Summary
Venous thromboembolism (VTE)‐BLEED, a decision tool for predicting major bleeding during chronic anticoagulation for VTE has not yet been validated in practice‐based conditions. We calculated the prognostic indices of VTE‐BLEED for major bleeding after day 30 and day 90, as well as for recurrent VTE and all‐cause mortality, in 4457 patients enrolled in the international, prospective XALIA study. The median at‐risk time was 190 days (interquartile range 106–360). The crude hazard ratio (HR) for major bleeding after day 30 was 2·6 [95% confidence interval (CI) 1·3–5·2] and the treatment‐adjusted HR was 2·3 (95% CI 1·1–4·5) for VTE‐BLEED high (versus low) risk patients: the corresponding values for major bleeding after day 90 were 3·8 (95% CI 1·6–9·3) and 3·2 (95% CI 1·3–7·7), respectively. The predictive value of VTE‐BLEED was similar in selected patients with unprovoked VTE or those treated with rivaroxaban. High VTE‐BLEED score was associated with higher incidence of all‐cause mortality (treatment‐adjusted HR 11, 95% CI 4·8–23), but not evidently with recurrent VTE (treatment‐adjusted HR 1·5; 95% CI 0·85–2·7). These results confirm the predictive value of VTE‐BLEED in practice‐based data in patients treated with rivaroxaban or conventional anticoagulation, supporting the hypothesis that VTE‐BLEED may be useful for making management decisions on the duration of anticoagulant therapy.
Keywords: venous thromboembolism, bleeding, anticoagulation therapy, rivaroxaban, prediction
Current international guidelines recommend discontinuing anticoagulant therapy for unprovoked acute pulmonary embolism (PE) or deep vein thrombosis (DVT) after the first 3 months of treatment only in patients considered at high risk of bleeding (Konstantinides et al, 2014; Kearon et al, 2016). However, it remains challenging to quantify the individual risk of major bleeding and although risk assessment models have been developed in the past years, they still, without exception, lack sufficient validation in cohort and management studies (Klok et al, 2015).
We recently derived and externally validated VTE‐BLEED, a simple 6‐variable risk score designed to predict the risk of major bleeding in patients with venous thromboembolism (VTE) on stable, long‐term anticoagulation (Table 1) (Klok et al, 2016). VTE‐BLEED was first derived from patients randomized to treatment with dabigatran in the RE‐COVER trials (Schulman et al, 2009, 2014). As the first validation step, the score was further evaluated in warfarin‐treated patients from the same trial programme. In a subsequent study, VTE‐BLEED was tested in both treatment arms of the HOKUSAI‐VTE trial, namely in edoxaban‐ and warfarin‐treated patients (Hokusai VTE Investigators, 2013). In both the derivation and validation studies, the odds ratio (OR) for major bleeding in patients categorized in the VTE‐BLEED high risk group compared to the low risk group ranged between 3·1 and 6·5, indicating a substantial increased risk in patients with two or more VTE‐BLEED points (Klok et al, 2016, 2017). These risk estimates remained the same when only fatal or intracranial bleeding events were considered (Klok et al, 2018). Based on these findings, VTE‐BLEED is the only available externally validated bleeding score for VTE patients that has been tested in patients receiving vitamin K antagonists and direct thrombin inhibitors as well as direct factor Xa inhibitors, across several relevant patient subcategories. Nonetheless, the score still needs validation in practice‐based conditions before it can be integrated in the decision algorithms of future management studies on the optimal duration of anticoagulant therapy in VTE patients.
Table 1.
The VTE‐BLEED score with original definition of variables (Klok et al, 2016)
| Factor | Score |
|---|---|
| Active cancera | 2 |
| Male with uncontrolled arterial hypertensionb | 1 |
| Anaemiac | 1·5 |
| History of bleedingd | 1·5 |
| Age ≥60 years old | 1·5 |
| Renal dysfunctione | 1·5 |
| Classification of patients with the VTE‐BLEED score | |
| Low bleeding risk | Total score <2 |
| High bleeding risk | Total score ≥2 |
Cancer diagnosed within 6 months before diagnosis of venous thromboembolism (VTE) (excluding basal‐cell or squamous‐cell carcinoma of the skin), recently recurrent or progressive cancer or any cancer that required anti‐cancer treatment within 6 months before the VTE was diagnosed.
Males with uncontrolled arterial hypertension were defined by values of systolic blood pressure ≥140 mmHg at baseline.
Haemoglobin <130 g/l in men or <120 g/l in women.
Including prior major or non‐major clinically relevant bleeding event, rectal bleeding, frequent nose bleeding, or haematuria.
An estimated glomerular filtration rate (eGRF) <60 ml/min defined the presence of renal dysfunction: eGRF was calculated at baseline with the Cockcroft‐Gault formula, which include serum creatinine, age, and body weight.
In the current study, we aimed to assess the predictive accuracy of VTE‐BLEED for major bleeding during long‐term anticoagulant therapy in patients with DVT with or without concurrent acute PE enrolled in the multicentre, international, prospective, non‐interventional XALIA study (Ageno & Turpie, 2016; Ageno et al, 2016). We additionally compared the incidences of recurrent VTE and death between the VTE‐BLEED low and high risk cohorts.
Patients and methods
Study setting and patients
The design of the XALIA study and patients' selection criteria were published elsewhere (Ageno et al, 2014). In short, patients with a confirmed DVT aged 18 years or older and with an indication (and no contraindication) for anticoagulant treatment duration of at least 3 months were eligible for inclusion. DVT was confirmed according to current diagnostic standards. Patients were treated with either rivaroxaban or conventional anticoagulation therapy, starting with a course of parenteral anticoagulants, usually followed by a vitamin K antagonist. The decision to treat a patient with rivaroxaban or conventional anticoagulants was at the attending physician's discretion. The institutional review boards of all participating hospitals approved the study and all patients provided written informed consent.
Patients were followed for at least 12 months or until they died. Major bleeding was defined as overt bleeding associated with a decrease in haemoglobin of 20 g/l or more, or requiring a transfusion of two or more units of blood (or red blood cell concentrates), occurred in a critical site (i.e. intracranial, intraspinal, intraocular, pericardial, intra‐articular, intramuscular with compartment syndrome, retroperitoneal), or contributed to death, based on the on the criteria of the International Society on Thrombosis and Haemostasis (ISTH) (Schulman & Kearon, 2005). Recurrent VTE was defined as new‐onset symptoms of DVT or PE with the final diagnosis confirmed by objective tests according to the current standard or death in which PE could not be ruled out or an autopsy confirmed PE as the primary cause of death. All outcome events were adjudicated by the independent Clinical Events Committee whose members were unaware of the treatment assignment. Only treatment‐emergent events, i.e. events occurring up to 2 days after terminating active anticoagulant treatment, were included in this post‐hoc analysis.
The current study excluded all patients who (i) did not use anticoagulant treatment beyond the first 30 days, (ii) who died or experienced recurrent VTE or major bleeding during the first 30 days and (iii) those who received a vitamin K antagonist for 1–14 days or parenteral anticoagulation for 3–14 days before they were switched to rivaroxaban (‘early switchers’) (Klok et al, 2016).
VTE‐BLEED
The 6‐variable VTE‐BLEED score (Table 1) was calculated for all patients from the baseline variables. Renal insufficiency was defined as an estimated glomerular filtration rate (eGFR) <60 ml/min, and uncontrolled arterial hypertension as a systolic blood pressure ≥140 mmHg. A score of ≥2 points served to identify those patients at a predicted high risk of bleeding (Klok et al, 2016).
Primary aim
The primary aim of the current analysis was to validate VTE‐BLEED in both the overall XALIA study population and in each treatment arm of XALIA separately. We calculated the prognostic indices for VTE‐BLEED for major bleeding occurring after day 30 and after day 90 as proxy for long‐term stable anticoagulation. Also, a subgroup analysis of patients with unprovoked VTE was planned. Unprovoked VTE was defined as the absence of recent surgery (<3 months), recent trauma/fracture (<3 months), pregnancy or post‐partum (<3 months), post‐thrombotic syndrome, central venous catheter, recent immobilization and/or use of oral contraceptives or hormone replacement therapy (Ageno et al, 2014).
Secondary aims
The secondary aims of the current analysis included evaluation of the main study outcome in patient subgroups stratified by age, sex and presence of cancer (Turpie et al, 2017). Further, the predictive value of VTE‐BLEED for both recurrent VTE and all‐cause mortality was evaluated after day 30. Also, a sensitivity analysis was performed in a subgroup of patients treated with either 20 mg rivaroxaban once daily or a vitamin K antagonist, excluding patients treated with reduced dosed rivaroxaban or long‐term parenteral anticoagulation.
Statistical analysis
For the presentation of the baseline characteristics, continuous variables are described with means and standard deviation, and categorical variables are presented as proportions (n/N) and percentage (%). The absolute number and incidence proportions of major bleeding, recurrent VTE and all‐cause mortality after day 30 (and day 90) are presented. The at‐risk period for events was defined as the period between Day 31 (or Day 91) and treatment stop plus 2 days. Missing values of the baseline characteristics necessary to calculate VTE‐BLEED were replaced using multivariate imputation by chained equations (full imputation using a single chain with 200 imputations, 500 burn‐in samples and 500 updates between imputations) (Schafer, 1997). Crude ORs for each increase of one point in VTE‐BLEED were calculated for the overall population and the predefined subgroups. A logistic model with VTE‐BLEED and treatment arm as explanatory variables was applied to present adjusted ORs as well. Cox regression analyses were performed to calculate the hazard ratio (HR) of patients categorized as high risk of bleeding by VTE‐BLEED for all outcomes. A Cox regression model using VTE‐BLEED and treatment group as covariates was applied to calculate adjusted HRs. The diagnostic quality of the VTE‐BLEED score was further evaluated using receiver operator characteristic (ROC) curve derived c‐statistics.
Lastly, decision curve analyses were performed in order to estimate the range of threshold probabilities in which VTE‐BLEED may provide a net benefit over standard management considering only major bleeding events (Fitzgerald et al, 2015; Klok et al, 2017). The assumptions included treatment according to VTE‐BLEED works better than two extreme scenarios, considering that ‘all’ or ‘none of’ patients are treated, and assuming that anticoagulation in patients classified in the high‐risk VTE‐BLEED group will be discontinued. The net benefit (y‐axis of the graph) of the decision curve analyses indicates the number of additional true positives, i.e. patients with bleeding who were correctly classified as high risk, identified by VTE‐BLEED (0·01 = 1 additional true positive high‐risk patients out of 100 patients) without additional false positive cases. In the present analysis, we studied the estimated net benefit of VTE‐BLEED with the strategies of ‘assuming all patients at high risk’ or ‘assuming all patients at low risk’ within a range of threshold probabilities. SAS 9.4 software (SAS Institute, Cary, NC, USA) was used for the analysis of data. A two‐sided P < 0·05 was considered to be statistically significant. Due to the retrospective character of the study no formal hypothesis test was performed and all P‐values should be interpreted descriptively.
Results
Study patients
From the 5142 patients included in XALIA, 368 were excluded because they were classified as ‘early switchers’, six because they did not take study medication and 311 because they experienced one of the study outcomes or stopped treatment before day 30 after VTE diagnosis, leaving 4457 patients for the primary analysis. Of these, 804 patients (18%) were treated after day 30 and for at least a part of the observation period, with low (less than 20 mg once daily) doses of rivaroxaban, or received prolonged parenteral treatment with heparin. The remaining 3653 patients (82%), who received the ‘standard’ therapeutic rivaroxaban dose of 20 mg once daily or an International Normalised Ratio‐targeted (2·0–3·0) vitamin K antagonist after day 30, formed the population of the sensitivity analysis. Baseline characteristics of the study population are shown in Table 2. Of all patients in the primary analysis, 63% were classified into the VTE‐BLEED low‐risk group. This was 72% for patients in the rivaroxaban cohort and 53% in the standard of care cohort.
Table 2.
Baseline characteristics of study patients
| Patients for primary analysis (n = 4457) | |
|---|---|
| Age (years), mean (SD) | 60 (17) |
| Female, n (%) | 2065 (46) |
| Length of at‐risk period (days), median (IQR) | 190 (106–360) |
| DVT only, n (%) | 4022 (90) |
| DVT plus PE, n (%) | 435 (9·8) |
| Unprovoked DVT, n (%) | 2860 (64) |
| Previous VTE, n (%) | 1032 (23) |
| Active cancer, n (%) | 500 (11) |
| First available eGFR, n (%) | |
| <30 ml/min | 63 (1·4) |
| 30–50 ml/min | 224 (5·0) |
| ≥50 ml/min | 2569 (58) |
| Missing | 1601 (36) |
| Haemoglobin (g/l) | |
| Mean (SD) | 140 (17) |
| Missing, n (%) | 1731 (39) |
| Systolic blood pressure (mmHg) | |
| Mean (SD) | 137 (19) |
| Missing, n (%) | 2179 (49) |
| Previous major bleeding episode, n (%) | 91 (2·0) |
DVT, deep vein thrombosis; eGFR, estimated glomerular filtration rate; IQR, interquartile range; PE, pulmonary embolism.
Adverse events
Of all 4457 patients available for the primary analysis, 39 patients (0·88%) experienced a major bleeding event after day 30 during a median at‐risk time of 190 days [interquartile range (IQR) 106–360 days]. This percentage was 0·45% in the rivaroxaban‐treated group and 1·4% in the standard of care group. Major bleeding after day 90 was diagnosed in 0·68% of all patients. A total of 55 (1·2%) patients suffered recurrent VTE on anticoagulant treatment and 84 (1·9%) died (Table 3).
Table 3.
Occurrence of adverse events during anticoagulation of 4457 patients available for the primary analysis. Fatal pulmonary embolism included unexplained deaths
| Adverse event | Treatment arm | Incidence, n (%) | |
|---|---|---|---|
| Low risk | High risk | ||
| Major bleeding after day 30 | Rivaroxaban | 6 (0·35) | 5 (0·70) |
| Standard of care | 9 (0·84) | 19 (2·1) | |
| Overall | 15 (0·53) | 24 (1·5) | |
| Major bleeding after day 90 | Rivaroxaban | 2 (0·13) | 3 (0·48) |
| Standard of care | 6 (0·63) | 16 (1·9) | |
| Overall | 8 (0·31) | 19 (1·31) | |
| Recurrent VTE after day 30 | Rivaroxaban | 14 (0·80) | 5 (0·71) |
| Standard of care | 13 (1·3) | 23 (2·5) | |
| Overall | 27 (0·96) | 28 (1·7) | |
| All‐cause mortality after day 30 | Rivaroxaban | 3 (0·19) | 6 (0·81) |
| Standard of care | 6 (0·54) | 69 (7·4) | |
| Overall | 9 (0·32) | 75 (4·6) | |
Primary outcome
The absolute incidence of major bleeding after day 30 was 0·53% [15/2818, 95% confidence interval (CI) 0·32–0·88] in the low‐risk VTE group and 1·5% (24/1629, 95% CI 0·98–2·2) in the high‐risk group for a crude OR of 1·4 (95% CI 1·2–1·7) per increase of the VTE‐BLEED score by one point. This crude OR was 1·5 (95% CI 1·1–2·2) in the rivaroxaban group and 1·3 (95% CI 1·1–1·6) in the standard of care group, respectively (Table 4). The c‐statistic was 0·68 (95% CI 0·59–0·77) in the overall cohort, 0·69 (95% CI 0·55–0·84) in the rivaroxaban group, and 0·64 (95% CI 0·52–0·75) in the standard of care group. The crude HR for major bleeding after day 30 was 2·6 (95% CI 1·3–5·2) and the treatment‐adjusted HR was 2·3 (95% CI 1·1–4·5) comparing the high‐risk versus the low‐risk VTE‐BLEED group.
Table 4.
Primary study outcome (major bleeding after day 30 during anticoagulation of 4457 patients available for the primary analysis)
| Overall | Rivaroxaban | Standard of care | Unprovoked VTE | |
|---|---|---|---|---|
| Number of patients in low risk group (absolute risk) | 2818 (63%) | 1770 (72%) | 1048 (53%) | 1765 (62%) |
| Number of patients in high risk group (absolute risk) | 1629 (37%) | 6977 (28%) | 9322 (47%) | 1091 (38%) |
| Crude OR for 1‐point score increase (95% CI) | 1·4 (1·2–1·7) | 1·5 (1·1–2·2) | 1·3 (1·1–1·6) | 1·3 (1·0–1·8) |
| Adjusted OR for 1‐point score increase (95% CI) | 1·4 (1·1–1·6) | n.a. | n.a. | 1·3 (1·0–1·7) |
| Crude HR ≥2 points (95% CI) | 2·6 (1·3–5·2) | 1·9 (0·56–6·5) | 2·5 (1·0–5·8%) | 1·9 (0·77–4·8) |
| Adjusted HR ≥2 points (95% CI) | 2·3 (1·1–4·5) | n.a. | n.a. | 1·7 (0·69–4·4) |
| PPV (95% CI) | 1·5% (0·98–2·2%) | 0·72% (0·31–1·7%) | 2·1% (1·3–3·3%) | 1·1% (0·63–1·9%) |
| NPV (95% CI) | 99·5% (99–100%) | 99·7% (99–100%) | 99·1% (98–100%) | 99·5% (99–100%) |
| c‐statistic (95% CI) | 0·68 (0·59–0·77) | 0·69 (0·55–0·84) | 0·64 (0·52–0·75) | 0·64 (0·51–0·70) |
95% CI, 95% confidence interval; HR, hazard ratio; n.a., not applicable; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value; VTE, venous thromboembolism.
The overall crude and treatment‐adjusted OR per point increase for major bleeding after day 90 were 1·5 (95% CI 1·2–1·9) and 1·4 (95% CI 1·1–1·7), whereas the crude and treatment‐adjusted HR were 3·8 (95% CI 1·6–9·3) and 3·2 (95% CI 1·3–7·7 comparing the high‐risk vs. the low‐risk VTE‐BLEED group; Table 5), respectively. The c‐statistic for major bleeding after day 90 was 0·71 (95% CI 0·61–0·82).
Table 5.
Secondary study outcome in the 4457 patients available for primary analysis
| Major bleeding after day 90 | Recurrent VTE after day 30 | All‐cause mortality after day 30 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR per point increase (95% CI) | c‐statistic (95% CI) | Crude HR ≥2 points (95% CI) | Adjusted HR ≥2 points (95% CI) | Adjusted OR per point increase (95% CI) | c‐statistic (95% CI) | Crude HR ≥2 points (95% CI) | Adjusted HR ≥2 points (95% CI) | Adjusted OR per point increase (95% CI) | c‐statistic (95% CI) | Crude HR ≥2 points (95% CI) | Adjusted HR ≥2 points (95% CI) | |
| All patients | 1·4 (1·1–1·7) | 0·71 (0·61–0·82) | 3·8 (1·6–9·3) | 3·2 (1·3–7·7) | 1·2 (1·0–1·4) | 0·60 (0·52–0·68) | 1·7 (1·0–3·1) | 1·5 (0·9–2·7) | 2·1 (1·8–2·5) | 0·88 (0·84–0·91) | 14 (6·3–30) | 11 (4·8–23) |
| Patients with unprovoked VTE | 1·5 (1·1–2·0) | 0·71 (0·58–0·84) | 3·2 (1·1–9·5) | 2·9 (1·0–8·7) | 1·2 (1·0–1·5) | 0·60 (0·49–0·70) | 1·7 (0·8–3·5) | 1·5 (0·7–3·2) | 2·1 (1·7–2·5) | 0·86 (0·81–0·92) | 13 (4·4–39) | 10 (3·4–30) |
c‐statistic is based on unadjusted model.
95% CI, 95% confidence interval; HR, hazard ratio; OR, odds ratio; VTE, venous thromboembolism.
The predictive value of VTE‐BLEED in patients with unprovoked VTE was comparable to that of the overall population with a c‐statistic of 0·64 (0·51–0·70; Table 4) for the primary outcome and 0·71 (95% CI 0·58–0·84) for major bleeding after day 90. The adjusted OR per point increase of VTE‐BLEED for major bleeding after day 30 and after day 90 were 1·3 (95% CI 1·0–1·7) and 1·5 (95% CI 1·1–2·0), respectively (Tables 4 and 5).
Secondary outcomes
VTE‐BLEED predicted major bleeding after day 30 was similar in subgroups stratified by age and sex, with wider confidence intervals due to the smaller patient numbers (Table 6). Increases in the score appeared to be less predictive for patients with malignancy, probably because all were (by definition) categorized as ‘high risk’.
Table 6.
Subgroup analyses of primary outcome (major bleeding after day 30) in the 4457 Patients available for primary analysis
| Patients available for primary analysis | ||||||
|---|---|---|---|---|---|---|
| Male patients | Female patients | Cancer | No cancer | Age <60 years | Age ≥60 years | |
| Crude OR for 1‐point score increase (95% CI) | 1·4 (1·1–1·7) | 1·6 (1·2–2·1) | 1·0 (0·61–1·7) | 1·5 (1·2–2·0) | 1·5 (0·85–2·7) | 1·4 (1·1–1·9) |
| Adjusted OR for 1‐point score increase (95% CI) | 1·3 (1·0–1·6) | 1·5 (1·1–2·0) | 1·0 (0·60–1·7) | 1·5 (1·1–1·9) | 1·3 (0·75–2·4) | 1·4 (1·0–1·8) |
| Crude HR ≥2 points (95% CI) | 2·2 (0·89–5·2) | 3·6 (1·2–10) | n.a. | 2·0 (0·91–4·5) | 4·2 (0·89–20) | 2·5 (1·0–6·0) |
| Adjusted HR ≥2 points (95% CI) | 1·8 (0·72–4·4) | 3·3 (1·1–9·8) | n.a. | 1·8 (0·82–4·1) | 3·3 (0·68–15·9) | 1·7 (0·7–4·4) |
| C‐statistic (95% CI) | 0·66 (0·55–0·77) | 0·71 (0·56–0·86) | 0·53 (0·33–0·73) | 0·65 (0·54–0·76) | 0·61 (0·42–0·80) | 0·64 (0·53–0·76) |
95% CI, 95% confidence interval; HR, hazard ratio; n.a., not applicable; OR, odds ratio.
Using the same threshold of ≥2 points as for prediction of major bleeding, patients in the high risk VTE‐BLEED group had a notable significant treatment‐adjusted HR for overall mortality (11; 95% CI 4·8–23; Table 5). Risk estimates for recurrent VTE after day 30 remained non‐significant. As with the primary analysis, these point estimates of the OR and HR for all‐cause mortality and recurrent VTE were comparable for patients with unprovoked VTE.
Sensitivity analysis
Of the patients evaluated in the sensitivity analysis, 68% were categorized in the VTE‐BLEED low‐risk group. This was 73% for patients in the rivaroxaban 20 mg once daily cohort and 60% in the vitamin K antagonist cohort. A total of 25 patients (0·69%) experienced a major bleeding event after day 30 during a median at‐risk time of 192 days (IQR 110–367). This percentage was 0·41% in the rivaroxaban 20 mg once daily group and 1·1% in the vitamin K antagonist group (Table SI). Major bleeding after day 90 occurred in 0·51% of all patients. VTE‐BLEED was associated with comparable risk estimates for the primary and secondary outcomes and subgroup analysis to the primary analysis except for overall mortality (Tables SI, SII and SIII). All analyses were repeated in a dataset restricted to patients with no missing data, with comparable results (data not shown).
Decision curve analysis
The decision curve analysis showed that management decision based on the categorization of patients into two risk classes by VTE‐BLEED, i.e. continue anticoagulation in low‐risk and discontinue in high‐risk patients, was (considering 30‐day major bleeding events only) better than discontinuing the treatment in all patients (assumption that all are at high bleeding risk) or in none (assumption that all are at low bleeding risk) up to a threshold probability of 0·5–1·5% for the risk of major bleeding after Day 30 (Fig 1). For example, following Fig 1, in a population with an expected 0·9% absolute incidence of major bleeding on treatment after Day 30, using (compared with not using) VTE‐BLEED would allow the identification of three additional true major bleedings per 1000 subjects, without increasing the number of false positive predictions and weighing for the relative harm of a false positive and false negative result.
Figure 1.
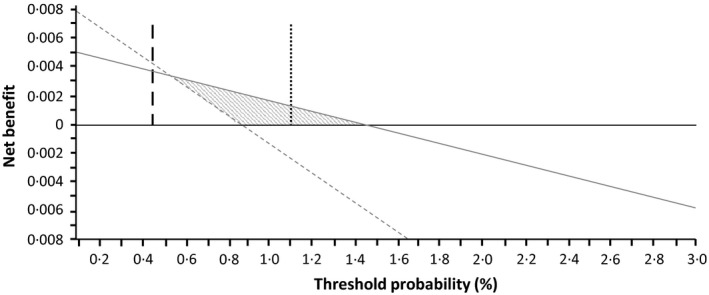
Decision curve analysis. Grey line represents scenario where VTE‐BLEED is used to treat low risk patients but not high risk patients; Horizontal black line represents scenario where all patients are assumed to be of low risk and none are treated with discontinuation of anticoagulants (all receive anticoagulants); dashed grey line represents scenario in which all patients are assumed to be a high risk and all are treated with anticoagulant discontinuation (none receive anticoagulants); vertical long dashed black line represents prevalence of major bleeding in Rivaroxaban‐treated patients in the sensitivity analysis; vertical dotted dashed black line represents prevalence of major bleeding in vitamin K antagonist‐treated patients in the sensitivity analysis. Grey shaded area represents the range of threshold probabilities for which use of VTE‐BLEED is associated with a net clinical benefit over not using the score. Importantly, risks of VTE are not taken into account. VTE, venous thromboembolism.
Discussion
Our main finding is that in practice‐based conditions, VTE‐BLEED can differentiate VTE patients with higher from those with lower risk of major bleeding during long‐term anticoagulation treatment. This conclusion is based on the observed significant ORs for each point increase in the score as well as the relevant HRs after application of the dichotomized VTE‐BLEED threshold of ≥2 (versus <2) points. The prognostic indices were comparable for the sub‐analyses of major bleeding occurring after day 90, between treatment with rivaroxaban and vitamin K antagonists, and both for the overall study population as well as for selected patients with unprovoked VTE, who comprised 64% of the overall study population. Moreover, the c‐statistics for major bleeding after day 90 was >0·70 for patients with unprovoked VTE, for whom accurate prediction of major bleeding on long‐term anticoagulant therapy is most relevant. In general, VTE‐BLEED appears to be useful for a range of threshold probabilities between 0·5% and 1·5% during at‐risk time in XALIA, which roughly translates to a yearly risk of major bleeding between 1·1% and 3·4%, assuming constant risks. This risk is a realistic estimation for treatment with direct oral anticoagulants (DOAC) (lower limit) and vitamin K antagonists (higher limit), emphasizing the potential relevance of VTE‐BLEED for day‐to‐day clinical practice.
We identified two notable differences between the current study and the previous derivation and validation studies (Klok et al, 2016, 2017), which were performed in the setting of multinational randomized controlled trials. First, where ~25% of patients were categorized in the high‐risk group in previous studies, this was considerably higher in the XALIA patients (37%) (Klok et al, 2016, 2017). Of note, XALIA patients treated with rivaroxaban were less often categorized as high risk (28%) than patients treated with conventional anticoagulants (47%; OR 0·44, 95% CI 0·39–0·50). Second, the prognostic indices of VTE‐BLEED in the XALIA study were qualitatively better for the patients treated with a DOAC than for those subjected to conventional treatment, in contrast to what was described earlier (Klok et al, 2016, 2017). The key explanation for both observations is that this was not a randomized trial and patients with cancer in XALIA were more often treated with conventional treatment than with rivaroxaban (19% vs. 6%, P < 0·001), where patients with cancer constituted less than 5% of all patients enrolled in the RE‐COVER trials and HOKUSAI study (Schulman et al, 2009, 2014; Hokusai VTE Investigators 2013). Patients with cancer are, by definition, categorized as VTE‐BLEED high risk and the majority of cases are treated with parenteral anticoagulants. Until now, VTE‐BLEED was never tested for long‐term treatment with low molecular weight heparins. Importantly, the fact that the prognostic indices observed in patients with unprovoked VTE and the sensitivity analysis remained relevant, underlines the validity of our conclusion.
Although the negative predictive value of a VTE‐BLEED score <2·0 points was very high (>99·5% for primary analysis), the positive predictive value (PPV) of VTE‐BLEED in XALIA was low, with values ranging between 0·72% and 2·1% (Table 4). This limited PPV is explained by the relatively low incidence of major bleeding during the study period (after day 30): 0·41% for rivaroxaban and 1·1% for conventional treatment with vitamin K antagonists. Interestingly and despite the fact that XALIA was an observational study with less strict selection criteria than the phase 3 trials with DOACs, these incidences of major bleeding compare well to those observed in the RE‐COVER trials (0·54% and 1·0% per 6 months for dabigatran and warfarin, respectively) and HOKUSAI study (0·82% and 1·0% per 3–12 months for edoxaban and warfarin, respectively) (Schulman et al, 2009, 2014; Hokusai VTE Investigators 2013; Klok et al, 2016, 2017). Even so, considering the 1·1% incidence of major bleeding in the sensitivity analysis on warfarin treatment, the incidences of major bleeding in XALIA do seem representative in the perspective of the established yearly 2·7% incidence‐rate of major bleeding in patients on long‐term vitamin K antagonist treatment (Linkins et al, 2003). The strength of VTE‐BLEED in this ‘real‐world’ cohort of patients thus lies predominantly in the identification of the majority of patients with a very low risk of major bleeding, in whom anticoagulants could be continued safely beyond the first 3 months.
We also found that VTE‐BLEED high‐risk patients had a higher incidence of all‐cause mortality than low‐risk patients (Table 5). This effect can be very likely explained by the clustering of patients with more comorbid conditions in the high‐risk group, such as cancer and renal insufficiency. This higher incidence of mortality could not be explained by higher incidences of fatal bleeding, because this complication occurred only rarely: no fatal bleeding events occurred in the rivaroxaban‐treated patients versus two in patients of the standard anticoagulation group. Interestingly, VTE‐BLEED high‐risk patients were not at a higher risk of recurrent VTE. Nonetheless, the hazards for VTE recurrence on treatment in patients with high and low VTE‐BLEED score (HR 1·5; 95% CI 0·72–3·2 for patients with unprovoked VTE) in this study (Table 5) indicate that one cannot exclude the lack of any association with recurrent VTE off treatment. The present decision curve analysis can therefore not fully consider the spectrum of risks associated with anticoagulant discontinuation.
How are our findings relevant for clinical practice? First of all, this analysis supports the predictive value of VTE‐BLEED reported in the derivation and validation studies (Klok et al, 2016, 2017). It also demonstrates the good performance of the score in patients treated with rivaroxaban and, importantly, supports the generalizability of VTE‐BLEED beyond clinical trial conditions. Current evidence therefore points towards the conclusion that therapeutic‐dose anticoagulant treatment is safe in patients with unprovoked VTE categorized as ‘low risk’ by VTE‐BLEED. On the other hand, optimal long‐term management of VTE‐BLEED high‐risk patients, i.e. to continue anticoagulant treatment or not, needs to be determined in future randomized controlled trials.
The strongpoint of this analysis is the unique design of XALIA, being a large non‐interventional study with a comparator arm, involving extensive baseline as well as follow‐up data, and most importantly, subjecting all events to blinded adjudication. The limitations of our study include that some data required to calculate VTE‐BLEED was missing, which was solved by performing imputation techniques. Further, the prevalence of acute PE among the study patients was low, and no patients with isolated acute PE were enrolled. Although the authors have no reason to consider this a major bias, i.e. no differences were found in the predictive value of VTE‐BLEED in PE versus DVT patients in previous studies (Klok et al, 2016, 2017), it remains to be proven that our current findings could be translated to patient populations involving PE patients. Lastly, even though we were able to study over 4500 patients, this was a post‐hoc analysis and subgroup analyses were performed in considerably smaller patient numbers. This resulted in wider 95% confidence intervals that, on some occasions, crossed the line ‘of no difference’, although point estimates of the OR and HR remained in the same order of magnitude for all sub‐analyses across all predefined study groups.
In conclusion, the current analysis confirms the accuracy of VTE‐BLEED in high‐quality practice‐based data in patients treated with rivaroxaban or warfarin. These data support the hypothesis that VTE‐BLEED may be useful for making management decisions on the duration of anticoagulant therapy, although our findings should be interpreted with caution due to the design of the study. Where long‐term anticoagulant treatment seems to be safe and appropriate in patients with unprovoked VTE categorized as ‘low risk’ by VTE‐BLEED, involving 70–75% of all patients, further outcome studies should determine the optimal long‐term therapeutic management of VTE‐BLEED high risk patients.
Authorship statement
All authors have contributed significantly to this manuscript and take responsibility for the analyses.
Disclosures
Frederikus Klok reports research grants from Bayer, Bristol‐Myers Squibb, Boehringer‐Ingelheim, Daiichi‐Sankyo, MSD and Actelion. Stefano Barco has received congress and travel payments from Daiichi‐Sankyo and financial support for the printing costs of his PhD thesis from Pfizer BV, CSL Behring bv, Sanquin Plasma Products, Boehringer Ingelheim bv, Aspen Netherlands and Bayer bv. Alexander GG Turpie has received speaker's honoraria and consultancy fees from, and participated in scientific advisory boards for, Bayer HealthCare Pharmaceuticals and Janssen Research & Development, LLC. Sylvia Haas has received consultancy fees from Aspen Pharmacare, Bayer HealthCare Pharmaceuticals, Bristol‐Myers Squibb, Daiichi Sankyo, Pfi zer Inc, and Sanofi SA. Reinhold Kreutz has received consultancy fees from Bayer HealthCare Pharmaceuticals, Berlin‐Chemie Menarini, Daiichi Sankyo, Lundbeck Ltd, and Servier Laboratories Ltd, and speaker's honoraria from Bayer HealthCare Pharmaceuticals, Bristol‐Myers Squibb, and Daiichi Sankyo. Lorenzo G Mantovani has received consultancy fees from Bayer HealthCare Pharmaceuticals and Daiichi Sankyo, and research support from Boehringer Ingelheim, Janssen‐Cilag Ltd, and Pfizer Inc. Martin Gebel and Joerg‐Peter Bugge are salaried employees at Bayer. Mathias Herpers is a salaried employee of a company contracted by Bayer. Stavros Konstantinides reports having received consultancy and lecture honoraria from Bayer HealthCare, Boehringer Ingelheim, Daiichi‐Sankyo, and Pfizer – Bristol‐Myers Squibb; payment for travel accommodation/meeting expenses from Bayer HealthCare; and institutional grants from Boehringer Ingelheim, Bayer HealthCare, and Daiichi Sankyo. Walter Ageno has received speaker's honoraria from, and participated in scientific advisory boards for, Boehringer Ingelheim, Bayer, Bristol‐Myers Squibb/Pfizer,Daiichi Sankyo, Aspen, Stago, CSL Behring, Sanofi, Portola and has received research support from Bayer. Martin Gebel and Joerg‐Peter Bugge are salaried employees at Bayer. Mathias Herpers was assigned by Bayer to conduct the analyses.
Supporting information
Table SI. Occurrence of adverse events during anticoagulation in the sensitivity analysis.
Table SII. Primary study outcome (major bleeding after day 30 during anticoagulation) in the sensitivity analysis.
Table SIII. Secondary study outcomes in the sensitivity analysis.
Acknowledgements
The work of Frederikus Klok, Stefano Barco and Stavros Konstantinides was supported by the German Federal Ministry of Education and Research (BMBF 01EO1003 and 01EO1503). All authors are responsible for the contents of this publication.
References
- Ageno, W. & Turpie, A.G. (2016) Spotlight on real‐world evidence for the treatment of DVT: XALIA. Thrombosis and Haemostasis, 116, S41–S49. [DOI] [PubMed] [Google Scholar]
- Ageno, W. , Mantovani, L.G. , Haas, S. , Kreutz, R. , Haupt, V. , Schneider, J. & Turpie, A.G. (2014) XALIA: rationale and design of a non‐interventional study of rivaroxaban compared with standard therapy for initial and long‐term anticoagulation in deep vein thrombosis. Thrombosis Journal, 12, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ageno, W. , Mantovani, L.G. , Haas, S. , Kreutz, R. , Monje, D. , Schneider, J. , van Eickels, M. , Gebel, M. , Zell, E. & Turpie, A.G. (2016) Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep‐vein thrombosis (XALIA): an international, prospective, non‐interventional study. The Lancet Haematology, 3, e12–e21. [DOI] [PubMed] [Google Scholar]
- Fitzgerald, M. , Saville, B.R. & Lewis, R.J. (2015) Decision curve analysis. JAMA, 313, 409–410. [DOI] [PubMed] [Google Scholar]
- Hokusai VTE Investigators . (2013) Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. New England Journal of Medicine, 369, 1406–1415. [DOI] [PubMed] [Google Scholar]
- Kearon, C. , Akl, E.A. , Ornelas, J. , Blaivas, A. , Jimenez, D. , Bounameaux, H. , Huisman, M. , King, C.S. , Morris, T.A. , Sood, N. , Stevens, S.M. , Vintch, J.R.E. , Wells, P. , Woller, S.C. & Moores, L. (2016) Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest, 149, 315–352. [DOI] [PubMed] [Google Scholar]
- Klok, F.A. , Kooiman, J. , Huisman, M.V. , Konstantinides, S. & Lankeit, M. (2015) Predicting anticoagulant‐related bleeding in patients with venous thromboembolism: a clinically oriented review. European Respiratory Journal, 45, 201–210. [DOI] [PubMed] [Google Scholar]
- Klok, F.A. , Hosel, V. , Clemens, A. , Yollo, W.D. , Tilke, C. , Schulman, S. , Lankeit, M. & Konstantinides, S.V. (2016) Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. European Respiratory Journal, 48, 1369–1376. [DOI] [PubMed] [Google Scholar]
- Klok, F.A. , Barco, S. & Konstantinides, S.V. (2017) External validation of the VTE‐BLEED score for predicting major bleeding in stable anticoagulated patients with venous thromboembolism. Thrombosis and Haemostasis, 117, 1164–1170. [DOI] [PubMed] [Google Scholar]
- Klok, F.A. , Barco, S. & Konstantinides, S.V. (2018) Evaluation of VTE‐BLEED for predicting intracranial or fatal bleedings in stable anticoagulated patients with venous thromboembolism. European Respiratory Journal, 51, 1800077. [DOI] [PubMed] [Google Scholar]
- Konstantinides, S.V. , Torbicki, A. , Agnelli, G. , Danchin, N. , Fitzmaurice, D. , Galie, N. , Gibbs, J.S. , Huisman, M.V. , Humbert, M. , Kucher, N. , Lang, I. , Lankeit, M. , Lekakis, J. , Maack, C. , Mayer, E. , Meneveau, N. , Perrier, A. , Pruszczyk, P. , Rasmussen, L.H. , Schindler, T.H. , Svitil, P. , Vonk Noordegraaf, A. , Zamorano, J.L. , Zompatori, M. & Task Force for the, Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cariology . (2014) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. European Heart Journal, 35, 3033–3069, 3069a–3069k. [DOI] [PubMed] [Google Scholar]
- Linkins, L.A. , Choi, P.T. & Douketis, J.D. (2003) Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta‐analysis. Annals of Internal Medicine, 139, 893–900. [DOI] [PubMed] [Google Scholar]
- Schafer, J.L. (1997) Analysis of Incomplete Multivariate Data. Chapman & Hall, New York. [Google Scholar]
- Schulman, S. , Kearon, C. & on behalf of the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . (2005) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. Journal of Thrombosis and Haemostasis, 3, 692–694. [DOI] [PubMed] [Google Scholar]
- Schulman, S. , Kearon, C. , Kakkar, A.K. , Mismetti, P. , Schellong, S. , Eriksson, H. , Baanstra, D. , Schnee, J. & Goldhaber, S.Z. (2009) Dabigatran versus warfarin in the treatment of acute venous thromboembolism. New England Journal of Medicine, 361, 2342–2352. [DOI] [PubMed] [Google Scholar]
- Schulman, S. , Kakkar, A.K. , Goldhaber, S.Z. , Schellong, S. , Eriksson, H. , Mismetti, P. , Christiansen, A.V. , Friedman, J. , Le Maulf, F. , Peter, N. & Kearon, C. (2014) Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation, 129, 764–772. [DOI] [PubMed] [Google Scholar]
- Turpie, A. , Gebel, M. , van Eickels, M. , Schneider, J. , Monje, D. , Kreutz, R. , Haas, S. , Mantovani, L. & Ageno, W. (2017) Subgroup analysis of patients with cancer in XALIA: a Noninterventional Study of Rivaroxaban versus Standard Anticoagulation for VTE. TH Open, 01, e33–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Occurrence of adverse events during anticoagulation in the sensitivity analysis.
Table SII. Primary study outcome (major bleeding after day 30 during anticoagulation) in the sensitivity analysis.
Table SIII. Secondary study outcomes in the sensitivity analysis.


