Abstract
Background:
To investigate the value of positron emission tomography (PET) and PET/computed tomography (CT) using fluorine-18-fluorodeoxyglucose (18F-FDG) in the diagnosis, staging, restaging and recurrence monitoring of Ewing sarcoma family of tumors (ESFTs), a meta-analysis was performed through systematically searching PubMed, Embase, and Cochrane Central library to retrieve articles.
Methods:
After screening and diluting out the articles that met inclusion criteria to be used for statistical analysis the pooled evaluation indexes including sensitivity, specificity, and diagnostic odd ratio (DOR) as well as the summary receiver operating characteristic curve (SROC) were calculated involving diagnostic data (true positive, false positive, false negative, and true negative) extracted from original studies.
Results:
Screening determined that out of 2007, 23 studies involving a total of 524 patients were deemed viable for inclusion in the meta-analysis. The results of the analysis showed that the sensitivity and specificity were at 86% and 80%, respectively. Additionally, a satisfactory accuracy of 18F-FDG PET and PET/CT was observed in detecting ESFT recurrence, lung metastasis, and osseous metastasis.
Conclusion:
This meta-analysis suggests that 18F-FDG PET and PET/CT with an extremely high accuracy could be considered a valuable method for detecting distant metastasis and post-operational recurrence of ESFT, which might have a profound impact on the development of treatment protocols for ESFT.
Keywords: 18F-FDG PET, Ewing sarcoma family of tumors, meta-analysis, metastasis, PET/CT, recurrence
1. Introduction
The Ewing sarcoma family of tumors (ESFT) includes a series of small, round-cell malignancies that are characterized by varying degrees of neuroectodermal differentiation.[1] Ewing sarcoma (ES) of bone is the most common type of ESFT and the second most common primary bone sarcoma in children and adolescents.[2,3] Moreover, extra skeletal ES, Askin tumor (ES arising in the chest) and primitive neuroectodermal tumors (PNET) also belong to ESFT.[1] Combined therapy, including high-dose chemotherapy, surgery and/or radiotherapy, has dramatically improved the survival of localized ESFT.[4] Nevertheless, ESFTs have a tendency to distantly metastasize and locally relapse. Approximately 25% of ES cases have detectable metastases at presentation; on the other hand, approximately 30%∼40% of patients will develop local or distant recurrence after treatment, which would modify the outcome with a poor prognosis.[5–8] Consequently, accurate initial staging, restaging and recurrence monitoring play a crucial role in the treatment strategy for ESFT.
Traditional imaging modalities used to assess ESFT include plain film, computed tomography (CT), magnetic resonance imaging, bone marrow biopsy and aspiration, bone scintigraphy, and fluorine-18-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) or PET/CT. ESFT possess increased rate of glycolysis. After intravenous administration of 18F-FDG, a labeled glucose analog, accumulates in the malignant cells of ESFT and is avidly retained. By detecting sites with high 18F-FDG uptake, PET could identify malignant ESFT lesions. Hawkins et al[9] suggested that 18F-FDG PET could be applied to assess the histological response of ESFT after treatment and thus predict the outcome. More recently, hybrid 18F-FDG PET/CT further combined morphologic and metabolic information and achieved better diagnostic performance. Although guidelines proposed by National Comprehensive Cancer Network (NCCN) and the Children's Oncology Group Bone Tumor Committee both recommend 18F-FDG PET as a considerable complementary tool after the diagnosis of ES, the optimal or standardized combination of staging tools is still unclear.[10,11]
Multiple trials have attempted to quantitatively measure the accuracy of 18F-FDG PET and PET/CT in the diagnosis, staging and recurrence monitoring of ESFT, but their results are inconclusive. A previous meta-analysis suggested that 18F-FDG PET and PET/CT have high pooled sensitivity and specificity (0.96 and 0.92, respectively) in diagnosing ESFT. However, this statistical analysis only included 5 studies and did not differentiate sites of metastatic lesions or assess the accuracy on lesion-based analysis.[12] Meanwhile, more eligible studies were published in recent years. To draw a more precise conclusion on this topic, a systematic review and meta-analysis were conducted systematically involving published studies.
2. Materials and methods
This investigation was conducted based on “the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)” statement.[14] Ethical approval and patient consent were not necessary, as the analysis was performed based on data available in published literature.
2.1. Article search and study selection
Previously published studies were collected using 2 approaches. First, PubMed, Embase and the Cochrane Library were systematically searched using the following keywords:
-
1)
“Ewing” or “sarcoma” and
-
2)
“PET” or “positron emission tomography”.
No language or publication time limitation was imposed. The last search was updated on February 28, 2018. Subsequently, the bibliographies of relevant articles (reviews, editorials, included trials and meta-analyses) were screened by hand to retrieve additional studies.
Clinical studies appraising the performance of 18F-FDG PET and PET/CT in the diagnosis, staging and recurrence monitoring of patients with ESFT were eligible for inclusion in the meta-analysis. The studies that provided data to calculate the sensitivity and specificity were further included in the statistical analysis. For articles containing overlapping data, the one presenting the most comprehensive data or that was published the most recently was chosen.
The exclusion criteria were as follows:
-
1)
letters, reviews, editorials and other non-original articles;
-
2)
trials with fewer than 5 patients with ESFT;
-
3)
congress proceedings; and
-
4)
animal experiment.
Two reviewers (LFX and ZQY) independently and repeatedly evaluated the retrieved articles using the aforementioned criteria. Ineligible articles were first excluded by screening titles and abstracts. Then, the full texts of remaining studies were downloaded and reviewed in detail.
2.2. Data extraction and quality assessment
To reduce the bias, 3 reviewers (LFX, ZQY, and DJL) independently performed this process. Any discrepancies were resolved by consensus. For each included trial, the following basic information was recorded: the first author's surname, year of publication, original country, study design, number, age and gender of participants, 18F-FDG PET or PET/CT, tracer dose, methods of image interpretation and reference tests. With respect to trials eligible for inclusion in the meta-analysis, examinations or lesions were classified as true positive (TP), false positive (FP), true negative (TN) or false negative (FN) cases according to their 18F-FDG statuses and the true outcome verified by reference tests. The numbers of TPs, FPs, TNs, and FNs on lesion-based or examination-based analysis were entered into a standardized Excel file.
A quality assessment tool for the diagnostic accuracy studies (QUADAS) was used to evaluate the methodological quality of the included studies.[13] This tool contains 14 items, and each one was described for 1 score. Any discrepancies were resolved by consensus from a third investigator (DJL).
2.3. Statistical analysis
All statistical analyses were performed with Meta-disc 1.4. The heterogeneity across studies was evaluated using I-square; for an I-square <50%, the between-study heterogeneity was considered not significant. To quantitatively assess the performance of 18F-FDG PET or PET/CT in the diagnosis, staging and recurrence monitoring of ESFT, a random effect model was applied to calculate the following pooled outcome estimates: sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic odd ratio (DOR) (with 95% confidence interval) on examination-based or lesion-based analysis. Moreover, the summary receiver operating characteristic curve (SROC) with sensitivity as the x-coordinate and 1-specificity as the y-coordinate was constructed. The Q∗-index (the point where sensitivity and specificity are equal on SROC) and the area under SROC (AUC) could reflect the diagnostic accuracy of 18F-FDG PET and PET/CT.
3. Results
3.1. Search results and study characteristics
The search of electronic databases and references yielded a total of 2007 titles and abstracts. After careful selection, 23 articles published in English were included in the meta-analysis.[15–36] The detailed article search and study selection process were listed in Figure 1.
Figure 1.
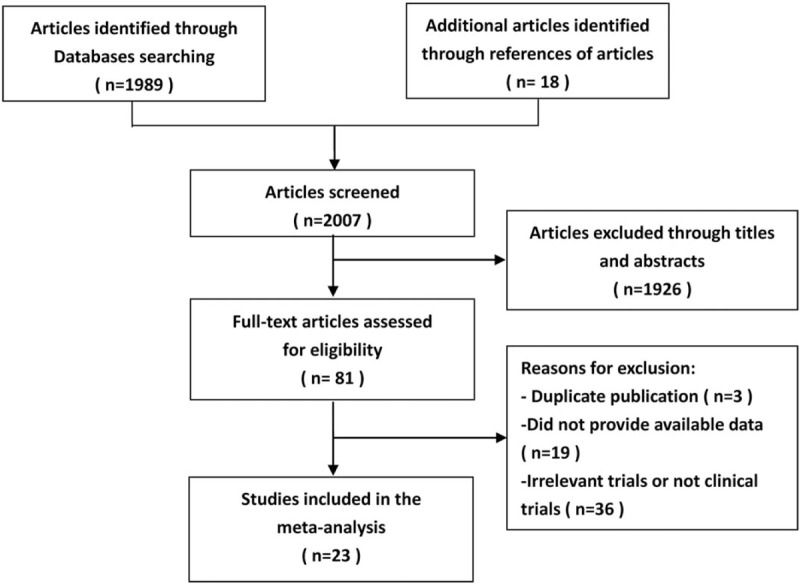
Selection flow chart for studies included in the systematic review and meta-analysis.
Several studies sharing overlapped participants presented data for different subgroup analyses. Therefore, all of these studies were included in our research. The extracted data were presented in Tables 1 and 2. As for the methodological quality, the review authors’ QUADAS judgments of included studies were presented in Figure 2.
Table 1.
Main characteristics of the included studies.
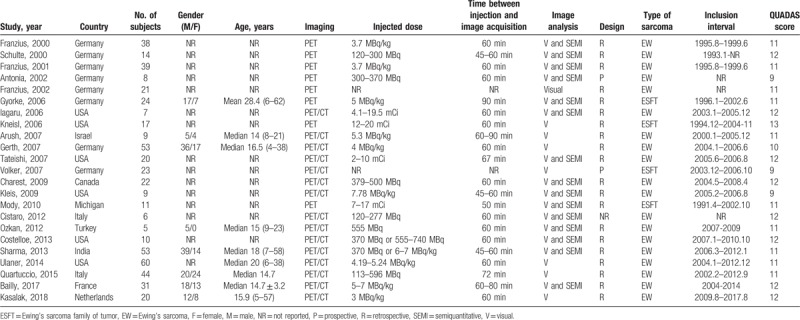
Table 2.
Diagnosis accuracy data on each examination– or lesion–based analysis.
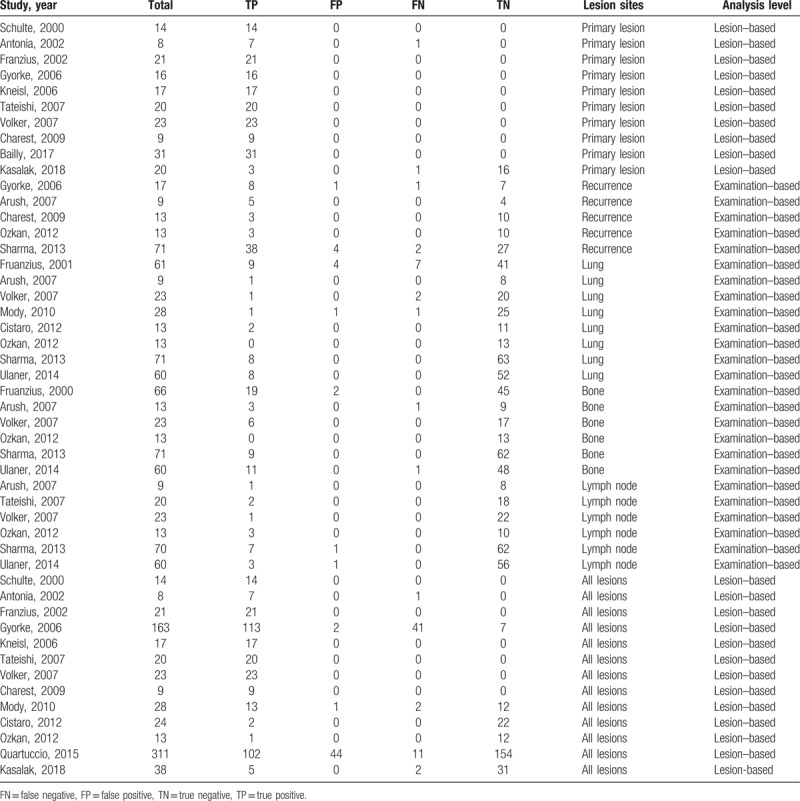
Figure 2.
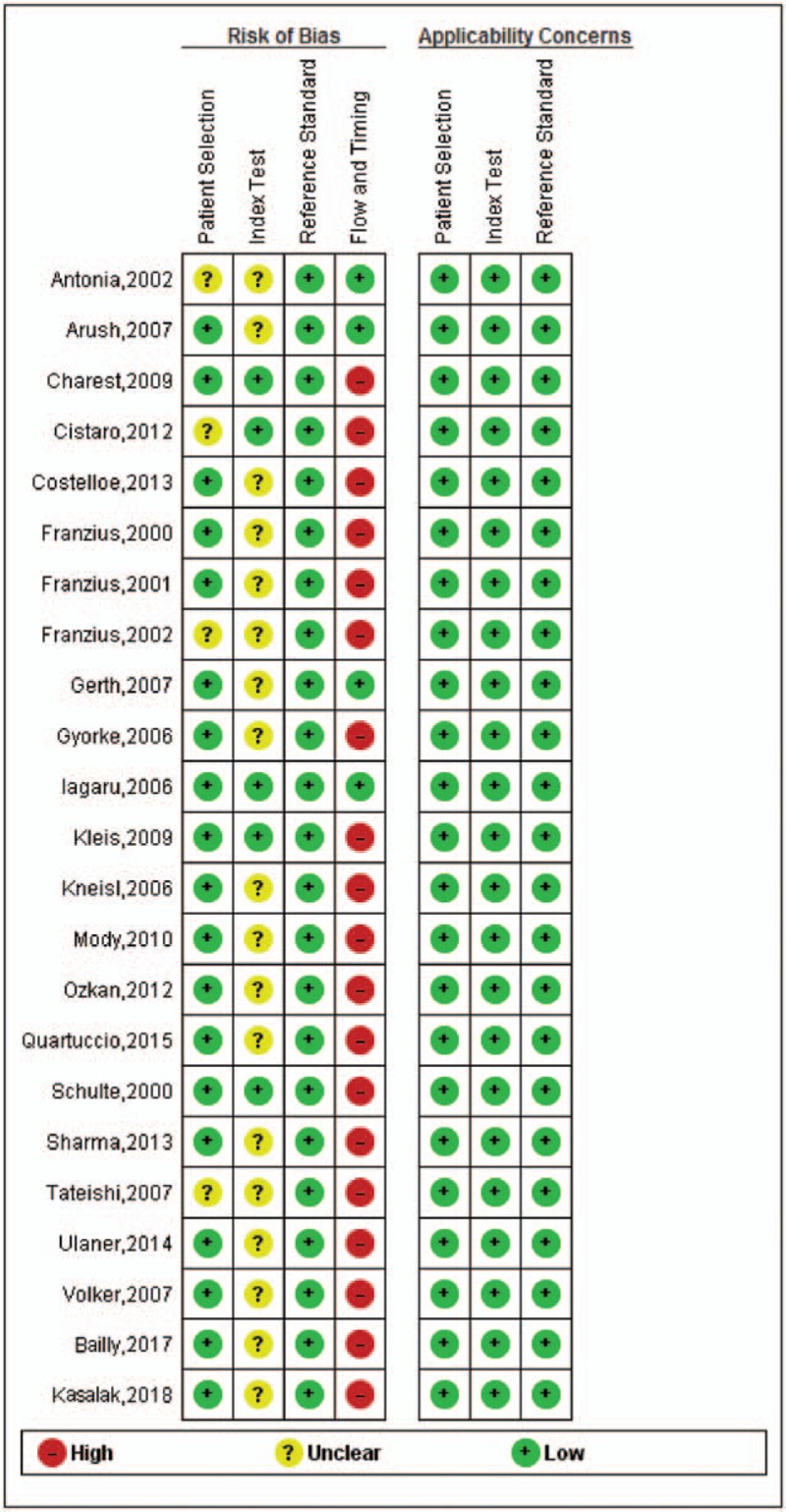
Review authors’ QUADAS judgments about each domain presented as percentages across included studies.
3.2. Lesion-based analysis
A total of 13 trials,[16,18–20,22,25–27,29–31,34,36] involving 689 lesions, were available to investigate the diagnostic performance of 18F-FDG PET and PET/CT in ESFT. The pooled results were as follows: sensitivity of 86% (95% CI of 82%–89%), specificity of 80% (95% CI of 75%–85%), PLR of 3.92 (95% CI of 3.08–4.98), NLR of 0.19 (95% CI of 0.12–0.30) and DOR of 29.22 (95% CI of 16.49–51.78). There was no significant between-study heterogeneity for the sensitivity (I-square = 0.001%). Meanwhile, the Q∗-index and AUC were 0.8474 and 0.9147, respectively (Fig. 3). Meanwhile, 9 studies[16,18–20,22,25–27,35] involving a total of 170 ESFT described SUVmax uptake in the primary lesions at initial staging, all being 18F-FDG-avid.
Figure 3.
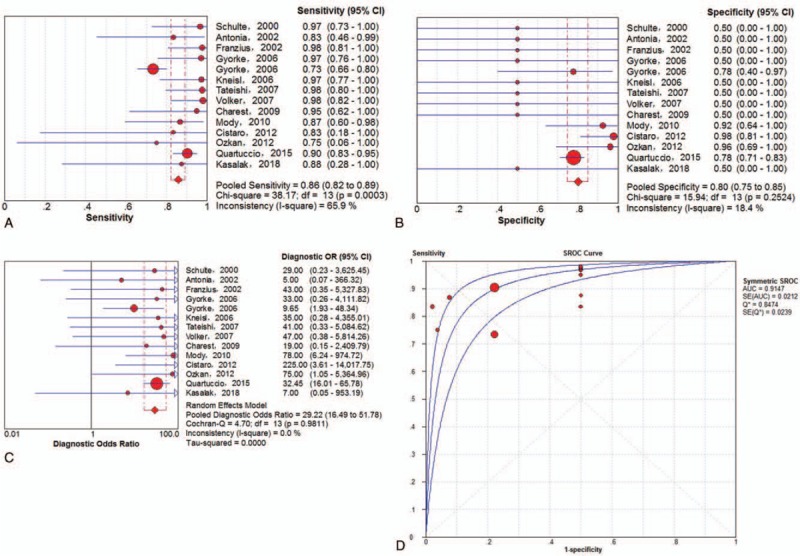
Performance of 18F-FDG PET/CT to detect all ESFT lesions on lesion-based analysis: a. pooled sensitivity, b. pooled specificity, c. pooled diagnostic odds ratio, and d. SROC with the Q∗-index. SROC = summary receiver operating characteristic curve.
3.3. Examination-based analysis
3.3.1. Recurrence
A total of 5 trials[20,23,27,31,33] involving 123 examinations addressed ESFT recurrence (including local and distant recurrence) using 18F-FDG PET or PET/CT. The threshold effect was found in the provided examination-based data (Spearman correlation coefficient = 0.895; P value = .040). The pooled results for 18F-FDG PET and PET/CT to detect ESFT recurrence were as follows: sensitivity of 93% (95% CI of 83%–98%), specificity of 90% (95% CI of 80%–96%), PLR of 8.53 (95% CI of 4.12–17.65), NLR of 0.09 (95% CI of 0.04–0.21), and DOR of 109.98 (95% CI of 30.66–394.54). There was no significant between-study heterogeneity for the included outcome estimates (all I-squares = 0). Meanwhile, the Q∗-index and AUC were 0.9129 and 0.9656, respectively (Fig. 4).
Figure 4.
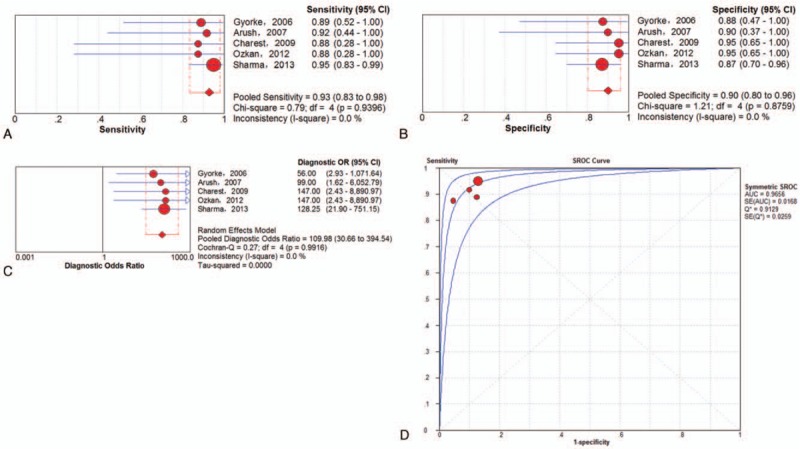
Performance of 18F-FDG PET and PET/CT to detect recurrence of ESFT on an examination-based analysis: a. pooled sensitivity, b. pooled specificity, c. pooled diagnostic odds ratio, and d. SROC with the Q∗-index. ESFT = Ewing's sarcoma family of tumor, 18F-FDG = fluorine-18-fluorodeoxyglucose, PET/CT = positron emission tomography/computed tomography, SROC = summary receiver operating characteristic curve.
Subgroup analysis was performed exclusively on the basis of PET/CT data. A total of 4 trials[23,27,31,33] involving 106 examinations addressed ESFT recurrence using 18F-FDG PET/CT. The pooled results for 18F-FDG PET to detect ESFT recurrence were as follows: sensitivity of 94% (95% CI of 83%–98%), specificity of 91% (95% CI of 80%–97%), PLR of 8.82 (95% CI of 4.00–19.45), NLR of 0.08 (95% CI of 0.03–0.22) and DOR of 128.49 (95%CI of 31.15–529.99). There was no significant between-study heterogeneity for the included outcome estimates (all I-squares = 0). Meanwhile, the Q∗-index and AUC were 0.9198 and 0.9700, respectively (Fig. 5).
Figure 5.
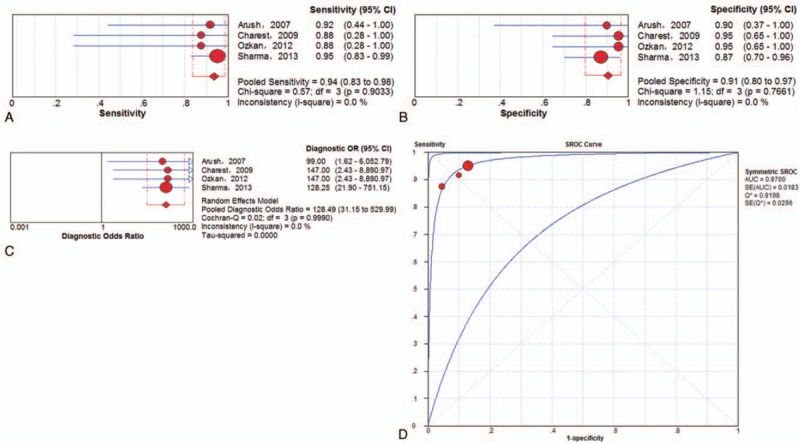
Performance of 18F-FDG PET/CT to detect recurrence of ESFT on an examination-based analysis: a. pooled sensitivity, b. pooled specificity, c. pooled diagnostic odds ratio, and d. SROC with the Q∗-index. SROC = summary receiver operating characteristic curve.
3.3.2. Lung metastasis
On the examination-based level, a total of 8 trials[5,17,23,26,29–31,33] involving 278 examinations investigated lung metastasis of ESFT using 18F-FDG PET or PET/CT. The pooled results for 18F-FDG PET and PET/CT to detect ESFT recurrence were as follows: sensitivity of 72% (95% CI of 57%–84%), specificity of 97% (95% CI of 94%–99%), PLR of 13.51 (95% CI of 6.22–29.34), NLR of 0.38 (95% CI of 0.20–0.74) and DOR of 60.55 (95% CI of 14.41–254.39). There was no significant between-study heterogeneity for the included outcome estimates. The Q∗-index and AUC were 0.8861 and 0.9467, respectively (Fig. 6).
Figure 6.
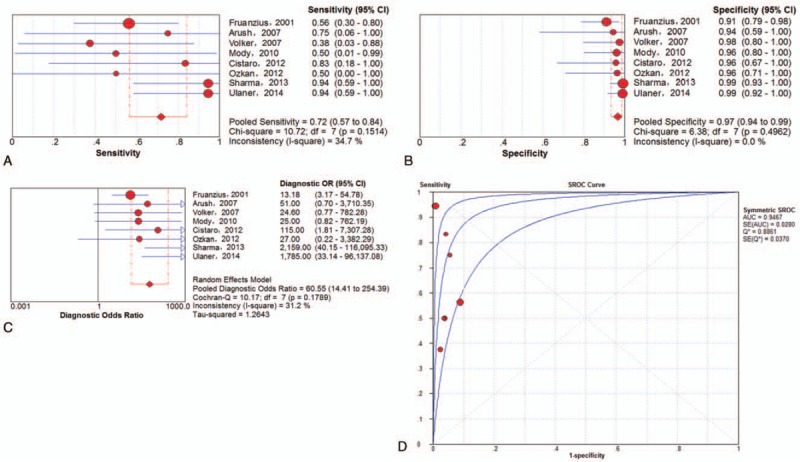
Performance of 18F-FDG PET and PET/CT to detect lung metastasis of ESFT on examination-based analysis: a. pooled sensitivity, b. pooled specificity, c. pooled diagnostic odds ratio, and d. SROC with the Q∗-index. ESFT = Ewing's sarcoma family of tumor, 18F-FDG = fluorine-18-fluorodeoxyglucose, PET/CT = positron emission tomography/computed tomography, SROC = summary receiver operating characteristic curve.
Subgroup analysis was also performed exclusively on the basis of PET/CT data. On the examination-based level, a total of 6 trials[5,23,26,30,31,33] involving 189 examinations investigated lung metastasis of ESFT using 18F-FDG PET/CT. The pooled results for 18F-FDG PET and PET/CT to detect ESFT recurrence were as follows: sensitivity of 82% (95% CI of 63%–94%), specificity of 98% (95% CI of 95%–100%), PLR of 32.04 (95% CI of 9.85–104.24), NLR of 0.24 (95% CI of 0.06–0.89) and DOR of 160.70 (95% CI of 30.68–841.61). There was no significant between-study heterogeneity for the included outcome estimates. The Q∗-index and AUC were 0.9807 and 0.9972, respectively (Fig. 7).
Figure 7.
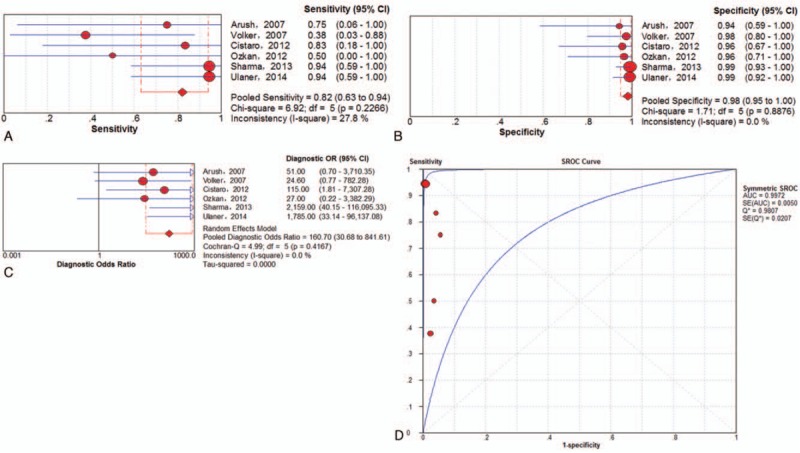
Performance of 18F-FDG PET/CT to detect lung metastasis of ESFT on examination-based analysis: a. pooled sensitivity, b. pooled specificity, c. pooled diagnostic odds ratio, and d. SROC with the Q∗-index. ESFT = Ewing's sarcoma family of tumor, 18F-FDG = fluorine-18-fluorodeoxyglucose, PET/CT = positron emission tomography/computed tomography, SROC = summary receiver operating characteristic curve.
3.3.3. Bone metastasis
A total of 6 trials[5,15,23,26,31,33] involving 246 examinations addressed osseous metastasis of ESFT using 18F-FDG PET or PET/CT. The pooled results for 18F-FDG PET and PET/CT to detect bone metastasis of ESFT were as follows: sensitivity of 91% (95% CI of 80% to 97%), specificity of 98% (95% CI of 94%–99%), PLR of 26.60 (95% CI of 11.06–63.97), NLR of 0.15 (95% CI of 0.05–0.39) and DOR of 347.37 (95% CI of 78.50–1537.18). There was no significant between-study heterogeneity for the included outcome estimates. The Q∗-index and AUC were 0.9492 and 0.9859, respectively (Fig. 8).
Figure 8.
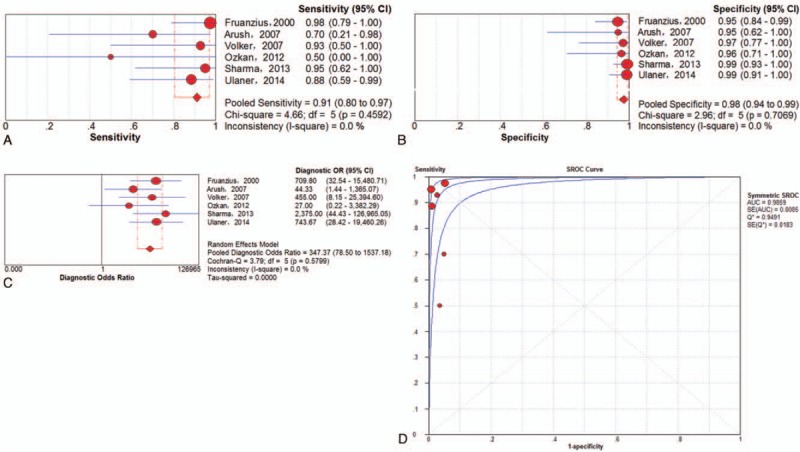
Performance of 18F-FDG PET and PET/CT to detect osseous metastasis of ESFT on an examination-based analysis: a. pooled sensitivity, b. pooled specificity, c. pooled diagnostic odds ratio, and d. SROC with the Q∗-index. ESFT = Ewing's sarcoma family of tumor, 18F-FDG = fluorine-18-fluorodeoxyglucose, PET/CT = positron emission tomography/computed tomography, SROC = summary receiver operating characteristic curve.
Subgroup analysis was performed exclusively on the basis of PET/CT data. A total of 5 trials[5,23,26,31,33] involving 180 examinations addressed osseous metastasis of ESFT using 18F-FDG PET/CT. The pooled results for 18F-FDG PET and PET/CT to detect bone metastasis of ESFT were as follows: sensitivity of 88% (95% CI of 72%–96%), specificity of 98% (95% CI of 95%–100%), PLR of 39.34 (95% CI of 10.99–140.84), NLR of 0.19 (95% CI of 0.08–0.45) and DOR of 279.65 (95% CI of 51.19–1527.81) (Fig. 9). There was no significant between-study heterogeneity for the included outcome estimates. The Q∗-index and AUC were 0.9780 and 0.9965, respectively (Fig. 9).
Figure 9.
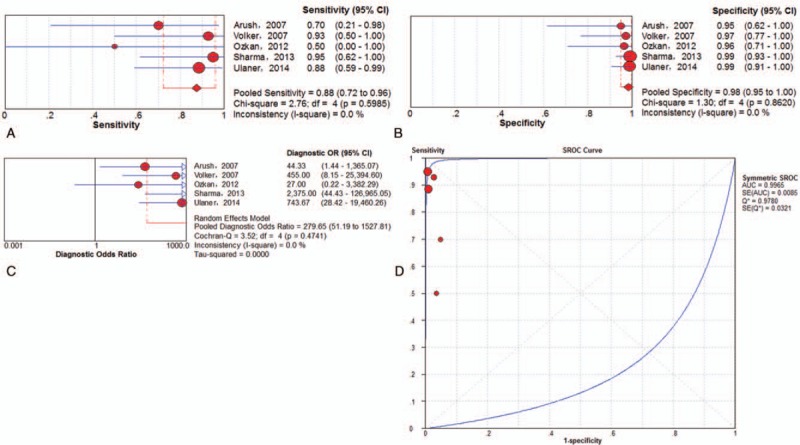
Performance of 18F-FDG PET/CT to detect osseous metastasis of ESFT on an examination-based analysis: a. pooled sensitivity, b. pooled specificity, c. pooled diagnostic odds ratio, and d. SROC with the Q∗-index. ESFT = Ewing's sarcoma family of tumor, 18F-FDG = fluorine-18-fluorodeoxyglucose, PET/CT = positron emission tomography/computed tomography, SROC = summary receiver operating characteristic curve.
3.3.4. Lymph metastasis
Only 6 studies[5,23,25,26,31,33] involving 17 TP examinations and no FN examinations were included in this study. This result should be interpreted cautiously.
4. Discussion
Results of the diagnostic accuracy of 18F-FDG PET and PET/CT in ESFT remain inconclusive. One previous study [37] tried to further clarify this issue, but none of the included studies specifically aimed at ESFT and did not statistically analyze the retrieved data. To the best of our knowledge, there is no meta-analysis comprehensively evaluating the performance of 18F-FDG PET and PET/CT in the diagnosis, staging and recurrence monitoring of ESFT. In this meta-analysis, this issue was statistically investigated by analyzing data collected from 23 studies on the lesion- or examination-based level.
ESFT is rather rare. Approximately 225 cases of ESFT in patients younger than 20 years of age are diagnosed in North America annually.[38] No blood, urine or imaging tests can specifically identify ESFT, and the gold standard for diagnosing ESFT is still pathological examination. In 2000, Franzius et al first reported the value of PET in the diagnosis of Ewing's sarcoma.[15] On examination-based analysis, no FN cases and only 2 FP cases were observed (n = 66), and the sensitivity, specificity, and accuracy were 100%, 96%, and 97%, respectively. Previous studies suggested that the SUVmax of ESFT is normally high.[39] In our collected data, a total of 139 primary ESFT lesions retrieved from 8 studies were classified as 18F-FDG-avid. However, to be clear, as overlapping SUV exist in different sarcoma types, including benign lesions, 18F-FDG PET and PET/CT cannot distinctly differentiate between ESFT and other malignant tumors, and they are mainly used as staging tools.
Although the prognosis of ESFT has greatly improved, approximately 30%∼40% of patients will develop recurrent disease after treatment. The outcome of those patients is very poor and has an overall 5-year survival of less than 15%.[40,41] Detecting patients with recurrence and improving the diagnostic accuracy at early stage may prompt effective treatment. For examination-based data, the high accuracy of 18F-FDG PET and PET/CT for detecting recurrence (local or distant) of ESFT was demonstrated; the sensitivity, specificity, and DOR were 0.93, 0.90, and 109.98, respectively, which is similar to the conclusions for other recurrent malignant tumors.[42,43] Especially, the sensitivity, specificity, and DOR of 18F-FDG PET/CT solely for detecting recurrence (local or distant) of ESFT were 0.94, 0.91, and 128.49.
18F-FDG PET/CT should be performed before a diagnostic biopsy site is chosen in patients with a high clinical suspicion of aggressive, advanced tumor. One retrospective study[44] involving 51 advanced lung cancer patients aimed to evaluate the safety and efficacy of 18F-FDG PET/CT in bone metastases lesions. No serious complications were encountered, which demonstrated that PET/CT-guided percutaneous biopsy of 18F-FDG-avid bone metastases is an effective and safe method that yields a high diagnostic success rate. Salem U and his co-authors reported that a high SUVmax of pre-therapeutic and post-therapeutic FDG PET/CT correlated with overall survival and that a pre-therapeutic SUVmax >11.6 decreased the overall survival and progression-free survival in patients with ES. They revealed that 18F-FDG PET/CT can be used as a prognostic indicator of survival in primary ES.[45] The most crucial indicator for an adverse prognosis of ESFT is the presence of metastatic disease. Approximately 25% of patients with ESFT already have detectable metastasis at diagnosis. Cotterill SJ et al analyzed 975 Ewing sarcoma patients from the European Intergroup Cooperative Group and observed that their survival rate had a good correlation with the number and location of metastatic lesions.[46] Therefore, the prognostic staging and restaging of ESFT plays a vital role in developing treatment plans and eventually ameliorates the outcome of patients. Although several imaging modalities, including chest CT, bone scintigraphy, 18F-FDG PET, and PET/CT, are recommended in the guidelines, the best imaging strategy remains unclear. An advantage of 18F-FDG PET and PET/CT is that they can be used to identify systematic metastasis. In this investigation, we further performed examination analyses according to the metastatic sites.
Most metastatic ESFT lesions occur in lung.[38] Compared to patients with bone-only metastases or a combination of lung and bone metastasis, patients with lung involvement have a better prognosis.[46] Bone is the second most frequently involved site for metastatic ESFT lesions.[38] DOR, as an indicator of diagnostic accuracy, reflects the association between the relevant disease, and test results by calculating a single special ratio that combines the sensitivity and specificity. A higher DOR (range from 0 to infinity) stands for a more favorable diagnostic accuracy. In this respect, this meta-analysis revealed that diagnosing lung metastasis of ESFT using 18F-FDG-PET and PET/CT does have a good accuracy with DORs of 60.55 (95% CI of 14.41–254.39) as for bone metastasis with DORs of 347.37 (95% CI of 78.50–1537.18). A similar trend was observed on SROC with AUCs of 0.9467 and 0.9859 for lung and bone metastases, respectively. Considering the high accuracy of 18F-FDG PET and PET/CT in detecting the osseous metastasis of ESFT, bone scintigraphy may be omitted from the staging of ESFT. Metastasis to the bone marrow, lymph nodes, and other sites, although uncommon, is also observed in patients with ESFT. In retrieved patients, all metastatic lesions in lymph nodes were identified.
Several subgroup-analyses were made based exclusively on PET/CT data about diagnosis of recurrence, lung metastases, and osseous metastases. Although seemingly the results of PET/CT were better compared with PET, we could not draw a distinct conclusion due to the overlapping confidence intervals.
The reliability and innovation of this research rely on the large sample size, uniform statistical processing, and elaborate subgroup analysis, which will help us obtain a more comprehensive understanding of the performance of 18F-FDG PET and PET/CT in the diagnosis, staging, and recurrence monitoring of ESFT. However, some limitations in this study merited consideration. First, this is a meta-analysis and systemic review; we analyzed the questions on the study level instead of on the patient level. Consequently, underestimation/overestimation was unavoidable. Second, there was methodological variability in the included trials, such as the method for measuring the 18F-FDG uptake, cutoff for determining the lesion positivity, reference standards tests and duration of follow-up. In addition, some studies evaluated 18F-FDG PET, while others evaluated PET/CT. Last but not least, several subgroup analyses were based on a small number of studies, which could reduce their power. We made our best effort to extract data on the sensitivity and specificity from 3 levels of analysis, but in some subgroups, sufficient data were unavailable for quantitative statistics.
5. Conclusion
In conclusion, this systemic review and meta-analysis indicated high sensitivity for 18F-FDG PET and PET/CT in identifying primary ESFT lesions. Moreover, 18F-FDG PET and PET/CT, with extremely high accuracy, could be considered valuable methods for detecting metastasis and post-operational recurrence, which might have a profound impact on the development of treatment protocols for ESFT. Nevertheless, considering the possibility of false-positive and false-negative cases, pathological examination or long-term follow-up should be performed for 18F-FDG-avid ESFT lesions. Large-scale, randomized, prospective trials are still needed to further warrant current conclusions.
Author contributions
Conceptualization: Tao Huang, Feng Li, Fanxiao Liu, Jinlei Dong.
Data curation: Qingyu Zhang, Fanxiao Liu, Jinlei Dong.
Formal analysis: Tao Huang, Qingyu Zhang, Fanxiao Liu, Jinlei Dong.
Funding acquisition: Jinlei Dong.
Investigation: Qingyu Zhang, Fanxiao Liu, Jinlei Dong.
Methodology: Tao Huang, Qingyu Zhang, Fanxiao Liu, Jinlei Dong.
Project administration: Qingyu Zhang, Fanxiao Liu, Jinlei Dong.
Resources: Qingyu Zhang, Fanxiao Liu, Jinlei Dong.
Software: Tao Huang, Qingyu Zhang, Fanxiao Liu, Jinlei Dong.
Supervision: Jinlei Dong.
Validation: Qingyu Zhang, Fanxiao Liu, Jinlei Dong.
Visualization: Qingyu Zhang, Fanxiao Liu, Jinlei Dong.
Writing – original draft: Tao Huang, Feng Li, Zexing Yan, Yupeng Ma, Fei Xiong, Xia Cai, Qingyu Zhang, Fanxiao Liu, Jinlei Dong.
Writing – review & editing: Tao Huang, Feng Li, Zexing Yan, Yupeng Ma, Fei Xiong, Xia Cai, Qingyu Zhang, Fanxiao Liu, Jinlei Dong.
Fanxiao Liu orcid: 0000-0002-1412-849X.
Footnotes
Abbreviations: 18F-FDG = fluorine-18-fluorodeoxyglucose, AUC = the area under SROC, CT = computed tomography, DOR = diagnostic odd ratio, ES = Ewing sarcoma, ESFT = Ewing sarcoma family of tumors, FN = false negative, FP = false positive, NLR = negative likelihood ratio, PET = positron emission tomography, PLR = positive likelihood ratio, SROC = summary receiver operating characteristic curve, TN = true negative, TP = true positive.
This study was funded by the Key R & D program in Shandong Province (Jinlei Dong, NO. 2016GSF201214) and the China Scholarship Council (CSC), which supported 2 of the authors (Fanxiao Liu, CSC, No. 201808080126; Fei Xiong, CSC, No. 201706920036).
The authors have no conflicts of interest to disclose.
References
- [1].Navid F, Billups C, Liu T, et al. Second cancers in patients with the Ewing sarcoma family of tumours. Eur J Cancer 2008;44:983–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ludwig JA. Ewing sarcoma: historical perspectives, current state-of-the-art, and opportunities for targeted therapy in the future. Curr Opin Oncol 2008;20:412–8. [DOI] [PubMed] [Google Scholar]
- [3].ESMO/European Sarcoma Network Working Group Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25suppl 3:iii113–23. [DOI] [PubMed] [Google Scholar]
- [4].Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with Ewing's sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol 2015;39:189–95. [DOI] [PubMed] [Google Scholar]
- [5].Ulaner GA, Magnan H, Healey JH, et al. Is methylene diphosphonate bone scan necessary for initial staging of Ewing sarcoma if 18F-FDG PET/CT is performed. AJR Am J Roentgenol 2014;202:859–67. [DOI] [PubMed] [Google Scholar]
- [6].Potratz J, Dirksen U, Jurgens H, et al. Ewing sarcoma: clinical state-of-the-art. Pediatr Hematol Oncol 2012;29:1–1. [DOI] [PubMed] [Google Scholar]
- [7].Ferrari S, Luksch R, Hall KS, et al. Post-relapse survival in patients with Ewing sarcoma. Pediatr Blood Cancer 2015;62:994–9. [DOI] [PubMed] [Google Scholar]
- [8].Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol 2010;11:184–92. [DOI] [PubMed] [Google Scholar]
- [9].Hawkins DS, Schuetze SM, Butrynski JE, et al. [18F] Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol 2005;23:8828–34. [DOI] [PubMed] [Google Scholar]
- [10].Meyer JS, Nadel HR, Marina N, et al. Imaging guidelines for children with Ewing sarcoma and osteosarcoma: a report from the Children's Oncology Group Bone Tumor Committee. Pediatr Blood Cancer 2008;51:163–70. [DOI] [PubMed] [Google Scholar]
- [11].National Comprehensive Cancer Network. Ewing's sarcoma family of tumors. In: NCCN clinical practice guidelines in oncology (NCCN Guidelines®): bone cancer, version 1 2014. [Google Scholar]
- [12].Treglia G, Salsano M, Stefanelli A, et al. Diagnostic accuracy of 18F-FDG-PET and PET/CT in patients with Ewing sarcoma family tumours: a systematic review and a meta-analysis. Skeletal Radiol 2012;41:249–56. [DOI] [PubMed] [Google Scholar]
- [13].Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moher D, Liberati A, Tetzlaff J, et al. Reprint--preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther 2009;89:873–80. [PubMed] [Google Scholar]
- [15].Franzius C, Sciuk J, Daldrup-Link HE, et al. FDG-PET for detection of osseous metastases from malignant primary bone tumours: comparison with bone scintigraphy. Eur J Nucl Med 2000;27:1305–11. [DOI] [PubMed] [Google Scholar]
- [16].Schulte M, Brecht-Krauss D, Heymer B, et al. Grading of tumors and tumorlike lesions of bone: evaluation by FDG PET. J Nucl Med 2000;41:1695–701. [PubMed] [Google Scholar]
- [17].Franzius C, Daldrup-Link HE, Sciuk J, et al. FDG-PET for detection of pulmonary metastases from malignant primary bone tumors: comparison with spiral CT. Ann Oncol 2001;12:479–86. [DOI] [PubMed] [Google Scholar]
- [18].Dimitrakopoulou-Strauss A, Strauss LG, Heichel T, et al. The role of quantitative (18)F-FDG PET studies for the differentiation of malignant and benign bone lesions. J Nucl Med 2002;43:510–8. [PubMed] [Google Scholar]
- [19].Franzius C, Daldrup-Link HE, Wagner-Bohn A, et al. FDG-PET for detection of recurrences from malignant primary bone tumors: comparison with conventional imaging. Ann Oncol 2002;13:157–60. [DOI] [PubMed] [Google Scholar]
- [20].Gyorke T, Zajic T, Lange A, et al. Impact of FDG PET for staging of Ewing sarcomas and primitive neuroectodermal tumours. Nucl Med Commun 2006;27:17–24. [DOI] [PubMed] [Google Scholar]
- [21].Iagaru A, Chawla S, Menendez L, et al. 18F-FDG PET and PET/CT for detection of pulmonary metastases from musculoskeletal sarcomas. Nucl Med Commun 2006;27:795–802. [DOI] [PubMed] [Google Scholar]
- [22].Kneisl JS, Patt JC, Johnson JC, et al. Is PET useful in detecting occult nonpulmonary metastases in pediatric bone sarcomas. Clin Orthop Relat Res 2006;450:101–4. [DOI] [PubMed] [Google Scholar]
- [23].Arush MW, Israel O, Postovsky S, et al. Positron emission tomography/computed tomography with 18Fluoro-deoxyglucose in the detection of local recurrence and distant metastases of pediatric sarcoma. Pediatr Blood Cancer 2007;49:901–5. [DOI] [PubMed] [Google Scholar]
- [24].Gerth HU, Juergens KU, Dirksen U, et al. Significant benefit of multimodal imaging: PET/CT compared with PET alone in staging and follow-up of patients with Ewing tumors. J Nucl Med 2007;48:1932–9. [DOI] [PubMed] [Google Scholar]
- [25].Tateishi U, Hosono A, Makimoto A, et al. Accuracy of 18F fluorodeoxyglucose positron emission tomography/computed tomography in staging of pediatric sarcomas. J Pediatr Hematol Oncol 2007;29:608–12. [DOI] [PubMed] [Google Scholar]
- [26].Volker T, Denecke T, Steffen I, et al. Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J Clin Oncol 2007;25:5435–41. [DOI] [PubMed] [Google Scholar]
- [27].Charest M, Hickeson M, Lisbona R, et al. FDG PET/CT imaging in primary osseous and soft tissue sarcomas: a retrospective review of 212 cases. Eur J Nucl Med Mol Imaging 2009;36:1944–51. [DOI] [PubMed] [Google Scholar]
- [28].Kleis M, Daldrup-Link H, Matthay K, et al. Diagnostic value of PET/CT for the staging and restaging of pediatric tumors. Eur J Nucl Med Mol Imaging 2009;36:23–36. [DOI] [PubMed] [Google Scholar]
- [29].Mody RJ, Bui C, Hutchinson RJ, et al. FDG PET imaging of childhood sarcomas. Pediatr Blood Cancer 2010;54:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cistaro A, Lopci E, Gastaldo L, et al. The role of 18F-FDG PET/CT in the metabolic characterization of lung nodules in pediatric patients with bone sarcoma. Pediatr Blood Cancer 2012;59:1206–10. [DOI] [PubMed] [Google Scholar]
- [31].Ozkan E, Soydal C, Araz M, et al. Clinical experience of 18F-FDG PET/CT in soft tissue and osseous sarcomas. UHOD—Uluslararasi Hematoloji-Onkoloji Dergisi 2012;22:163–9. [Google Scholar]
- [32].Costelloe CM, Chuang HH, Chasen BA, et al. Bone windows for distinguishing malignant from benign primary bone tumors on FDG PET/CT. J Cancer 2013;4:524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sharma P, Khangembam BC, Suman KC, et al. Diagnostic accuracy of 18F-FDG PET/CT for detecting recurrence in patients with primary skeletal Ewing sarcoma. Eur J Nucl Med Mol Imaging 2013;40:1036–43. [DOI] [PubMed] [Google Scholar]
- [34].Quartuccio N, Fox J, Kuk D, et al. Pediatric bone sarcoma: diagnostic performance of (1)(8)F-FDG PET/CT versus conventional imaging for initial staging and follow-up. AJR Am J Roentgenol 2015;204:153–60. [DOI] [PubMed] [Google Scholar]
- [35].Bailly C, Leforestier R, Campion3 L, et al. Prognostic value of FDG-PET indices for the assessment of histological response to neoadjuvant chemotherapy and outcome in pediatric patients with Ewing sarcoma and osteosarcoma. PLoS One 2017;12:e0183841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kasalak O, Glaudemans WJM, Overbosch J, et al. Can FDG-PET/CT replace blind bone marrow biopsy of the posterior iliac crest in Ewing sarcoma. Skeletal Radiol 2018;47:363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu F, Zhang Q, Zhu D, et al. Performance of positron emission tomography and positron emission tomography/computed tomography using fluorine-18-fluorodeoxyglucose for the diagnosis, staging, and recurrence assessment of bone sarcoma: a systematic review and meta-analysis. Medicine 2015;94:e1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bernstein M, Kovar H, Paulussen M, et al. Ewing's sarcoma family of tumors: current management. Oncologist 2006;11:503–19. [DOI] [PubMed] [Google Scholar]
- [39].Peller PJ. Role of positron emission tomography/computed tomography in bone malignancies. Radiol Clin North Am 2013;51:845–64. [DOI] [PubMed] [Google Scholar]
- [40].Leavey PJ, Mascarenhas L, Marina N, et al. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: a report from the Children's Oncology Group. Pediatr Blood Cancer 2008;51:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stahl M, Ranft A, Paulussen M, et al. Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer 2011;57:549–53. [DOI] [PubMed] [Google Scholar]
- [42].Xiao Y, Wei J, Zhang Y, et al. Positron emission tomography alone, positron emission tomography-computed tomography and computed tomography indiagnosing recurrent cervical carcinoma: a systematic review and meta-analysis. Arch Med Sci 2014;10:222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gao S, Li S, Yang X, et al. 1818FDG PET-CT for distant metastases in patients with recurrent head and neck cancer after definitive treatment. A meta-analysis. Oral Oncol 2014;50:163–7. [DOI] [PubMed] [Google Scholar]
- [44].Guo W, Hao B, Chen HJ, et al. PET/CT-guided percutaneous biopsy of FDG-avid metastatic bone lesions in patients with advanced lung cancer: a safe and effective technique. Eur J Nucl Med Mol Imaging 2017;44:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Salem U, Amini B, Chuang HH, et al. 18F-FDG PET/CT as an indicator of survival in Ewing sarcoma of bone. J Cancer 2017;8:2892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol 2000;18:3108–14. [DOI] [PubMed] [Google Scholar]


