śpiewak M, Kowalik-Mikołajewska B, Aniszewska M, et al. Is liver biopsy still needed in children with chronic viral hepatitis. World J Gastroenterol 2015;21:12141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26. [DOI] [PubMed] [Google Scholar]
- [9].Vallet-Pichard A, Mallet V, Pol S. FIB-4: a simple, inexpensive and accurate marker of fibrosis in HCV-infected patients. Hepatology 2006;44:769author reply 769-770. [DOI] [PubMed] [Google Scholar]
- [10].Imanieh MH, Hakimzadeh M, Dehghani SM, et al. Aspartate aminotransferase to platelet ratio index and severity of hepatic fibrosis in children. Comp Clin Path 2015;24:1611–5. [Google Scholar]
- [11].Trivedi PJ, Bruns T, Cheung A, et al. Optimising risk stratification in primary biliary cirrhosis: AST/platelet ratio index predicts outcome independent of ursodeoxycholic acid response. J Hepatol 2014;60:1249–58. [DOI] [PubMed] [Google Scholar]
- [12].Adamsson ES, Borné Y, Melander O, et al. Red blood cell distribution width is associated with incidence of atrial fibrillation. J Intern Med 2014;275:84–92. [DOI] [PubMed] [Google Scholar]
- [13].Yonemoto S, Hamano T, Fujii N, et al. Red cell distribution width and renal outcome in patients with non-dialysis-dependent chronic kidney disease. PLoS One 2018;13:e0198825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wei J, Yang RX, Ye Q, et al. Higher risk of myocardial injury in chest pain patients with elevated red blood cell distribution width. Clin Chim Acta 2018;481:121–5. [DOI] [PubMed] [Google Scholar]
- [15].Cheng S, Han F, Wang Y, et al. The red distribution width and the platelet distribution width as prognostic predictors in gastric cancer. BMC Gastroenterol 2017;17:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cengiz M, Candir BA, Yilmaz G, et al. Is increased red cell distribution width an indicating marker of nonalcoholic steatohepatitis and fibrotic stage. World J Gastroenterol 2013;19:7412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xu WS, Qiu XM, Ou QS, et al. Red blood cell distribution width levels correlate with liver fibrosis and inflammation: a noninvasive serum marker panel to predict the severity of fibrosis and inflammation in patients with hepatitis B. Medicine (Baltimore) 2015;94:e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yilmaz B, Aydin H, Can G, et al. The relationship between fibrosis level and blood neutrophil to lymphocyte ratio in inactive hepatitis B carriers. Eur J Gastroenterol Hepatol 2014;26:1325–8. [DOI] [PubMed] [Google Scholar]
- [19].Liu X, He L, Han J, et al. Association of neutrophil-lymphocyte ratio and T lymphocytes with the pathogenesis and progression of HBV-associated primary liver cancer. PLoS One 2017;12:e0170605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tanaka A, Ma X, Yokosuka O, et al. Autoimmune liver diseases in the Asia-Pacific region: proceedings of APASL symposium on AIH and PBC 2016. Hepatol Int 2016;10:909–15. [DOI] [PubMed] [Google Scholar]
- [21].Leuschner U. Primary biliary cirrhosis--presentation and diagnosis. Clin Liver Dis 2003;7:741–58. [DOI] [PubMed] [Google Scholar]
- [22].Poupon R. Primary biliary cirrhosis: a 2010 update. J Hepatol 2010;52:745–58. [DOI] [PubMed] [Google Scholar]
- [23].Lammers WJ, Kowdley KV, van Buuren HR. Predicting outcome in primary biliary cirrhosis. Ann Hepatol 2014;13:316–26. [PubMed] [Google Scholar]
- [24].European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67:145–72. [DOI] [PubMed] [Google Scholar]
- [25].Mueller M, Kratzer W, Oeztuerk S, et al. Percutaneous ultrasonographically guided liver punctures: an analysis of 1961 patients over a period of ten years. BMC Gastroenterol 2012;12:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol 2010;8:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology 2000;31:1005–13. [DOI] [PubMed] [Google Scholar]
- [28].Degott C, Zafrani ES, Callard P, et al. Histopathological study of primary biliary cirrhosis and the effect of ursodeoxycholic acid treatment on histology progression. Hepatology 1999;29:1007–12. [DOI] [PubMed] [Google Scholar]
- [29].Alizadeh A, Mansour-Ghanaei F, Roozdar A, et al. Laboratory tests, liver vessels color doppler sonography, and FibroScan findings in patients with nonalcoholic fatty liver disease: an observation study. J Clin Imaging Sci 2018;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- [31].Zhao Z, Liu J, Wang J, et al. Platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) are associated with chronic hepatitis B virus (HBV) infection. Int Immunopharmacol 2017;51:1–8. [DOI] [PubMed] [Google Scholar]
- [32].Sebastiani G, Alberti A. How far is noninvasive assessment of liver fibrosis from replacing liver biopsy in hepatitis C. J Viral Hepat 2012;19suppl:18–32. [DOI] [PubMed] [Google Scholar]
- [33].Tsai E, Lee TP. Diagnosis and evaluation of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, including noninvasive biomarkers and transient elastography. Clin Liver Dis 2018;22:73–92. [DOI] [PubMed] [Google Scholar]
- [34].Lou Y, Wang M, Mao W. Clinical usefulness of measuring red blood cell distribution width in patients with hepatitis B. PLoS One 2012;7:e37644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen B, Ye B, Zhang J, et al. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. PLoS One 2013;8:e68780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Taefi A, Huang CC, Kolli K, et al. Red cell distribution width to platelet ratio, a useful indicator of liver fibrosis in chronic hepatitis patients. Hepatol Int 2015;9:454–60. [DOI] [PubMed] [Google Scholar]
- [37].Nyblom H, Björnsson E, Simrén M, et al. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int 2006;26:840–5. [DOI] [PubMed] [Google Scholar]
- [38].Alkhouri N, Morris-Stiff G, Campbell C, et al. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 2012;32:297–302. [DOI] [PubMed] [Google Scholar]
- [39].He Y, Liu C, Zeng Z, et al. Red blood cell distribution width: a potential laboratory parameter for monitoring inflammation in rheumatoid arthritis. Clin Rheumatol 2018;37:161–7. [DOI] [PubMed] [Google Scholar]
- [40].Poludasu S, Marmur JD, Weedon J, et al. Red cell distribution width (RDW) as a predictor of long-term mortality in patients undergoing percutaneous coronary intervention. Thromb Haemost 2009;102:581–7. [DOI] [PubMed] [Google Scholar]
- [41].Tahtaci M, Yurekli OT, Bolat AD, et al. Increased mean platelet volume is related to histologic severity of primary biliary cirrhosis. Eur J Gastroenterol Hepatol 2015;27:1382–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang H, Xu H, Wang X, et al. Red blood cell distribution width to platelet ratio is related to histologic severity of primary biliary cirrhosis. Medicine (Baltimore) 2016;95:e3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Joshita S, Umemura T, Ota M, et al. AST/platelet ratio index associates with progression to hepatic failure and correlates with histological fibrosis stage in Japanese patients with primary biliary cirrhosis. J Hepatol 2014;61:1443–5. [DOI] [PubMed] [Google Scholar]


