Abstract
Rationale:
Cardiac inflammatory myofibroblastic tumor (IMT) is a rare primary cardiac tumor which is currently considered as a low-grade neoplasm. The tumor has a predilection in infants and adolescents and primarily occurs as an endocardial-based cavitary mass. However, cardiac IMT that only involves the interventricular septum in middle-aged adults is extremely rarely reported. Considering its infrequency, we report a rare clinical case, with the aim of sharing our experience during the diagnostic procedures.
Patient concerns:
A 45-year-old, previously healthy female, with no medical history was admitted to the outpatient clinic due to the identification of an abnormal radiographic finding during a routine health examination.
Diagnosis:
Transthoracic echocardiography (TTE) revealed a 3.5 cm × 4.0 cm × 4.5 cm heterogeneous mass in the interventricular septum. Color Doppler echocardiography detected sparse blood flow signals inside the mass. Magnetic resonance imaging (MRI) confirmed a hyperintense T2-weighted, isointense T1-weighted mass. Three-dimensional (3D) TTE demonstrated a spherical mass in the middle part of the interventricular septum. Postoperative histopathological examinations revealed a mesenchymal tumor composed of scattered spindle myofibroblasts with a myxoid atypia, associated with infiltration of lymphocytes and plasma cells.
Interventions:
Complete tumor resection was successfully performed via median sternotomy under general anesthesia.
Outcomes:
After surgery, the patient recovered successfully. The patient was in good general health without any clinical symptoms. The echocardiographic examination at the 12-month follow-up period revealed normal function of the heart, and there was no evidence of tumor recurrence.
Lessons:
To our knowledge, cardiac IMT only the involving interventricular septum in a middle-aged adult has never been previously reported before. Echocardiography plays a critical role in establishing the primary diagnosis of cardiac IMT and evaluating regular follow-up examinations. Complete surgical resection of the mass is considered the first-line treatment despite the absence of symptoms.
Keywords: cardiac tumor, echocardiography, inflammatory myofibroblastic tumor, three-dimensional echocardiography
1. Introduction
Primary cardiac tumors are exceedingly rare with an autopsy frequency of only 0.001%–0.03%.[1] Histologically, almost 90% of these tumors are benign, of which 50% of are myxomas; remaining benign tumors included: fibroelastomas (26%), fibromas (6%), lipomas (4%) and others.[2] Inflammatory myofibroblastic tumor (IMT) is a rare proliferative lesion which is characterized by myofibroblast proliferation associated with inflammatory infiltrates including plasmocytes, lymphocytes, and histiocytes.[3] IMT was first reported, in 1939, to be located in the lung, and was subsequently identified in every major organ of the body. Cardiac IMT is extremely rare, mainly seen in infants and adolescents; it primarily occurred as an endocardial-based cavitary mass.[4] Although any site in the heart may be involved, IMT only involving the interventricular septum is rarely reported. Due to the rarity of cardiac IMT, the diagnosis of this tumor is troublesome. Herein, we describe an unusual form of an IMT in the interventricular septum in a middle-aged woman with nonspecific clinical symptom.
2. Case history
A 45-year-old previously healthy female with no medical history was admitted to outpatient clinic due to an abnormal radiographic finding detected during a routine health examination. Physical examination was unremarkable. All laboratory testing results were normal. Upon cardiac auscultation, normal heart sounds and no pathological heart murmurs were found. Electrocardiography revealed a normal QRS axis. Transthoracic echocardiography (TTE) showed a hyperechoic mass (3.5 cm × 4.0 cm × 4.5 cm) in the interventricular septum and revealed a preserved left ventricular function. This heterogeneous mass had a clear boundary (Fig. 1A). Color Doppler echocardiography detected sparse blood flow signals inside the mass (Fig. 1B). Three-dimensional (3D) TTE demonstrated a spherical mass in the middle part of interventricular septum (Fig. 1C). Magnetic resonance imaging (MRI) confirmed the location of the mass and a clear boundary of this hyperintense T2-weighted, isointense T1-weighted mass (Fig. 2) and precontrast T1-weight imaging showed that the mass was significantly enhanced by early and late contrast infusion and that the mass showed inhomogeneous enhancement. Whole body MRI was performed but no other abnormalities were found.
Figure 1.
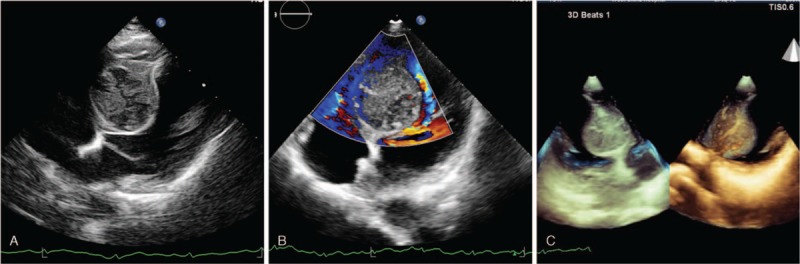
A. Transthoracic echocardiography revealed a large heterogeneous mass at the interventricular septum, with a clear boundary. B. Color Doppler echocardiography detected sparse blood flow signals inside the mass. C. Three-dimensional echocardiography demonstrated a spherical mass in the middle part of the interventricular septum.
Figure 2.
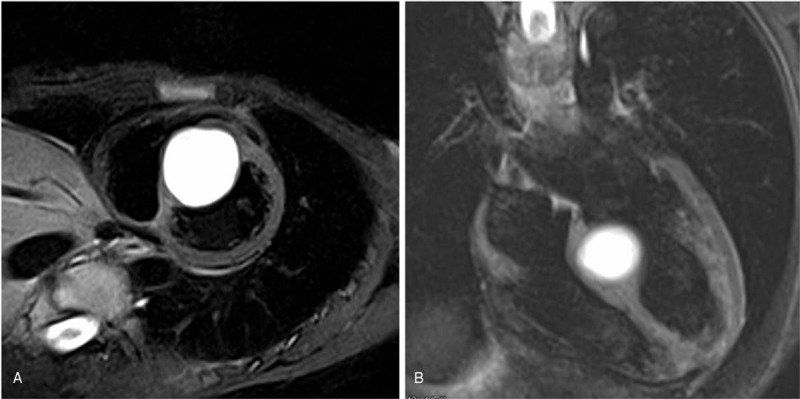
Magnetic resonance imaging scan confirmed the location and clear boundary of this mass.
Tumor extirpation was successfully performed via median sternotomy under general anesthesia. On gross examination, the excised mass measured 3.0 cm × 4.0 cm and was a cystic solid. It had an intact capsule and appeared grayish white (Fig. 3). Histopathological examinations revealed a mesenchymal tumor composed of scattered spindle myofibroblasts within a myxoid atypia and infiltration of lymphocytes and plasma cells (Fig. 4A). Cells were positive for desmin (Fig. 4B), SMA (Fig. 4C), CDK4, S-100 (partly positive), WT-1 (partly positive). Moreover, they were negative for myogenin, myoD1, EMA, PCK, CR, HMB45, ALK-1, CD34, CD1a, NF, CD21, GFAP, Collagen IV, and leu-7. A fluorescent in situ hybridization (FISH) detection confirmed the absence of AKL-1 translocation and MDM2 amplification. Thus, a diagnosis of cervical IMT was confirmed.
Figure 3.
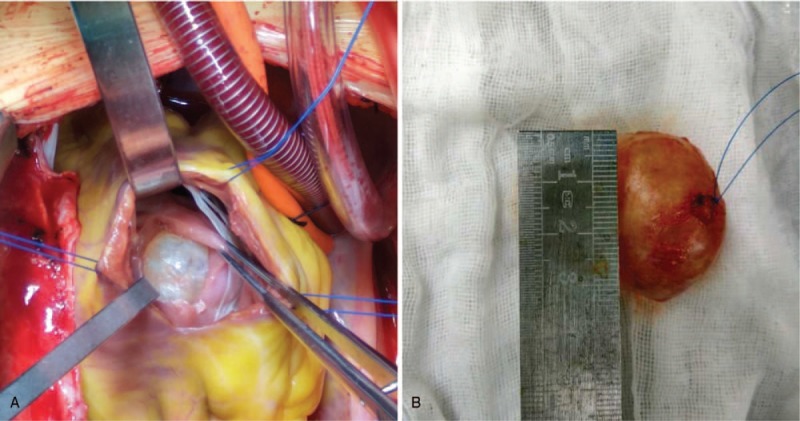
The excised mass is a cystic solid, with an intact capsule appearing grayish white.
Figure 4.
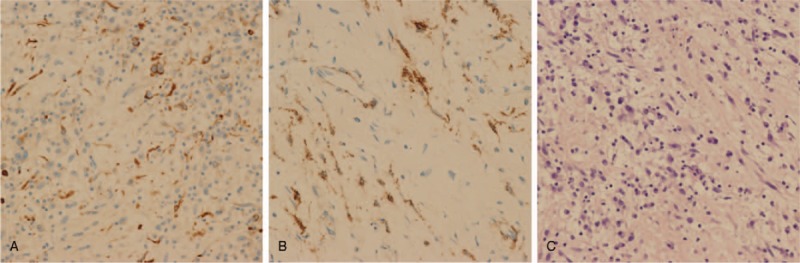
A. Histopathological examinations revealed a mesenchymal tumor composed of scattered spindle myofibroblasts within myxoid atypia, with infiltration of lymphocytes and plasma cells. B. Cells were positive for desmin. C. Cells were positive for SMA.
After surgery, the patient recovered successfully. The patient was in good general condition without any clinical symptoms. The echocardiographic examination at 12-month follow-up revealed normal function of the heart, and there was no evidence of tumor recurrence (Fig. 5). The patient signed written informed consent, and the study protocol was approved by the Ethics Committee of West China Hospital of Sichuan University.
Figure 5.
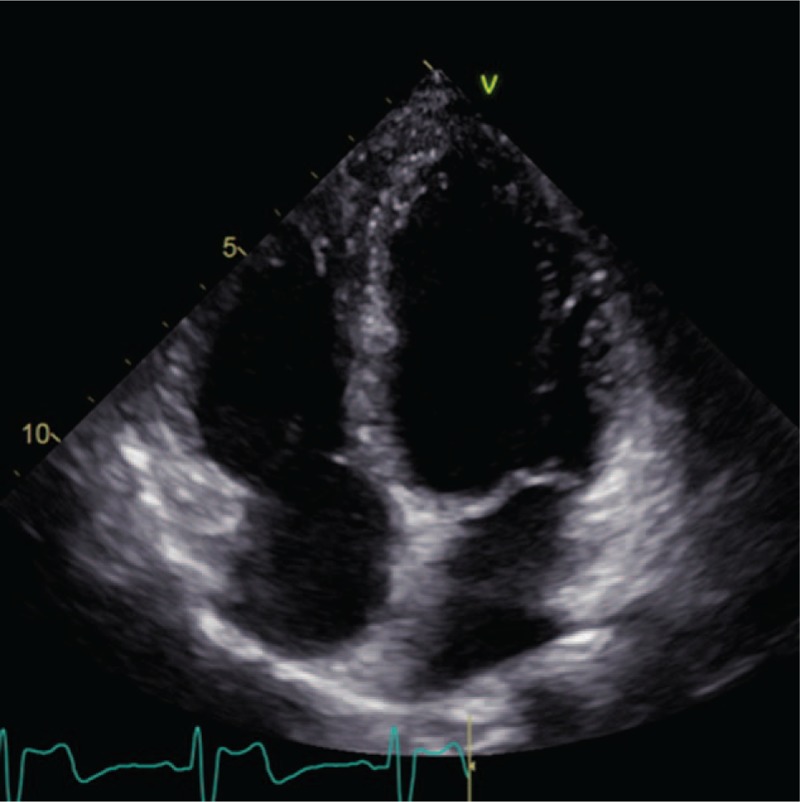
Echocardiography exhibited a recovered interventricular septum at the 12-month follow-up period.
3. Discussion
IMTs are rarely involved in heart accounting for less than 5% of primary cardiac tumors. Cardiac IMTs have a predilection for patients less than 20 years old; less than 10 cases reported occurred in older adults,[5] which makes the current case much rarer. Due to the rarity of the disease, the natural history and pathogenesis of cardiac IMT remain unclear. In general, the clinical symptoms and signs of cardiac IMT are associated with tumor size, location, and rate of growth. One-third of patients with cardiac IMT presented with a constitutional syndrome consisting of fever, weight loss, anemia, thrombocytopenia, hypergammaglobulinemia, and an elevated erythrocyte sedimentation rate.[4,6] Cardiovascular manifestations are the most common way patients present: decreased exercise tolerance, syncope, chest pain, transit ischemic attack, arrhythmia, and sudden death.[4,7,8] After partial or complete resection of the tumor, the clinical outcomes appear to be satisfactory.[9,10] In the current case, although the mass was located in the interventricular septum, the rhythm of the heart was normal suggesting the conduction system was not involved. However, IMT is currently regarded as a neoplasm of intermediate biologic potential, with a 2% risk of metastasis and a 25% of recurrence rate.[11,12] Thus, we completely resected the tumor to prevent further local invasion, which may result in sudden unexpected death.
Diagnosing IMT can be difficult due to the wide morphological and immunohistochemical spectrum and rarity of the disease. Previous literature has demonstrated right atrium and right ventricle predominance. Furthermore, according to gross findings, cardiac IMTs are mainly pedunculated endocardial-based tumors.[4,8,13] In contrast to previous reports, the tumor in the current case is only located in the interventricular septum with a solid gross appearance. The diagnostic approach should start with a thorough history and physical examination. However, symptoms are mainly dependent on the location of the tumor, it is extremely difficult to distinguish between a benign and a malignant lesion. Especially in asymptomatic patients, present day imaging techniques, such as echocardiography, MRI, and computed tomography (CT) are indispensable to make a rapid and precise diagnosis of a cardiac tumor. Echocardiography is regarded as an ideal imaging technique in cardiac disease since it is simple, noninvasive, widely available and has a relatively low cost. Reddy et al [14] suggested that 3D TTE has an incremental value in the assessment of right ventricular masses allowing for the better identification of the characteristics of the masses compared with CT. Better yet, 3D TTE can provide the measurement of the real dimensions of cardiac tumors and various visual field on different angles and be cropped on any expected plane to evaluate any region of interest contained in the volume acquired.[15,16] In addition, approximately 60 cases of cardiac IMT have been reported, of which 4 were recurrent.[17–20] Among the 4 cases, 2 died of the recurrent IMT.[19,20] Given the recurrence and intermediate biologic potential of cardiac IMT, regular echocardiography was recommended during follow-up. MRI is a complementary technique that provides further information and is used to decide the stage and plan of treatment. Ultimately, the diagnosis of cardiac IMT required a resection specimen postoperatively for histopathologic confirmation, which is the definitive means of diagnosing the cardiac tumor. If necessary, FISH can be performed for further differential diagnosis.
Surgery is considered as the optimal treatment in patients with symptomatic resectable tumors, whereas the role of surgery in patients with asymptomatic cardiac tumors is less clear.[3] This is because cardiac fibromas can remain dormant for many years and even regress. However, due to fatal arrhythmias, surgery is often recommended despite the absence of symptoms. Thus, for cardiac IMTs, complete surgical resection remains the first-line treatment, whenever feasible.[5]
As mentioned earlier, IMT is regarded as a neoplasm of intermediate biologic potential, with 25% of recurrence and a 2% of metastasis.[12] Subtotal tumor is considered as a risk factor for recurrence.[3] According to a review study, cardiac IMT was associated with less frequent recurrence and the ratio reached about 7% in 57 patients. Of the 57 patients, 14 (24.6%) died for several reasons.[6] However, the data may underreport mortality for lack of standardized follow-up. In another review, the author reported that 74% of patients remained asymptomatic after complete surgical resection.[5]
In conclusion, we described a rare cardiac IMT only involving the interventricular septum in a middle-aged adult which has scarcely been reported before. Echocardiography is widely available and is the mainstay for the primary diagnosis of cardiac IMT and regular follow-up examinations. Complete surgical resection of the mass is the first-line treatment even if there is an absence of symptoms.
Author contributions
Conceptualization: Ming-dan Deng, Jun-yang Han, and Hong Tang.
Data curation: Ming-dan Deng and Jun-yang Han.
Formal analysis: Ming-dan Deng and Hong Tang.
Investigation: Ming-dan Deng and Hong Tang.
Methodology: Ming-dan Deng, Jun-yang Han, and Hong Tang.
Project administration: Ke Lin.
Resources: Jun-yang Han, Ke Lin, and Hong Tang.
Software: Ke Lin.
Validation: Ming-dan Deng and Jun-yang Han.
Visualization:Ming-dan Deng, Ke Lin, and Hong Tang.
Writing – original draft: Ming-dan Deng.
Writing – review, and editing: Hong Tang.
Footnotes
Abbreviations: 3D = three-dimensional, FISH = fluorescent in situ hybridisation, IMT = inflammatory myofibroblastic tumor, MRI = magnetic resonance imaging, TTE = transthoracic echocardiography.
M-dD and J-yH contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Butany J, Nair V, Naseemuddin A, et al. Cardiac tumours: diagnosis and management. Lancet Oncol 2005;6:219–28. [DOI] [PubMed] [Google Scholar]
- [2].Elbardissi AW, Dearani JA, Daly RC, et al. Survival after resection of primary cardiac tumors: a 48-year experience. Circulation 2008;118:S7–15. [DOI] [PubMed] [Google Scholar]
- [3].Mizia-Malarz A, Sobol-Milejska G, Buchwald J, et al. Inflammatory myofibroblastic tumor of the heart in the infant: review of the literature. J Pediatr Hematol/Oncol 2016;38:e298–302. [DOI] [PubMed] [Google Scholar]
- [4].Burke A, Li L, Kling E, et al. Cardiac inflammatory myofibroblastic tumor: a “benign” neoplasm that may result in syncope, myocardial infarction, and sudden death. Am J Surg Pathol 2007;31:1115–22. [DOI] [PubMed] [Google Scholar]
- [5].Xu B, Fraser RS, Renaud C, et al. Inflammatory myofibroblastic tumor of the aortic valves causing sudden cardiac death: a case report and review of the literature. Pediatr Dev Pathol 2014;17:231–9. [DOI] [PubMed] [Google Scholar]
- [6].Eilers AL, Nazarullah AN, Shipper ES, et al. Cardiac inflammatory myofibroblastic tumor: a comprehensive review of the literature. World J PediatrCongenit Heart Surg 2014;5:556–64. [DOI] [PubMed] [Google Scholar]
- [7].Rose AG, McCormick S, Cooper K, et al. Inflammatory pseudotumor (plasma cell granuloma) of the heart. Report of two cases and literature review. Arch Pathol Lab Med 1996;120:549–54. [PubMed] [Google Scholar]
- [8].Li L, Burke A, He J, et al. Sudden unexpected death due to inflammatory myofibroblastic tumor of the heart: a case report and review of the literature. Int J Legal Med 2011;125:81–5. [DOI] [PubMed] [Google Scholar]
- [9].Luo W, Teng P, Ni Y. A rare cardiac inflammatory myofibroblastic tumor involving aortic valve. J Cardiothorac Surg 2017;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Butany J, Dixit V, Leong SW, et al. Inflammatory myofibroblastic tumor with valvular involvement: a case report and review of the literature. Cardiovasc Pathol 2007;16:359–64. [DOI] [PubMed] [Google Scholar]
- [11].Coffin CM, Watterson J, Priest JR, et al. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 1995;19:859–72. [DOI] [PubMed] [Google Scholar]
- [12].Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509–20. [DOI] [PubMed] [Google Scholar]
- [13].Anvari MS, Soleimani A, Abbasi A, et al. Inflammatory myofibroblastic tumor of the right ventricle causing tricuspid valve regurgitation. Tex Heart Instit J 2009;36:164–7. [PMC free article] [PubMed] [Google Scholar]
- [14].Reddy VK, Faulkner M, Bandarupalli N, et al. Incremental value of live/real time three-dimensional transthoracic echocardiography in the assessment of right ventricular masses. Echocardiography (Mount Kisco, NY) 2009;26:598–609. [DOI] [PubMed] [Google Scholar]
- [15].Zaragoza-Macias E, Chen MA, Gill EA. Real time three-dimensional echocardiography evaluation of intracardiac masses. Echocardiography (Mount Kisco, NY) 2012;29:207–19. [DOI] [PubMed] [Google Scholar]
- [16].Espinola-Zavaleta N, Morales GH, Vargas-Barron J, et al. Three-dimensional transesophageal echocardiography in tumors of the heart. J Am Soc EchocardiogrV 15 2002;972–9. [DOI] [PubMed] [Google Scholar]
- [17].Hartyanszky IL, Kadar K, Hubay M. Rapid recurrence of an inflammatory myofibroblastic tumor in the right ventricular outflow tract. Cardiol Young 2000;10:271–4. [DOI] [PubMed] [Google Scholar]
- [18].Park HO, Yang JH, Kim SH, et al. Autotransplantation of the heart for recurrent inflammatory myofibroblastic tumor. J Korean Med Sci 2017;32:1548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Andersen ND, DiBernardo LR, Linardic CM, et al. Recurrent inflammatory myofibroblastic tumor of the heart. Circulation 2012;125:2379–81. [DOI] [PubMed] [Google Scholar]
- [20].Yang X, Xiao C, Liu M, et al. Cardiac inflammatory myofibroblastic tumor: does it recur after complete surgical resection in an adult? J Cardiothorac Surg 2012;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]


