Abstract
The prevalence of obesity is high among reproductive-age women and is associated with impaired reproductive function. Obesity is multifactorial in origin, yet many cases of obesity result from overconsumption of a diet high in fat. Excess dietary fat increases both adipose and nonadipose tissue lipid content and, through lipotoxicity, leads to cell dysfunction and death. High dietary fat intake, with or without the development of obesity, impairs female hypothalamic-pituitary-ovarian (HPO) axis functionality and fertility. Based on the current evidence, it appears the reproductive dysfunction involves increased leptin and insulin signaling at the various levels of the HPO axis, as well as changes in peroxisome proliferator-activated receptor γ actions and increased inflammation, yet other mechanisms may also be involved. This review summarizes the current body of knowledge on impaired female reproductive function after high-fat diet exposure, as well as discusses proposed mechanisms through which this may occur.
High-fat diet exposure leads to female reproductive dysfunction. This review discusses the current state of knowledge and potential mechanisms for the dysfunction.
Obesity is associated with adverse reproductive outcomes, including menstrual dysfunction, anovulation, infertility, miscarriage, and pregnancy complications (1, 2). As obesity is common in women of reproductive age, with 38.3% of women older than 20 years classified as obese (1), the implications of reproductive dysfunction are a major problem facing society. In addition, assisted reproduction is less effective in overweight and obese women, with lower pregnancy and live birth rates, as well as increased incidence of pregnancy loss (2–4). Therefore, many assisted reproduction clinics use body mass index (BMI) cutoffs for treatment, with an average cutoff of 38.4 kg/m2 (5). Obesity has numerous causes and is multifactorial, yet many suffer from diet-induced obesity (DIO), in which a chronic positive energy balance leads to excess adiposity. Although overconsumption of any macronutrient can lead to excessive adiposity, diets high in fat are of interest. The definition of a high-fat diet (HFD) in humans varies from as low as 30% to as high as 75% of caloric intake (6, 7–13). Most American women consume an HFD as the average fat intake is approximately 35% of caloric intake (14), whereas it is recommended to consume between 20% and 35% of calories as dietary fat (15). An HFD in the context of overnutrition leads to increased lipid deposition in both adipose and nonadipose tissues (16). When the capacity of nonadipose tissues for lipid storage is met, lipotoxicity results, cellular function suffers, and cell death occurs (17).
The relationship between high dietary fat intake and female reproductive dysfunction warrants exploration of how an HFD alters female reproductive function. Few studies have been performed in humans examining the impact of administering a diet with differing fat contents on reproductive health (18, 19). Much of the work to further elucidate the mechanisms behind altered reproductive function in females has been conducted in animal models of DIO. These studies have multiple methodological differences that may confound the data and limit the conclusions that can currently be drawn. The length of HFD exposure, percent fat in both the HFD and the control diet, and types of fats in the diets (both within HFDs and between HFDs and controls) vary widely. These differences alter the type of HFD exposure and thus may alter the reproductive function and/or response. As the manipulation of dietary components other than fat will differentially affect fertility, studies only manipulating the dietary fat content were considered when writing this review. The purpose of this review is to discuss effects of an HFD on reproductive function in females, with a specific focus on ovarian dysfunction.
Impaired Hypothalamic-Pituitary-Ovarian Axis Function After HFD Exposure
Estrous cycle and fertility rates
An HFD has been shown to negatively affect the estrous cycle with a high incidence of anovulation (16, 20–30). Interestingly, when the insulin receptor was knocked out in the theca interstitial cells of the ovary (24) or in gonadotropin-releasing hormone (GnRH) neurons (28), mice were protected from the HFD-induced impaired estrous cycle. As the tissues of the hypothalamic-pituitary-ovarian (HPO) axis have been shown to remain insulin sensitive when insulin resistance develops systemically with HFD feeding (25, 28, 31), it is likely that overstimulation of insulin signaling in the ovary and hypothalamus is involved. This protection of estrous cycle dysfunction was also observed when proliferator-activated receptor γ (PPARγ) was knocked out in the brain of mice exposed to an HFD (21), suggesting alteration in PPARγ’s actions is also involved. Further speculation on the role of insulin signaling and PPARγ’s actions is discussed later in the review.
In addition to altered estrous cycle, fertility rates are also decreased with an HFD (24, 25, 28, 32–35). In mice that had regular estrous cycles after HFD exposure, there were no differences in fertility rates, but the animals had fewer ovulated oocytes and fewer pups per litter (36). The impaired fertility outcomes appear to be worse when the obese phenotype is present in addition to the HFD exposure (33).
HFD and HPO axis changes
The effect of an HFD on serum luteinizing hormone (LH) levels is unclear. Some studies report decreases (23), some show no change (21, 27), and most indicate increases (24, 25, 28, 37) in LH levels. One study showed that LH levels were only increased in rats fed an HFD diet for 120 days and not 180 days (26), suggesting the increase may be transient and eliminated after longer exposure. As there may be a transient effect on LH, perhaps the differences observed stem from different lengths of HFD feeding (ranging from 7 days to 24 weeks) or differences in fat content in HFDs (ranging from 30% to 60% of calories) and control diets (ranging from 2% to 18% of calories or not reported). When the insulin receptor was knocked out in either the pituitary or GnRH neurons in mouse models, LH levels following HFD exposure were completely or partially returned to that of the controls, respectively (25, 28), suggesting that increased insulin signaling in the pituitary or hypothalamus may be involved in the increased LH levels. There is no effect on follicle-stimulating hormone (FSH) levels following HFD exposure (21, 25–28, 36). Other related hormones have not been as readily assessed. Progesterone levels have been shown to increase after HFD exposure in one study (26) and have no change in another (27). Based on the current evidence, it appears that there are increased LH levels following an HFD, yet other hormones appear to be unaffected.
Ovarian morphology and function
Several studies have examined the impact of an HFD on follicular development in the ovary. In general, there is a decrease in primordial follicles after exposure to an HFD (20, 33, 38), but one study did not observe any change (21). Exposure to an HFD has been found to increase follicular atresia (20, 36, 39). The effect on primary, secondary, and antral follicles and corpora lutea is not as clear, with some studies observing an increase (20, 38), some no change (21, 22, 33), and others a decrease (21, 23–25, 34, 36) in the different follicle pools. There are several plausible explanations as to why the changes in follicle counts are not uniform across studies. First, there is a wide range in fat content of the HFDs and control diets, ranging from 45% to 60.9% and 4.8% to 18% fat, respectively. Second, the length of HFD exposure varies widely across studies, ranging from 4 to 24 weeks. Third, methodological differences in counting may confound the data, with some studies counting follicles in every fifth ovarian section to some only counting one representative section. Finally, species-specific differences may be involved in the different follicle counts as both rats and mice (as well as several substrains of mice) have been used to assess HFD impact of follicle development. Furthermore, several studies use various knockout (KO) mice (21, 24, 25), in which their changes in follicle numbers may be due to genetic differences in the animal and not the HFD. Granulosa cell function appears to be normal as anti-Müllerian hormone levels, an indirect measure of their function, are unaltered with HFD exposure (33). Although the exact changes in follicular development in HFD studies are inconclusive, it is clear that folliculogenesis is altered with HFD exposure as nearly all studies see alterations in at least one follicular pool (20, 21, 23–25, 33, 34, 36, 38, 39). Likely there is a depletion in the primordial follicle pool and increased follicular atresia following HFD exposure. These changes can potentially shorten the reproductive life span of the female, resulting in premature ovarian insufficiency with HFD exposure, as once the primordial follicle reserve is depleted, so is the reproductive capacity of the female (20, 40–44).
Increased lipid levels in the ovary have also been observed in response to an HFD. Wistar rats maintained on a 59% HFD for 180 days had increased lipid droplets in the ovary (26). Increased lipid content and abnormal accumulation patterns were observed in the germinal vesicle oocytes after a 60% HFD for 6 weeks in C57BL/6J mice (45). In addition, lipid accumulation increased in both the cumulus cells and oocytes from CBA mice that were fed a 40% HFD for 4 weeks (16). In these mice, the increased lipid content in the cumulus cells and oocytes was associated with increased lipotoxicity [increased markers of endoplasm reticulum (ER) stress, decreased mitochondrial membrane potential, and increased apoptosis] and increased rates of anovulation (16). In those animals that did ovulate, fewer oocytes were fertilized than in the controls (16). Markers of ER stress in human follicular fluid were shown to be positively correlated with BMI in women, suggesting dietary fat may drive this increase in humans as well (16). Furthermore, mouse oocytes matured in human follicular fluid with high lipid content had elevated levels of lipids, markers of ER stress, and slower maturation to metaphase II compared with those matured in low lipid concentrations (46), implying the elevated lipid content is driving these changes. Although little work has been conducted in humans examining oocyte lipid accumulation, several studies provide evidence that elevated lipid content does affect oocyte quality. One study with over 43,000 assisted reproduction cases found that increasing obesity was associated with an increase in failure to achieve pregnancy only when autologous oocytes were used, not with donor oocytes (47), suggesting obesity impairs oocyte quality, perhaps in relation to elevated lipid content. Supporting the role of elevated lipids, others have observed associations with lipid content in the follicular fluid of women undergoing assisted reproduction and poor cumulus oocyte complex morphology or oocyte quality (48, 49). There is also evidence that increasing BMI is associated with increasing triglycerides in the follicular fluid (50), and in a human granulosa cell line, treatment with saturated fatty acids induced apoptosis (51). It is important to consider that different species have been shown to have different lipid contents in their oocytes (52–54), and thus the threshold for lipid accumulation is likely different in different species. However, it appears that excess lipid exposure above certain threshold impairs oocyte quality.
Alterations in ovarian steroid production have been observed after HFD exposure. Aromatase messenger RNA expression in the ovary is downregulated after HFD feeding (21, 36). Along with decreases in aromatase expression, decreased serum estradiol levels have been observed in some studies (23, 36) but not others (21, 24, 26, 27, 55–57).
HFD, inflammation, and HPO dysfunction
Obesity is associated with a chronic low-grade inflammatory state that is involved in the development of associated metabolic complications (58–60). Few studies have examined ovarian inflammation after an HFD, but all of these have observed increased inflammation as a result (26, 33, 55).
C57BL/6J mice fed a 60% HFD had increased macrophage presence in their ovaries after both 10 and 32 weeks (33). Interestingly, macrophage infiltration in the ovary was increased regardless of whether the animal became obese (33). Not only were there signs of increased inflammation in the ovary, but there were also increased systemic proinflammatory cytokines (33). The periovarian adipose tissue and the ovaries have been assessed for immune cell infiltration and inflammation in C57BL/6J mice exposed to a 60% HFD for 7 months (55). Mice exposed to an HFD had an increased adipocyte size and increased infiltrating immune cells in the periovarian adipose tissue (55). Their ovaries had increased expression of several inflammatory markers, including Il1b, Il6, Tnfα, Tnfα’s receptor genes p55 and p75, Ccl2, Ikbkb, and Rela, and a trend for increased Nos2 and RELA protein (55). Rats maintained on a 59% HFD for 180 days had elevated protein levels of both IL1B and TNFα in their ovaries compared with rats maintained on an 11% fat diet (26). Earlier in this HFD exposure, at 120 days, the levels of these inflammatory proteins were not increased in the ovary, suggesting that it takes a longer exposure to the HFD to see elevation of these inflammatory markers in the rat ovary (26). It is likely that the increased inflammatory state of the ovary and its surrounding tissue is involved in the development of impaired ovarian function.
Potential Mechanisms for Reproductive Dysfunction After HFD
Maintained insulin signaling in the ovary and pituitary in the face of systemic insulin resistance
The chronic consumption of an HFD leads to the development of obesity and metabolic complications, including insulin resistance in liver and muscle. Many animal studies assessing the reproductive impact of an HFD observed increased serum insulin levels (24–26, 28, 31, 34, 35, 61), elevated blood glucose levels (24–26, 33–35, 45, 56), and systemically impaired insulin sensitivity (24–26, 28, 31, 45, 61). However, the tissues of the HPO axis appear to have a different threshold for insulin signaling than liver and muscle (31). Intriguingly, it appears that insulin signaling in the ovary and pituitary stimulates only the phosphoinositide 3-kinase (PI3K) pathway and not extracellular signal-related kinase (31). There is evidence that the ovaries and pituitary remain insulin sensitive following an HFD while systemic insulin resistance develops (25, 31). Thus, it is possible that the elevated serum insulin levels observed after HFD exposure are constantly stimulating the insulin pathways in the ovaries and pituitary, leading to reproductive dysfunction.
Insulin is involved in many aspects of female reproductive function, such as ovarian steroidogenesis (with LH), proliferation, gene expression, and central control of reproduction (62–66. Several insulin receptor (INSR) KO studies have been conducted in the various tissues of the HPO axis in the context of HFD feeding (24, 25, 28). When the INSR was knocked out in GnRH neurons (28), pituitary (25), or theca interstitial cells of the ovary (24), the KO mice gained weight with HFD exposure as well as had elevated insulin levels, impaired glucose tolerance (24, 25, 28), systemic insulin resistance (24, 25), elevated leptin levels (24, 25, 28), and increased LH levels (24, 28) similar to HFD wild-type (WT) mice. However, all three of the INSR KO models had partially restored fertility, with levels intermediate between the controls and the WT HFD mice (24, 25, 28). In the GnRH INSR KO mice fed an HFD, GnRH pulse amplitude was similar to the controls but increased in the WT HFD mice (28). The WT HFD mice had elevated LH levels that were partially restored in the KO HFD mice, implying that the altered GnRH pulse amplitude, possibly due to elevated insulin signaling in GnRH neurons, leads to increased LH levels in HFD exposure, which may be involved in the resulting impaired fertility (28). These three INSR KO studies provide convincing evidence that elevated insulin signaling in the HPO axis after HFD exposure is involved in the impaired fertility that is observed in WT mice. However, it is unlikely insulin signaling is the only factor involved as fertility was only partially restored, and other studies do not see the development of insulin resistance but still see altered reproductive function (27, 30, 55, 67). For example, when DBA/2J and C57BL/6J mice were fed a 45% HFD for 23 weeks, the DBA/2J mice, which became obese, only had minor insulin resistance, whereas the C57BL/6J mice, which did not become obese, had more severe insulin resistance (34). Interestingly, only the DBA/2J mice had impaired fertility and elevated leptin levels (34), suggesting the impaired fertility is related to elevated leptin, not insulin. Yet the cumulative effect of knocking out the insulin receptor across all tissues of the HPO axis has yet to be done simultaneously, and thus the combined effect is currently unknown.
Nevertheless, other studies have not reported altered insulin levels and/or glucose levels in response to an HFD (27, 30, 55, 67), and still others have observed the development of insulin resistance in the ovaries after an HFD (26, 61). There may be species-specific differences in ovarian insulin sensitivity, as Akamine et al. (26) observed insulin resistance developing in the ovaries of rats after a 59% HFD for 180 days, whereas other studies showing the maintenance of insulin sensitivity in the ovary have been conducted in mice (24, 25, 28, 31). Purcell et al. (61) examined insulin-mediated glucose uptake in the cumulus cells and oocytes from mice fed a 58% fat diet in an ex vivo culture system and observed insulin-stimulated glucose uptake was greatly reduced. However, the other studies did not assess insulin-mediated glucose uptake, instead focusing on downstream insulin signaling (25, 31). As insulin signaling has many targets (68), perhaps a resistance does develop to insulin-mediated glucose uptake, but other signaling pathways of insulin are active. As 5-aminoimidazole-4-carboxamide ribonucleotide, an insulin sensitizer that increases GLUT4 translocation, did not have an effect on HFD-related infertility (30), this seems plausible.
Yet, it appears that other activators of the insulin signaling cascade may be involved, as C57BL/6J mice exposed to a 60% HFD had decreased INSR and IRS1 in the ovary, yet there were signs of activation downstream in the insulin signaling cascade (56). Kit ligand (Kitlg) expression, another activator of the PI3K pathway, was increased in the ovary, suggesting that KITLG is activating this pathway in the ovaries of HFD-fed mice (56). The insulin receptor KO studies clearly identify a role of insulin signaling. There is evidence that both KITLG and insulin are activating PI3K signaling in obesity (69), and thus the combined activation is likely occurring in response to an HFD.
Increased PI3K signaling may be involved in the increased activation of primordial follicles that is seen with HFD exposure (20, 33, 38), as PI3K signaling is involved in primordial follicle activation (70–72), and ovarian KITLG is involved in the regulation of folliculogenesis (73, 74). Leptin is also elevated after an HFD (21, 23–25, 31, 33, 34, 36, 37) and activates PI3K signaling (65) and thus may also be contributing to its activation. Furthermore, insulin and KITLG have been shown to have an additive effect, increasing follicle activation in cultured rat ovaries (75), and excess stimulation of the PI3K-AKT-Foxo3 signaling pathway has been shown to increase the rate of primordial follicle activation (76–78). Likely, this combined activation is occurring in HFD exposure, leading to the depletion of the primordial follicle pool and increased follicular atresia that are observed with HFD feeding (20, 33, 36, 38, 39).
PPARγ signaling
PPARγ is a member of the nuclear receptor superfamily and binds to peroxisome proliferator response elements as a heterodimer, with the retinoid X receptors controlling the expression of genes involved in a variety of metabolic processes, including lipid metabolism and insulin sensitivity (79–81). Abnormal ligand binding, as occurs with dietary fats, alters PPARγ’s conformation and its regulation of gene expression, allowing for modification of PPARγ signaling (79–82). Evidence of PPARγ’s involvement in HFD-induced reproductive dysfunction was observed in C57BL/6 mice that were treated with three different insulin-sensitizing drugs for 4 days after being fed a 40% fat diet for 16 weeks (30). Only one of the drugs, rosiglitazone, had a positive impact on reproductive function, improving days to plug, anovulation rates, and ovulation rates (30). Rosiglitazone is a PPARγ agonist (30, 83), forming a complex with PPARγ that then binds to peroxisome proliferator response elements and alters transcription of genes involved in glucose metabolism, lipid metabolism, and immune function (83, 84), resulting in improved insulin sensitivity. The improved reproductive function with rosiglitazone treatment suggests there is dysfunction of PPARγ signaling related to reproductive function with HFD exposure, which may be linked to alterations in insulin signaling.
The role of PPARγ in HFD-induced subfertility has been assessed by knocking out PPARγ in the brain and placing KO and WT mice on a 60% HFD (21). The HFD WT and KO mice gained weight, had elevated leptin levels, and had a decreased number of primary and secondary follicles. However, the KO mice were protected from leptin resistance, impaired estrous cycles, and decreased ovarian aromatase expression that was observed in the WT HFD mice (21). These data suggest a role of neuronal PPARγ signaling in HFD reproductive dysfunction (21). As fatty acids are inducers of the PPARs (85–87), it is plausible that the elevated dietary fat content is altering PPARγ gene regulation, which is then corrected by knocking it out in the brain or through rosiglitazone treatment. HFD-fed mice with the PPARγ KO had elevated leptin levels but were protected from leptin resistance, suggesting that leptin resistance may be involved (21).
Elevated leptin
Elevated leptin levels are commonly seen after HFD exposure (21, 23–25, 31, 33, 34, 36, 37) and may be involved in the resulting reproductive dysfunction. DBA/2J mice exposed to a 45% HFD had higher rates of anovulation and lower pregnancy rates associated with a central defect. The animals were found to have a decreased expression of GnRH and LEPR-B, increased expression of neuropeptide Y (NPY), and elevated serum leptin levels in the hypothalamus (34). Under physiologic conditions, leptin suppresses hypothalamic NPY levels (88, 89) and NPY inhibits GnRH pulsatility, and it is likely that the elevated leptin levels from the HFD exposure lead to increased NPY activity in the hypothalamus, subsequently altering GnRH pulsatility (34). Insulin and leptin signaling meet at the levels of PI3K activation (65), so perhaps they work together to exert the dysfunction. However, this proposed mechanism has been assessed in only one strain of mice, and therefore implications to other strains and species may be limited.
Data from the leptin-deficient or leptin receptor–deficient female mice suggest that it is the leptin resistance, not necessarily the obesity, that is involved in the development of infertility in these mice (89–93). Specifically, leptin resistance in the agouti-related peptide neurons of the arcuate nucleus, as seen in leptin receptor knockout specific to these neurons, is involved in the development of infertility (90). Leptin administration to leptin-deficient mice restores fertility, whereas weight loss does not (89), suggesting the development of leptin resistance or impaired leptin signaling is central to infertility.
A more direct effect of leptin signaling was observed in normally cycling C57BL/6J mice on a 60% HFD (36). An HFD exposure led to increased body weight, leptin levels, and atretic follicles, as well as decreased antral follicles, aromatase expression, and estradiol levels. HFD mice were subfertile with smaller litter sizes and fewer ovulated oocytes than the controls (36). Furthermore, the elevated leptin levels increased cocaine- and amphetamine-regulated transcript expression through the leptin receptor in the ovary, which then suppressed aromatase expression and thus estradiol levels (36). It appears that the cocaine- and amphetamine-regulated transcript–mediated suppression of aromatase is through a general suppression of intracellular cyclic adenosine monophosphate levels and decreased mitogen-activated protein kinase 3/1 activation, but this suppression is not specific to FSH–regulated aromatase expression (36).
Thus, leptin appears to play a role both centrally and peripherally in the reproductive dysfunction observed in HFD-induced obesity.
Ovarian mitochondrial dysfunction
Disturbances in ovarian mitochondrial function, related to lipotoxicity, are likely involved in reproductive dysfunction. Mitochondria are maternally inherited, and their appropriate quality and quantity in the oocyte are needed for proper fertilization and development (94, 95). Increased lipid content in the ovary after an HFD has been observed (16, 26, 45) and leads to lipotoxicity (ER stress, mitochondrial dysfunction, and apoptosis) (16) and reproductive dysfunction (16, 26). Furthermore, when Chinese hamster ovary cells are exposed to excess palmitate, they display mitochondrial dysfunction, a sign of lipotoxicity (96).
Other studies supporting this theory exist in different models of obesity. In a genetic mouse model, obese females have elevated lipid levels and ER stress in their cumulus oocyte complexes and reduced mitochondrial membrane potential in ovulated oocytes (97). C57BL/6J mice fed a diet with 20% lard and sweetened condensed milk had increased mitochondrial membrane potential and reactive oxygen species production in both oocytes and zygotes, altered mitochondrial distribution, and increased mitochondrial DNA copy number in the oocytes (95). These changes in oocyte and zygote mitochondria were accompanied by reduced ability to develop into blastocysts (95). In ICR mice fed a 59% fat and 17% sucrose diet, both the cumulus cells and oocytes had increased mitochondrial stress and mitochondrial DNA copy numbers (98). C57BL/6J mice fed the same diet had increased lipid levels in the oocyte, altered mitochondrial distribution and structure, and impaired mitochondrial function (99, 100). Furthermore, these mitochondrial defects (abnormal morphology, increased presence of lipid droplets, and impaired mitochondrial metabolism) induced by an HFD appear to be carried into both skeletal muscle and oocytes of future generations never exposed to an HFD (101). The F1, F2, and F3 mice never exposed to an HFD had not only impaired mitochondrial function but also impaired insulin resistance (101), suggesting what the mother consumes can affect the metabolic outcomes both physiologically and of the germ line for subsequent generations, potentiating the impaired metabolic phenotype, regardless of the consumption of a normal balanced diet.
Thus, increased ovarian lipid leading to mitochondrial dysfunction is likely involved in HFD reproductive dysfunction.
Ovarian kisspeptins
The Kiss1 system is critical for regulation of GnRH neurons (102), and there is some evidence that Kiss1 may also be important in the ovary (29, 103–105). Rats maintained on a 40% HFD from weaning had reduced levels of Kiss1 in the ovaries during proestrus and estrus (29). In rats on a standard diet, it has been shown that Kiss1 in the ovaries peak in the afternoon of proestrus (106), so the reduction in Kiss1 on the HFD could play a role in the impaired cycling observed in the HFD rats in this study (29). Yet LH levels were not assessed and the preovulatory LH surge has been shown to be responsible for the increase in Kiss1 in the ovary on the afternoon of proestrus (106). Another study examining Kiss1 and Kiss1r expression in the ovary in C57BL/6J mice on a 60% HFD found no change in expression (107). Thus, the kisspeptin system may be involved, but based on the current evidence, its changes are likely driven by other alterations.
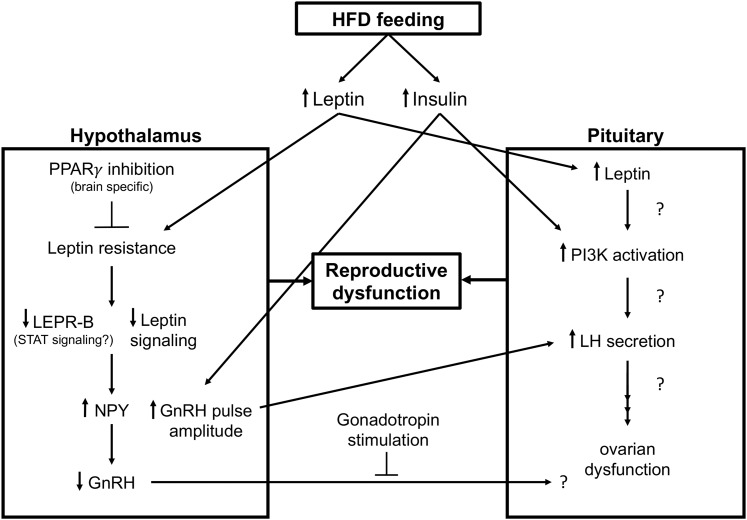
Based on the current evidence, there is also a strong case for the role of insulin, PPARγ, and leptin being altered with HFD exposure at multiple levels of the HPO axis and is involved in the resulting reproductive dysfunction (Fig. 1). It is likely a combined effect of these pathways, possibly converging at PI3K activation, as well as others that have yet to be identified.
Figure 1.
Proposed mechanisms for HFD-induced central reproductive dysfunction. HFD feeding leads to the development of increased leptin and insulin levels systemically. Elevated leptin levels lead to leptin resistance in the hypothalamus, causing decreased leptin signaling and its ability to inhibit NPY, as seen by an increase in NPY, which inhibits GnRH and likely affects pituitary function as addition of gonadotropin stimulus in this model restored fertility. Inhibiting PPARγ’s actions in the brain protects against leptin resistance and reproductive dysfunction. Elevated insulin levels in the hypothalamus increase GnRH pulse amplitude and lead to increased LH secretion from the pituitary. Alterations in hypothalamic function from both increased leptin and insulin signaling contribute to reproductive dysfunction. Increased insulin and likely increased leptin in the pituitary increase PI3K activation, which alters LH levels and contributes to reproductive dysfunction. LEPR-B, leptin receptor type B.
Is it the HFD or Obesity That Leads to Reproductive Dysfunction?
High dietary fat intake, even without the obese phenotype, is likely to induce metabolic and reproductive dysfunction. Therefore, it is important to consider that HFD exposure alone can result in impaired fertility even in lean individuals. Several studies examining an HFD and female reproductive function are able to shed light on this issue.
In one study, C57BL/6J mice were placed on a 60% HFD for 32 weeks followed by a 5-month breeding trial. Only some of the animals on the HFD gained weight and were considered obese, whereas other mice remained the same weight as the control mice (33). When fertility and ovarian function were assessed, subfertility and impaired ovarian function (depleted ovarian reserve, increased markers of inflammation) were seen in both groups of mice exposed to the HFD, regardless of the obese phenotype, suggesting that it is the dietary fat, not obesity, driving reproductive dysfunction (33). In another study, BALB/c mice were exposed to a 60.9% HFD but after 12 weeks did not weigh more than the controls (38). Although the HFD-fed mice were not obese, they displayed changes in follicle counts suggesting impaired folliculogenesis, had a slower maturation rate of germinal vesicle oocytes, and had lower numbers of metaphase II oocytes than the control mice (38). Finally, CBA mice that were exposed to a 40% HFD for 4 weeks only weighed 2 g more than the control mice but had impaired oocyte quality, increased lipid accumulation in the cumulus cells and oocytes, and impaired ovulation and fertilization (16). Collectively, these data suggest that it is the high dietary fat exposure, not obesity, that drives impaired HPO function.
However, other studies suggest that the development of obesity from the HFD drives reproductive dysfunction. When two strains of mice were fed a 45% HFD, only the DBA/2J mice developed obesity and impaired fertility (as assessed by pregnancy rate), whereas the C57BL/6J mice did not become obese and had pregnancy rates similar to their controls (34). Yet, the lower fat content of this HFD may not have been enough to see differences between the HFD and control mice as other studies that did see impaired fertility in C57BL/6J mice used a 60% HFD (33, 36, 39, 45, 55, 56, 107). Furthermore, DBA/2J mice have been shown to have lower levels of kiss1 in their arcuate nucleus and rostral periventricular region of the third ventricle than C57BL/6J mice, which becomes more pronounced with high-fat, high-sucrose exposure (108). As the kiss1 system is important in the regulation of GnRH neurons (102), this inherent difference between the strains may also explain the differences in fertility outcomes. National Institutes of Health Swiss mice fed a 60% HFD for 12 weeks followed by breeding trials were separated into two groups based on their fertility status (35). The HFD-fed mice that were fertile weighed less than those that were infertile but still weighed ∼34% more than the control mice (35). Outbred National Institutes of Health Swiss mice were used in this study because they are more similar to the genetic diversity present in human populations. Thus, it is plausible that the differences in fertility outcomes with the HFD were primarily due to genetic differences and not adiposity. These data collectively suggest that perhaps more of the increased adiposity in response to the HFD is involved in reproductive dysfunction, yet the limitations to these studies constrain the weight of this evidence.
When both diet-resistant (DR) rats and those susceptible to DIO were exposed to a 45% fat diet for 6 weeks, the DIO rats weighed more than the chow-fed controls, but the DR did not (23). The HFD decreased the number of rats having regular estrous cycles and suppressed the LH surge in both groups, yet the effect was more pronounced in the DIO animals (23). The DR and DIO mice have been created through selective breeding (23), and thus innate differences in these strains may be driving the observed difference instead of increased adiposity. However, it does appear that there is an impact of HFD on HPO axis functionality, but it becomes more exaggerated with the addition of obesity.
When considering if it is the dietary fat exposure or the obese phenotype, it is worthwhile to consider if the detrimental effects of the HFD on reproductive function can be reversed once the HFD is removed. This was examined in C57BL/6J mice, which, after 6 weeks of a 60% HFD, were switched back to the control diet (13% fat) for 8 weeks (45). The abnormal oocyte lipid accumulation observed after the 6-week HFD was not reversed. Oocyte quality was also diminished compared with the control mice (45). Interestingly, after the diet reversal, the metabolic disturbances, including increased body weight, that were observed after 6 weeks on the HFD were returned to that of the controls (45). These data suggest that there is sustained harm to the ovary, and it is likely that the HPO axis from the HFD (or its induced obesity) may be impossible to reverse.
Implications for Humans
Few studies have been conducted in humans focusing solely on dietary fat intake and reproductive dysfunction. Based on the limited research, it does appear that an increased dietary fat intake is associated with altered reproductive function.
In a controlled feeding study, a small group of healthy premenopausal women was fed a 40% fat diet for four menstrual cycles, followed by a 20% fat diet for four menstrual cycles (19). When the women were on the lower fat diet, they tended to have longer menstrual cycles than when on the HFD (19). Another controlled feeding study switched a small cohort of South African women to a Western-style diet by introducing meat into their diet to raise their typical fat intake from 30% to 35% (while all other aspects of their diet remained the same) for 2 months (18). This intervention resulted in increased FSH levels, decreased estradiol levels, and increased length of the follicular phase of their cycle (18). Although limited conclusions can be drawn from these studies as they had small sample sizes and limited data, they provide some evidence that changing the amount or type of dietary fat (from plant to animal derived) has an impact on the menstrual cycle in women. As one main difference between animal and plant fat is that animal fats are typically higher in saturated fat, changes in the type of fat in the diet may be specifically involved.
In prospective studies, the negative impact of dietary fat intake has been less clear. It is important to consider that with self-reported dietary intake data, especially with “unhealthy” dietary components, large errors in self-reporting, specifically underreporting of data, may be confounding these studies.
One prospective study examined dietary fat intake, as reported by food frequency questionnaires, and reproductive hormone concentrations in 259 regularly menstruating women (109). Positive associations between testosterone levels and total fat intake and polyunsaturated fatty acid intake were identified when these fat components were substituted for either total energy or carbohydrates (109). There were no associations identified with any measure of dietary fat intake and sporadic anovulation, but few cases were observed, and likely this study was underpowered to detect these associations (109). Another study examined follicular fluid and embryos obtained from 236 women undergoing assisted reproduction and looked for associations between the oxidative stress in the follicular fluid, embryo development, and dietary fat intake (as determined by food frequency questionnaires) (110). For the markers of oxidative stress, malondialdehyde and total antioxidant capacity, only the intake of polyunsaturated fatty acids was positively associated with malondialdehyde levels (110). In addition, the oocyte maturation rate was positively associated with total antioxidant capacity levels, and the percentage of nonfragmented embryos was negatively associated with calories from fat and total fat intake (110). A large prospective study of the Nurse Health Study II cohort examined the association between dietary fat intake and ovulatory infertility over a 9-year period in 18,555 premenopausal women with no history of infertility (111). This study did identify a few weak associations but observed only 438 cases of ovulatory infertility in the 9-year period and was likely underpowered. In energy- and age-adjusted models, the authors found that total fat, saturated fat, and monounsaturated fat intakes were inversely associated with the risk of ovulatory infertility but no longer were significant when a variety of potentially confounding variables were added into multivariate models (111). In 240 infertile women, total fat intake was positively associated with the number of retrieved oocytes and inversely associated with high embryo quality (112).
Further speculation of an HFD and its implications for humans can be derived from the polycystic ovary syndrome (PCOS) literature. PCOS is the most common endocrine disorder in women (109, 110), and there is an increased prevalence of obesity and central adiposity in women with PCOS (111). Women with PCOS are subfertile, anovulatory, and hyperandrogenemic; have increased miscarriage rates; and have a dysfunctional endometrium, which are made worse in the presence of excessive adiposity (109, 111). Although there is a worsening of PCOS with increased adiposity, the role of dietary fat intake is less clear, with some studies showing women with PCOS consuming higher amounts of fat (113–115), lower amounts of fat (116), or the same amount as controls (117–119). Although the intake of dietary fat in this population is unsettled, feeding women with PCOS either a high-fat or low-fat meal decreased testosterone levels. However, they remained lower 2 hours longer after the high-fat meal (120), suggesting dietary fat intake may affect steroidogenesis in these women. Although direct speculation of the role of dietary fat in this population on reproductive outcomes is lacking, clearly the elevated adiposity is involved in the pathogenesis of PCOS, again suggesting a link between dietary fat and female reproductive function.
Although limited data exist from human studies, it does appear that the amount of fat in the diet is associated with changes in reproductive function, including altered menstrual cycle length, changes in reproductive hormone levels, and embryo quality in assisted reproductive technology cycles. These changes are likely to affect female fertility and warrant further experimental exploration. Although some of the studies suggest a positive correlation between dietary fat intake and fertility, the correlation is weak, and more studies assessing total caloric intake and type of consumed fat are needed.
Summary and Conclusions
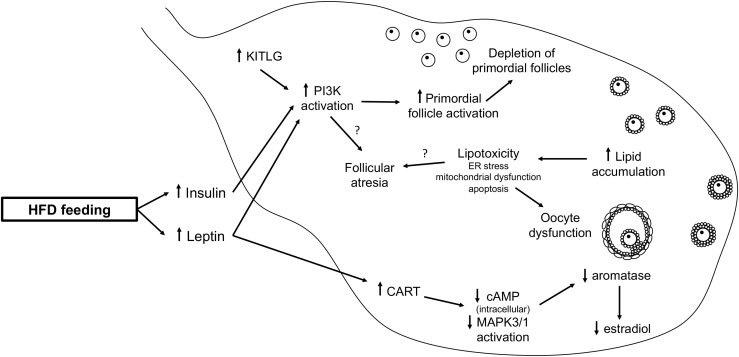
An HFD impairs female reproductive function, both with and without obesity; yet, when the obese phenotype is present, the effect is worsened. An HFD likely increases insulin and leptin signaling in the HPO axis, leading to increased PI3K activity. PPARγ activity is also involved both centrally (Fig. 1) and peripherally (Fig. 2) after HFD exposure. More research is needed to further determine the mechanisms by which an HFD leads to female reproductive dysfunction.
Figure 2.
HFD-induced ovarian dysfunction. In the ovary, elevated leptin signaling increases CART, which decreases intracellular cAMP levels and MAPK3/1 activation, leading to decreased aromatase and estradiol production. Elevated levels of leptin, insulin, and KITLG increase PI3K activation, which leads to increased primordial follicle activation, resulting in the depletion of the ovarian reserve. Increased lipid accumulation in the ovary leads to lipotoxicity. Both increased PI3K signaling and lipotoxicity may contribute to increased follicle atresia. These alterations in ovarian function contribute to the reproductive dysfunction. cAMP, cyclic adenosine monophosphate; CART, cocaine- and amphetamine-regulated transcript; MAPK3/1, mitogen-activated protein kinase 3/1.
Acknowledgments
The authors thank Dr. Nanette F. Santoro and Dr. T. Rajendra Kumar for their critical review of the manuscript.
This work was supported by an American Association of Obstetricians and Gynecologists Foundation Research Grant (to M.E.S.-W.).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- BMI
body mass index
- DIO
diet-induced obesity
- DR
diet-resistant
- ER
endoplasmic reticulum
- FSH
follicle stimulating hormone
- GnRH
gonadotropin-releasing hormone
- HFD
high-fat diet
- HPO
hypothalamic-pituitary-ovarian
- INSR
insulin receptor
- KITLG
Kit ligand
- KO
knockout
- LH
luteinizing hormone
- NPY
neuropeptide Y
- PCOS
polycystic ovary syndrome
- PI3K
phosphoinositide 3-kinase
- PPARγ
proliferator-activated receptor γ
- WT
wild-type.
References
- 1. Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015;(219):1–8. [PubMed] [Google Scholar]
- 2. Bellver J, Ayllón Y, Ferrando M, Melo M, Goyri E, Pellicer A, Remohí J, Meseguer M. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil Steril. 2010;93(2):447–454. [DOI] [PubMed] [Google Scholar]
- 3. Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421–439. [DOI] [PubMed] [Google Scholar]
- 4. Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology—a systematic review. Hum Reprod Update. 2007;13(5):433–444. [DOI] [PubMed] [Google Scholar]
- 5. Turner-McGrievy GM, Grant BL. Prevalence of body mass index and body weight cut-off points for in vitro fertilization treatment at U.S. clinics and current clinic weight loss strategy recommendations. Hum Fertil (Camb). 2015;18(3):215–219. [DOI] [PubMed] [Google Scholar]
- 6. Schwingshackl L, Hoffmann G. Comparison of effects of long-term low-fat vs high-fat diets on blood lipid levels in overweight or obese patients: a systematic review and meta-analysis. J Acad Nutr Diet. 2013;113(12):1640–1661. [DOI] [PubMed] [Google Scholar]
- 7. Stocks T, Taylor MA, Angquist L, Macdonald IA, Arner P, Holst C, Oppert JM, Martinez JA, Rössner S, Polak J, Langin D, Saris WH, Astrup A, Sørensen TI. Change in proportional protein intake in a 10-week energy-restricted low- or high-fat diet, in relation to changes in body size and metabolic factors. Obes Facts. 2013;6(3):217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tremblay AJ, Lamarche B, Guay V, Charest A, Lemelin V, Couture P. Short-term, high-fat diet increases the expression of key intestinal genes involved in lipoprotein metabolism in healthy men. Am J Clin Nutr. 2013;98(1):32–41. [DOI] [PubMed] [Google Scholar]
- 9. Numao S, Kawano H, Endo N, Yamada Y, Takahashi M, Konishi M, Sakamoto S. Short-term high-fat diet alters postprandial glucose metabolism and circulating vascular cell adhesion molecule-1 in healthy males. Appl Physiol Nutr Metab. 2016;41(8):895–902. [DOI] [PubMed] [Google Scholar]
- 10. Osterberg KL, Boutagy NE, McMillan RP, Stevens JR, Frisard MI, Kavanaugh JW, Davy BM, Davy KP, Hulver MW. Probiotic supplementation attenuates increases in body mass and fat mass during high-fat diet in healthy young adults. Obesity (Silver Spring). 2015;23(12):2364–2370. [DOI] [PubMed] [Google Scholar]
- 11. Boutagy NE, Neilson AP, Osterberg KL, Smithson AT, Englund TR, Davy BM, Hulver MW, Davy KP. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity (Silver Spring). 2015;23(12):2357–2363. [DOI] [PubMed] [Google Scholar]
- 12. Silver HJ, Kang H, Keil CD, Muldowney JA III, Kocalis H, Fazio S, Vaughan DE, Niswender KD. Consuming a balanced high fat diet for 16 weeks improves body composition, inflammation and vascular function parameters in obese premenopausal women. Metabolism. 2014;63(4):562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holloway CJ, Cochlin LE, Emmanuel Y, Murray A, Codreanu I, Edwards LM, Szmigielski C, Tyler DJ, Knight NS, Saxby BK, Lambert B, Thompson C, Neubauer S, Clarke K. A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects. Am J Clin Nutr. 2011;93(4):748–755. [DOI] [PubMed] [Google Scholar]
- 14. U.S. Department of Agriculture Agricultural Research Service . Nutrient intakes from food and beverages: mean amounts consumed per individual, by gender and age, what we eat in America, National Health and Nutrition Examination Survey 2013–2014. 2016. https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1314/Table_1_NIN_GEN_13.pdf. Accessed May 16, 2017.
- 15. Vannice G, Rasmussen H. Position of the academy of nutrition and dietetics: dietary fatty acids for healthy adults. J Acad Nutr Diet. 2014;114(1):136–153. [DOI] [PubMed] [Google Scholar]
- 16. Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151(11):5438–5445. [DOI] [PubMed] [Google Scholar]
- 17. Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14(3):281–287. [DOI] [PubMed] [Google Scholar]
- 18. Hill P, Garbaczewski L, Haley N, Wynder EL. Diet and follicular development. Am J Clin Nutr. 1984;39(5):771–777. [DOI] [PubMed] [Google Scholar]
- 19. Reichman ME, Judd JT, Taylor PR, Nair PP, Jones DY, Campbell WS. Effect of dietary fat on length of the follicular phase of the menstrual cycle in a controlled diet setting. J Clin Endocrinol Metab. 1992;74(5):1171–1175. [DOI] [PubMed] [Google Scholar]
- 20. Wang N, Luo LL, Xu JJ, Xu MY, Zhang XM, Zhou XL, Liu WJ, Fu YC. Obesity accelerates ovarian follicle development and follicle loss in rats. Metabolism. 2014;63(1):94–103. [DOI] [PubMed] [Google Scholar]
- 21. Fernandez MO, Sharma S, Kim S, Rickert E, Hsueh K, Hwang V, Olefsky JM, Webster NJ. Obese neuronal PPARγ knockout mice are leptin sensitive but show impaired glucose tolerance and fertility. Endocrinology. 2016;158(1):121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lai H, Jia X, Yu Q, Zhang C, Qiao J, Guan Y, Kang J. High-fat diet induces significant metabolic disorders in a mouse model of polycystic ovary syndrome. Biol Reprod. 2014;91(5):127. [DOI] [PubMed] [Google Scholar]
- 23. Balasubramanian P, Jagannathan L, Mahaley RE, Subramanian M, Gilbreath ET, Mohankumar PS, Mohankumar SM. High fat diet affects reproductive functions in female diet-induced obese and dietary resistant rats. J Neuroendocrinol. 2012;24(5):748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu S, Divall S, Nwaopara A, Radovick S, Wondisford F, Ko C, Wolfe A. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes. 2013;63(4):1270–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brothers KJ, Wu S, DiVall SA, Messmer MR, Kahn CR, Miller RS, Radovick S, Wondisford FE, Wolfe A. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010;12(3):295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akamine EH, Marçal AC, Camporez JP, Hoshida MS, Caperuto LC, Bevilacqua E, Carvalho CR. Obesity induced by high-fat diet promotes insulin resistance in the ovary. J Endocrinol. 2010;206(1):65–74. [DOI] [PubMed] [Google Scholar]
- 27. Hussain MA, Abogresha NM, Hassan R, Tamany DA, Lotfy M. Effect of feeding a high-fat diet independently of caloric intake on reproductive function in diet-induced obese female rats. Arch Med Sci. 2016;4(4):906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DiVall SA, Herrera D, Sklar B, Wu S, Wondisford F, Radovick S, Wolfe A. Insulin receptor signaling in the GnRH neuron plays a role in the abnormal GnRH pulsatility of obese female mice. PLoS One. 2015;10(3):e0119995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou Q, Chen H, Yang S, Li Y, Wang B, Chen Y, Wu X. High-fat diet decreases the expression of Kiss1 mRNA and kisspeptin in the ovary, and increases ovulatory dysfunction in postpubertal female rats. Reprod Biol Endocrinol. 2014;12:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Minge CE, Bennett BD, Norman RJ, Robker RL. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reverses the adverse effects of diet-induced obesity on oocyte quality. Endocrinology. 2008;149(5):2646–2656. [DOI] [PubMed] [Google Scholar]
- 31. Wu S, Divall S, Wondisford F, Wolfe A. Reproductive tissues maintain insulin sensitivity in diet-induced obesity. Diabetes. 2011;61(1):114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shaw MA, Rasmussen KM, Myers TR. Consumption of a high fat diet impairs reproductive performance in Sprague-Dawley rats. J Nutr. 1997;127(1):64–69. [DOI] [PubMed] [Google Scholar]
- 33. Skaznik-Wikiel ME, Swindle DC, Allshouse AA, Polotsky AJ, McManaman JL. High-fat diet causes subfertility and compromised ovarian function independent of obesity in mice. Biol Reprod. 2016;94(5):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tortoriello DV, McMinn J, Chua SC. Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology. 2004;145(3):1238–1247. [DOI] [PubMed] [Google Scholar]
- 35. Bermejo-Alvarez P, Rosenfeld CS, Roberts RM. Effect of maternal obesity on estrous cyclicity, embryo development and blastocyst gene expression in a mouse model. Hum Reprod. 2012;27(12):3513–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma X, Hayes E, Prizant H, Srivastava RK, Hammes SR, Sen A. Leptin-induced CART (cocaine- and amphetamine-regulated transcript) is a novel intraovarian mediator of obesity-related infertility in females. Endocrinology. 2016;157(3):1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soulis G, Kitraki E, Gerozissis K. Early neuroendocrine alterations in female rats following a diet moderately enriched in fat. Cell Mol Neurobiol. 2005;25(5):869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sohrabi M, Roushandeh AM, Alizadeh Z, Vahidinia A, Vahabian M, Hosseini M. Effect of a high fat diet on ovary morphology, in vitro development, in vitro fertilisation rate and oocyte quality in mice. Singapore Med J. 2015;56(10):573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151(8):4039–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. [DOI] [PubMed] [Google Scholar]
- 41. Zhang XM, Li L, Xu JJ, Wang N, Liu WJ, Lin XH, Fu YC, Luo LL. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene. 2013;523(1):82–87. [DOI] [PubMed] [Google Scholar]
- 42. Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development. 2015;142(15):2554–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cordts EB, Christofolini DM, Dos Santos AA, Bianco B, Barbosa CP. Genetic aspects of premature ovarian failure: a literature review. Arch Gynecol Obstet. 2010;283(3):635–643. [DOI] [PubMed] [Google Scholar]
- 44. Wood MA, Rajkovic A. Genomic markers of ovarian reserve. Semin Reprod Med. 2013;31(6):399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev. 2015;27(4):716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang X, Wu LL, Chura LR, Liang X, Lane M, Norman RJ, Robker RL. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil Steril. 2012;97(6):1438–1443. [DOI] [PubMed] [Google Scholar]
- 47. Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R; SART Writing Group . Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod. 2010;26(1):245–252. [DOI] [PubMed] [Google Scholar]
- 48. Niu Z, Lin N, Gu R, Sun Y, Feng Y. Associations between insulin resistance, free fatty acids, and oocyte quality in polycystic ovary syndrome during in vitro fertilization. J Clin Endocrinol Metab. 2014;99(11):E2269–E2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jungheim ES, Macones GA, Odem RR, Patterson BW, Lanzendorf SE, Ratts VS, Moley KH. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil Steril. 2011;95(6):1970–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, Lane M, Norman RJ. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab. 2009;94(5):1533–1540. [DOI] [PubMed] [Google Scholar]
- 51. Mu YM, Yanase T, Nishi Y, Tanaka A, Saito M, Jin CH, Mukasa C, Okabe T, Nomura M, Goto K, Nawata H. Saturated FFAs, palmitic acid and stearic acid, induce apoptosis in human granulosa cells. Endocrinology. 2001;142(8):3590–3597. [DOI] [PubMed] [Google Scholar]
- 52. McEvoy TG, Coull GD, Broadbent PJ, Hutchinson JS, Speake BK. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J Reprod Fertil. 2000;118(1):163–170. [PubMed] [Google Scholar]
- 53. Genicot G, Leroy JLMR, Soom AV, Donnay I. The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes. Theriogenology. 2005;63(4):1181–1194. [DOI] [PubMed] [Google Scholar]
- 54. Dunning KR, Russell DL, Robker RL. Lipids and oocyte developmental competence: the role of fatty acids and β-oxidation. Reproduction. 2014;148(1):R15–R27. [DOI] [PubMed] [Google Scholar]
- 55. Nteeba J, Ortinau LC, Perfield JW II, Keating AF. Diet-induced obesity alters immune cell infiltration and expression of inflammatory cytokine genes in mouse ovarian and peri-ovarian adipose depot tissues. Mol Reprod Dev. 2013;80(11):948–958. [DOI] [PubMed] [Google Scholar]
- 56. Nteeba J, Ross JW, Perfield JW II, Keating AF. High fat diet induced obesity alters ovarian phosphatidylinositol-3 kinase signaling gene expression. Reprod Toxicol. 2013;42:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhuo Y, Zhou D, Che L, Fang Z, Lin Y, Wu D. Feeding prepubescent gilts a high-fat diet induces molecular changes in the hypothalamus-pituitary-gonadal axis and predicts early timing of puberty. Nutrition. 2014;30(7-8):890–896. [DOI] [PubMed] [Google Scholar]
- 58. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–150. [DOI] [PubMed] [Google Scholar]
- 59. Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132(6):2169–2180. [DOI] [PubMed] [Google Scholar]
- 60. Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. 2015;2015:508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Purcell SH, Chi MM, Lanzendorf S, Moley KH. Insulin-stimulated glucose uptake occurs in specialized cells within the cumulus oocyte complex. Endocrinology. 2012;153(5):2444–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fontana R, Della Torre S. The deep correlation between energy metabolism and reproduction: a view on the effects of nutrition for women fertility. Nutrients. 2016;8(2):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998;83(6):2001–2005. [DOI] [PubMed] [Google Scholar]
- 64. Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140(4):489–504. [DOI] [PubMed] [Google Scholar]
- 65. Plum L, Schubert M, Brüning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16(2):59–65. [DOI] [PubMed] [Google Scholar]
- 66. Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289(5487):2122–2125. [DOI] [PubMed] [Google Scholar]
- 67. Tortoriello DV, McMinn JE, Chua SC. Increased expression of hypothalamic leptin receptor and adiponectin accompany resistance to dietary-induced obesity and infertility in female C57BL/6J mice. Int J Obes. 2006;31(3):395–402. [DOI] [PubMed] [Google Scholar]
- 68. Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6(1):a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nteeba J, Ganesan S, Madden JA, Dickson MJ, Keating AF. Progressive obesity alters ovarian insulin, phosphatidylinositol-3 kinase, and chemical metabolism signaling pathways and potentiates ovotoxicity induced by phosphoramide mustard in mice. Biol Reprod. 2017;96(2):478–490. [DOI] [PubMed] [Google Scholar]
- 70. Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319(5863):611–613. [DOI] [PubMed] [Google Scholar]
- 71. Reddy P, Adhikari D, Zheng W, Liang S, Hämäläinen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, Liu K. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet. 2009;18(15):2813–2824. [DOI] [PubMed] [Google Scholar]
- 72. Liu L, Rajareddy S, Reddy P, Jagarlamudi K, Du C, Shen Y, Guo Y, Boman K, Lundin E, Ottander U, Selstam G, Liu K. Phosphorylation and inactivation of glycogen synthase kinase-3 by soluble kit ligand in mouse oocytes during early follicular development. J Mol Endocrinol. 2007;38(1):137–146. [DOI] [PubMed] [Google Scholar]
- 73. Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140(9):4262–4271. [DOI] [PubMed] [Google Scholar]
- 74. Driancourt MA, Reynaud K, Cortvrindt R, Smitz J. Roles of KIT and KIT LIGAND in ovarian function. Rev Reprod. 2000;5(3):143–152. [DOI] [PubMed] [Google Scholar]
- 75. Kezele PR, Nilsson EE, Skinner MK. Insulin but not insulin-like growth factor-1 promotes the primordial to primary follicle transition. Mol Cell Endocrinol. 2002;192(1-2):37–43. [DOI] [PubMed] [Google Scholar]
- 76. Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301(5630):215–218. [DOI] [PubMed] [Google Scholar]
- 77. Jagarlamudi K, Liu L, Adhikari D, Reddy P, Idahl A, Ottander U, Lundin E, Liu K. Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS One. 2009;4(7):e6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development. 2007;134(1):199–209. [DOI] [PubMed] [Google Scholar]
- 79. Barish GD, Narkar VA, Evans RM. PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116(3):590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10(4):355–361. [DOI] [PubMed] [Google Scholar]
- 81. Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;99(5):557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Poulsen Ll, Siersbæk M, Mandrup S. PPARs: fatty acid sensors controlling metabolism. Semin Cell Dev Biol. 2012;23(6):631–639. [DOI] [PubMed] [Google Scholar]
- 83. Mudaliar S, Henry RR. New oral therapies for type 2 diabetes mellitus: the glitazones or insulin sensitizers. Annu Rev Med. 2001;52:239–257. [DOI] [PubMed] [Google Scholar]
- 84. Lemay DG, Hwang DH. Genome-wide identification of peroxisome proliferator response elements using integrated computational genomics. J Lipid Res. 2006;47(7):1583–1587. [DOI] [PubMed] [Google Scholar]
- 85. Keller H, Dreyer C, Medin J, Mahfoudi A, Ozato K, Wahli W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc Natl Acad Sci USA. 1993;90(6):2160–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA. 1997;94(9):4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sanderson LM, de Groot PJ, Hooiveld GJ, Koppen A, Kalkhoven E, Müller M, Kersten S. Effect of synthetic dietary triglycerides: a novel research paradigm for nutrigenomics. PLoS One. 2008;3(2):e1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A. , Mackellar W, Rosteck PR Jr, Schoner DS, Tinsley FC, Zhang X-Y, Heiman M. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377(6549):530–532. [DOI] [PubMed] [Google Scholar]
- 89. Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12(3):318–320. [DOI] [PubMed] [Google Scholar]
- 90. Egan OK, Inglis MA, Anderson GM. Leptin signaling in AgRP neurons modulates puberty onset and adult fertility in mice. J Neurosci. 2017;37(14):3875–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Singireddy AV, Inglis MA, Zuure WA, Kim JS, Anderson GM. Neither signal transducer and activator of transcription 3 (STAT3) or STAT5 signaling pathways are required for leptin’s effects on fertility in mice. Endocrinology. 2013;154(7):2434–2445. [DOI] [PubMed] [Google Scholar]
- 92. Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AWK, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG Jr. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421(6925):856–859. [DOI] [PubMed] [Google Scholar]
- 93. Bates SH, Myers MG Jr. The role of leptin receptor signaling in feeding and neuroendocrine function. Trends Endocrinol Metab. 2003;14(10):447–452. [DOI] [PubMed] [Google Scholar]
- 94. Reynier P, May-Panloup P, Chrétien MF, Morgan CJ, Jean M, Savagner F, Barrière P, Malthièry Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7(5):425–429. [DOI] [PubMed] [Google Scholar]
- 95. Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5(4):e10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47(12):2726–2737. [DOI] [PubMed] [Google Scholar]
- 97. Wu LL, Russell DL, Wong SL, Chen M, Tsai TS, St John JC, Norman RJ, Febbraio MA, Carroll J, Robker RL. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development. 2015;142(4):681–691. [DOI] [PubMed] [Google Scholar]
- 98. Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7(11):e49217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Boots CE, Boudoures A, Zhang W, Drury A, Moley KH. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum Reprod. 2016;31(9):2090–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Boudoures AL, Chi M, Thompson A, Zhang W, Moley KH. The effects of voluntary exercise on oocyte quality in a diet-induced obese murine model. Reproduction. 2015;151(3):261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Saben JL, Boudoures AL, Asghar Z, Thompson A, Drury A, Zhang W, Chi M, Cusumano A, Scheaffer S, Moley KH. Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Reports. 2016;16(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92(3):1235–1316. [DOI] [PubMed] [Google Scholar]
- 103. Wahab F, Atika B, Shahab M, Behr R. Kisspeptin signalling in the physiology and pathophysiology of the urogenital system. Nat Rev Urol. 2015;13(1):21–32. [DOI] [PubMed] [Google Scholar]
- 104. Fernandois D, Na E, Cuevas F, Cruz G, Lara HE, Paredes AH. Kisspeptin is involved in ovarian follicular development during aging in rats. J Endocrinol. 2015;228(3):161–170. [DOI] [PubMed] [Google Scholar]
- 105. Gaytán F, Gaytán M, Castellano JM, Romero M, Roa J, Aparicio B, Garrido N, Sánchez-Criado JE, Millar RP, Pellicer A, Fraser HM, Tena-Sempere M. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Endocrinol Metab. 2008;296(3):E520–E531. [DOI] [PubMed] [Google Scholar]
- 106. Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, Dieguez C, Aguilar E, Sánchez-Criado JE, Pellicer A, Pinilla L, Gaytan F, Tena-Sempere M. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology. 2006;147(10):4852–4862. [DOI] [PubMed] [Google Scholar]
- 107. Merhi Z, Thornton K, Bonney E, Cipolla MJ, Charron MJ, Buyuk E. Ovarian kisspeptin expression is related to age and to monocyte chemoattractant protein-1. J Assist Reprod Genet. 2016;33(4):535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152(4):1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mumford SL, Chavarro JE, Zhang C, Perkins NJ, Sjaarda LA, Pollack AZ, Schliep KC, Michels KA, Zarek SM, Plowden TC, Radin RG, Messer LC, Frankel RA, Wactawski-Wende J. Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am J Clin Nutr. 2016;103(3):868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kazemi A, Ramezanzadeh F, Nasr-Esfahani MH, Saboor Yaraghi AA, Ahmadi M. Does dietary fat intake influence oocyte competence and embryo quality by inducing oxidative stress in follicular fluid? Iran J Reprod Med. 2013;11(12):1005–1012. [PMC free article] [PubMed] [Google Scholar]
- 111. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Dietary fatty acid intakes and the risk of ovulatory infertility. Am J Clin Nutr. 2007;85(1):231–237. [DOI] [PubMed] [Google Scholar]
- 112. Kazemi A, Ramezanzadeh F, Nasr-Esfahani MH. Relationship between dietary fat intake, its major food sources and assisted reproduction parameters. J Reprod Infertil. 2014;15(4):214–221. [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang J, Liu Y, Liu X, Xu L, Zhou L, Tang L, Zhuang J, Guo W, Hu R. High intake of energy and fat in southwest Chinese women with PCOS: a population-based case-control study. PLoS One. 2015;10(5):e0127094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Turner-McGrievy G, Davidson CR, Billings DL. Dietary intake, eating behaviors, and quality of life in women with polycystic ovary syndrome who are trying to conceive. Hum Fertil (Camb). 2014;18(1):16–21. [DOI] [PubMed] [Google Scholar]
- 115. Barr S, Hart K, Reeves S, Sharp K, Jeanes YM. Habitual dietary intake, eating pattern and physical activity of women with polycystic ovary syndrome. Eur J Clin Nutr. 2011;65(10):1126–1132. [DOI] [PubMed] [Google Scholar]
- 116. Altieri P, Cavazza C, Pasqui F, Morselli AM, Gambineri A, Pasquali R. Dietary habits and their relationship with hormones and metabolism in overweight and obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2012;78(1):52–59. [DOI] [PubMed] [Google Scholar]
- 117. Douglas CC, Norris LE, Oster RA, Darnell BE, Azziz R, Gower BA. Difference in dietary intake between women with polycystic ovary syndrome and healthy controls. Fertil Steril. 2006;86(2):411–417. [DOI] [PubMed] [Google Scholar]
- 118. Toscani MK, Mario FM, Radavelli-Bagatini S, Spritzer PM. Insulin resistance is not strictly associated with energy intake or dietary macronutrient composition in women with polycystic ovary syndrome. Nutr Res. 2011;31(2):97–103. [DOI] [PubMed] [Google Scholar]
- 119. Álvarez-Blasco F, Luque-Ramírez M, Escobar-Morreale HF. Diet composition and physical activity in overweight and obese premenopausal women with or without polycystic ovary syndrome. Gynecol Endocrinol. 2011;27(12):978–981. [DOI] [PubMed] [Google Scholar]
- 120. Katcher HI, Kunselman AR, Dmitrovic R, Demers LM, Gnatuk CL, Kris-Etherton PM, Legro RS. Comparison of hormonal and metabolic markers after a high-fat, Western meal versus a low-fat, high-fiber meal in women with polycystic ovary syndrome. Fertil Steril. 2009;91(4):1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]