ABSTRACT
Introduction: Pain mechanisms in fibromyalgia syndrome (FMS) are not clearly understood. Growing evidence appears to suggest a role for small fiber polyneuropathy (SFPN) in some FMS patients, as measured by epidermal nerve fiber density (ENFD). We aimed to better characterize and distinguish the subset of patients with both fibromyalgia and small fiber, early or mild sensory polyneuropathy (FM‐SFSPN). Methods: 155 FMS patients with neuropathic symptoms completed a Short Form McGill Questionnaire and visual analog scale in addition to having skin biopsies, nerve conduction studies (NCS), and serologic testing. Results: Sural and medial plantar (MP) response amplitudes correlated with ENFD, with markers of metabolic syndrome being more prevalent in this subset of patients. Pain intensity and quality did not distinguish patients. Discussion: The FM‐SFSPN subset of patients may be identified through sural and MP sensory NCS and/or skin biopsy but cannot be identified by pain features and intensity. Muscle Nerve 58: 625–630, 2018
Keywords: fibromyalgia, nerve conduction studies, pain, skin biopsy, small fiber neuropathy
Abbreviations
- ACR
American College of Rheumatology
- DR
distal reduced
- ENF
epidermal nerve fiber
- ENFD
epidermal nerve fiber density
- FM
fibromyalgia
- FMS
fibromyalgia syndrome
- FM‐SFSPN
fibromyalgia and small fiber, early or mild sensory neuropathy
- HDL
high density lipoprotein
- HgbA1C
hemoglobin A1C
- MP
medial plantar
- NCS
nerve conduction studies
- PoTS
postural tachycardia syndrome
- PR
proximal reduced
- QSART
quantitative sudomotor axon reflex testing
- ROC
receiver operating characteristic
- SFPN
small fiber polyneuropathy
- SFSPN
small fiber, early or mild sensory polyneuropathy
- VAS
visual analog scale
Fibromyalgia syndrome (FMS) is a well‐recognized and common disorder of chronic widespread pain characterized by specific criteria set out by the American College of Rheumatology (ACR). In 2010, these criteria were revised to remove the need for tender points on exam, leaving specific, clinical features that were deemed vital to the diagnosis: (i) widespread pain (widespread pain index of ≥7 regions); (ii) at least moderate severity (symptom severity score ≥5) of pain, fatigue, sleep disruption, and cognitive symptoms; (iii) duration of symptoms ≥3 months; and (iv) absence of a disorder that would otherwise explain the disorder. Criteria are also satisfied if only 3–6 regions are affected by pain but symptoms are more severe (severity score ≥9).1 Therefore, patient‐reported metrics are central to the diagnosis. The term myalgia suggests the disorder derives at least in part from muscle. However, the co‐existence of neuropathic features of pain and a burgeoning literature implicating small fiber axonal loss in the setting of fibromyalgia (FM), has raised the question of whether the pain in FMS is actually a neuropathic phenomenon.2, 3, 4, 5, 6, 7
Small fiber polyneuropathy (SFPN) refers to selective loss of unmyelinated C and thinly myelinated Aδ fibers that mediate pain, heat, and cold sensation, respectively. As these fibers are not detected on nerve conduction studies (NCS) and physical exam findings may often be minimal or normal,8 the diagnosis relies heavily on the demonstration of reduced epidermal nerve fiber (ENF) density (ENFD) on skin biopsy.9, 10, 11 While reduced ENFD is not absolutely specific for SFPN, it is currently the best objective measure. SFPN is classically slowly progressive and length dependent in onset, although non‐length dependent forms exist.12 Some patients present with an abrupt, generalized onset of SFPN injury, deemed small fiber ganglionopathy or non‐length dependent small fiber neuropathy.12 Both of these entities of small fiber axonal loss can be associated with potentially treatable etiologies.13 Therefore, identification of a small fiber or early sensory neuropathy in the setting of widespread pain is important and carries clinical management implications.
Recent studies have demonstrated that a substantial proportion of patients who carry the diagnosis of FMS have reduced ENFD at the distal leg, as diagnosed by punch biopsy.2, 3, 4, 5, 6, 14, 15 Indeed, up to 50–61% of patients with FMS may have undiagnosed SFPN.3, 15 Many studies have captured the neuropathic nature of fibromyalgia pain, using such measures as the Neuropathic Pain Symptom Inventory, the visual analog scale (VAS), the Michigan Neuropathy Screening Instrument, the Utah Early Neuropathy, the Pain DETECT questionnaire, and the Leeds Assessment of Neuropathic Symptoms and Signs Questionnaire. In addition to having neuropathic clinical features, some of these patients have been described as having inflammatory changes on skin biopsies including IgG deposits in the dermis and vessel walls, and the presence of tumor‐necrosis factor‐α and interleukin‐6.15 This implicates a potential pathophysiologic mechanism for small nerve fiber loss mimicking FMS.
SFPN is diagnosed using skin biopsy among other diagnostic tools. However, skin biopsy has been shown to be complementary with medial plantar (MP) nerve action potentials in the assessment of distal, early or mild large fiber sensory polyneuropathy.16 This finding supports the idea that there is overlap in small fiber and early, large fiber, sensory neuropathy, both of which may be sufficient to result in nonspecific neuropathic pain. Therefore, it is relevant to determine if specific pain characteristics of FMS can be distinguished from pain in small fiber, early or mild sensory polyneuropathy (SFSPN).
In this report, we sought to determine whether pain quality would identify patients with FMS who also had SFSPN (FM‐SFSPN) as assessed by skin biopsy. We sought to corroborate previous findings that distal nerve action potential amplitudes would correlate with ENFD and we evaluated for common serologic differences between the groups.
MATERIALS AND METHODS
Patients with a diagnosis of FMS made by a rheumatologist who were referred to the Ohio State University neurology clinic for assessment of neuropathic symptoms (paresthesias, burning, tingling, or prickling) were retrospectively assessed through an institutional review board approved protocol. Duration of FMS pain, beyond the required 3‐month duration for diagnosis, was not assessed to avoid ascertainment bias. Patients underwent standard clinical assessment that included neurologic examination; pain assessment (Short Form McGill Pain Questionnaire); electrodiagnostic study, including testing of distal (sural and plantar) nerve action potentials; quantitative sudomotor axon reflex testing (QSART); skin biopsy; and serologic studies.
Patients were included in the assessment if the diagnosis of FMS was independently confirmed using the 2010 ACR criteria. Because ACR criteria requires the absence of a disorder that would otherwise explain FMS symptoms, patients were excluded if they had exam findings suggestive of large or mixed fiber peripheral neuropathy. Therefore, in our study population, ankle jerks were preserved, strength was normal and proprioception at the toes was preserved. Similarly, patients were excluded if there was any established history of myopathy, neuropathy, polyradiculopathy, plexopathy, or other a priori neurologic diagnosis. Patients with a known co‐morbid condition that would predispose to neuropathy were also excluded (e.g., diabetes mellitus, connective tissue disorder, etc).
The Short‐Form McGill Pain Questionnaire includes 15 different descriptors of pain quality.17 Pain severity was graded as mild, moderate, or severe. Of the 15 descriptors, 11 are subjective and 4 were affective. A third component of this questionnaire is a VAS pain score (maximum 10 points).
Patients underwent skin biopsy, QSART, and NCS, including bilateral sural and MP evoked responses. In the results, the average amplitude of both sides as well as the lower of the 2 values are reported. The site selected for QSART was the distal foot. Skin biopsies were analyzed by the Therapath Neuropathology Laboratory. A biopsy was deemed positive for SFPN if the ENFD at the distal calf was reduced below the 5th percentile for age‐, gender‐, and body mass index‐adjusted norms.
To distinguish patients with length‐dependent SFPN from nonlength dependent SFPN, we designated the former group as “distal reduced” (DR; ENFD ≤ 5th percentile at the calf) and the latter group as “proximal reduced” (PR; ENFD ≤ 5th percentile at the thigh but normal at the calf). Patients with ENFD > 5th percentile at both sites were designated as biopsy negative. DR patients, but not PR patients, were included in the pain analysis in an effort to standardize the comparison group. Both DR and PR groups were included in correlative analyses. Patients were referred to as: (i) FMS before designation with skin biopsy/NCS; (ii) FM if skin biopsy and NCS were negative; and (iii) FM‐SFSPN if ENFD was reduced.
Because the study was retrospective, the serologic studies that were performed were not standardized but based on the judgment of the evaluating clinician. However, there were some serologic studies that were required for inclusion: comprehensive metabolic profile, complete blood count with differential, metrics of dysglycemia (hemoglobin A1C [HgbA1C], 2‐h glucose tolerance testing, fasting glucose, fasting lipid profile) and serum protein electrophoresis/immunofixation. If all patients in the analysis had other serologic testing (e.g., glutamic acid decarboxylase antibody titers), this was also included in the analysis. Univariate statistical analysis was performed using 2‐tailed Student's t‐test, with P < 0.05 as a threshold for statistical significance.
RESULTS
Demographic data for all FMS patients is detailed in Table 1. Of the 155 enrolled patients, 93 (60%) were biopsy negative, 43 (28%) DR positive, and 19 (12%) PR positive. The mean age of patients was 49.4 years with a range of 18–87 years. A total of 68% of participants were female, with no significant gender difference between biopsy positive and biopsy negative patients. The grouped mean MP and sural nerve action potential amplitudes were above the age‐adjusted cutoffs for the lower limit of normal in our laboratory and others.18, 19 ENFD at the calf and thigh demonstrated a wide range in the FMS group with the distal site reduced in relation to the proximal site.
Table 1.
Demographic data for all FMS patients
| Variable | n | Mean ± SD | Median | (min, max) |
|---|---|---|---|---|
| Age | 155 | 49.4 ± 12.4 | 49 | (18, 87) |
| MP (low, μV)* | 155 | 8.7 ± 6.4 | 8 | (0, 48.5) |
| MP (avg, μV)* | 155 | 9.2 ± 6.7 | 9 | (0, 53.3) |
| Sural (low, μV)* | 155 | 18.0 ± 7.8 | 16 | (2.1, 43.5) |
| Sural (avg, μV)* | 155 | 18.8 ± 7.8 | 16.8 | (2.4, 43.5) |
| ENFD (calf) | 155 | 7.0 ± 3.2 | 6.9 | (0.27, 15.3) |
| ENFD (thigh) | 155 | 10.1 ± 3.3 | 9.71 | (2.02, 18.7) |
| Subjective pain score | 155 | 12.2 ± 8.1 | 11 | (0, 33) |
| Affective pain score | 155 | 2.7 ± 3.0 | 2 | (0, 12) |
| VAS pain score | 155 | 5.2 ± 3.0 | 5 | (0,10) |
| Total pain score | 155 | 20.1 ± 12.0 | 18.5 | (0, 50) |
Min, minimum; max, maximum; avg, average.
MP nerve action potential amplitude was reduced below threshold values in 31.6% of patients (43/155). Both the MP and sural nerve action potential amplitudes correlated with distal ENFD at a statistically significant level. The correlation was stronger for the MP response amplitude than for the sural response amplitude (Table 2). A receiver operating characteristic (ROC) curve of MP and sural amplitudes as a function of predictors of reduced ENFD or positive skin biopsy is shown in Figure 1. Sensitivity of MP amplitude was estimated at 60% for abnormal skin biopsy with a specificity of 82% in patients with FMS. Based on these data, the positive predictive value of reduced ENFD at the calf based on an abnormal MP response is also 60% and the negative predictive value is 82% in the population of FM‐SFSPN with DR positive skin biopsy. QSART at the distal foot was not significantly different between DR positive and biopsy negative groups.
Table 2.
Correlation coefficients and P‐values for MP/sural nerve action potential amplitudes and ENFD at the calf or thigh
| Biopsy negative (n = 93) | DR (n = 43) | P‐Value | |
|---|---|---|---|
| BUN | 12.13 | 14.5 | 0.023 |
| GTT 1h | 130.5 | 176.7 | 0.011 |
| GTT 2h | 102.97 | 131.7 | 0.031 |
| HDL | 50.74 | 45.13 | 0.07 |
| HgbA1C | 5.4 | 6.07 | 0.034 |
| GAD65 AB | 0.2825 | 0 | 0.05 |
| Serum IFE IgM | 132.97 | 101.44 | 0.03 |
Avg, average.
Figure 1.
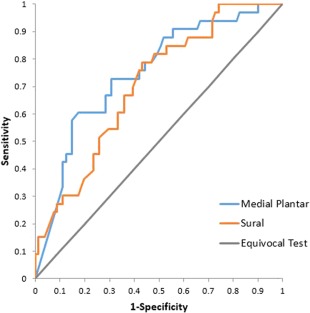
Sensitivity, specificity, positive predictive value, and negative predictive value of MP nerve action potential amplitude as predictor for reduced ENFD in FMS patients. An ROC curve is demonstrated for both MP and sural nerve action potential amplitudes, indicating better performance of MP amplitudes. [Color figure can be viewed at http://wileyonlinelibrary.com]
Both total pain scores and pain descriptors were measured as a function of skin biopsy (DR positive) and separately as a function of MP nerve action potential amplitude (Supplementary Tables S1 and S2, which are available online). Pain scores did not differ significantly between FM‐SFSPN (21.5) and FM patients (19.0). This was true across pain subtypes including subjective, affective pain scores, VAS pain scores, and total pain scores. No significant findings were seen with regards to pain scores in FMS patients relative to MP nerve action potential amplitudes. With respect to pain qualitators, none reached significance when assessed as a function of abnormal skin biopsy. The specific pain descriptors of “sharp” and “splitting” pain were more likely to identify FM‐SFSPN patients. Pain descriptors did not help distinguish FM‐SFPN with and without reduced distal nerve action potential amplitudes.
A significantly greater number of FM‐SFSPN patients had indices of glucose intolerance (abnormal 2‐h glucose tolerance testing, HgbA1C) (Table 3). Low high density lipoproteins (HDLs), another index of metabolic syndrome, did not attain statistical significance (P > 0.05). No significant differences were found in other serum indices, namely complete blood count, renal function, or hepatic function.
Table 3.
Serologic testing with significant differences between biopsy positive/ DR and biopsy negative groups
| rho | P‐Value | |
|---|---|---|
| Low MP amplitude/calf density | 0.46 | <0.0001 |
| Low MP amplitude/thigh density | 0.1 | 0.2501 |
| Low sural amplitude/calf density | 0.39 | <0.0001 |
| Low sural amplitude/thigh density | 0.06 | 0.5095 |
| Avg MP amplitude/calf density | 0.45 | <0.0001 |
| Avg MP amplitude/thigh density | 0.08 | 0.3485 |
| Avg sural amplitude/calf density | 0.38 | <0.0001 |
| Avg sural amplitude/thigh density | 0.07 | 0.4284 |
BUN, blood urea nitrogen; GTT, glucose tolerance test; GAD65 AB, glutamic acid decarboxylase 65 antibody; IFE, immunofixation.
DISCUSSION
The paradigm in our understanding of FMS pain has shifted over the past decade from one of only central sensitization to one of peripheral nervous system injury. Recent evidence points to somatic small fiber dysfunction. Spontaneous activity and peripheral sensitization of silent (type 1B) C fiber nociceptors was found in a substantial proportion of patients with FMS.20 Phenotypically, the problem is compounded by the notable overlap in clinical complaints between patients with such varying diagnostic labels as FM, SFPN, postural tachycardia syndrome (PoTS), and systemic exercise intolerance disorder, formerly referred to as chronic fatigue syndrome. Further adding to this diagnostic uncertainty is the lack of a typical “stocking‐glove” phenotype in nonlength dependent or focal (burning mouth syndrome, complex regional pain syndrome, etc.) SFPN. Whether SFPN is an initiating event leading to FMS, a finding in a subset of FMS patients, or a coincident but separate disorder remains unclear.21, 22
Our study provided several key insights: (i) lower extremity sensory nerve action potential amplitudes correlated well with ENFD; (ii) pain intensity did not correlate well with ENFD; (iii) the quality and quantity of pain did not distinguish FM‐SFSPN from FM; (iv) patients with FM‐SFSPN are more likely to have abnormal glucose metabolism and possibly metabolic syndrome than those with FM; (v) differences in QSART at the distal foot were not identified between groups.
Lower Extremity Sensory Nerve Action Potential Amplitudes Correlated Well with ENFD
Significant correlations were seen for sural and MP response amplitudes with ENFD at the calf, but not the thigh. This is consistent with previous reports in which MP amplitudes correlated with skin biopsy findings.16, 23, 24 Unlike previous studies, we specifically did not stratify for neuropathy because patients did not have exam evidence for distal large fiber dysfunction (although they all fulfilled 2010 ACR criteria for FMS). The implication of this correlation in FM‐SFSPN is that early or mild subclinical loss of distal sensory axons occurs in a subset of patients with FMS. Even in the absence of objective examination evidence of distal large sensory fiber loss, skin biopsy and MP studies can improve diagnostic yield for distal sensory neuropathy in FMS patients.
Pain Intensity Did Not Correlate Well with Reduced ENFD
In our cohort, pain scores correlated with neither ENFD nor MP amplitudes. This is consistent with other reports. In their cohort of 30 FMS patients who underwent skin biopsy, Kosimidis and colleagues found no correlation between scores on the Neuropathic Pain Symptom Inventory and ENFD.6 This does not suggest that SFSPN is not painful, but rather that the experience of pain does not correlate well with axon loss.2, 3, 7, 24 Axon injury to small nerve fibers may incite a cascade of pain‐generating events such as neurogenic inflammation, changes in ion flow in sensory neurons or up‐regulation of pain generating receptors or voltage gated channels that are independent of the amount of axon loss that has occurred.25 The lack of correlation of axon loss with pain may also relate to the complexities of the pain pathway or subjective experience of pain.
Quality and Quantity of Pain Did Not Distinguish FM‐SFSPN from FM
Qualitative ‘neuropathic’ pain descriptors such as ‘sharp’ and ‘splitting’ did not have predictive value in distinguishing FM‐SFSPN from FM. Giannoccaro and colleagues evaluated 20 consecutive patients with FMS and divided them into 2 categories, those with neuropathic like symptoms (paresthesias, burning, tingling, and prickling) and those without. Interestingly, only 40% of those in the neuropathic group had decreased ENFD.5 Some authors have clustered pain phenotypes, reporting more pressure pain, pain attacks and thermal sensitivity in patients with FMS than those with diabetic neuropathy.26 Despite this tendency, the authors also pointed out considerable overlap in phenotype. The “neuropathic nature” of FMS pain is supported by a report in which patients with FMS were found to have pain of dysesthetic, evoked, paroxysmal, and thermal quality, in comparison to patients with rheumatoid arthritis.7 These results support that, although FMS pain goes along with specific neuropathic descriptors, it is not specific enough to distinguish FM from FM‐SFSPN.
Patients with FM‐SFSPN Are More Likely to Have Abnormal Glucose Metabolism and Possibly Metabolic Syndrome
Glucose tolerance testing and HgbA1c, but not low HDL, were significantly more common in the FM‐SFSPN than the FM groups. This is in contrast to a previous study in which 13 FM‐SFPN patients had normal HgbA1C values and most (8 of 11) had normal 2‐h glucose tolerance test results.3 Two larger studies found different results. Loevinger and colleagues found a nearly sixfold increase in incidence of metabolic syndrome among 109 women with FMS compared with 46 healthy control women. Higher triglyceride and hemoglobin A1c levels as well as increased waist circumference were also noted in the FMS group.27 Another study of 33 FMS women reported elevated levels of biochemical markers of metabolic syndrome, leptin, in FMS patients independent of adipose status.28
Differences in QSART at the Distal Foot Were Not Identified between Groups
Our study found no significant differences on QSART testing between FM‐SFSPN and FM patients. In the Oaklander series, autonomic function testing results were comparable between both FMS and control subjects.3 In a smaller cohort from Italy, 5/6 patients with FMS demonstrated reduced innervation to sweat glands and erector pili muscles at the distal leg and thigh in patients, all of whom showed morphologic changes in autonomic nerve fibers. Four of the 6 patients had autonomic symptoms.5 Myofascial pain similar in character to FMS is described in patients with PoTS, a disorder defined by its autonomic manifestations.29 Whether an overlap of FM, FM‐SFSPN, and PoTS exists requires further clarification.
Our study faced certain limitations. The retrospective design precluded a control arm without FMS. This makes it difficult to establish SFSPN as an independent factor in the generation of pain. It also makes it more difficult to seriously consider muscle pain within the spectrum of neuropathic pain descriptors especially in the absence of clear exam findings or correlation with ENFD. Indeed, an interpretation of this data is that the pain is indistinguishable because it derives from the same source in both FM and FM‐SFSPN, and this source is unrelated to the SFSPN. This is compounded by the fact that FMS remains a clinical diagnosis based on subjective features. Although we cannot rule out this interpretation, the high incidence of DR positive patients and reduced MP amplitude (32%) makes this interpretation less tenable in our view. We would be reluctant to dismiss the interpretation that: (i) SFSPN may account for a proportion of individuals labeled as FM; or that (ii) myalgia is neuropathic given the neurologic exam is insensitive for mild sensory and small fiber axonal loss and pain has not been shown to correlate with axon loss. Rather than invoking SFSPN as causation for the larger defined group of FMS, we would propose that the clinical distinction between these entities is nonspecific and, therefore, less reliable than ancillary testing.
In conclusion, we have recapitulated the findings of others in identifying a subset of patients with FMS who have concomitant SFSPN. In addition, we identified MP amplitude as a useful electrophysiologic correlate to ENFD, and thus, a potential means to separate FM‐SFSPN from FM patients. The data in this report suggest that a syndrome of widespread pain indistinguishable from FM‐SFSPN. Pain characteristics may not be helpful in distinguishing the 2 entities but skin biopsy, evaluating for ENFD, and conduction studies of distal sensory nerves may help the clinician to distinguish FMS from neuropathy. The other consideration that is raised, but not addressed, by this study is whether SFSPN in the FMS population is intrinsically different from SFSPN in non‐FMS populations. Future studies including skin biopsy and NCS data on individuals without pain will help to clarify this question. The detection of SFSPN may have management implications if a reversible etiology such as glucose intolerance is detected.
Ethical Publication Statement: The authors confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information 1
Conflicts of Interest: All authors have no relevant financial disclosures or conflicts of interest to report.
REFERENCES
- 1. Ablin JN, Wolfe F. A Comparative evaluation of the 2011 and 2016 criteria for fibromyalgia. J Rheumatol 2017;44:1271–1276. [DOI] [PubMed] [Google Scholar]
- 2. Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel‐Schneider S, Schmid A, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013; 136(Pt 6):1857–1867. [DOI] [PubMed] [Google Scholar]
- 3. Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small‐fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013;154:2310–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caro XJ, Winter EF. Evidence of abnormal epidermal nerve fiber density in fibromyalgia: clinical and immunologic implications. Arthritis Rheumatol 2014;66:1945–1954. [DOI] [PubMed] [Google Scholar]
- 5. Giannoccaro MP, Donadio V, Incensi A, Avoni P, Liguori R. Small nerve fiber involvement in patients referred for fibromyalgia. Muscle Nerve 2014;49:757–759. [DOI] [PubMed] [Google Scholar]
- 6. Kosmidis ML, Koutsogeorgopoulo L, Alexopoulos H, Mamali I, Vlachoyiannopoulos PG, Voulgarelis M, et al. Reduction of Intraepidermal Nerve Fiber Density (IENFD) in the biopsies of patients with fibromyalgia: a controlled study. J Neurol Sci 2014;347:143–147. [DOI] [PubMed] [Google Scholar]
- 7. Martínez‐Lavin M, López S, Medina M, Nava A. Use of the Leeds assessment of neuropathic symptoms and signs questionnaire in patients with fibromyalgia. Semin Arthritis Rheum 2003;32:407–411. [DOI] [PubMed] [Google Scholar]
- 8. Hovaguimian A, Gibbons CH. Diagnosis and treatment of pain in small‐fiber neuropathy. Curr Pain Headache Rep 2011;15:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010;15:202–207. [DOI] [PubMed] [Google Scholar]
- 10. Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008;131(Pt 7):1912–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gibbons CH, Griffin JW, Polydefkis M, Bonyhay I, Brown A, Hauer PE, et al. The utility of skin biopsy for prediction of progression in suspected small fiber neuropathy. Neurology 2006;66:256–258. [DOI] [PubMed] [Google Scholar]
- 12. Holland NR, Crawford TO, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Small‐fiber sensory neuropathies: clinical course and neuropathology of idiopathic cases. Ann Neurol 1998;44:47–59. [DOI] [PubMed] [Google Scholar]
- 13. Chai J, Herrmann DN, Stanton M, Barbano RL, Logigian EL. Painful small‐fiber neuropathy in Sjögren syndrome. Neurology 2005;65:925–927. [DOI] [PubMed] [Google Scholar]
- 14. Levine T, Saperstein DS, Levine A, Hackshaw KV, Lawson V. Small fiber neuropathy in patients meeting diagnostic criteria for fibromyalgia. J Neurol Disord 2016,4:7. [Google Scholar]
- 15. Kim SH. Skin biopsy findings: implications for the pathophysiology of fibromyalgia. Med Hypoth 2007;69:141–144. [DOI] [PubMed] [Google Scholar]
- 16. Herrmann DN, Ferguson ML, Pannoni V, Barbano RL, Stanton M, Logigian EL. Plantar nerve AP and skin biopsy in sensory neuropathies with normal routine conduction studies. Neurology 2004;63:879–885. [DOI] [PubMed] [Google Scholar]
- 17. Melzack R. The short‐form McGill Pain Questionnaire. Pain 1987;30:191–197. [DOI] [PubMed] [Google Scholar]
- 18. Nodera H, Logigian EL, Herrmann DN. Class of nerve fiber involvement in sensory neuropathies: clinical characterization and utility of the plantar nerve action potential. Muscle Nerve 2002;26:212–217. [DOI] [PubMed] [Google Scholar]
- 19. Løseth S, Nebuchennykh M, Stålberg E, Mellgren SI. Medial plantar nerve conduction studies in healthy controls and diabetics. Clin Neurophysiol 2007;118:1155–1161. [DOI] [PubMed] [Google Scholar]
- 20. Serra J, Collado A, Solà R, Antonelli F, Torres X, Salgueiro M, et al. Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol 2014;75:196–208. [DOI] [PubMed] [Google Scholar]
- 21. Gemignani F. Fibromyalgia syndrome and small‐fiber neuropathy: comment on the article by Caro and Winter. Arthritis Rheumatol 2014;66:3526–3527. [DOI] [PubMed] [Google Scholar]
- 22. Gemignani F, Giovanelli M, Vitetta F, Santilli D, Bellanova MF, Brindani F, et al. Non‐length dependent small fiber neuropathy. A prospective case series. J Peripher Nerv Syst 2010;15:57–62. [DOI] [PubMed] [Google Scholar]
- 23. Lawson V, Mongiovi P, Arnold WD. Limitations of skin biopsy in the assessment of isolated small fiber neuropathy. Neurology 2012;78(Meeting Abstracts 1):P03.207. [Google Scholar]
- 24. Ebadi H, Perkins BA, Katzberg HD, Lovblom LE, Bril V. Evaluation of proxy tests for SFSN: evidence for mixed small and large fiber dysfunction. PLoS One 2012;7:e42208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koroschetz J, Rehm SE, Gockel U, Brosz M, Freynhagen R, Tölle TR, et al. Fibromyalgia and neuropathic pain‐differences and similarities. A comparison of 3057 patients with diabetic painful neuropathy and fibromyalgia. BMC Neurol 2011;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Møller AT1, Jensen TS. Neurological manifestations in Fabry's disease. Nat Clin Pract Neurol 2007;3:95–106. [DOI] [PubMed] [Google Scholar]
- 27. Loevinger BL, Muller D, Alonso C, Coe CL. Metabolic syndrome in women with chronic pain. Metabolism 2007;56:87–93. [DOI] [PubMed] [Google Scholar]
- 28. Homann D, Carvalho HM, Stefanello JM, Góes SM, Lopes AL, de Oliveira ÁR, et al. Hyperleptinemia independent of body adiposity in women with fibromyalgia. Rheumatol Int 2014;34:1593–1598. [DOI] [PubMed] [Google Scholar]
- 29. Thieben MJ, Sandroni P, Sletten DM, Benrud‐Larson LM, Fealey RD, Vernino S, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc 2007;2:308–313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information 1


