Abstract
We investigated the prognostic ability of preoperative monocyte–lymphocyte ratio for oncologic outcomes in non-metastatic clear cell renal cell carcinoma of ≤7 cm on preoperative computed tomography (CT).
We retrospectively reviewed 1637 patients who underwent radical or partial nephrectomy for solid renal masses ≤7 cm (2005–2014). We included 1137 patients after exclusion of benign pathology, non-clear cell, morbidity affecting inflammatory markers, metastasis, regional lymphadenopathy, positive margin, and follow up <12 months. According to cutoff values of 0.21, we had high ≥0.21 and low <0.21 preoperative monocyte–lymphocyte ratio groups. Mann–Whitney U and chi-squared tests were used for continuous and Dichotomous variables. Univariate and multivariate Cox regression analysis were used to predict factors affecting recurrence and survival. Kaplan–Meier curve was used for survival analysis.
At a median age of 56 years with a median follow up of 65 months, 51 patients had a recurrence (4.5%). There were no statistical differences between the high and low monocyte–lymphocyte ratio groups as regard the pathological characters (P > .005). Monocyte–lymphocyte ratio was a predictor for recurrence-free and cancer-specific survivals (hazard risk [HR] 2.17, P = .012 and HR 4.06, P = .004, respectively). A higher monocyte–lymphocyte ratio was significantly associated with worse, both 10-year recurrence-free (90.2% vs 94.9%) and cancer-specific survival (89.5% vs 98.8%) (Log-rank, P = .002 and P < .001, respectively).
The preoperative monocyte–lymphocyte ratio is an independent prognostic marker for recurrence-free and cancer-specific survivals after curative surgery for non-metastatic clear cell renal cell carcinoma of ≤7 cm on preoperative CT.
Keywords: lymphocytes, monocytes, non-metastatic clear cell renal cell carcinoma, partial nephrectomy, radical nephrectomy
1. Introduction
In the last few decades, the incidence of renal cell carcinoma (RCC) is slightly increasing with clear cell RCC (ccRCC) is the most common variant (60–70%).[1] Due to the widespread practice of abdominal imaging, most of newly diagnosed cases are organ confined (up to 80%) with the surgical resection is considered as the gold standard treatment. However, recurrence may take place (up to 20%) with a pronounced deterioration in cancer specific survival (CSS).[2] Several prognostic models have been exploited to adopt the best management and follow up options, as once metastasis takes place, the 5-year survival will be below 20%.[3]
Nowadays, most prognostic tools depend on postoperative pathologic findings. However, there are diverse outcomes even for cases who share similar clinico-pathological characters, so the evolution of tumor associated immunohistochemical and blood-based biomarkers can help more accurate prognosis.[4,5]
RCC is an immunogenic cancer that showed response to immunologic therapy.[6,7] Immunologic dysfunction has frequently accused of oncogenesis and progression of RCC as a quite proportion of patients with ccRCC harbor mutations of Janus Kinase (JAK3) gene which mediates cytokine signaling and T-cell function.[8,9] Besides, the cancer associated imbalance in antigen-presenting cells (i.e., dendritic cells) which normally play an antitumor effect by capturing tumor-specific antigen to regional lymphatics where tumor-specific T-cells become ready for action.[7]
In the tumor microenvironment (TME), both lymphocytes and monocytes are representing host immunity and tumor aggressiveness respectively for many cancers including RCC.[10,11] Lymphocyte–monocyte ratio (LMR) has been proved to have an independent association with various tumors.[12,13] Most of the articles about the preoperative inflammatory biomarkers studied the prognostic impact on metastatic RCC.[14,15] Despite the all mentioned above, there is a lack of sufficient data in the literature as regarding the monocyte–lymphocyte ratio (MLR) as a pretreatment prognostic model that help the stratification of non-metastatic ccRCC. Thus, the aim of our study is to measure prognostic value of preoperative MLR in non-metastatic ccRCC ≤7 cm on preoperative computed tomography (CT).
2. Methods
2.1. Patient
Based on our Institutional Review Board guidelines of the Yonsei University Health System (project number: 4–2018–0215) that conform to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013), we retrospectively reviewed the renal tumor database of 1637 patients who underwent radical or partial nephrectomy for solid renal masses ≤7 cm on preoperative CT between 2005 and 2014. We finally included 1137 patients with clinically localized unilateral ccRCC (pT1–3N0M0) after exclusion of cases with benign pathology, non-clear cell type, preoperative morbidity affecting inflammatory markers (i.e., chronic liver disease, immunosuppression, hematologic and non-hematologic malignancies, autoimmune diseases, and chronic inflammatory diseases), metastasis, lymph nodes involvement in preoperative CT scan, positive margin, short postoperative follow up <12 month, and those with missing data of the preoperative laboratory parameters within 1 month prior to surgery as shown in the flow chart (Fig. 1).
Figure 1.

A flow chart showing the algorithm of database selection after application of inclusion and exclusion criteria. MLR = monocyte–lymphocyte ratio.
All patients were evaluated for metastasis and lymph node status by CT, chest radiographs, and bone scans preoperatively. No patient received adjuvant treatment. Blood monocyte and lymphocyte counts were obtained within a month prior surgery. Pathological outcomes were based on previous pathological reports without reanalysis because most of the pathological slides had been interpreted by a single genitourinary pathologist at our institute. Tumors were classified based on TNM system of the 2010 American Joint Committee on Cancer (AJCC)[16] and Fuhrman grading.[17]
2.2. Follow-up evaluations
Follow-up examinations consisting of physical examination, serum chemistry evaluation, chest radiography, and abdominal-pelvic CT were performed semi-annually for the first 3 years and annually thereafter. Recurrence was defined as the first detection of either a local or distant recurrence. To assess CSS, the survival status and the cause of death were investigated using the national cancer registry database and institutional electronic medical records.
2.3. Statistical analysis
MLR was evaluated as a dichotomized variable by dividing cases into 2 groups (high MLR and low MLR). Using the Shapiro–Wilk test, we rejected the hypothesis that continuously coded clinical variables (i.e., age, body mass index, follow-up duration) are normally distributed (P < .001). Thus, they were presented as the median and interquartile range (IQR). Mann–Whitney U and chi-squared tests were used to compare MLR groups with continuous and dichotomized variables, respectively.
The endpoints of the study were the recurrence-free survival (RFS) and CSS. For survival analysis, Kaplan–Meier with the log-rank test was used. The relative risk was assessed by 95% confidence intervals (CI) of hazard risk (HR). The variables that achieved statistical significance in the univariate analysis were subsequently enrolled in the multivariate analysis with Cox proportional hazards regression model with a backward variable selection approach. All tests were 2-sided, with statistical significance set at (P < .05). Statistical analysis was performed using SPSS version 18 (SPSS Inc., Chicago, IL).
3. Results
3.1. The optimal cutoff for MLR
As there were no clinically known cutoff values for MLR, the optimal cutoff value was determined as (0.21), which was calculated by the receiver operating curve (ROC) analysis. The areas under the curve (AUCs) based on end-points of RFS and CSS were 0.585 (sensitivity = 60.8%, specificity = 59.3%) and 0.669 (sensitivity = 73.9%, specificity = 61%), respectively, as shown in (Fig. 2). MLR was evaluated as a dichotomized variable by dividing cases into 2 groups (MLR <0.21 and MLR ≥0.21).
Figure 2.

Optimal cutoff levels for MLR were applied at 0.21 using receiver operating curve. (A) Considering recurrence-free survival as a state variable. (B) Considering cancer-specific survival as a state variable. AUC = area under the curve, MLR = monocyte–lymphocyte ratio.
3.2. Associations of clinico-pathologic features with MLR levels
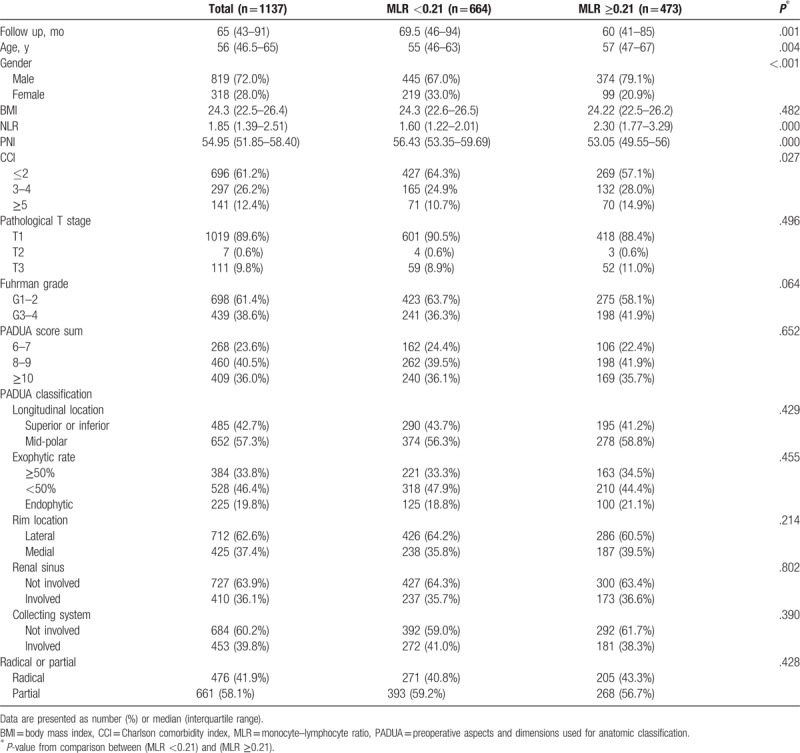
A total of 1137 patients met our study inclusion criteria. In the 819 men and 318 women, median age at surgery was 56 years (IQR 46.5–65). The median counts of monocytes and lymphocytes were 0.38 × 109/L (IQR 0.30–0.48) and 2 × 109/L (IQR 1.6–2.4), respectively. Median MLR value was 0.19 (IQR 0.15–0.25). Total of 664 (58.4%) had MLR <0.21 and 473 (41.6%) had MLR ≥0.21. Clinico-pathologic features for patients with preoperative MLR <0.21 and ≥0.21 are provided in Table 1. Patients with MLR ≥0.21 were more likely to be men with an older age and have worse Charlson comorbidity indexes (CCI) score (all, P < .05). There were no significant differences in pathologic and radiologic features between the 2 groups. However, patients with MLR ≥0.21 tended to have a greater nuclear grade (P = .064).
Table 1.
Patient and tumor characteristics according to preoperative monocyte–lymphocyte ratio.
3.3. Associations with patient outcome and MLR levels
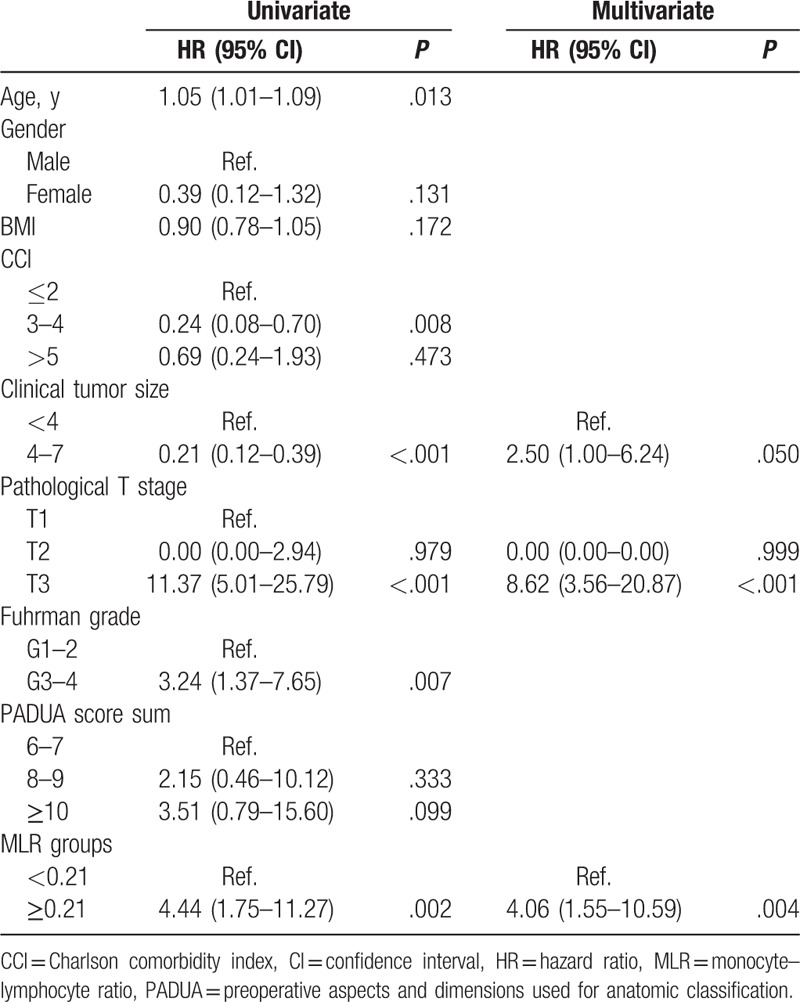
In univariate analyses, MLR ≥0.21 was associated with 2.4-fold increased risk or recurrence (P = .003) and with 4.4-fold increased risk of death from the disease (P = .002). The significant factors in univariate analysis were used to determine the influence on both RFS and CSS by multivariate analyses with a backward variable selection. MLR ≥0.21 was an independent predictor of RFS (HR 2.17, P = .012), among other predictors such as CCI score (HR 2.58, P = .004), greater clinical tumor size (HR 3.92, P < .001) and greater pathological T stage (HR 4.78, P < .001), while other factors like age, Fuhrman grade, and Preoperative aspects and dimensions used for anatomic classification (PADUA) score were excluded at earlier steps in the backward variable selection (Table 2). As regard CSS, multivariate analyses revealed that MLR ≥0.21 was an independent predictor of CSS (HR 4.06, P = .004). Meanwhile, greater clinical tumor size (HR 2.50, P = .050) and greater pathological T stage (HR 8.62, P < .001) were considered to be independent indictors for CSS (Table 3).
Table 2.
Univariate and multivariate analyses for factors predicting recurrence-free survival.

Table 3.
Univariate and multivariate analyses for factors predicting cancer-specific survival.
The median follow-up after surgery was 65 months (IQR 43–91), during that time, 51 patients experienced a disease recurrence at a median 63 months following surgery (IQR 30–105). Moreover, 67 died at a median 43 months after surgery (IQR 23–72) with 23 represent cancer-specific death at a median 38 months after surgery (IQR 22–70). The RFS rates in patients with MLR <0.21 was 97.2% at 5 years and 94.9% at 10 years, significantly higher than the 93.7% at 5 years and 90.2% at 10 years in patients with MLR ≥0.21 (log-rank, P = .002; Fig. 3A). The 5 years and 10 years for CSS rates were significantly higher in MLR <0.21 than in MLR ≥0.21 (96.9% and 90.8%) versus (93.7% and 79.7%) (Logrank, P = .001; Fig. 3B).
Figure 3.
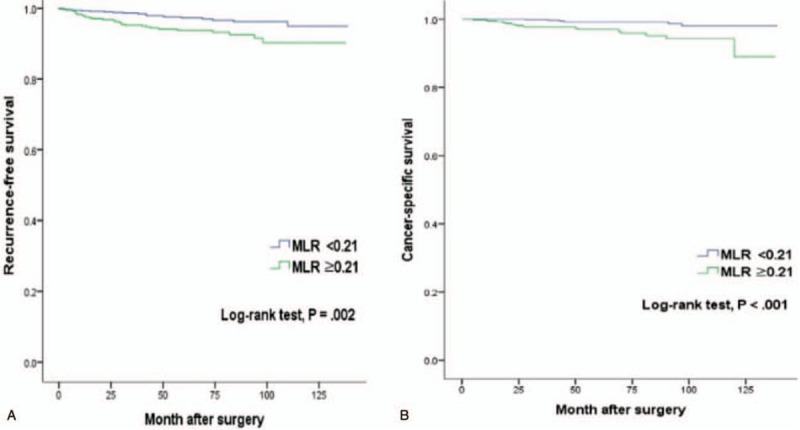
Kaplan–Meier curves according to preoperative MLR. (A) Recurrence-free survival. (B) Cancer-specific survival. MLR = monocyte–lymphocyte ratio.
4. Discussion
The reliability of the blood-based biomarkers for prediction of tumor progression is still under thorough investigations.[18,19] We showed that a greater preoperative MLR was independently correlated with poor RFS and CSS for patients with non-metastatic ccRCC ≤7 cm in the preoperative CT.
Tumor progression and its poor outcome can be greatly influenced by inflammation, considering the report that main immune cells present in the TME of ccRCC are macrophages.[20] Blood monocytes differentiate into tumor-associated macrophages (TAMs) that concentrate by chemokines in TME.[21] TAMs give rise to M2 macrophages which have a pro-tumor effect by stimulating angiogenesis, tumor cell growth, metastatic niche formation, and counteracting the T-lymphocytes mediated antitumor mechanism.[22,23] Based on in this, antitumor immunotherapies have been developed since early 1990s to antagonize the trophic effect of TAMs in TME.[24]
Higher blood monocytes count reflects macrophages load in the TME, which has proven to have a predictive role for poor clinical outcome in ccRCC.[25] In the contrary, high lymphocytes density in TME represents an immunologic anti-tumor reaction, and therefore, peripheral leucopenia is an indicator of a worse outcome of various tumors including ccRCC.[11] Moreover in RCC, there are associated poor quality of CD8+ T as well as defects in JAK3/STAT5/6 intracellular cytokine mediated signaling pathway leads to arrest of T-lymphocytes towards their terminal differentiation.[8,9] In RCC patients, dendritic cells were scanty in the peripheral blood while being more concentrated in RCC tissue as compared with healthy kidney tissue.[7]
The MLR was previously described in the literature as an independent prognostic factor in non-metastatic ccRCC. An Austrian study that was considered including the largest sample size of 687 cases demonstrated a 2.3-fold increased risk of cancer specific death with Low (LMR <3) when compared with high (LMR ≥3). However, there was no statistically significant association with RFS.[26] Further Chinese study included 430 cases showed the correlation of preoperative LMR with both RFS and overall survival.[27] Lucca et al[28] studied 430 patients and concluded that MLR with cutoff value of 0.4 have the most relevant prognostic ability when compared with the neutrophil to lymphocyte ratio, the platelet to lymphocyte ratio, and the prognostic nutritional index. Our study showed that higher preoperative MLR has a higher tendency to have a greater Fuhrman nuclear grade which is consistent with the systematic review and meta-analysis by Gu et al.[29]
The strength points in our study is that the MLR is a cost-effective, readily available biomarker which can be added to already valid or upcoming stratification scores predicting prognosis and assisting decision-making. We included the largest sample size of patients among studies that handled the prognostic role of hematologic scoring systems in RCC especially the MLR.[30,31] All cases derived from a single center and were subjected to the same standardized protocol of blood sampling, laboratory techniques, surgical procedure, pathological evaluation, and follow-up strategy.
We only included clinical tumor size ≤7 cm, which may be considered as a drawback of the study. However, this established the homogeneity of our cohort as tumor size may influence white blood cells count and survival. Another drawback is the retrospective nature of the study design and selection bias. One of the common limitations of such studies is that the median value of inflammatory markers and may show racial differences of the target population[32] as compared with other series come from different parts in the world with the subsequent differences in the cutoff values which can be enforced by using different statistical tools.[28] Further limitation is the need to add other biomarkers to enhance predictability, and there should be further studies on the inflammatory cells in the TME of the tumor itself with following up the postoperative circulating levels.[20,33] Besides, there may be other conditions influencing this correlation.[34]
Recent reviews have shown that various cancers have an influence on MLR such as colorectal carcinoma, soft tissue sarcoma, Hodgkin lymphoma, and other non-hematological malignancies.[12,13,29] Peripheral lymphopenia also predicts poor prognosis in other malignancies such as esophageal carcinoma.[35] That is the reason why we have excluded many cases with preexisting preoperative conditions (i.e., chronic inflammatory conditions, malignancies, immunosuppression, preoperative lymph node involvement, metastasis, or autoimmune disease) that may alter the circulating inflammatory cells count in peripheral blood and we included one pathologic type of RCC to avoid heterogeneity of study cohort. In conclusion, MLR can help preoperative evaluation and predicting outcome for patients with non-metastatic clear cell renal cell carcinoma of ≤7 cm on preoperative CT after curative surgery.
Author contributions
Conceptualization: Ahmed Elghiaty, Won Sik Jang, Young Deuk Choi, Won Sik Ham.
Data curation: Ahmed Elghiaty, Jongchan Kim, Ji Eun Heo.
Formal analysis: Ahmed Elghiaty, Jee Soo Park.
Methodology: Ahmed Elghiaty, Jongchan Kim, Jee Soo Park, Ji Eun Heo, Won Sik Ham.
Software: Jee Soo Park.
Supervision: Koon Ho Rha, Young Deuk Choi, Won Sik Ham.
Validation: Jongchan Kim, Won Sik Jang.
Writing – original draft: Ahmed Elghiaty, Ji Eun Heo.
Writing – review & editing: Won Sik Jang, Koon Ho Rha, Young Deuk Choi, Won Sik Ham.
Footnotes
Abbreviations: CCI = Charlson comorbidity indexes, ccRCC = clear-cell renal cell carcinoma, CSS = cancer-specific survival, CT = computed tomography, HR = hazard risk, IQR = interquartile range, LMR = lymphocyte–monocyte ratio, MLR = monocyte–lymphocyte ratio, RCC = renal cell carcinoma, RFS = recurrence-free survival, TAMs = tumor-associated macrophages, TME = tumor microenvironment.
Funding: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C1095).
The authors have no conflicts of interest to disclose.
References
- [1].Shuch B, Amin A, Armstrong AJ, et al. Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Eur Urol 2015;97:67–85. [DOI] [PubMed] [Google Scholar]
- [2].Sun M, Thuret R, Abdollah F, et al. Age-adjusted incidence, mortality, and survival rates of stage-specific renal cell carcinoma in North America: a trend analysis. Eur Urol 2011;59:135–41. [DOI] [PubMed] [Google Scholar]
- [3].Figlin RA. Renal cell carcinoma: management of advanced disease. J Urol 1999;161:381–7. discussion 386-393. [DOI] [PubMed] [Google Scholar]
- [4].Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 2002;168:2395–400. [DOI] [PubMed] [Google Scholar]
- [5].Michaelson MD, Stadler WM. Predictive markers in advanced renal cell carcinoma. Semin Oncol 2013;40:459–64. [DOI] [PubMed] [Google Scholar]
- [6].Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014;192:5451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gigante M, Blasi, Loverre A, et al. Dysfunctional DC subsets in RCC patients: ex vivo correction to yield an effective anti-cancer vaccine. Mol Immunol 2009;46:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].de Martino M, Gigante M, Cormio L, et al. JAK3 in clear cell renal cell carcinoma: mutational screening and clinical implications. Urol Oncol 2013;31:930–7. [DOI] [PubMed] [Google Scholar]
- [9].Cavalcanti E, Gigante M, Mancini V, et al. JAK3/STAT5/6 pathway alterations are associated with immune deviation in CD8+ T cells in renal cell carcinoma patients. J Biomed Biotechnol 2010;2010:935764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149–63. [DOI] [PubMed] [Google Scholar]
- [11].Saroha S, Uzzo RG, Plimack ER, et al. Lymphopenia is an independent predictor of inferior outcome in clear cell renal carcinoma. J Urol 2013;189:454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer 2014;110:435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ni XJ, Zhang XL, Ou-Yang QW, et al. An elevated peripheral blood lymphocyte-to-monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS One 2014;9:e111886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ramsey S, Lamb GW, Aitchison M, et al. Evaluation of an inflammation based prognostic score in patients with metastatic renal cancer. Cancer 2007;109:205–12. [DOI] [PubMed] [Google Scholar]
- [15].Gu L, Ma X, Xie Y, et al. Pretreatment lymphocyte to monocyte ratio is an independent prognostic factor in metastatic clear cell renal cell carcinoma. Clin Genitourin Cancer 2017;15:369–77. [DOI] [PubMed] [Google Scholar]
- [16].Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- [17].Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982;6:655–63. [DOI] [PubMed] [Google Scholar]
- [18].Wald G, Barnes KT, Bing MT, et al. Minimal changes in the systemic immune response after nephrectomy of localized renal masses. Urol Oncol 2014;32:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brookman-May S, May M, Ficarra V, et al. Does preoperative platelet count and thrombocytosis play a prognostic role in patients undergoing nephrectomy for renal cell carcinoma? Results of a comprehensive retrospective series. World J Urol 2013;31:1309–16. [DOI] [PubMed] [Google Scholar]
- [20].Motoshima T, Miura Y, Wakigami N, et al. Phenotypical change of tumor-associated macrophages in metastatic lesions of clear cell renal cell carcinoma. Med Mol Morphol 2018;51:57–63. [DOI] [PubMed] [Google Scholar]
- [21].Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol 2012;33:119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mantovani A, Schioppa T, Porta C, et al. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 2006;25:315–22. [DOI] [PubMed] [Google Scholar]
- [23].Mantovani A, Marchesi F, Malesci A, et al. Tumor associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Foucher ED, Blanchard S, Preisser L, et al. IL-34 induces the differentiation of human monocytes into immunosuppressive macrophages. antagonistic effects of GM-CSF and IFNγ. PLoS One 2013;8:e56045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xu L, Zhu Y, Chen L, et al. Prognostic value of diametrically polarized tumor associated macrophages in renal cell carcinoma. Ann Surg Oncol 2014;21:3142–50. [DOI] [PubMed] [Google Scholar]
- [26].Hutterer GC, Stoeckigt C, Stojakovic T, et al. Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urol Oncol 2014;32:1041–8. [DOI] [PubMed] [Google Scholar]
- [27].Chang Y, Fu Q, Xu L, et al. Prognostic value of preoperative lymphocyte to monocyte ratio in patients with nonmetastatic clear cell renal cell carcinoma. Tumor Biol 2016;37:4613–20. [DOI] [PubMed] [Google Scholar]
- [28].Lucca I, de Martino M, Hofbauer SL, et al. Comparison of the prognostic value of pretreatment measurements of systemic inflammatory response in patients undergoing curative resection of clear cell renal cell carcinoma. World J Urol 2015;33:2045–52. [DOI] [PubMed] [Google Scholar]
- [29].Gu L, Li H, Chen L, et al. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget 2016;7:31926–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Grimes N, Tyson M, Hannan C, et al. A systematic review of the prognostic role of hematologic scoring systems in patients with renal cell carcinoma undergoing nephrectomy with curative intent. Clin Genitourin Cancer 2016;14:271–6. [DOI] [PubMed] [Google Scholar]
- [31].Boissier R, Campagna J, Branger N, et al. The prognostic value of the neutrophil-lymphocyte ratio in renal oncology: a review. Urol Oncol 2017;35:135–41. [DOI] [PubMed] [Google Scholar]
- [32].Wallace K, Lewin DN, Sun S, et al. Tumor-infiltrating lymphocytes and colorectal cancer survival in African American and Caucasian patients. Cancer Epidemiol Biomarkers Prev 2018;27:755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ohno Y, Nakashima J, Ohori M, et al. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol 2012;187:411–7. [DOI] [PubMed] [Google Scholar]
- [34].Unlu M, Karaman M, Ay SA, et al. The comparative effects of valsartan and amlodipine on vascular microinflammation in newly diagnosed hypertensive patients. Clin Exp Hypertens 2013;35:418–23. [DOI] [PubMed] [Google Scholar]
- [35].Feng JF, Liu JS, Huang Y. Lymphopenia predicts poor prognosis in patients with esophageal squamous cell carcinoma. Medicine (Baltimore) 2014;93:e257. [DOI] [PMC free article] [PubMed] [Google Scholar]


