Abstract
In this paper, we review the history of the concept of neuroplasticity as it relates to the understanding of neuropsychiatric disorders, using schizophrenia as a case in point. We briefly review the myriad meanings of the term neuroplasticity, and its neuroscientific basis. We then review the evidence for aberrant neuroplasticity and metaplasticity associated with schizophrenia as well as the risk for developing this illness, and discuss the implications of such understanding for prevention and therapeutic interventions. We argue that the failure and/or altered timing of plasticity of critical brain circuits might underlie cognitive and deficit symptoms, and may also lead to aberrant plastic reorganization in other circuits, leading to affective dysregulation and eventually psychosis. This “dysplastic” model of schizophrenia can suggest testable, etiology and treatment-relevant questions for the future.
Since the seminal special issue of Development and Psychopathology in 1994 (Cicchetti & Tucker, 1994), major advances have taken place in research into brain plasticity and critical periods of development as they inform neuropsychiatric disorders. In this paper, we review the current concept of neuroplasticity as well as the expanding evidence of its aberrations in major psychiatric disorders, with a special focus on schizophrenia and the evolution of risk for this illness. We also examine the potential translational applications of our understanding of neuroplasticity, and how we may harness this in the service of treatment, and prevention of serious mental disorders.
1. Historical overview and definitions
The great neurologists of the 19th century, including Ramon Cajal, the father of modern neuroscience, thought that once developed, the adult brain is unlikely to change with experience (Ramon y Cajal, 1894). Cajal, however, later suggested that memories might be formed by strengthening the connections between existing neurons (Stahnisch & Nitsch, 2002). Hughlings Jackson, the father of modern neurology, proposed the hierarchical nature of how the nervous system is organized, and made the distinction between negative symptoms which result from loss of nervous function, and positive symptoms which may represent a failed attempt to compensate for such functional loss by disinhibited activity of lower brain regions (Berrios, 2001). William James, the noted American psychologist, who was inspired by Jackson’s work, was among the first to suggest that the brain is not as immutable as previously thought. In his book The Principles of Psychology, James wrote, "Organic matter, especially nervous tissue, seems endowed with a very extraordinary degree of plasticity." (James, 1890). This view was ignored for several decades. In 1949, Donald Hebb (Hebb, 1949) proposed an idea later referred to as "hebbian learning"; i.e., when two neurons repeatedly or persistently fire together, some change takes place in one or both cells such that the efficiency of neuronal activity is increased. This adage “neurons that fire together wire together” became the corner-stone of the concept of neuroplasticity, which refers to how the brain changes--organizes and reorganizes--in response to experience. While brain shows plasticity throughout an individual’s lifetime, its capacity for change may be higher at certain times than others; this led to the concept of Critical Periods.
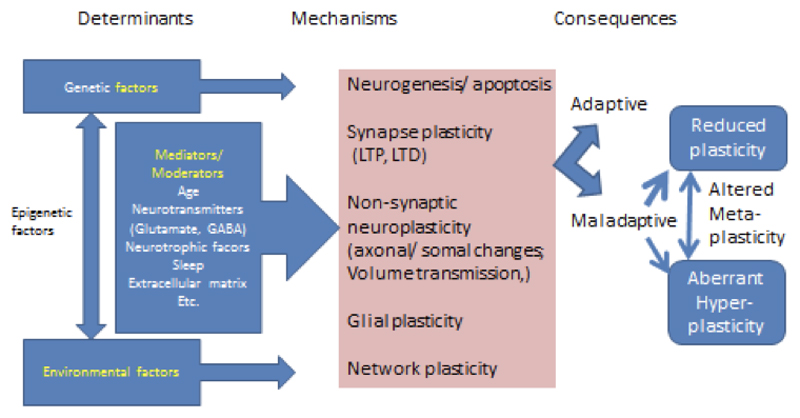
Brain plasticity has been defined in a number of different ways (Figure 1). First, plasticity can encompass both synaptic plasticity and non-synaptic plasticity. Synaptic plasticity is the ability of a synapse between two neurons to change in strength over time, perhaps due to modifications in synaptic potentials or receptors that transmit chemical signals. Modification of synaptic strength is mediated by Long Term Potentiation (LTP), a phenomenon whereby repeated signal transmission between two neurons leads to long-lasting enhancement (Lomo, 2003). By contrast, nonsynaptic plasticity is a modification of the intrinsic excitability of the neuron, mediated through changes in structures such as the soma, the axon, or the dendrites. This may happen through neuronal transmission that happens outside of synapses (e.g. by extracellular diffusion processes), using processes such as volume transmission (Vizi, 1979) or via glial and vascular changes (Markham & Greenough, 2004).
Figure 1.
Determinants, mechanisms and consequences of brain plasticity
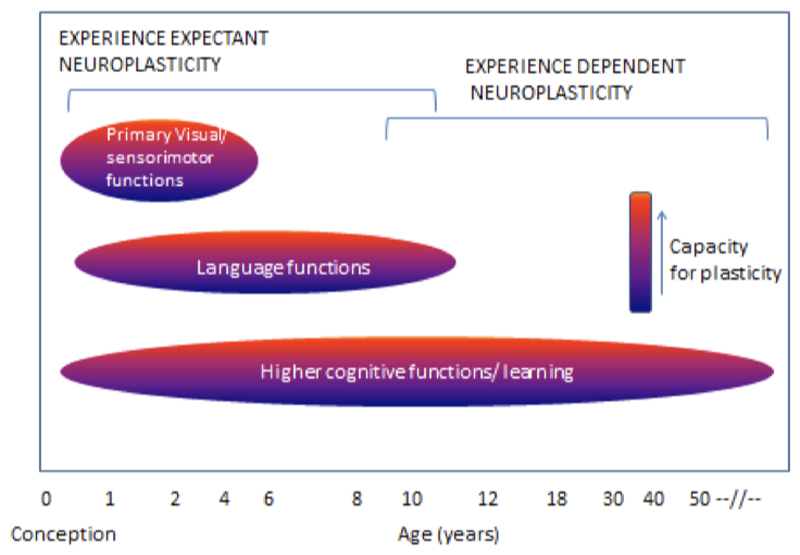
Neuroplasticity may occur in at least two (not mutually exclusive) developmental contexts (Figure 2). Very early in development, experience and its resulting neuronal activity can shape neuronal response properties irrespective of an organism’s attention to a stimulus. This process of experience-expectant neuroplasticity (Hubel & Wiesel, 1959) shapes neural representations to reflect statistical regularities in inputs (e.g. from one eye vs. another, and in the environment). Such plasticity is often conceptualized to occur within a finite window, an early “critical period.” Maladaptive experiences or insults to the developing brain during these critical periods can have lasting behavioral consequences. By contrast, experience-dependent neuroplasticity (Klintsova & Greenough, 1999) occurs throughout development. This process involves changes in neuronal activity in relation to experience, leading to lasting neural representations.
Figure 2.
Critical windows of neuroplasticity
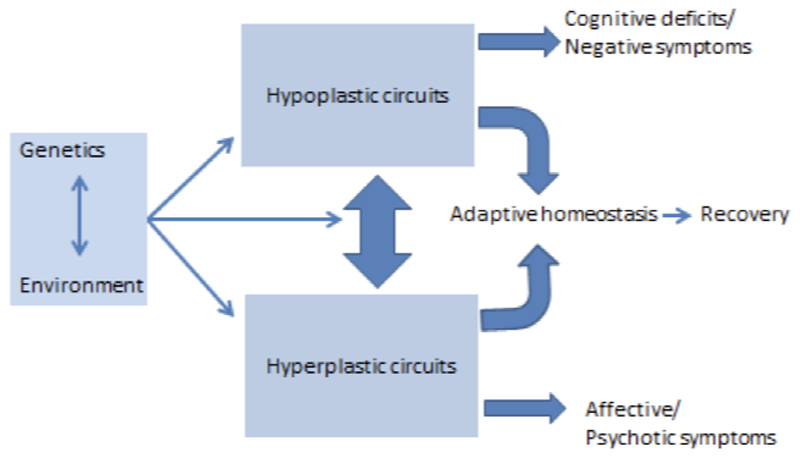
Based on the nature of experience and the state of the organism, the brain can be reshaped in either adaptive or maladaptive ways. Aberrant plasticity can have profound impact on neuronal activity (Papa et al., 2014; Pirttimaki & Parri, 2013) and may be triggered in pathological conditions such as Alzheimer disease and Huntington disease (Oberman & Pascual-Leone, 2013). Maladaptive excessive plasticity has also been implicated in addiction, PTSD and depression (Pittenger, 2013). As we will argue in this paper, the cardinal features of schizophrenia may arise either from diminished plasticity or pathological excessive plasticity. Through the lens of premorbid and prodromal risk states for schizophrenia, we propose – in an extension of Jackson’s model – that failure of plasticity in key brain circuits may result in cognitive and deficit symptoms, while an aberrant hyperplastic response to such deficits might underlie psychosis and emotional dysregulation (Figure 3).
Figure 3.
Schematic model representing possible ways in which plasticity processes may be impaired in schizophrenia
2. Neurobiological processes underlying plasticity
The nervous system is variably plastic throughout the lifespan. In this section, we will review the mechanisms of neuroplasticity as they relate to neurogenesis and apoptosis, synaptic formation and pruning, synaptic modulation, non-synaptic processes, and neuronal support cells.
2.1. Neurogenesis
The earliest stages of nervous system development include generation of neurons and glial cells from stem cell progenitors, neural differentiation, and neuronal migration to other locations. After neuronal migration, growth of axons and dendrites occurs through extension of growth cones at their tips. This process is influenced by cell-cell adhesion molecules and other external molecular signals (Alberts et al., 2002). Next, neurons compete for access to neurotrophic factors, and about half of them die through programmed cell death, also called apoptosis (Alberts et al., 2002). Synapses begin to form between neurons, mediated by release of neurotrophic factors by target tissues. Additionally, neurogenesis continues during adulthood in the dentate gyrus of the hippocampus and the subventricular zone (Benarroch, 2013; Lledo, Alonso & Grubb, 2006).
2.2. Synaptic plasticity and role of glutamatergic neurotransmission
Synaptic plasticity is the capacity of synapses to change their strength in response to changes in their activity. Long-term potentiation (LTP), a key mechanism underlying synaptic plasticity, is the strengthening of the transmission across two neurons with repeated stimulation of a synapse, reflected in changes to the amplitude of the postsynaptic potential (Zhang & Linden, 2003). LTP underlies memory formation and learning and occurs in many brain regions, notably the hippocampus. In early phase LTP, which occurs in the first several hours, large quantities of calcium ions are released and protein kinases are activated (Bliss & Collingridge, 1993). In late phase LTP, gene transcription occurs, and proteins are synthesized over the course of hours to days (Lynch, 2004). The neurotrophic factor BDNF plays an important role in this phase. The molecular mechanisms underlying LTP include activation of N-methyl D-aspartate (NMDA) receptors, which serve as coincidence detectors when two neurons fire simultaneously, allowing flow of ions into the neuron. NMDA antagonists block LTP and learning (Morris et al., 1986).
Long-term depression (LTD) refers to a long-lasting decrease in synaptic strength. Like LTP, it also involves glutamate signaling on NMDA and AMPA receptors (Collingridge et al., 2010). In contrast to LTP, it is induced by long-lasting low frequency stimulation, rather than brief high frequency stimulation (Collingridge et al., 2010). Mechanisms of LTD may include reductions in glutamate release due to both presynaptic and postsynaptic changes, removal of AMPA receptors from the synapse, or changes in the conductance properties of receptors (Collingridge et al., 2010; Malenka, 2003). LTD, like LTP, may also be involved in neuropsychiatric disease. Stress enhances LTD in the hippocampus through activation of NMDA receptors (Kim, Foy & Thompson, 1996), which may help explain stress-related impairments in memory formation. Additionally, LTD has been hypothesized to be involved in synaptic refinement processes during development (Collingridge et al., 2010). Thus, disruptions of LTD may lead to aberrant plasticity during development.
2.3. Non-synaptic plasticity and role of glia
Non-synaptic plasticity includes a wide range of processes affecting the intrinsic excitability of neurons (Daoudal & Debanne, 2003). Mechanisms may include changes to the neuronal soma (cell body), dendrites, axons, and components of the neuronal membrane, such as resting and voltage-gated ion channels (Mozzachiodi et al., 2008). Non-synaptic plasticity may impact serotonin, acetylcholine, metabotropic glutamate, kainite, and NMDA receptors, voltage-gated calcium channels, and cellular signaling (Daoudal & Debanne, 2003; Zhang & Linden, 2003). Volume transmission (Bach-Y-Rita and Mills 1993), another aspect of non-synaptic plasticity, involves both activation of extrasynaptic receptors, and induction of activity by diffusion of molecules from the extracellular fluid into synaptic clefts. The role of non-synaptic plasticity in memory and learning is still unclear.
Glial cells are non-neuronal cells that help maintain and support neurons, providing structural support and insulation, among other functions. While glial cells were generally considered as ‘support cells’, Glia can dynamically respond to environmental input and influence neuronal function by releasing neurotransmitters (gliotransmitters). Astrocytes, a type of glial cell, wrap their membranous projections around synapses and release substances such as neurotransmitters (Paixao & Klein, 2010), and D-serine, which influences LTP and LTD (Henneberger et al., 2010). Glutamate transporters on astrocytes remove excess glutamate from the extracellular space, preventing the excitotoxicity that can result from excessive glutamate stimulation of the synapse (Rothstein, 1996). Genetic or molecular changes that alter glutamate transporters in glia result in impairment of LTP (Filosa et al., 2009) and LTD (Omrani et al., 2009).
2.4. Neurotrophins and other trophic proteins
Neurotrophins are signaling proteins that prompt neurons to grow and differentiate, and thus they are essential to neurodevelopment and neural plasticity. Several major neurotrophins have been studied in depth: brain-derived growth factor (BDNF), nerve growth factor (NGF), neurotrophin 3 (NT-3), and neurotrophin-4 (NT-4). The most investigated neurotrophin, BDNF, influences synaptic regulation and growth (Kleim et al., 2006) and neuronal migration and differentiation (Huang et al., 1999). BDNF is involved in late-phase LTP (Tartaglia et al., 2001) and may work partly by enhancing the response of synapses to tetanic stimulation (Figurov et al., 1996).
2.5. Sleep, electrophysiology, and neuroplasticity
An important mediator of synaptic plasticity is sleep. According to the sleep homeostasis hypothesis, the extensive learning experiences and synaptic strengthening that occur during wakeful states, results in synaptic fatigue at a cellular level, which is restored during sleep (Tononi & Cirelli, 2014). Interestingly, sleep spindles are thought to play a role in synaptic changes and sleep-dependent memory consolidation (Fogel et al., 2012). Spindles are non-rapid eye movement (NREM) sleep EEG rhythms (7-14 Hz). Spindle associated spike discharges have been shown to induce LTP-like synaptic plasticity, thus playing an important role in sleep-dependent memory consolidation (Rosanova & Ulrich, 2005). Moreover, a simultaneous EEG-fMRI study showed that the functional connectivity of the hippocampal formation with the neocortex was the strongest during stage-2 NREM sleep when spindles were present (Andrade et al., 2011).
Electrophysiology has been used to assess LTP in the human cortex in the waking state as well. In normal individuals, repetitive auditory stimulation or visual stimulation has been associated with increases in the amplitude of the auditory and visual evoked potentials, respectively (Clapp et al., 2012; Clapp et al., 2005a), suggesting the induction of LTP. This type of LTP generally lasts more than an hour and can be blocked by NMDA receptors in animal models (Clapp et al., 2012). As will be discussed in detail later, the combination of TMS and EEG is now being used to identify deficits of long-term potentiation in neuropsychiatric disease.
2.6. Network plasticity
The existence of cortical network plasticity is supported by functional neuroimaging studies of cortical remapping during learning. When a specific motor task is practiced repeatedly, the amount of motor cortex activated during performance of that task widens in comparison with performance of different, unpracticed tasks (Karni et al., 1995). However, it is not known whether this cortical remapping of a learned task is permanent or a temporary part of the learning process. A recent expansion-normalization model (Kilgard, 2012) suggests that changes in cortical mapping during learning are transient states that facilitate the learning process. Through a temporary increase in the availability of neurons to engage in a novel task, the optimal neural circuitry for the task can then be recruited and refined (Kilgard, 2012).
Studies of brain injury also demonstrate the brain’s adaptive cortical plasticity. Following a stroke, brain regions adjacent to the injured region are recruited to perform the tasks formerly performed by the injured region (Xerri et al., 1998). Active rehabilitation training may enhance this process of cortical reorganization (Nudo & Milliken, 1996). Over time, this task-related activation decreases and becomes restricted to fewer regions, implying an initial compensatory expansion of activation, followed by cortical re-organization (Ward et al., 2003).
2.7. Genes, environment and plasticity
Genetic variation can influence plasticity processes, including neurogenesis and LTP. For example, mutations in the DISC1 gene, which is associated with schizophrenia, can disrupt hippocampal neurogenesis, leading to creation of neurons with abnormal morphology or premature axonal and dendritic development (Duan et al., 2007; Eisch et al., 2008). Epigenetic mechanisms can also impact neuroplasticity. Epigenetics refers to heritable changes in gene expression that do not involve changes in actual DNA sequences. Three major mechanisms of epigenetic changes include DNA methylation, histone modification (such as acetylation), and non-coding RNAs (Hsieh & Eisch, 2010). Non-coding RNAs have been shown to regulate the proliferation of neural stem cells, either stimulating division of neural progenitor cells or promoting the apoptosis of cells (Iyengar et al., 2014). Deficiency in Gadd45b, a gene that promotes DNA demethylation, has been associated with deficits in neurogenesis and dendritic growth of neurons (Ma et al., 2009). Late-phase LTP is dependent on gene transcription, which in turn depends on epigenetic processes. Thus, deletion of the gene coding for CREB binding protein, which activates transcription through histone acetylation, results in impairments of late-phase LTP in animal models (Alarcon et al., 2004). In a related fashion, histone deacetylase inhibitors can enhance the induction of LTP by promoting gene transcription (Levenson & Sweatt, 2005).
LTP and LTD can also be impaired by various genetic mutations and deletions. For example, deletions in the genes coding for GluN2A subunit of the NMDA receptor (Kannangara et al., 2014) and subunits of CaMKII (Malenka & Nicoll, 1999) result in impairments in LTP. Numerous other genes that have been linked to LTP and LTD, including CREB1 (Bourtchuladze et al., 1994), mTor (mammalian target of rapamycin) (Hoeffer & Klann, 2010), and GSK-3B, which codes for glycogen synthase kinase -3 beta (Bradley et al., 2012),
The Val66Met polymorphism in the BDNF gene results in the substitution of the amino acid valine with methionine, and is associated with changes in cortical morphology and hippocampal activity. The Met allele has been associated with volumetric reductions in the hippocampus and prefrontal cortex (Pezawas et al., 2004; Szeszko et al., 2005), and poorer performance in episodic memory tasks in normal individuals (Egan et al., 2003). This polymorphism may affect plasticity by impairing NMDA-receptor mediated LTP (Ninan et al., 2010).
Environmental factors can substantially impact neurogenesis. Maternal infectious exposure in rats is associated with decreased neurogenesis in the dentate gyrus of the hippocampus (Cui et al., 2009) and diminished cognitive performance in offspring after birth (Jiang et al., 2013). This correlation may be mediated by immune system activation (De Miranda et al., 2010). Prenatal exposure to substances of abuse, such as alcohol, can also lead to dysmorphic brain development (Gil-Mohapel et al., 2010). In addition, rodent models of chronic stress demonstrate decreases in hippocampal progenitor cells (Hsieh & Eisch, 2010; Pham et al., 2003).
While many variables have been observed to inhibit adult neurogenesis, other factors may enhance it. Deep brain stimulation, antidepressants, and exercise have been shown to increase adult neurogenesis in rodent models (Eisch et al., 2008). While promising, most research on this topic has been conducted on animal models. New techniques are being developed for in vivo visualization of neurogenesis in humans, such as the use of metabolic biomarkers to identify neural stem cells using proton magnetic resonance spectroscopy (Manganas et al., 2007). Further technical advances may allow for direct study of neurogenesis in human neuropsychiatric illness.
2.8. Critical periods and timing of onset and closure of neuroplasticity
Environment can shape brain function substantively across the lifespan. Plasticity is at its greatest during key epochs early in development (critical periods) and this presents developmental psychopathologists with new avenues for understanding vulnerability of the brain and intervening in a timely manner (Cicchetti & Toth, 2009). Studies of critical periods in the visual cortex have shown that among other processes, maturation of specific GABA circuits may determine the onset of certain critical periods. Timing and duration of critical periods may be modifiable by pharmacological manipulation of these and similar circuits (Takesian & Hensch, 2013). The onset of critical periods may be triggered by factors such as polysialic acid (PSA) and neural cell adhesion molecule (NCAM), which limit function of parvalbumin containing GABA circuits. Neural networks refined by experience are then actively stabilized by extracellular milieu factors, such as perineural nets (PNNs) which serve as “brakes” for pruning processes (Wang & Fawcett, 2012). An understanding of such factors is likely to shed light on disorders of neuroplasticity such as schizophrenia, and motivate potentially novel treatment targets (Bitanihirwe & Woo, 2014).
2.9. Effects of age: plasticity across the lifespan
The brain maintains some plasticity throughout life, but the capacity to change, which is at its peak early in life, gradually declines with age after young adulthood. The degree, slope and timing of such decline, however, varies between individuals, is determined by both genetic and environmental factors, and may underlie risk for neuropsychiatric disorders (Oberman & Pascual-Leone, 2013).
2.10. Metaplasticity
The concept of metaplasticity refers to the processes that regulate synaptic plasticity; that is, the ability of a synapse to engage in long-term potentiation or long-term depression can itself be modulated in a dynamic fashion (Abraham & Bear, 1996). Metaplasticity involves a priming stimulus which alters the subsequent response of a neuron to a plasticity-inducing stimulus. An important feature of metaplasticity is a time gap between the priming signal that stimulates metaplastic mechanisms, and subsequent events that induce synaptic plasticity. Several mechanisms may enable metaplasticity. NMDA receptor activation, which induces LTP, also inhibits subsequent LTP for some time afterwards (Abraham, 2008). Another mechanism may be metabotropic glutamate receptor activation, which enhances the induction of LTP in the hippocampus (Cohen et al., 1999). Lastly, mechanisms of non-synaptic plasticity (i.e., intrinsic plasticity) may also be categorized as a type of metaplasticity (Abraham, 2008). One possible function of metaplasticity may be to protect against excitotoxic damage to neurons that could occur through unopposed LTP (Abraham, 2008).
2.11. Summary
The brain maintains plasticity throughout life, though in varying degrees at the different epochs of age. This remarkable ability of the brain is orchestrated by the inherent properties of neurons, synapses and glia, by neurotransmitter systems such as glutamate, GABA and neurotrophic factors. As a result, cortical reorganization occurs in response to learning and to injury throughout life. The extent to which the brain can dynamically change with learning and exogenous exposures is determined by genetic, epigenetic and environmental factors; such plastic change could be adaptive, or represent maladaptive cascades secondary to genetic and environmental inputs, as we will see in the next section.
3. Plasticity alterations in schizophrenia
Schizophrenia is a common, chronic complex illness typically beginning in adolescence and characterized by positive symptoms (hallucinations, delusions and disorganized thinking), negative symptoms (social withdrawal, affect flattening and motivational deficits), and impaired cognition across several domains (attention, executive function, memory and social cognition) (Tandon et al 2008). It is widely held that schizophrenia is a developmental brain disorder, involving several processes affecting brain plasticity: early (neurogenesis, neural migration, synaptogenesis) (Murray et al., 1988; Weinberger, 1987), and late in brain development (synaptic pruning, myelination) (Feinberg, 1982; Keshavan, Anderson & Pettegrew, 1994; Murray et al., 1988; Weinberger, 1987). We herein review extant literature on alterations in schizophrenia that bear upon these neuroplasticity processes. There is evidence for both diminished plasticity as well as aberrant excessive plasticity, as our review will show.
3.1. Neurons and synapses
Schizophrenia has been associated with a number of neuropathological abnormalities, which may also reflect deficiencies in plasticity. While neuroimaging studies demonstrate subtle reductions in gray matter volume in schizophrenia, post-mortem studies indicate that this reduction is due to loss of cortical neuropil and dendritic arborization, rather than loss of neurons (Selemon et al., 2003). The most consistent neuropathological findings in schizophrenia are reduced density of dendritic spines (Glantz & Lewis, 2000; Harrison, 1999) and smaller cell bodies of neurons in the dorsolateral prefrontal cortex (Pierri et al., 2001) and the hippocampus (Bennett, 2011). Adolescence, when schizophrenia typically begins, is a period during which the synaptic density is normally pruned by 50% (Anderson et al., 1995; Woo, 2013). Consequently, it has been suggested that dysfunctional or excessive synaptic pruning in the prefrontal cortex during adolescence may serve to diminish plasticity in schizophrenia (Keshavan et al., 1994).
3.2. Altered neurotransmission
NMDA receptors and glutamatergic pathways play a crucial role in modulating synaptic plasticity (Butefisch et al., 2000); they have also been implicated in schizophrenia (Moghaddam & Javitt, 2012; Woo, 2013) based on studies of NMDA antagonists causing psychotic and cognitive symptoms and electrophysiological changes in healthy individuals (Javitt et al., 1996), and diminished cortical NMDA receptor subunit expression in individuals with schizophrenia (Harrison, Law & Eastwood, 2003). NMDA receptor hypofunction may cause glutamatergic excess and damage to pyramidal neurons, which may manifest as loss of dendritic arborization (Woo, 2013), leading to diminished neuroplasticity.
Impairments in GABAergic systems may also disrupt plasticity in schizophrenia. Inhibitory parvalbumin-containing neurons promote the normal maturation of neuronal circuits, and are abnormal in schizophrenia (Woo, 2013). In post-mortem brains of schizophrenia patients, parvalbumin-containing neurons demonstrate diminished expression of GAD-67, an enzyme that helps synthesize the inhibitory neurotransmitter GABA (Akbarian et al., 1995). Thus, in patients with schizophrenia, these neurons may fail to regulate synaptic pruning. GABA activity may be decreased in certain brain regions in schizophrenia (Barr et al., 2013) which may lead to reduced cortical plasticity (Butefisch et al., 2000; Voineskos et al., 2013) and abnormal pruning. Additionally, maturation of the extracellular matrix (ECM), comprising perineuronal nets, may be critical for termination of synaptic pruning processes (Woo, 2014); failure of such maturation may lead to “runaway” pruning.
3.3. Glial alterations
Alterations in microglia, a type of neuronal support cell, may also contribute to impaired plasticity in schizophrenia. Both imaging and post-mortem studies have observed increased activation of microglia in patients with schizophrenia. This has been noted in both the frontal cortex and the hippocampus (Doorduin et al., 2009). In an analysis of publically available gene pathways related to glial cell function from the Psychiatric Genomics Consortium data, the glia-oligodendrocyte pathway was specifically associated with schizophrenia, indicating how oligodendrocyte dysfunction may contribute to the myelination abnormalities seen in schizophrenia (Duncan et al., 2014).
3.4. Alterations in Neurotrophins
Deficits in neurotrophins, particularly BDNF, may underlie diminished plasticity in schizophrenia. Levels of BDNF are lower in schizophrenia than in healthy controls and are associated with severity of both positive (Pillai et al., 2010) and negative symptoms (Chen et al., 2014). As discussed earlier, the Val66Met polymorphism may impair the cellular transport of BDNF. Data on the association of this polymorphism with schizophrenia is inconsistent. One meta-analysis found that homozygosity for the infrequent Met/Met genotype was associated with elevated risk of schizophrenia (Gratacos et al., 2007), though other meta-analyses have not confirmed this finding (Kanazawa et al., 2007; Naoe et al., 2007).
BDNF appears to have an intricate relationship with the dopamine neurotransmitter system. While BDNF mediates expression of D1 and D5 dopamine receptors, removal of dopaminergic neurons in the midbrain is associated with diminished levels of BDNF, suggesting that these neurons influence BDNF gene expression (Favalli et al., 2012). BDNF levels may rise with antipsychotic treatment, though again, evidence is inconsistent (Favalli et al., 2012; Grillo et al., 2007).
3.5. Abnormal sleep spindles and EEG findings
Patients with schizophrenia demonstrate significant reductions in density, number and coherence of sleep spindles. Synchronous oscillations of neural circuits during spindle sleep have been thought to contribute to learning-related synaptic plasticity. Motor procedural learning during sleep, normally seen in healthy individuals, is impaired in schizophrenia, and this deficit is related to spindle reductions. (Wamsley et al., 2012). Spindle reductions appear to be related to cognitive impairments in early course patients with schizophrenia (Keshavan et al., 2011b).
Some EEG abnormalities seen in schizophrenia may reflect impaired plasticity. For example, prepulse inhibition of the startle response refers to a decrease in the amplitude of the startle response that occurs when the startling stimulus is preceded by a weak stimulus. In schizophrenia, the startle response does not decrease as much as it does in healthy controls (Braff, Geyer & Swerdlow, 2001), implying failure of habituation to a stimulus. This diminished habituation may reflect abnormal plasticity, in that the brain is unable to efficiently adapt to environmental change. Mismatch negativity, which represents an evoked response to an unexpected deviant stimulus, is also impaired in schizophrenia, and has been thought to reflect abnormal NMDA mediated short-term plasticity.
3.6. Diminished LTP and LTD-like network plasticity
As reviewed in the earlier section, functional MRI studies have demonstrated evidence of cortical plasticity in humans. This cortical remapping is observed in both, healthy individuals following motor learning tasks, as well as, in subjects with brain injury. It is also believed to underlie important perceptual and motor learning abilities (Reed et al., 2011). The cellular substrate of such cortical map plasticity is hypothesized to be related to the better demonstrated synaptic plasticity (Buonomano & Merzenich, 1998). Reduced neuroplasticity in schizophrenia could lead to deficit states such as cognitive deficits, negative symptoms and functional disability (Green et al., 2004) (Sergi et al., 2007) (Fett et al., 2011). Evidence to support reduced cortical plasticity in schizophrenia comes from novel neuroimaging experiments that incorporate brain stimulation and electroencephalography (EEG).
Transcranial magnetic stimulation (TMS) has been used to study in vivo cortical plasticity in schizophrenia. This method uses focal magnetic fields to penetrate the cranium. The resultant electric currents then depolarize the underlying cortex, thus inducing action potentials in targeted brain regions (Kobayashi & Pascual-Leone, 2003). The output of cortical activation (in this case motor cortex) is measured using electromyographic (EMG) recordings of hand muscle contractions. The most common method has been to compare motor evoked potentials (MEP) and motor thresholds (MT) before and after repetitive brain stimulation – with repetitive TMS (rTMS) or transcranial direct current stimulation (tDCS) which uses direct currents to shift the resting membrane potentials of underlying neurons (Nitsche & Paulus, 2000). These techniques use synaptic plasticity-inducing protocols that result in cortical excitability changes mirroring LTP (high-frequency rTMS and anodal tDCS) or LTD (low-frequency rTMS and cathodal tDCS).
Compared to healthy controls, reduced LTD-like plasticity has been reported in schizophrenia patients by demonstrating lack of expected changes in MEP (reduction in amplitude) and MT (increase) as induced by low-frequency rTMS, delivered to the premotor (Oxley et al., 2004) and motor (Fitzgerald et al., 2004) cortices, as well as by cathodal tDCS delivered to the motor cortex (Hasan et al., 2012b). Interestingly, these deficits were also demonstrated in recordings from the non-stimulated hemisphere (Hasan et al., 2012a), suggesting an association between LTD-like cortical plasticity and inter-hemispheric connectivity.
Possible impairments in LTP-like plasticity have also been demonstrated using similar study designs. Lesser enhancement of MEP was observed after anodal tDCS to the contralateral motor cortex in chronic schizophrenia patients relative to recent-onset patients and healthy controls (Hasan et al., 2011). Frantseva et al used a different strategy to measure LTP-like plasticity by pairing (within 25 milliseconds) median nerve electric stimulation with TMS over the contralateral motor cortex, in what is referred to as paired associative stimulation. Schizophrenia patients showed lesser facilitation of MEPs, when compared to healthy individuals (Frantseva et al., 2008). Interestingly, these patients also demonstrated motor learning deficits and there was a significant association between the measure of LTP and motor skill learning. Use-dependent plasticity is another TMS-measure that may reflect LTP-like plasticity (Classen et al., 1998). Here, the spontaneous direction of TMS-induced thumb movements is first measured. Subjects are then trained with 30-minutes of motoric practice of thumb movements in a direction that is opposite (by 180 degrees) to the actual thumb movements. Post-practice thumb movement direction elicited by TMS is then evaluated. Using this experiment, Daskalakis et al found that schizophrenia patients had significantly attenuated motor reorganization compared to healthy subjects (Daskalakis et al., 2008).
Stimulus-specific plasticity paradigms using event related potentials have also been used to quantify occipital (visual) and temporal (auditory) lobe LTP-like plasticity (Clapp et al., 2005a; Clapp et al., 2005b). Here, repetitive high frequency visual or auditory stimuli are used to produce a lasting facilitation of visual or auditory evoked potentials respectively. Using this paradigm, researchers have demonstrated lesser facilitation of visual (Cavus et al., 2012) and auditory (Mears & Spencer, 2012) evoked potentials in schizophrenia patients as compared to healthy controls.
Overall, these findings not only provide evidence for deficient cortical plasticity that represent both LTD and LTP- like synaptic plasticity, but importantly, also link these deficits to impairments in cognitive functions like learning and memory (Frantseva et al., 2008; Wamsley et al., 2012).
3.7. Genes, environment and impaired plasticity in schizophrenia
Schizophrenia is highly heritable. In recent years, several genetic loci with small to moderate effects have been identified in genome-wide association studies. Interestingly, not only do these genes regulate glutamatergic, GABAergic, and dopaminergic transmission, they also regulate several aspects of brain development and plasticity discussed above (Balu & Coyle, 2011). Alterations in the DISC1 gene, expressed during both prenatal and adult hippocampal neurogenesis (Jun et al., 2012) have demonstrated signs of maladaptive plasticity (mistargeted formation of synapses and reduced dendritic arborization) in mice (Kvajo et al., 2011; Pletnikov et al., 2008). Time-specific transient alterations (e.g., in utero) of DISC1 have shown to adversely affect postnatal maturation of prefrontal dopaminergic and GABAergic neurotransmission (Niwa et al., 2010). The gene NRG1, which codes for the protein neuregulin 1, is involved in adult neurogenesis. Neuregulin 1 has been shown to stimulate proliferation of hippocampus-derived neural progenitor cells (Lai & Feng, 2004), and partial deletions of this gene are associated with stress sensitivity in animal models (Chohan et al., 2014). Thus, genetic alterations in the capacity for neurogenesis may weaken the brain’s response to environmental stress, elevating the risk for development of neuropsychiatric disorders.
One novel line of work has used human induced pluripotent stem cells to examine alterations in neurogenesis in schizophrenia. In this method, fibroblasts are obtained from individuals and re-programmed into pluripotent stem cells. In one such study in patients with schizophrenia, these neurons displayed a significant decrease in the number of neurites and neuronal connectivity (Brennand et al., 2011). Abnormalities in gene expression were also observed, such as decreased expression of the protein PSD95 and increased expression of NRG1. Of the several hundred genes that demonstrated abnormal expression, 13% were reported to be abnormal in schizophrenia in previous publications (Brennand et al., 2011).
Environmental factors may mediate the dendritic spine reductions observed in schizophrenia. Chronic stress and prenatal stress have been correlated with reduced dendritic arborization in animal models (Markham, Mullins & Koenig, 2013), while environmental enrichment and learning are associated with increased dendritic arborization (O'Malley et al., 2000). In summary, plasticity in schizophrenia may be abnormal due to genetically mediated changes in NMDA receptor function, GABA-mediated inhibition, and neurogenesis. These abnormalities eventually lead to observable neuropathological abnormalities in dendritic spine density and complexity. Epigenetic factors may mediate the impact of environmental factors on plasticity processes via non-coding RNAs (Spadaro & Bredy, 2012).
3.8. Aberrant excessive neuroplasticity in schizophrenia?
In contrast to diminished plasticity, aberrant excessive synaptic plasticity in neural networks may underlie positive symptoms of schizophrenia; this may result from dysregulated metaplasticity secondary to either genetically controlled reduced synaptic plasticity in key cortical regions or environmental effects like stress or substance abuse. For instance, Hoffman has suggested that social withdrawal or “deafferentation” may trigger the initial active phase of schizophrenia (Hoffman, 2007) by plastic reorganization by the ‘social brain’ to generate spurious meaning from social cues that may manifest as hallucinations or delusions (Hoffman, 2008). This may reflect metaplastic effects (Abraham, 2008) on social brain regions. Indeed, animal experiments suggest that social isolation enhances the surface trafficking of NMDA receptors in dendritic spines of principal neurons in the amygdala, thus leading to aberrant plasticity and emotion dysregulation (Gan et al., 2014). These findings are also in sync with the observation that sensory deafferentation induces hyperplastic brain changes that may be mediated either by removal of GABA-related cortical inhibition or by LTP-like mechanisms (Ziemann, Hallett & Cohen, 1998).
Support for aberrant excessive plasticity also comes from neuroimaging studies examining the dysconnection hypothesis of schizophrenia (Stephan, Friston & Frith, 2009). Diffusion tensor imaging (DTI) has revealed greater white matter connectivity in schizophrenia patients with auditory hallucinations, in contrast to those without in the arcuate fasciculus – which connects the primary auditory cortex with language areas, and the cingulate bundle – a part of the limbic cortex (Hubl et al., 2004). This aberrant connectivity could underlie the abnormal coactivation of regions that normally process external auditory stimuli, and language-related areas (Dierks et al., 1999). Moreover, patients with both auditory and visual hallucinations show higher white matter connectivity in the pathways connecting the visual areas to the hippocampal formation (Amad et al., 2014) and the amygdala (Ford et al., 2014), when compared to patients with only auditory hallucinations. Similarly, patients with auditory hallucinations show increased resting state functional connectivity between the hippocampal formation and the language regions (Sommer et al., 2012). These findings partially support earlier observations of heightened regional hippocampal blood flow in schizophrenia patients at rest and during a cognitive task (Medoff et al., 2001) and emerging evidence on correlations between hippocampal volumes and psychotic symptoms (Mathew et al., 2014). In another study that combined resting state and task (working memory) based functional imaging, patients with schizophrenia and their relatives demonstrated hyperactivation (reduced task related suppression) and hyperconnectivity of the default mode network (Whitfield-Gabrieli et al., 2009) when compared to healthy subjects. These abnormalities were associated with severity of psychopathology and cognitive deficits (Whitfield-Gabrieli et al., 2009). Finally, structural MRI studies have demonstrated significantly increased cortical thickness in regions related to self-monitoring – the left insular cortex, cingulate gyrus, and dorsal middle frontal gyrus and hippocampal formation in schizophrenia patients with auditory hallucinations than those without (Amad et al., 2014; van Swam et al., 2012). Interestingly, auditory hallucinations have been linked to a failure to activate areas concerned with the monitoring of inner speech (McGuire et al., 1995); impaired corollary discharges – neural signals that coincide with self-generated thoughts/movements (Crapse & Sommer, 2008), may underlie increased cortical activity to self-induced sensory stimuli observed in patients with schizophrenia (Whitford et al., 2011). Intriguingly, it has been speculated that the mirror neuron system (MNS) plays a role in the generation of these corollary discharges (Prather et al., 2008; Tchernichovski & Wallman, 2008). Indeed, MNS activity is reduced in schizophrenia (Kato et al., 2011; Mehta et al., 2013). These deficits were also found to be associated with social cognitive deficits in these patients (Mehta et al., 2012; Mehta et al., 2013).
The 22q11 microdeletion syndrome is the strongest known link between any genetic anomaly and schizophrenia, with as many as 30% developing symptoms of schizophrenia (Pulver et al., 1994). Mouse models of the 22q11 microdeletion syndrome show a dramatic enhancement in short and long term potentiation of synaptic transmission in an age dependent manner in the hippocampus of these mice, as compared to the wild-type mice (Earls et al., 2010). The 22q11 microdeletion may lead to a reduction in the Dgcr8 gene, resulting in an elevated Serca2 expression causing abnormally excessive synaptic plasticity (Earls & Zakharenko, 2013). Another mechanism through which 22q11 microdeletion syndrome manifests is haploinsufficiency of the transcription factor TBX1. This transcription factor interacts with several signaling pathways, including β-catenin– a protein that functions as the “molecular glue” to keep synapses together (Papangeli & Scambler, 2013). Recent evidence from mice experiments has demonstrated that excessive hippocampal beta-catenin can potentially lead to ‘sticky synapses’ that have impaired LTD-like plasticity which result in impaired cognitive flexibility (Mills et al., 2014). This process may yield itself as a mechanistic basis to understand the inflexible nature of persistent delusions in schizophrenia.
As reviewed earlier, mutations in the DISC1 gene are considered risk factors for schizophrenia (Harrison & Weinberger, 2005). DISC1 knockdown models have demonstrated an accelerated hippocampal neurogenesis, as well as an increased dendritic development and synapse formation. These aberrant morphological changes result in an accelerated formation of functional GABAergic and glutamatergic synaptic inputs to new neurons, as well as enhanced excitability of the hippocampal neurons (Duan et al., 2007). DISC1 thus appear to play a critical regulator of the aberrant excessive synaptic plasticity observed in schizophrenia. Yet another animal model of schizophrenia that employs phospholipase C-β1 knockout mice has also demonstrated significantly enhanced adult hippocampal neurogenesis in these mice when compared with the wild-type littermates (Manning et al., 2012).
Together, the above synthesis of evidence for aberrant excessive synaptic plasticity in limbic regions against a background of reduced cortical plasticity provides a framework to understand different symptom dimensions of schizophrenia within the broad purview of the “Dysplastic” model.
3.9. Summary
Several lines of evidence point to diminished neuronal plasticity in widespread brain regions in schizophrenia, including reductions in dendritic and glial density, altered function of glutamatergic, GABAergic and neurotrophic function and in vivo evidence of diminished LTP and LTD-like plasticity. While these changes may account for the core deficit symptoms of schizophrenia, positive symptoms might result from excessive neuroplasticity causing aberrant reorganization in limbic circuits. Genetic, epigenetic and environmental factors, as discussed below, may influence the nature, extent, timing and persistence of such abnormalities.
4. Aberrant plasticity and the schizophrenia risk state
Schizophrenia has been linked to a plethora of genetic as well as socioenvironmental risk factors (Morgan, McKenzie & Fearon, 2008; Shah, Mizrahi & McKenzie, 2011; Sullivan, 2005) which may impact (either directly or indirectly) the kinds of neuroplastic processes described in previous sections. The extant neurodevelopmental hypotheses of schizophrenia (Fatemi & Folsom, 2009; Keshavan, 1999; McGrath et al., 2003; Murray, 1994) suggest that developmental brain changes may occur during the prenatal or in post-natal life extending into adolescence or early adulthood. Such alterations could profoundly alter early brain developmental processes such as neuronal proliferation, migration, apoptosis and synaptogenesis, and/or later processes of synaptic pruning and myelination. These processes are further impacted by hormonal changes, and exogenous insults such as trauma, neglect and substance abuse during childhood or adolescence (Keshavan, 1999; Paus, Keshavan & Giedd, 2008; Piper et al., 2012). As the combination of ‘hits’ individuals encounter increases, their brains are likely to become successively prone to more distressing symptoms and worsening functional impairment (Owen et al., 2011). The role of certain chronic risk factors (such as negative life events, daily hassles or substance misuse) appears to be additive and cumulative (Collip, Myin-Germeys & van Os, 2008). We suggest that it is not just the cumulative adverse exposures per se but repeated exposure that plays a role via altered brain plasticity in promoting liability to brain changes, symptoms and impairment.
4.1. Trajectory of premorbid psychopathology in high risk subjects may be linked to critical periods of vulnerability
If insults to core neurobiological processes contribute to the schizophrenia phenotype many years later, the neurodevelopmental hypothesis holds that such processes are both unfolding and especially susceptible to perturbation during critical periods. These periods may include specific windows of prenatal life, childbirth, childhood, and adolescence at which particular risk exposures have been identified. At the earliest stages of conception, for example, advanced paternal age – presumably through de novo mutations – appears to confer susceptibility (Malaspina, 2001). During the prenatal period, maladaptive infectious exposure may influence maternal immune response and/or fetal physiology in an experience-expectant fashion to contribute to a modest but still significant risk for psychosis (Brown & Derkits, 2010). In childhood and adolescence, experience-dependent factors such as abuse, use of psychotropic substances and bullying also increase risk (Addington et al., 2013; Shah et al., 2012; van Dam et al., 2012).
The concept of combinations of exposures/insults at specific critical periods maps onto observations that the timing and course of development may differ across brain regions or circuits (Lewis & Akil, 1997). For example, synaptic density in the visual cortex reaches adult levels by preschool age (Toga, Thompson & Sowell, 2006) whereas higher order disruption in executive function has repeatedly been localized to the prefrontal cortex, an area that is among the last to complete maturation (Gogtay, 2008). If endogenous or exogenous insults lead to disrupted neural processes that compromise neuroplasticity, then, the phenotypic manifestations may reflect the neural circuits maximally affected by failed, aberrant or excessive plasticity.
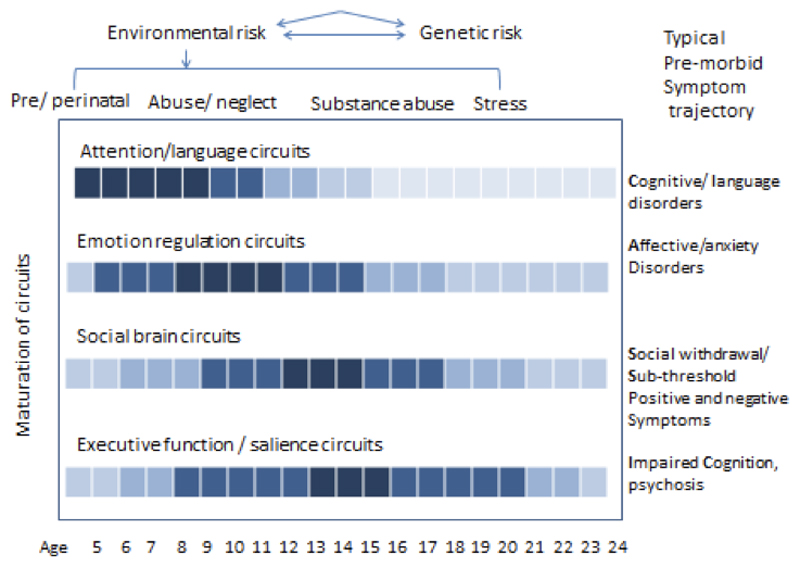
The earliest detectable phases of psychotic illness (i.e. the component signs and symptoms with which it is consistently associated) tend to emerge after, rather than before, the accumulation of critical periods of exposure and brain maturation. This fact has allowed researchers to focus new attention on the trajectory of the premorbid and sub-threshold stages in children and adolescents at high clinical and/or familial risk for schizophrenia. Individuals at familial high risk (FHR) have an 8-12% chance of converting to psychosis over the lifespan; in contrast, in clinical studies, distressed and help-seeking “clinical high-risk” (CHR) subjects have a 15-40% rate of conversion over a 2-3 year period (Fusar-Poli et al., 2013; Tandon, Keshavan & Nasrallah, 2008). However, a broad spectrum of non-psychotic psychopathology is substantively more common in the premorbid phase. In an ongoing prospective study of familial high-risk relatives (Shah et al, in preparation), we have observed that Cognitive/Learning disorders appear to emerge earliest, followed by Anxiety and affective disorders, before Social withdrawal and Sub-threshold psychotic-like symptoms and Impaired Cognition set in (Figure 4). The emergence of such a “CLASSIC” trajectory may reflect the fact that the neural circuits underlying attention and sensorimotor function mature (i.e. show diminishing plasticity) earliest, followed by the maturation of limbic / striatal and eventually higher association brain regions such as the prefrontal cortex. However, as will be discussed further, the sequence, timing and slope of such trajectory may vary greatly between individuals, in relation to genetic and environmental factors.
Figure 4.
Trajectory of phenotypic manifestations among individuals deemed to be at high risk for schizophrenia and the genetic and environmental predisposing factors
4.2. Neuroplasticity may be altered in subjects at risk for psychosis
We herein review the relatively limited evidence suggesting that neurodevelopmental processes involved in schizophrenia etiopathogenesis reflect not just an altered trajectory of otherwise determined brain development, but disruptions to neuroplastic processes themselves.
(i). Grey matter changes
While few neuropathological data exist, progressive gray matter loss seen in high-risk subjects indirectly suggests alterations in brain plasticity. CHR individuals who later developed psychosis had decreased GM volumes in some neuroanatomical structures compared with both, controls and non-converters, as well as GM reductions over time (Borgwardt et al., 2007; Pantelis et al., 2003). However, in young high-risk siblings who did not develop serious psychopathology, GM deficits normalize by late adolescence, suggesting that normal plasticity is associated with resilience (Gogtay et al., 2007; Mattai et al., 2011). Genetic and environmental factors may determine variation in trajectories of high risk individuals (Peper et al., 2007). For example, more severe GM loss over time is seen in familial high-risk subjects exposed to cannabis compared with those without such exposure (Welch et al., 2013). Interaction between cortical thickness and the well-studied COMT val158met polymorphism provides another example of differential susceptibility: while Val/Val homozygosity was related to steeper grey matter loss in adolescence in probands and their siblings, it in fact attenuated cortical thinning in healthy controls (Raznahan et al., 2011).
(ii). Alterations in functional connectivity
fMRI studies in subjects at familial high risk subjects using nonlinear Dynamic Causal Modelling have shown reduced thalamocortical connectivity (Dauvermann et al., 2013). In a recent fMRI study using an emotion recognition paradigm, Gee et al (Gee et al., 2012) showed that relative to controls, CHR subjects showed increased amygdala and decreased vlPFC activation with age. This suggests that a failure of prefrontal cortex to regulate amygdala reactivity emerges during adolescence and young adulthood.
(ii). Altered glutamatergic neurotransmission
Altered glutamatergic function implicated in impaired brain development (Keshavan, 1999) may be related to aberrant neuroplasticity. Neuronal ‘dysconnectivity’, in this model, may be mediated by abnormal NMDA receptor function. Evidence for this model stems from structural and functional neuroimaging, electroencephalography, neurophysiology, neuropharmacology, genetics, network modeling, neuropathology, and post-mortem studies of patients with schizophrenia (Stephan et al., 2009). However, direct evidence of premorbid glutamatergic abnormalities is sparse. Of interest, recent MR spectroscopy reports have found abnormal glutamine/glutamate levels in familial and clinical high-risk subjects (de la Fuente-Sandoval et al., 2011; Fusar-Poli et al., 2011; Stone et al., 2009; Stone et al., 2010; Tandon et al., 2013).
Mismatch negativity (MMN), a promising biomarker in schizophrenia (Javitt et al., 1996), is significantly reduced in clinical high risk subjects compared to healthy control subjects and in those who convert to psychotic disorders (Perez et al., 2014). The MMN effect has been thought to reflect abnormal modulation of NMDA receptor-dependent plasticity (Baldeweg & Hirsch, 2014). Another physiological measure thought to reflect integrity of GABAergic circuits is that of gamma synchrony, known to be abnormal in schizophrenia. Recent evidence suggests abnormalities in gamma band responses to auditory stimuli in clinical high risk subjects (Perez et al., 2014). These observations support the view that impaired NMDA/GABA mediated plasticity may underlie the risk for schizophrenia.
(iii). BDNF
As discussed earlier, neurotrophins such as BDNF appear to be altered in schizophrenia (Buckley, Pillai & Howell, 2011). In familial high-risk groups, a verbal memory task undertaken during functional MR imaging found decreased activation during encoding and retrieval in multiple corticolimbic regions for Val/Val BDNF homozygotes at the rs6265 polymorphism (Baig et al., 2010). Interestingly, this risk allele does not suppress task-related frontal activation per se, since the same risk allele increased activation at the anterior cingulate cortex during a sentence completion task (Whalley et al., 2010). Altogether, these variable findings suggest impaired neuroplasticity in frontal brain functions depending on region and/or task.
4.3. Increased stress reactivity and emergent symptoms may be related to aberrant plasticity
Adolescence denotes a period of increasing physiologic response to stress which, if dysregulated, can alter the long-term set-point of neurobiologic pathways (Walker, Sabuwalla & Huot, 2004). One mechanism invoked to explain the linkage between stress and psychotic symptoms is that of sensitization, the notion that repeated exposures to stress will produce successively larger physiologic responses over time, in some cases becoming aberrant – particularly in those at risk (Collip et al., 2008). First, recurrent administration and withdrawal of amphetamine in peripubertal mice leads to long lasting alterations in neuroplasticity-related genes which then may increase dopamine dependent behaviors (Calabrese et al., 2013). Support for this theory is found in experiments in which dopamine response following amphetamine challenge is associated with psychotic symptoms; in time, exposure to even attenuated stressors can lead to excessive dopamine release (Laruelle & Abi-Dargham, 1999; Lieberman, Sheitman & Kinon, 1997). Second, dynamic changes in the hypothalamic-pituitary-adrenal (HPA) axis are believed to occur in response to internal and external stimuli and demands, whether adaptive or pathologic (Pariante, 2008). The number and significance of stressful life events increases as children enter adolescence (Gunnar & Quevedo, 2007; Gunnar & Talge, 2011); with this comes HPA axis alterations, including higher basal cortisol levels and more robust acute responses to stress (Lupien et al., 2009; Walker et al., 2013). Pituitary volume is elevated in the early phases of the illness (first episode and high-risk subjects who later convert) (Nordholm et al., 2013). Cortisol has also been repeatedly found to be elevated in psychosis (Borges, Gayer-Anderson & Mondelli, 2013) and in CHR subjects, particularly those who will later convert to psychosis (Sugranyes, Thompson & Corcoran, 2012; Walker et al., 2010). Finally, prevailing theories regard dopaminergic hyperactivity as a ‘final common pathway’ by which attenuated and (later) full-blown symptoms emerge in psychosis. It has been postulated that dopaminergic dysregulation in psychosis is sensitized (influenced by stress) through the HPA axis, since mesolimbic DA activity is known to be associated with cortisol release, symptom appearance, and relapse. Positron emission tomography studies link psychosocial stress with DA release abnormalities in healthy individuals (Pruessner et al., 2004; Wand et al., 2007), patients with schizophrenia, CHR and first-degree relatives (Brunelin et al., 2010; Lataster et al., 2013; Mizrahi et al., 2012).
4.4. Social deafferentation may predispose to plastic brain reorganization, leading to psychosis
Observations of social withdrawal long preceding psychotic symptoms, in the premorbid and prodromal phases of schizophrenia is consistent with Hoffman’s model, discussed earlier, which posits that the plastic brain reorganizes neural pathways following isolation to “produce spurious social meaning… in the form of complex, emotionally compelling hallucinations and delusions” (Hoffman, 2008). Social deafferentation suggests a chain of causation, beginning with the effect of environment on neurobiology, followed by the impact of neurobiological changes and plasticity on experience. This concept also identifies a critical period during which both social withdrawal and deafferentation might result in psychotic symptoms. Initial, although only preliminary, evidence for the hypothesis has been obtained in clinical high-risk patients (Hoffman, 2007).
4.5. Summary
The aberrant plasticity model and critical period concepts, as they relate to the premorbid and onset periods prior to psychosis, allow for the suggestion of an evolution of risk states that brings together various hypotheses of reduced plasticity predisposing to aberrant plastic reorganization of neural circuits. First, early brain insults due to prenatal or early life adversity may lead to reduced cortical glutamatergic neurons and impaired experience-dependent neuroplasticity. This fits with the picture of early cognitive and learning deficits, and social withdrawal and deafferentation seen in premorbid studies of adolescents at risk for schizophrenia. In turn, reduced glutamatergic tone would result in decreased synaptic and grey matter density by the time of early adolescence. Combined with increased exposure to stressful situations and decreased cognitive adaptive capacity to them, these alterations would lead to a maladaptive plasticity cascade, i.e. overactivation of the HPA axis (even beyond the normal HPA changes expected during adolescence) and dopaminergic stress responses that underlie affective dysregulation, risk for substance abuse and eventually psychosis. This integrative pathophysiologic model might explain the ‘CLASSIC’ trajectory of phenomena and symptomatology in high-risk populations.
5. Harnessing neuroplasticity for therapeutic (and prophylactic) gains
It is clear that observations of diminished as well as aberrant excessive plasticity motivate novel therapeutic as well as prophylactic therapeutic strategies in schizophrenia and the at-risk states for this illness. We herein provide examples of such emerging approaches.
Cognitive training
Cognitive training or cognitive remediation is an evolving form of intervention that allows us to intentionally harness neuroplastic processes related to learning for therapeutic purposes that target the disabling cognitive deficits of schizophrenia (Keshavan et al., 2014). A recent meta-analysis suggested that cognitive training resulted in modest gains on cognition and socio-occupational functioning with mean effect sizes of 0.45 and 0.42 respectively (Wykes et al., 2011). Besides, the benefits of some of these interventions are likely to last beyond treatment cessation (Eack et al., 2010a; Subramaniam et al., 2012; Wykes et al., 2003).
The underlying plastic changes with cognitive training have been explored by neuroimaging studies. Patients who received cognitive training showed less gray matter loss in the left parahippocampal and fusiform gyrus and greater gray matter increases in the left amygdala after two years of cognitive enhancement therapy, as compared to a non-specific supportive therapy (Eack et al., 2010b). Interestingly, patients with larger cortical thickness at baseline (higher cortical “reserve”) improved faster (Keshavan et al., 2011a). Computer based cognitive training has been shown to normalize the task-based activation of the prefrontal regions during a reality-monitoring task (Subramaniam et al., 2012), emotional-task based neural activations in the postcentral gyrus (Hooker et al., 2012) and attention/executive task based activations of the dorsolateral prefrontal cortex, anterior cingulate and frontopolar cortex (Haut, Lim & MacDonald, 2010). In addition, specific training of auditory discrimination and verbal memory and not a broadly administered cognitive training showed normalization of abnormally reduced sensory gating in schizophrenia patients as measured using magnetoencephalography (Popov et al., 2011). DTI studies provide additional evidence by revealing normalization of the interhemispheric connectivity between the bilateral prefrontal cortexes via the corpus callosum in patients who received cognitive remediation (Penades et al., 2013). While structural and functional cortical plasticity changes have been demonstrated with cognitive training, one study also showed an increase in serum BDNF levels (Vinogradov et al., 2009).
Brain stimulation approaches to improve symptoms by targeting cortical plasticity
Non-invasive brain stimulation strategies have been increasingly used to target specific regions of the brain, guided by existing neurobiological evidence of impaired (either reduced or excessive) activity in specific neural systems (Hasan et al., 2013; Rajji et al., 2013). Two symptom dimensions that have been commonly studied are negative symptoms – where high frequency TMS pulses are applied to activate the left dorsolateral prefrontal cortex, and auditory hallucinations – where low frequency TMS pulses are applied to inhibit the left temporoparietal cortex (Hoffman et al., 1999). In a meta-analysis of studies on high frequency rTMS delivered to the left DLPFC, it was shown that the rTMS improved negative symptoms of schizophrenia with a modest effect size of 0.43 (Dlabac-de Lange, Knegtering & Aleman, 2010). Similar findings were also replicated in a larger, more recent meta-analysis (Shi et al., 2014). This is still an evolving treatment modality, and one of the means to optimize the therapeutic benefit is by targeting different sites like deeper prefrontal cortices (Levkovitz et al., 2011) or even the cerebellar vermis (Demirtas-Tatlidede et al., 2010).
A recent meta-analysis of five randomized, double blind, sham-controlled studies reported that low frequency rTMS delivered to the left temporoparietal cortex improved auditory hallucinations with a modest effect size of 0.44 (Slotema et al., 2012). Furthermore, another study using the same investigation demonstrated a reduction in cerebral blood flow in the primary auditory cortex, left Broca’s area, and cingulate gyrus in patients who received 10-day rTMS sessions for auditory hallucinations (Kindler et al., 2013). Transcranial direct current stimulation (tDCS) has recently been shown to be beneficial in treating medication resistant auditory hallucinations in schizophrenia (Brunelin et al., 2012).
A third application of brain stimulation in schizophrenia that is gaining preliminary empirical support is in treating cognitive deficits (Guse, Falkai & Wobrock, 2010). A single session of 20-Hz rTMS delivered to bilateral DLPFC resulted in a potentiation of the frontal gamma oscillatory activity during a working memory task in healthy individuals (Barr et al., 2009). This sequence of bilateral rTMS administered in schizophrenia patients for 4-weeks, was compared to stimulation using sham rTMS in a randomized controlled trial. It was found that the group receiving true rTMS performed significantly better on a working memory task at the end of the 4-week trial (Barr et al., 2013). However, another study using unilateral (left) 10-Hz rTMS did not find similar benefits (Guse et al., 2013). Application of rTMS for cognitive enhancement is still in its infancy and requires more studies to standardize and optimize the treatment protocols. One way ahead may be to target modulation of mirror neuron regions to enhance social cognitive performance (Meherwan Mehta et al., 2013).
Medications
Antipsychotic medications are the most common form of therapeutic intervention in schizophrenia. Multiple studies have demonstrated that antipsychotic medications induce both anatomical- and molecular-level neuroplastic changes in the brain (Konradi & Heckers, 2001). Studies using rat hippocampal neuronal cultures have revealed adaptive changes in post-synaptic density proteins, dendritic spine morphology, BDNF expression and excitatory post-synaptic potentials (Critchlow et al., 2006; Pandya, Kutiyanawalla & Pillai, 2013; Park et al., 2013; Shim et al., 2012). Interestingly, typical and atypical antipsychotics have a differential regulation of synaptic plasticity by modulating activity of different post-synaptic proteins (Critchlow et al., 2006). At a molecular level, typical antipsychotic medication-induced plasticity changes are largely observed in the striatum and nucleus accumbens, whereas atypical antipsychotic drugs have a subtler and more widespread impact (Konradi & Heckers, 2001).
Such a pattern is partially corroborated by structural neuroimaging studies. Treatment with typical antipsychotic medications is associated with enlargement of the striatum and other structures in the basal ganglia and reduction in frontal, temporal and parietal cortical gray matter volume (Dazzan et al., 2005; Lieberman et al., 2005; Smieskova et al., 2009). Atypical antipsychotic medications are associated with enlargement of thalamus and cortical grey matter volumes (Dazzan et al., 2005; Deng et al., 2009; Scherk & Falkai, 2006), as well as, reduction in other cortical (medial frontal gyrus) volumes (Deng et al., 2009). Intriguingly, potential neuroplastic changes are seen considerably early. A pharmacological-MRI investigation in humans reported striatal volume changes and structural-functional decoupling in motor circuits within hours of administering D2-receptor blockers (Tost et al., 2010). It is however important to note that it is still unclear as to whether long-term cortical volume change is a function of antipsychotic medications or of disease progression (Andreasen et al., 2013).
The therapeutic efficacy of lithium may also be explained based on its ability to modulate synaptic plasticity. Chronic lithium treatment increases dendritic branching in hippocampal neurons (Shim et al., 2013), and also enhances LTP-like plasticity (Shim et al., 2012). Lithium in humans can cause a switch from LTD- to LTP-like plasticity using TMS (Voytovych, Krivanekova & Ziemann, 2012). Lithium’s effects on BDNF (Voytovych et al., 2012; Yasuda et al., 2009) and on the BCL2 family of genes that regulate apoptosis (Beech et al., 2014; Lowthert et al., 2012) may explain these observed effects.
Erythropoietin has important neurotrophic and immunomodulatory functions (Rabie & Marti, 2008), and has shown improvement in cognitive performance in a controlled trial in schizophrenia (Ehrenreich et al., 2007). Overall, these novel treatment strategies provide broader therapeutic avenues for clinicians to harness neuroplasticity in aiding patients with schizophrenia.
Other approaches
Physical exercise (wheel-running) in mice has shown to increase neurogenesis, dendritic proliferation, and long-term potentiation in the dentate gyrus of the hippocampus (van Praag, Kempermann & Gage, 1999). In humans, regular aerobic exercise increases hippocampal and cortical volumes and improves cognitive performance in the elderly (Erickson et al., 2011), as well as in early-middle adulthood (Killgore, Olson & Weber, 2013). Plausible mechanisms include increased blood flow and oxygenation to the hippocampus (Pereira et al., 2007), and greater production of BDNF (Vaynman, Ying & Gomez-Pinilla, 2004). Pajonk and colleagues have extended this work in patients with chronic schizophrenia. They found that 3-months of aerobic exercise, as opposed to control condition (playing table football) not only increased hippocampal volumes, but also resulted in greater N-acetylaspartate to creatine ratio in the hippocampus, and improved short-term memory of these patients (Pajonk et al., 2010).
Other novel therapeutic options that can enhance synaptic plasticity include enriched environment. Providing an enriched environment comprising of novel and complex sensory, cognitive, social and motor stimuli can boost key neural circuits to bring about adaptive behavioral change. This has been demonstrated in rodents, where enriched environments have resulted in neuroplasticity driven molecular, cellular and behavioral changes. These plasticity-harnessing benefits have been demonstrated in rodent models of Alzheimer’s dementia, schizophrenia and autism spectrum disorders (Hannan, 2014; Pang & Hannan, 2013). Integrating specific dimensions of environmental enrichment in rehabilitation programs for schizophrenia patients needs further study.
6. Conclusion
We have outlined evidence that the core manifestations of schizophrenia (positive, negative and cognitive symptoms) may be understood as resulting from aberrant neuroplasticity. As shown in patients with schizophrenia as well as at-risk populations, this “dysplasticity” may involve both hypoplasticity in key brain systems serving cognitive functions and goal-directed behavior, and hyperplasticity in neural systems governing salience detection, emotion processing and regulation as well as default mode systems. It is possible that the hyperplasticity is a maladaptive response in an effort to compensate for a primary impairment in plasticity, though the converse is also possible (Figure 3). Preventive and therapeutic interventions with medications, neuromodulation and psychosocial treatments may be directed both at reversing plasticity deficits as well as harnessing compensatory neuroplasticity in more adaptive channels. The conceptual model we have developed herein has heuristic value, and suggests several testable questions for future research. The increasing understanding of brain mechanisms underlying plasticity may suggest new ways of detecting preclinical disease, better biomarkers to guide treatment selection, and novel therapeutic targets.
A number of essential questions remain to be examined and answered by future generations of basic and clinical research: a) What specific mechanisms of altered plasticity contribute to schizophrenia? Are they primary rather than secondary to other pathophysiological processes, and what gene-environment interactions underlie such mechanisms? b) What underlies the variable trajectories among high-risk individuals, e.g. between those who to convert to psychosis vs. non-converters, and (in non-converters) between those who develop cognitive versus affective, anxiety, or substance misuse disorders? c) Among proband groups, do differences in plasticity capacities predict differential outcome trajectories? d) What are the best ways to noninvasively harness plasticity in the service of prevention and early intervention? e) Are the plasticity alterations unique to specific diagnoses, or they vary dimensionally across a broad spectrum of major psychiatric disorders? And finally, f) Can critical windows of neuroplasticity be reopened in patients who are already on a trajectory to major mental illness or who have an already-evident disorder?
Acknowledgments
This work was supported by NIMH grants MH 60902 and 92440 (MSK).
References
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nature Reviews: Neuroscience. 2008;9(5):387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends in Neurosciences. 1996;19(4):126–30. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Addington J, Stowkowy J, Cadenhead KS, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Cannon TD. Early traumatic experiences in those at clinical high risk for psychosis. Early Intervention in Psychiatry. 2013;7(3):300–5. doi: 10.1111/eip.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Archives of General Psychiatry. 1995;52(4):258–66. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/- mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42(6):947–59. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. New York: Garland Science; 2002. [Google Scholar]
- Amad A, Cachia A, Gorwood P, Pins D, Delmaire C, Rolland B, Mondino M, Thomas P, Jardri R. The multimodal connectivity of the hippocampal complex in auditory and visual hallucinations. Molecular Psychiatry. 2014;19(2):184–91. doi: 10.1038/mp.2012.181. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67(1):7–22. [Google Scholar]
- Andrade KC, Spoormaker VI, Dresler M, Wehrle R, Holsboer F, Samann PG, Czisch M. Sleep spindles and hippocampal functional connectivity in human NREM sleep. Journal of Neuroscience. 2011;31(28):10331–9. doi: 10.1523/JNEUROSCI.5660-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Liu D, Ziebell S, Vora A, Ho BC. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. American Journal of Psychiatry. 2013;170(6):609–15. doi: 10.1176/appi.ajp.2013.12050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig BJ, Whalley HC, Hall J, McIntosh AM, Job DE, Cunningham-Owens DG, Johnstone EC, Lawrie SM. Functional magnetic resonance imaging of BDNF val66met polymorphism in unmedicated subjects at high genetic risk of schizophrenia performing a verbal memory task. Psychiatry Research. 2010;183(3):195–201. doi: 10.1016/j.pscychresns.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Baldeweg T, Hirsch SR. Mismatch negativity indexes illness-specific impairments of cortical plasticity in schizophrenia: A comparison with bipolar disorder and Alzheimer's disease. International Journal of Psychophysiology. 2014 doi: 10.1016/j.ijpsycho.2014.03.008. In press. [DOI] [PubMed] [Google Scholar]
- Balu DT, Coyle JT. Neuroplasticity signaling pathways linked to the pathophysiology of schizophrenia. Neuroscience and Biobehavioral Reviews. 2011;35(3):848–70. doi: 10.1016/j.neubiorev.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Rajji TK, Voineskos AN, Blumberger DM, Arenovich T, Fitzgerald PB, Daskalakis ZJ. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biological Psychiatry. 2013;73(6):510–7. doi: 10.1016/j.biopsych.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Rusjan PM, Chen R, Fitzgerald PB, Daskalakis ZJ. Potentiation of gamma oscillatory activity through repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex. Neuropsychopharmacology. 2009;34(11):2359–67. doi: 10.1038/npp.2009.79. [DOI] [PubMed] [Google Scholar]
- Beech RD, Leffert JJ, Lin A, Sylvia LG, Umlauf S, Mane S, Zhao H, Bowden C, Calabrese JR, Friedman ES, Ketter TA, et al. Gene-expression differences in peripheral blood between lithium responders and non-responders in the Lithium Treatment-Moderate dose Use Study (LiTMUS) Pharmacogenomics Journal. 2014;14(2):182–91. doi: 10.1038/tpj.2013.16. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Adult neurogenesis in the dentate gyrus: general concepts and potential implications. Neurology. 2013;81(16):1443–52. doi: 10.1212/WNL.0b013e3182a9a156. [DOI] [PubMed] [Google Scholar]
- Bennett MR. Schizophrenia: susceptibility genes, dendritic-spine pathology and gray matter loss. Progress in Neurobiology. 2011;95(3):275–300. doi: 10.1016/j.pneurobio.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Berrios GE. The factors of insanities: J. Hughlings Jackson. Classic Text No. 47. History of Psychiatry. 2001;12(47 Pt 3):353–73. doi: 10.1177/0957154x0101204705. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Woo TU. Perineuronal nets and schizophrenia: The importance of neuronal coatings. Neuroscience & Biobehavioral Reviews. 2014;45C:85–99. doi: 10.1016/j.neubiorev.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Borges S, Gayer-Anderson C, Mondelli V. A systematic review of the activity of the hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology. 2013;38(5):603–11. doi: 10.1016/j.psyneuen.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, McGuire PK, Aston J, Berger G, Dazzan P, Gschwandtner U, Pflüger M, D'Souza M, Radue E-W, Riecher-Rössler A. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. British Journal of Psychiatry Supplement. 2007;51:s69–75. doi: 10.1192/bjp.191.51.s69. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79(1):59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Bradley CA, Peineau S, Taghibiglou C, Nicolas CS, Whitcomb DJ, Bortolotto ZA, Kaang BK, Cho K, Wang YT, Collingridge GL. A pivotal role of GSK-3 in synaptic plasticity. Frontiers in Molecular Neuroscience. 2012;5:13. doi: 10.3389/fnmol.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156(2–3):234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473(7346):221–5. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. The American journal of psychiatry. 2010;167(3):261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelin J, d'Amato T, van Os J, Costes N, Suaud Chagny M-F, Saoud M. Increased left striatal dopamine transmission in unaffected siblings of schizophrenia patients in response to acute metabolic stress. Psychiatry Research. 2010;181(2):130–5. doi: 10.1016/j.pscychresns.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, Suaud-Chagny MF, Saoud M, Mechri A, Poulet E. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. American Journal of Psychiatry. 2012;169(7):719–24. doi: 10.1176/appi.ajp.2012.11071091. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Pillai A, Howell KR. Brain-derived neurotrophic factor: findings in schizophrenia. Current Opinion in Psychiatry. 2011;24(2):122–7. doi: 10.1097/YCO.0b013e3283436eb7. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annual Review of Neuroscience. 1998;21:149–86. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(7):3661–5. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese F, Richetto J, Racagni G, Feldon J, Meyer U, Riva MA. Effects of withdrawal from repeated amphetamine exposure in peri-puberty on neuroplasticity-related genes in mice. Neuroscience. 2013;250:222–31. doi: 10.1016/j.neuroscience.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Cavus I, Reinhart RM, Roach BJ, Gueorguieva R, Teyler TJ, Clapp WC, Ford JM, Krystal JH, Mathalon DH. Impaired visual cortical plasticity in schizophrenia. Biological Psychiatry. 2012;71(6):512–20. doi: 10.1016/j.biopsych.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Lee SY, Chang YH, Chen SH, Chu CH, Wang TY, Chen PS, Lee IH, Yang YK, Hong JS, Lu RB. The BDNF Val66Met polymorphism and plasma brain-derived neurotrophic factor levels in Han Chinese patients with bipolar disorder and schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014;51:99–104. doi: 10.1016/j.pnpbp.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan TW, Boucher AA, Spencer JR, Kassem MS, Hamdi AA, Karl T, Fok SY, Bennett MR, Arnold JC. Partial Genetic Deletion of Neuregulin 1 Modulates the Effects of Stress on Sensorimotor Gating, Dendritic Morphology, and HPA Axis Activity in Adolescent Mice. Schizophrenia Bulletin. 2014 doi: 10.1093/schbul/sbt193. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: the coming of age of a discipline. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2009;50(1–2):16–25. doi: 10.1111/j.1469-7610.2008.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Tucker D. Development and self-regulatory structures of the mind. Development and Psychopathology. 1994;6:533–49. [Google Scholar]
- Clapp WC, Hamm JP, Kirk IJ, Teyler TJ. Translating long-term potentiation from animals to humans: a novel method for noninvasive assessment of cortical plasticity. Biological Psychiatry. 2012;71(6):496–502. doi: 10.1016/j.biopsych.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Kirk IJ, Hamm JP, Shepherd D, Teyler TJ. Induction of LTP in the human auditory cortex by sensory stimulation. European Journal of Neuroscience. 2005a;22(5):1135–40. doi: 10.1111/j.1460-9568.2005.04293.x. [DOI] [PubMed] [Google Scholar]
- Clapp WC, Zaehle T, Lutz K, Marcar VL, Kirk IJ, Hamm JP, Teyler TJ, Corballis MC, Jancke L. Effects of long-term potentiation in the human visual cortex: a functional magnetic resonance imaging study. Neuroreport. 2005b;16(18):1977–80. doi: 10.1097/00001756-200512190-00001. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. Journal of Neurophysiology. 1998;79(2):1117–23. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Coussens CM, Raymond CR, Abraham WC. Long-lasting increase in cellular excitability associated with the priming of LTP induction in rat hippocampus. Journal of Neurophysiology. 1999;82(6):3139–48. doi: 10.1152/jn.1999.82.6.3139. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nature Reviews: Neuroscience. 2010;11(7):459–73. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- Collip D, Myin-Germeys I, van Os J. Does the concept of "sensitization" provide a plausible mechanism for the putative link between the environment and schizophrenia? Schizophrenia Bulletin. 2008;34(2):220–5. doi: 10.1093/schbul/sbm163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nature Reviews: Neuroscience. 2008;9(8):587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchlow HM, Maycox PR, Skepper JN, Krylova O. Clozapine and haloperidol differentially regulate dendritic spine formation and synaptogenesis in rat hippocampal neurons. Molecular and Cellular Neurosciences. 2006;32(4):356–65. doi: 10.1016/j.mcn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Cui K, Ashdown H, Luheshi GN, Boksa P. Effects of prenatal immune activation on hippocampal neurogenesis in the rat. Schizophrenia Research. 2009;113(2–3):288–97. doi: 10.1016/j.schres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learning and Memory. 2003;10(6):456–65. doi: 10.1101/lm.64103. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Dysfunctional neural plasticity in patients with schizophrenia. Archives of General Psychiatry. 2008;65(4):378–85. doi: 10.1001/archpsyc.65.4.378. [DOI] [PubMed] [Google Scholar]
- Dauvermann MR, Whalley HC, Romaniuk L, Valton V, Owens DGC, Johnstone EC, Lawrie SM, Moorhead TWJ. The application of nonlinear Dynamic Causal Modelling for fMRI in subjects at high genetic risk of schizophrenia. Neuroimage. 2013;73:16–29. doi: 10.1016/j.neuroimage.2013.01.063. [DOI] [PubMed] [Google Scholar]
- Dazzan P, Morgan KD, Orr K, Hutchinson G, Chitnis X, Suckling J, Fearon P, McGuire PK, Mallett RM, Jones PB, Leff J, et al. Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: the AESOP study. Neuropsychopharmacology. 2005;30(4):765–74. doi: 10.1038/sj.npp.1300603. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, León-Ortiz P, Favila R, Stephano S, Mamo D, Ramírez-Bermúdez J, Graff-Guerrero A. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology. 2011;36(9):1781–91. doi: 10.1038/npp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miranda J, Yaddanapudi K, Hornig M, Villar G, Serge R, Lipkin WI. Induction of Toll-like receptor 3-mediated immunity during gestation inhibits cortical neurogenesis and causes behavioral disturbances. MBio. 2010;1(4):e00176–10. doi: 10.1128/mBio.00176-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Freitas C, Cromer JR, Safar L, Ongur D, Stone WS, Seidman LJ, Schmahmann JD, Pascual-Leone A. Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophrenia Research. 2010;124(1–3):91–100. doi: 10.1016/j.schres.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng MY, McAlonan GM, Cheung C, Chiu CP, Law CW, Cheung V, Sham PC, Chen EY, Chua SE. A naturalistic study of grey matter volume increase after early treatment in anti-psychotic naive, newly diagnosed schizophrenia. Psychopharmacology. 2009;206(3):437–46. doi: 10.1007/s00213-009-1619-z. [DOI] [PubMed] [Google Scholar]
- Dierks T, Linden DE, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W. Activation of Heschl's gyrus during auditory hallucinations. Neuron. 1999;22(3):615–21. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Dlabac-de Lange JJ, Knegtering R, Aleman A. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: review and meta-analysis. Journal of Clinical Psychiatry. 2010;71(4):411–8. doi: 10.4088/JCP.08r04808yel. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. Journal of Nuclear Medicine. 2009;50(11):1801–7. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130(6):1146–58. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Holmans PA, Lee PH, O'Dushlaine CT, Kirby AW, Smoller JW, Ongur D, Cohen BM. Pathway analyses implicate glial cells in schizophrenia. PloS One. 2014;9(2):e89441. doi: 10.1371/journal.pone.0089441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Greenwald DP, Hogarty SS, Keshavan MS. One-year durability of the effects of cognitive enhancement therapy on functional outcome in early schizophrenia. Schizophrenia Research. 2010a;120(1–3):210–6. doi: 10.1016/j.schres.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Cho RY, Prasad KM, Greenwald DP, Hogarty SS, Keshavan MS. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Archives of General Psychiatry. 2010b;67(7):674–82. doi: 10.1001/archgenpsychiatry.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls LR, Bayazitov IT, Fricke RG, Berry RB, Illingworth E, Mittleman G, Zakharenko SS. Dysregulation of presynaptic calcium and synaptic plasticity in a mouse model of 22q11 deletion syndrome. Journal of Neuroscience. 2010;30(47):15843–55. doi: 10.1523/JNEUROSCI.1425-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls LR, Zakharenko SS. A Synaptic Function Approach to Investigating Complex Psychiatric Diseases. Neuroscientist. 2013;20(3):257–71. doi: 10.1177/1073858413498307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hinze-Selch D, Stawicki S, Aust C, Knolle-Veentjer S, Wilms S, Heinz G, Erdag S, Jahn H, Degner D, Ritzen M, et al. Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Molecular Psychiatry. 2007;12(2):206–20. doi: 10.1038/sj.mp.4001907. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? Journal of Neuroscience. 2008;28(46):11785–91. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The Neurodevelopmental Hypothesis of Schizophrenia, Revisited. Schizophrenia Bulletin. 2009;35(3):528–48. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favalli G, Li J, Belmonte-de-Abreu P, Wong AH, Daskalakis ZJ. The role of BDNF in the pathophysiology and treatment of schizophrenia. Journal of Psychiatric Research. 2012;46(1):1–11. doi: 10.1016/j.jpsychires.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? Journal of Psychiatric Research. 1982;17(4):319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35(3):573–88. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381(6584):706–9. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Filosa A, Paixao S, Honsek SD, Carmona MA, Becker L, Feddersen B, Gaitanos L, Rudhard Y, Schoepfer R, Klopstock T, Kullander K, Rose CR, Pasquale EB, Klein R. Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nature Neuroscience. 2009;12(10):1285–92. doi: 10.1038/nn.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Marston NA, Oxley T, De Castella A, Daskalakis ZJ, Kulkarni J. Reduced plastic brain responses in schizophrenia: a transcranial magnetic stimulation study. Schizophrenia Research. 2004;71(1):17–26. doi: 10.1016/j.schres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Fogel S, Martin N, Lafortune M, Barakat M, Debas K, Laventure S, Latreille V, Gagnon JF, Doyon J, Carrier J. NREM Sleep Oscillations and Brain Plasticity in Aging. Frontiers in Neurology. 2012;3:176. doi: 10.3389/fneur.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Palzes VA, Roach BJ, Potkin SG, van Erp TG, Turner JA, Mueller BA, Calhoun VD, Voyvodic J, Belger A, Bustillo J, et al. Visual Hallucinations Are Associated With Hyperconnectivity Between the Amygdala and Visual Cortex in People With a Diagnosis of Schizophrenia. Schizophrenia Bulletin. 2014 doi: 10.1093/schbul/sbu031. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantseva MV, Fitzgerald PB, Chen R, Moller B, Daigle M, Daskalakis ZJ. Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill learning. Cerebral Cortex. 2008;18(5):990–6. doi: 10.1093/cercor/bhm151. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Bechdolf A, Taylor MJ, Bonoldi I, Carpenter WT, Yung AR, McGuire P. At risk for schizophrenic or affective psychoses? A meta-analysis of DSM/ICD diagnostic outcomes in individuals at high clinical risk. Schizophrenia bulletin. 2013;39(4):923–32. doi: 10.1093/schbul/sbs060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Stone JM, Broome MR, Valli I, Mechelli A, McLean MA, Lythgoe DJ, O'Gorman RL, Barker GJ, McGuire PK. Thalamic glutamate levels as a predictor of cortical response during executive functioning in subjects at high risk for psychosis. Archives of General Psychiatry. 2011;68(9):881–90. doi: 10.1001/archgenpsychiatry.2011.46. [DOI] [PubMed] [Google Scholar]
- Gan JO, Bowline E, Lourenco FS, Pickel VM. Adolescent social isolation enhances the plasmalemmal density of NMDA NR1 subunits in dendritic spines of principal neurons in the basolateral amygdala of adult mice. Neuroscience. 2014;258:174–83. doi: 10.1016/j.neuroscience.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Karlsgodt KH, van Erp TGM, Bearden CE, Lieberman MD, Belger A, Perkins DO, Olvet DM, Cornblatt BA, Constable T, Woods SW, et al. Altered age-related trajectories of amygdala-prefrontal circuitry in adolescents at clinical high risk for psychosis: a preliminary study. Schizophrenia Research. 2012;134(1):1–9. doi: 10.1016/j.schres.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Research Reviews. 2010;64(2):283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of General Psychiatry. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Gogtay N. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophrenia Bulletin. 2008;34(1):30–6. doi: 10.1093/schbul/sbm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Greenstein D, Lenane M, Clasen L, Sharp W, Gochman P, Butler P, Evans A, Rapoport J. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Archives of General Psychiatry. 2007;64(7):772–80. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- Gratacos M, Gonzalez JR, Mercader JM, de Cid R, Urretavizcaya M, Estivill X. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biological Psychiatry. 2007;61(7):911–22. doi: 10.1016/j.biopsych.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RS, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biological Psychiatry. 2004;56(5):301–7. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Grillo RW, Ottoni GL, Leke R, Souza DO, Portela LV, Lara DR. Reduced serum BDNF levels in schizophrenic patients on clozapine or typical antipsychotics. Journal of Psychiatric Research. 2007;41(1–2):31–5. doi: 10.1016/j.jpsychires.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The Neurobiology of Stress and Development. Annual Review of Psychology. 2007;58(1):145–73. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM. Neuroendocrine Measures in Developmental Research. In: Schmidt LA, Segalowitz SJ, editors. Cambridge: Cambridge University Press; 2011. pp. 343–64. [Google Scholar]
- Guse B, Falkai P, Gruber O, Whalley H, Gibson L, Hasan A, Obst K, Dechent P, McIntosh A, Suchan B, Wobrock T. The effect of long-term high frequency repetitive transcranial magnetic stimulation on working memory in schizophrenia and healthy controls--a randomized placebo-controlled, double-blind fMRI study. Behavioural Brain Research. 2013;237:300–7. doi: 10.1016/j.bbr.2012.09.034. [DOI] [PubMed] [Google Scholar]
- Guse B, Falkai P, Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. Journal of Neural Transmission. 2010;117(1):105–22. doi: 10.1007/s00702-009-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan AJ. Environmental enrichment and brain repair: harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathology and Applied Neurobiology. 2014;40(1):13–25. doi: 10.1111/nan.12102. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ, Eastwood SL. Glutamate receptors and transporters in the hippocampus in schizophrenia. Annals of the New York Academy of Sciences. 2003;1003:94–101. doi: 10.1196/annals.1300.006. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Molecular Psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Hasan A, Aborowa R, Nitsche MA, Marshall L, Schmitt A, Gruber O, Falkai P, Wobrock T. Abnormal bihemispheric responses in schizophrenia patients following cathodal transcranial direct stimulation. European Archives of Psychiatry and Clinical Neuroscience. 2012a;262(5):415–23. doi: 10.1007/s00406-012-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A, Nitsche MA, Herrmann M, Schneider-Axmann T, Marshall L, Gruber O, Falkai P, Wobrock T. Impaired long-term depression in schizophrenia: a cathodal tDCS pilot study. Brain Stimulation. 2012b;5(4):475–83. doi: 10.1016/j.brs.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Hasan A, Nitsche MA, Rein B, Schneider-Axmann T, Guse B, Gruber O, Falkai P, Wobrock T. Dysfunctional long-term potentiation-like plasticity in schizophrenia revealed by transcranial direct current stimulation. Behavioural Brain Research. 2011;224(1):15–22. doi: 10.1016/j.bbr.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Hasan A, Wobrock T, Rajji T, Malchow B, Daskalakis ZJ. Modulating neural plasticity with non-invasive brain stimulation in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2013;263(8):621–31. doi: 10.1007/s00406-013-0446-8. [DOI] [PubMed] [Google Scholar]
- Haut KM, Lim KO, MacDonald A., 3rd Prefrontal cortical changes following cognitive training in patients with chronic schizophrenia: effects of practice, generalization, and specificity. Neuropsychopharmacology. 2010;35(9):1850–9. doi: 10.1038/npp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. Organization of Behavior: A Neuropsychological Theory. New York: John Wiley; 1949. [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463(7278):232–6. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends in Neurosciences. 2010;33(2):67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RE. A social deafferentation hypothesis for induction of active schizophrenia. Schizophrenia Bulletin. 2007;33(5):1066–70. doi: 10.1093/schbul/sbm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RE. Auditory/Verbal hallucinations, speech perception neurocircuitry, and the social deafferentation hypothesis. Clinical EEG and Neuroscience. 2008;39(2):87–90. doi: 10.1177/155005940803900213. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Boutros NN, Berman RM, Roessler E, Belger A, Krystal JH, Charney DS. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated "voices". Biological Psychiatry. 1999;46(1):130–2. doi: 10.1016/s0006-3223(98)00358-8. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Bruce L, Fisher M, Verosky SC, Miyakawa A, Vinogradov S. Neural activity during emotion recognition after combined cognitive plus social cognitive training in schizophrenia. Schizophrenia Research. 2012;139(1–3):53–9. doi: 10.1016/j.schres.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Eisch AJ. Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: unraveling the genome to understand the mind. Neurobiology of Disease. 2010;39(1):73–84. doi: 10.1016/j.nbd.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98(6):739–55. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat's striate cortex. The Journal of Physiology. 1959;148:574–91. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Archives of General Psychiatry. 2004;61(7):658–68. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Iyengar BR, Choudhary A, Sarangdhar MA, Venkatesh KV, Gadgil CJ, Pillai B. Non-coding RNA interact to regulate neuronal development and function. Frontiers in Cellular Neuroscience. 2014;8:47. doi: 10.3389/fncel.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. Principles of Psychology. Dover Publications; 1890. [Google Scholar]
- Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(21):11962–7. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Zhu T, Zhao W, Shen J, Yu Y, Xu J, Chen X, Yu H. The persistent effects of maternal infection on the offspring's cognitive performance and rates of hippocampal neurogenesis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;44:279–89. doi: 10.1016/j.pnpbp.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Jun H, Mohammed Qasim Hussaini S, Rigby MJ, Jang MH. Functional role of adult hippocampal neurogenesis as a therapeutic strategy for mental disorders. Neural Plasticity. 2012;2012 doi: 10.1155/2012/854285. 854285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T, Glatt SJ, Kia-Keating B, Yoneda H, Tsuang MT. Meta-analysis reveals no association of the Val66Met polymorphism of brain-derived neurotrophic factor with either schizophrenia or bipolar disorder. Psychiatric Genetics. 2007;17(3):165–70. doi: 10.1097/YPG.0b013e32801da2e2. [DOI] [PubMed] [Google Scholar]
- Kannangara TS, Eadie BD, Bostrom CA, Morch K, Brocardo PS, Christie BR. GluN2A-/- Mice Lack Bidirectional Synaptic Plasticity in the Dentate Gyrus and Perform Poorly on Spatial Pattern Separation Tasks. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377(6545):155–8. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Kato Y, Muramatsu T, Kato M, Shibukawa Y, Shintani M, Mimura M. Magnetoencephalography study of right parietal lobe dysfunction of the evoked mirror neuron system in antipsychotic-free schizophrenia. PloS One. 2011;6(11):e28087. doi: 10.1371/journal.pone.0028087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. Journal of Psychiatric Research. 1999;33(6):513–21. doi: 10.1016/s0022-3956(99)00033-3. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. Journal of Psychiatric Research. 1994;28(3):239–65. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Eack SM, Wojtalik JA, Prasad KM, Francis AN, Bhojraj TS, Greenwald DP, Hogarty SS. A broad cortical reserve accelerates response to cognitive enhancement therapy in early course schizophrenia. Schizophrenia Research. 2011a;130(1–3):123–9. doi: 10.1016/j.schres.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Montrose DM, Miewald JM, Jindal RD. Sleep correlates of cognition in early course psychotic disorders. Schizophrenia Research. 2011b;131(1–3):231–4. doi: 10.1016/j.schres.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Vinogradov S, Rumsey J, Sherrill J, Wagner A. Cognitive training in mental disorders: update and future directions. American Journal of Psychiatry. 2014;171(5):510–22. doi: 10.1176/appi.ajp.2013.13081075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends in Neurosciences. 2012;35(12):715–22. doi: 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Olson EA, Weber M. Physical exercise habits correlate with gray matter volume of the hippocampus in healthy adult humans. Scientific Reports. 2013;3:3457. doi: 10.1038/srep03457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(10):4750–3. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler J, Homan P, Jann K, Federspiel A, Flury R, Hauf M, Strik W, Dierks T, Hubl D. Reduced neuronal activity in language-related regions after transcranial magnetic stimulation therapy for auditory verbal hallucinations. Biological Psychiatry. 2013;73(6):518–24. doi: 10.1016/j.biopsych.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nature Neuroscience. 2006;9(6):735–7. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Greenough WT. Synaptic plasticity in cortical systems. Current Opinion in Neurobiology. 1999;9(2):203–8. doi: 10.1016/s0959-4388(99)80028-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurology. 2003;2(3):145–56. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Konradi C, Heckers S. Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biological Psychiatry. 2001;50(10):729–42. doi: 10.1016/s0006-3223(01)01267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Drew LJ, Lepagnol-Bestel AM, Xiao L, Levy RJ, Blazeski R, Arguello PA, Lacefield CO, Mason CA, Simonneau M, et al. Altered axonal targeting and short-term plasticity in the hippocampus of Disc1 mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(49):E1349–58. doi: 10.1073/pnas.1114113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Feng L. Neuregulin induces proliferation of neural progenitor cells via PLC/PKC pathway. Biochemical and Biophysical Research Communications. 2004;319(2):603–11. doi: 10.1016/j.bbrc.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. Journal of Psychopharmacology. 1999;13(4):358–71. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Lataster J, Collip D, Ceccarini J, Hernaus D, Haas D, Booij L, van Os J, Pruessner J, Van Laere K, Myin-Germeys I. Familial Liability to Psychosis Is Associated With Attenuated Dopamine Stress Signaling in Ventromedial Prefrontal Cortex. Schizophrenia Bulletin. 2014;40(1):66–77. doi: 10.1093/schbul/sbs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nature Reviews: Neuroscience. 2005;6(2):108–18. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Rabany L, Harel EV, Zangen A. Deep transcranial magnetic stimulation add-on for treatment of negative symptoms and cognitive deficits of schizophrenia: a feasibility study. International Journal of Neuropsychopharmacology. 2011;14(7):991–6. doi: 10.1017/S1461145711000642. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Akil M. Cortical dopamine in schizophrenia: strategies for postmortem studies. Journal of Psychiatric Research. 1997;31(2):175–95. doi: 10.1016/s0022-3956(96)00057-x. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Sheitman BB, Kinon BJ. Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology. 1997;17(4):205–29. doi: 10.1016/S0893-133X(97)00045-6. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Archives of General Psychiatry. 2005;62(4):361–70. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nature Reviews: Neuroscience. 2006;7(3):179–93. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lomo T. The discovery of long-term potentiation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2003;358(1432):617–20. doi: 10.1098/rstb.2002.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowthert L, Leffert J, Lin A, Umlauf S, Maloney K, Muralidharan A, Lorberg B, Mane S, Zhao H, Sinha R, Bhagwagar Z, et al. Increased ratio of anti-apoptotic to pro-apoptotic Bcl2 gene-family members in lithium-responders one month after treatment initiation. Biology of Mood & Anxiety Disorders. 2012;2(1):15. doi: 10.1186/2045-5380-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews: Neuroscience. 2009;10(6):434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiological Reviews. 2004;84(1):87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323(5917):1074–7. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D. Paternal factors and schizophrenia risk: de novo mutations and imprinting. Schizophrenia Bulletin. 2001;27(3):379–93. doi: 10.1093/oxfordjournals.schbul.a006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Annals of the New York Academy of Sciences. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285(5435):1870–4. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, Maletic-Savatic M. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318(5852):980–5. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning EE, Ransome MI, Burrows EL, Hannan AJ. Increased adult hippocampal neurogenesis and abnormal migration of adult-born granule neurons is associated with hippocampal-specific cognitive deficits in phospholipase C-beta1 knockout mice. Hippocampus. 2012;22(2):309–19. doi: 10.1002/hipo.20900. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biology. 2004;1(4):351–63. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Mullins SE, Koenig JI. Periadolescent maturation of the prefrontal cortex is sex-specific and is disrupted by prenatal stress. Journal of Comparative Neurology. 2013;521(8):1828–43. doi: 10.1002/cne.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew I, Gardin TM, Tandon N, Eack S, Francis AN, Seidman LJ, Clementz B, Pearlson GD, Sweeney JA, Tamminga CA, Keshavan MS. Medial Temporal Lobe Structures and Hippocampal Subfields in Psychotic Disorders: Findings From the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Study. JAMA Psychiatry. 2014;71(7):769–77. doi: 10.1001/jamapsychiatry.2014.453. [DOI] [PubMed] [Google Scholar]
- Mattai AA, Weisinger B, Greenstein D, Stidd R, Clasen L, Miller R, Tossell JW, Rapoport JL, Gogtay N. Normalization of cortical gray matter deficits in nonpsychotic siblings of patients with childhood-onset schizophrenia. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(7):697–704. doi: 10.1016/j.jaac.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JJ, Féron FP, Burne THJ, Mackay-Sim A, Eyles DW. The neurodevelopmental hypothesis of schizophrenia: a review of recent developments. Annals of Medicine. 2003;35(2):86–93. doi: 10.1080/07853890310010005. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Silbersweig DA, Wright I, Murray RM, David AS, Frackowiak RS, Frith CD. Abnormal monitoring of inner speech: a physiological basis for auditory hallucinations. Lancet. 1995;346(8975):596–600. doi: 10.1016/s0140-6736(95)91435-8. [DOI] [PubMed] [Google Scholar]
- Mears RP, Spencer KM. Electrophysiological assessment of auditory stimulus-specific plasticity in schizophrenia. Biological Psychiatry. 2012;71(6):503–11. doi: 10.1016/j.biopsych.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11(5):543–50. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Mehta UM, Agarwal SM, Kalmady SV, Shivakumar V, Kumar CN, Venkatasubramanian G, Thirthalli J, Gangadhar BN, Pascual-Leone A, Keshavan MS. Enhancing putative mirror neuron activity with magnetic stimulation: a single-case functional neuroimaging study. Biological Psychiatry. 2013;74(3):e1–2. doi: 10.1016/j.biopsych.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Mehta UM, Basavaraju R, Thirthalli J, Gangadhar BN. Mirror neuron dysfunction-a neuro-marker for social cognition deficits in drug naive schizophrenia. Schizophrenia Research. 2012;141(2–3):281–3. doi: 10.1016/j.schres.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Mehta UM, Thirthalli J, Basavaraju R, Gangadhar BN, Pascual-Leone A. Reduced Mirror Neuron Activity in Schizophrenia and its Association With Theory of Mind Deficits: Evidence From a Transcranial Magnetic Stimulation Study. Schizophrenia Bulletin. 2013 doi: 10.1093/schbul/sbt155. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills F, Bartlett TE, Dissing-Olesen L, Wisniewska MB, Kuznicki J, Macvicar BA, Wang YT, Bamji SX. Cognitive flexibility and long-term depression (LTD) are impaired following β-catenin stabilization in vivo. Proceedings of the National Academy of Sciences. 2014;111(23):8631–6. doi: 10.1073/pnas.1404670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, Pruessner JC, Remington G, Houle S, Wilson AA. Increased stress-induced dopamine release in psychosis. Biological Psychiatry. 2012;71(6):561–7. doi: 10.1016/j.biopsych.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37(1):4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C, McKenzie K, Fearon P, editors. Society and Psychosis. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319(6056):774–6. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Mozzachiodi R, Lorenzetti FD, Baxter DA, Byrne JH. Changes in neuronal excitability serve as a mechanism of long-term memory for operant conditioning. Nature Neuroscience. 2008;11(10):1146–8. doi: 10.1038/nn.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM. Neurodevelopmental schizophrenia: the rediscovery of dementia praecox. British Journal of Psychiatry Supplement. 1994;(25):6–12. [PubMed] [Google Scholar]
- Murray RM, Lewis SW, Owen MJ, Foerster A. The Neurodevelopmental Origins of Dementia Praecox. London: Oxford: Heinemann Medical Books; 1988. [Google Scholar]
- Naoe Y, Shinkai T, Hori H, Fukunaka Y, Utsunomiya K, Sakata S, Matsumoto C, Shimizu K, Hwang R, Ohmori O, Nakamura J. No association between the brain-derived neurotrophic factor (BDNF) Val66Met polymorphism and schizophrenia in Asian populations: Evidence from a case-control study and meta-analysis. Neuroscience Letters. 2007;415(2):108–12. doi: 10.1016/j.neulet.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS, Chao MV. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. Journal of Neuroscience. 2010;30(26):8866–70. doi: 10.1523/JNEUROSCI.1405-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology. 2000;527(Pt 3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, Lu L, Tomisato S, Jaaro-Peled H, Seshadri S, Hiyama H, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65(4):480–9. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordholm D, Krogh J, Mondelli V, Dazzan P, Pariante C, Nordentoft M. Pituitary gland volume in patients with schizophrenia, subjects at ultra high-risk of developing psychosis and healthy controls: A systematic review and meta-analysis. Psychoneuroendocrinology. 2013:1–11. doi: 10.1016/j.psyneuen.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. Journal of Neurophysiology. 1996;75(5):2144–9. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- O'Malley A, O'Connell C, Murphy KJ, Regan CM. Transient spine density increases in the mid-molecular layer of hippocampal dentate gyrus accompany consolidation of a spatial learning task in the rodent. Neuroscience. 2000;99(2):229–32. doi: 10.1016/s0306-4522(00)00182-2. [DOI] [PubMed] [Google Scholar]
- Oberman L, Pascual-Leone A. Changes in plasticity across the lifespan: cause of disease and target for intervention. Progress in Brain Research. 2013;207:91–120. doi: 10.1016/B978-0-444-63327-9.00016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani A, Melone M, Bellesi M, Safiulina V, Aida T, Tanaka K, Cherubini E, Conti F. Up-regulation of GLT-1 severely impairs LTD at mossy fibre--CA3 synapses. J Physiol. 2009;587(Pt 19):4575–88. doi: 10.1113/jphysiol.2009.177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, O'Donovan MC, Thapar A, Craddock N. Neurodevelopmental hypothesis of schizophrenia. The British Journal of Psychiatry. 2011;198(3):173–5. doi: 10.1192/bjp.bp.110.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley T, Fitzgerald PB, Brown TL, de Castella A, Daskalakis ZJ, Kulkarni J. Repetitive transcranial magnetic stimulation reveals abnormal plastic response to premotor cortex stimulation in schizophrenia. Biological Psychiatry. 2004;56(9):628–33. doi: 10.1016/j.biopsych.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Paixao S, Klein R. Neuron-astrocyte communication and synaptic plasticity. Current Opinion in Neurobiology. 2010;20(4):466–73. doi: 10.1016/j.conb.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Kierer A, Muller S, Oest M, Meyer T, Backens M, et al. Hippocampal plasticity in response to exercise in schizophrenia. Archives of General Psychiatry. 2010;67(2):133–43. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Pandya CD, Kutiyanawalla A, Pillai A. BDNF-TrkB signaling and neuroprotection in schizophrenia. Asian Journal of Psychiatry. 2013;6(1):22–8. doi: 10.1016/j.ajp.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang TY, Hannan AJ. Enhancement of cognitive function in models of brain disease through environmental enrichment and physical activity. Neuropharmacology. 2013;64:515–28. doi: 10.1016/j.neuropharm.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–8. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Papa M, De Luca C, Petta F, Alberghina L, Cirillo G. Astrocyte-neuron interplay in maladaptive plasticity. Neuroscience & Biobehavioral Reviews. 2014;42:35–54. doi: 10.1016/j.neubiorev.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Papangeli I, Scambler P. The 22q11 deletion: DiGeorge and velocardiofacial syndromes and the role of TBX1. Wiley Interdiscip Rev Dev Biol. 2013;2(3):393–403. doi: 10.1002/wdev.75. [DOI] [PubMed] [Google Scholar]
- Pariante CM. Pituitary volume in psychosis: the first review of the evidence. Journal of Psychopharmacology. 2008;22(2 suppl):76–81. doi: 10.1177/0269881107084020. [DOI] [PubMed] [Google Scholar]
- Park SW, Lee CH, Cho HY, Seo MK, Lee JG, Lee BJ, Seol W, Kee BS, Kim YH. Effects of antipsychotic drugs on the expression of synaptic proteins and dendritic outgrowth in hippocampal neuronal cultures. Synapse. 2013;67(5):224–34. doi: 10.1002/syn.21634. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews: Neuroscience. 2008;9(12):947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penades R, Pujol N, Catalan R, Massana G, Rametti G, Garcia-Rizo C, Bargallo N, Gasto C, Bernardo M, Junque C. Brain effects of cognitive remediation therapy in schizophrenia: a structural and functional neuroimaging study. Biological Psychiatry. 2013;73(10):1015–23. doi: 10.1016/j.biopsych.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE. Genetic influences on human brain structure: a review of brain imaging studies in twins. Human Brain Mapping. 2007;28(6):464–73. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5638–43. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VB, Woods SW, Roach BJ, Ford JM, McGlashan TH, Srihari VH, Mathalon DH. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biological Psychiatry. 2014;75(6):459–69. doi: 10.1016/j.biopsych.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. Journal of Neuroscience. 2004;24(45):10099–102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. European Journal of Neuroscience. 2003;17(4):879–86. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Archives of General Psychiatry. 2001;58(5):466–73. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- Pillai A, Kale A, Joshi S, Naphade N, Raju MS, Nasrallah H, Mahadik SP. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. International Journal of Neuropsychopharmacology. 2010;13(4):535–9. doi: 10.1017/S1461145709991015. [DOI] [PubMed] [Google Scholar]
- Piper M, Beneyto M, Burne TH, Eyles DW, Lewis DA, McGrath JJ. The neurodevelopmental hypothesis of schizophrenia: convergent clues from epidemiology and neuropathology. Psychiatric Clinics of North America. 2012;35(3):571–84. doi: 10.1016/j.psc.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Pirttimaki TM, Parri HR. Astrocyte plasticity: implications for synaptic and neuronal activity. Neuroscientist. 2013;19(6):604–15. doi: 10.1177/1073858413504999. [DOI] [PubMed] [Google Scholar]
- Pittenger C. Disorders of memory and plasticity in psychiatric disease. Dialogues in Clinical NeuroSciences. 2013;15(4):455–63. doi: 10.31887/DCNS.2013.15.4/cpittenger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, Mori S, Moran TH, Ross CA. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Molecular Psychiatry. 2008;13(2):173–86. doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- Popov T, Jordanov T, Rockstroh B, Elbert T, Merzenich MM, Miller GA. Specific cognitive training normalizes auditory sensory gating in schizophrenia: a randomized trial. Biological Psychiatry. 2011;69(5):465–71. doi: 10.1016/j.biopsych.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature. 2008;451(7176):305–10. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. The Journal of Neuroscience. 2004;24(11):2825–31. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, Morrow B, Karayiorgou M, Antonarakis SE, Housman D, et al. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. Journal of Nervous and Mental Disease. 1994;182(8):476–8. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- Rabie T, Marti HH. Brain protection by erythropoietin: a manifold task. Physiology (Bethesda) 2008;23:263–74. doi: 10.1152/physiol.00016.2008. [DOI] [PubMed] [Google Scholar]
- Rajji TK, Rogasch NC, Daskalakis ZJ, Fitzgerald PB. Neuroplasticity-based brain stimulation interventions in the study and treatment of schizophrenia: a review. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 2013;58(2):93–8. doi: 10.1177/070674371305800206. [DOI] [PubMed] [Google Scholar]
- Ramon y, Cajal S. The Croonian Lecture: La Fine Structure des Centres Nerveux. Proceedings of the Royal Society of London. 1894;55:331–5. [Google Scholar]
- Raznahan A, Greenstein D, Lee Y, Long R, Clasen L, Gochman P, Addington A, Giedd JN, Rapoport JL, Gogtay N. Catechol-o-methyl transferase (COMT) val158met polymorphism and adolescent cortical development in patients with childhood-onset schizophrenia, their non-psychotic siblings, and healthy controls. Neuroimage. 2011;57(4):1517–23. doi: 10.1016/j.neuroimage.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, Kilgard MP. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70(1):121–31. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. Journal of Neuroscience. 2005;25(41):9398–405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD. Excitotoxicity hypothesis. Neurology. 1996;47(4 Suppl 2):S19–25. doi: 10.1212/wnl.47.4_suppl_2.19s. discussion S6. [DOI] [PubMed] [Google Scholar]
- Scherk H, Falkai P. Effects of antipsychotics on brain structure. Current Opinion in Psychiatry. 2006;19(2):145–50. doi: 10.1097/01.yco.0000214339.06507.d8. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Mrzljak J, Kleinman JE, Herman MM, Goldman-Rakic PS. Regional specificity in the neuropathologic substrates of schizophrenia: a morphometric analysis of Broca's area 44 and area 9. Arch Gen Psychiatry. 2003;60(1):69–77. doi: 10.1001/archpsyc.60.1.69. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Widmark C, Reist C, Erhart S, Braff DL, Marder SR, Green MF. Social cognition in schizophrenia: relationships with neurocognition and negative symptoms. Schizophrenia Research. 2007;90(1–3):316–24. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Shah J, Eack SM, Montrose DM, Tandon N, Miewald JM, Prasad KM, Keshavan MS. Multivariate prediction of emerging psychosis in adolescents at high risk for schizophrenia. Schizophrenia Research. 2012;141(2–3):189–96. doi: 10.1016/j.schres.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J, Mizrahi R, McKenzie K. The four dimensions: a model for the social aetiology of psychosis. The British Journal of Psychiatry. 2011;199:11–4. doi: 10.1192/bjp.bp.110.090449. [DOI] [PubMed] [Google Scholar]
- Shi C, Yu X, Cheung EF, Shum DH, Chan RC. Revisiting the therapeutic effect of rTMS on negative symptoms in schizophrenia: a meta-analysis. Psychiatry Research. 2014;215(3):505–13. doi: 10.1016/j.psychres.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim SS, Hammonds MD, Tatsuoka C, Feng IJ. Effects of 4-weeks of treatment with lithium and olanzapine on long-term potentiation in hippocampal area CA1. Neuroscience Letters. 2012;524(1):5–9. doi: 10.1016/j.neulet.2012.06.047. [DOI] [PubMed] [Google Scholar]
- Slotema CW, Aleman A, Daskalakis ZJ, Sommer IE. Meta-analysis of repetitive transcranial magnetic stimulation in the treatment of auditory verbal hallucinations: update and effects after one month. Schizophrenia Research. 2012;142(1–3):40–5. doi: 10.1016/j.schres.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, Radue EW, McGuire PK, Riecher-Rossler A, Borgwardt SJ. The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia?--a systematic review. Current Pharmaceutical Design. 2009;15(22):2535–49. doi: 10.2174/138161209788957456. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Clos M, Meijering AL, Diederen KM, Eickhoff SB. Resting state functional connectivity in patients with chronic hallucinations. PloS One. 2012;7(9):e43516. doi: 10.1371/journal.pone.0043516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro PA, Bredy TW. Emerging role of non-coding RNA in neural plasticity, cognitive function, and neuropsychiatric disorders. Frontiers in Genetics. 2012;3:132. doi: 10.3389/fgene.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahnisch FW, Nitsch R. Santiago Ramon y Cajal's concept of neuronal plasticity: the ambiguity lives on. Trends in Neurosciences. 2002;25(11):589–91. doi: 10.1016/s0166-2236(02)02251-8. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophrenia Bulletin. 2009;35(3):509–27. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, O'Gorman RL, Barker GJ, McGuire PK, OASIS Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biological Psychiatry. 2009;66(6):533–9. doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Stone JM, Howes OD, Egerton A, Kambeitz J, Allen P, Lythgoe DJ, O'Gorman RL, McLean MA, Barker GJ, McGuire P. Altered relationship between hippocampal glutamate levels and striatal dopamine function in subjects at ultra high risk of psychosis. Biological Psychiatry. 2010;68(7):599–602. doi: 10.1016/j.biopsych.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73(4):842–53. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugranyes G, Thompson JL, Corcoran CM. HPA-axis function, symptoms, and medication exposure in youths at clinical high risk for psychosis. Journal of Psychiatric Research. 2012;46(11):1389–93. doi: 10.1016/j.jpsychires.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF. The genetics of schizophrenia. PLoS Medicine. 2005;2(7):e212. doi: 10.1371/journal.pmed.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, Ashtari M, Napolitano B, Bilder RM, Kane JM, Goldman D, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Molecular Psychiatry. 2005;10(7):631–6. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Progress in Brain Research. 2013;207:3–34. doi: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
- Tandon N, Bolo NR, Sanghavi K, Mathew IT, Francis AN, Stanley JA, Keshavan MS. Brain metabolite alterations in young adults at familial high risk for schizophrenia using proton magnetic resonance spectroscopy. Schizophrenia Research. 2013;148(1-3):59–66. doi: 10.1016/j.schres.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, "just the facts" what we know in 2008. 2. Epidemiology and etiology. Schizophrenia Research. 2008;102(1-3):1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Tartaglia N, Du J, Tyler WJ, Neale E, Pozzo-Miller L, Lu B. Protein synthesis-dependent and -independent regulation of hippocampal synapses by brain-derived neurotrophic factor. Journal of Biological Chemistry. 2001;276(40):37585–93. doi: 10.1074/jbc.M101683200. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Wallman J. Behavioural neuroscience: neurons of imitation. Nature. 2008;451(7176):249–50. doi: 10.1038/451249a. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29(3):148–59. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Braus DF, Hakimi S, Ruf M, Vollmert C, Hohn F, Meyer-Lindenberg A. Acute D2 receptor blockade induces rapid, reversible remodeling in human cortical-striatal circuits. Nature Neuroscience. 2010;13(8):920–2. doi: 10.1038/nn.2572. [DOI] [PubMed] [Google Scholar]
- van Dam DS, van der Ven E, Velthorst E, Selten JP, Morgan C, de Haan L. Childhood bullying and the association with psychosis in non-clinical and clinical samples: a review and meta-analysis. Psychological Medicine. 2012;42(12):2463–74. doi: 10.1017/S0033291712000360. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2(3):266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Swam C, Federspiel A, Hubl D, Wiest R, Boesch C, Vermathen P, Kreis R, Strik W, Dierks T. Possible dysregulation of cortical plasticity in auditory verbal hallucinations-A cortical thickness study in schizophrenia. Journal of Psychiatric Research. 2012;46(8):1015–23. doi: 10.1016/j.jpsychires.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. European Journal of Neuroscience. 2004;20(10):2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biological Psychiatry. 2009;66(6):549–53. doi: 10.1016/j.biopsych.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi ES. Presynaptic modulation of neurochemical transmission. Progress in Neurobiology. 1979;12(3-4):181–290. doi: 10.1016/0301-0082(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Voineskos D, Rogasch NC, Rajji TK, Fitzgerald PB, Daskalakis ZJ. A review of evidence linking disrupted neural plasticity to schizophrenia. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 2013;58(2):86–92. doi: 10.1177/070674371305800205. [DOI] [PubMed] [Google Scholar]
- Voytovych H, Krivanekova L, Ziemann U. Lithium: a switch from LTD- to LTP-like plasticity in human cortex. Neuropharmacology. 2012;63(2):274–9. doi: 10.1016/j.neuropharm.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. Journal of Abnormal Psychology. 2010;119(2):401–8. doi: 10.1037/a0018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Sabuwalla Z, Huot R. Pubertal neuromaturation, stress sensitivity, and psychopathology. Development and Psychopathology. 2004;16(4):807–24. doi: 10.1017/s0954579404040027. [DOI] [PubMed] [Google Scholar]
- Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA, Heinssen R, Mathalon DH, Perkins DO, Seidman LJ. Cortisol levels and risk for psychosis: Initial findings from the North American Prodrome Longitudinal Study. Biological psychiatry. 2013;74(6):410–7. doi: 10.1016/j.biopsych.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker MA, Shinn AK, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biological Psychiatry. 2012;71(2):154–61. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, Kuwabara H, Kumar A. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32(11):2310–20. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Research. 2012;349(1):147–60. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126(Pt 11):2476–96. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Archives of General Psychiatry. 1987;44(7):660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Welch KA, Moorhead TW, McIntosh AM, Owens DGC, Johnstone EC, Lawrie SM. Tensor-based morphometry of cannabis use on brain structure in individuals at elevated genetic risk of schizophrenia. Psychological Medicine. 2013;43(10):2087–96. doi: 10.1017/S0033291712002668. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Baig BJ, Hall J, Job DE, McIntosh AM, Cunningham-Owens DG, Johnstone EC, Lawrie SM. Effects of the BDNF val66met polymorphism on prefrontal brain function in a population at high genetic risk of schizophrenia. American Journal of Medical Genetics. Part B: Neuropsychiatric genetics. 2010;153B(8):1474–82. doi: 10.1002/ajmg.b.31128. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(4):1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Mathalon DH, Shenton ME, Roach BJ, Bammer R, Adcock RA, Bouix S, Kubicki M, De Siebenthal J, Rausch AC, Schneiderman JS, et al. Electrophysiological and diffusion tensor imaging evidence of delayed corollary discharges in patients with schizophrenia. Psychological Medicine. 2011;41(5):959–69. doi: 10.1017/S0033291710001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU. Neurobiology of Schizophrenia Onset. Current Topics in Behavioral Neurosciences. 2013 doi: 10.1007/7854_2013_243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. American Journal of Psychiatry. 2011;168(5):472–85. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Wykes T, Reeder C, Williams C, Corner J, Rice C, Everitt B. Are the effects of cognitive remediation therapy (CRT) durable? Results from an exploratory trial in schizophrenia. Schizophrenia Research. 2003;61(2–3):163–74. doi: 10.1016/s0920-9964(02)00239-6. [DOI] [PubMed] [Google Scholar]
- Xerri C, Merzenich MM, Peterson BE, Jenkins W. Plasticity of primary somatosensory cortex paralleling sensorimotor skill recovery from stroke in adult monkeys. Journal of Neurophysiology. 1998;79(4):2119–48. doi: 10.1152/jn.1998.79.4.2119. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Molecular Psychiatry. 2009;14(1):51–9. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nature Reviews: Neuroscience. 2003;4(11):885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. Journal of Neuroscience. 1998;18(17):7000–7. doi: 10.1523/JNEUROSCI.18-17-07000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]