The cell-biological program termed the epithelial-to-mesenchymal transition (EMT) has been invoked as a critical component of the metastatic process. Contrastingly, Fischer et al.1 recently reported that in two genetically engineered mouse models of mammary tumour development, carcinoma cells could metastasize without activating EMT programs. However, as detailed below, we find their evidence that EMT programs were not expressed in these primary tumours to be insufficient. Therefore, the contribution of EMT to carcinoma metastasis could not be ruled out in their analysis. There is a Reply to this Comment by Fischer, K. R. et al. Nature 547, http://dx.doi.org/10.1038/nature22817 (2017).
It has been well-established that the EMT is not a single, stereotypical program2–4. Instead, it represents a group of related cell-biological programs, each of which confers certain mesenchymal traits on epithelial cells. Its variability stems from the facts that (1) it can be induced by expression of multiple alternative transcription factors5–9; (2) cells activating an EMT program often proceed only part-way towards a fully mesenchymal state, acquiring only a fraction of various mesenchymal markers2,10,11 and such partial and reversible activation of EMT has been shown to be critical for metastasis12–15; and (3) EMT programs may be manifested in different ways in different tissues2–4,12. These considerations help to illuminate the EMT programs analysed in the publications by Fischer et al.1, who concluded that metastasis from primary tumours occurred in the absence of EMT activation.
Fischer et al.1 employed the Cre/CreER lineage-tracing method to track carcinoma cells that have undergone EMT activation. In such a genetic tracing protocol, the promoter of interest drives the expression of the Cre recombinase, which in turn inflicts a stable genetic mark on the genome of a cell and its lineal descendants. A derivation of this basic protocol involves a CreER protein, which only functions when a ligand of the oestrogen receptor (ER), in this case tamoxifen, is present. Hence, the marking of a cell depends on the concomitant presence of CreER expression and experimentally supplemented tamoxifen. In the present case, the authors used a Cre-activatable GFP transgene; accordingly, transient actions of Cre/CreER would permanently turn off an RFP marker and activate a GFP reporter. By monitoring the expression of GFP, Fischer et al.1 would therefore be able to determine whether an ancestor of these cells had expressed Cre/CreER.
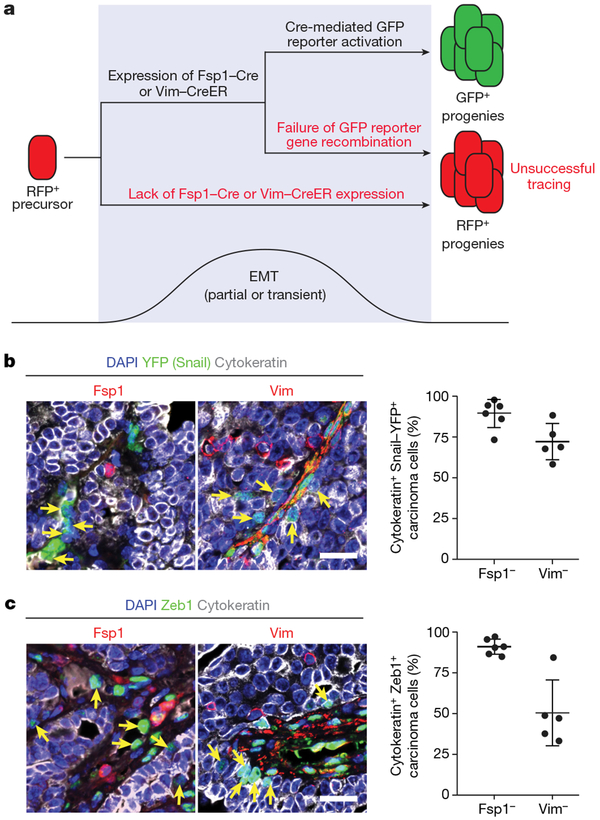
In order for the lineage tracing system to effectively mark carcinoma cells that have undergone EMT activation, it needs to meet, in our view, at least two criteria (Fig. 1a). First, the Cre/CreER driver needs to be expressed in most if not all of the cells that transiently undergo EMT activation. Second, once expressed, the Cre/CreER protein needs to activate the GFP reporter in most if not all of the carcinoma cells where Cre/CreER is expressed.
Figure 1 |. Fspl and vimentin are not universal markers of EMT programs in the MMTV-PyMT breast cancer model.
a, Schematic illustration showing the two criteria for a lineage tracing system to faithfully report EMT. b, c, Tumour sections from Snai1YFF/+;MMTV-PyMT animals5 were stained with DAPI as well as for cytokeratin (an epithelial marker) and either Fsp1 or vimentin together with YFP (b), which marks Snail-expressing cells, or the EMT transcription factor Zeb1 (c). Error bars represent mean ± s.d. Expression of Snail (a, green) and Zeb1 (b, green) is seen in cells that exhibit no evidence of Fsp1 or vimentin expression (red) as indicated by the arrows. Quantifications are shown in the right panels. A minimum of three different microscopic fields were counted per tumour for each marker combination. Scale bars, 20 μm.
Fischer et al.1 chose to use transgenic Cre/CreER lines driven by the promoters of two genes, Fspl and Vim, that are known to be expressed in certain versions of the EMT program. For most oftheir analyses, they used an Fspl-cre transgene. The authors portrayed Fspl as a “critical gatekeeping gene” of EMT initiation, based on a publication showing that Fsp1 is required in order for renal proximal tubular epithelial cells to undergo an EMT in vitro in response to TGFβ16. If this portrayal of the gatekeeper role of Fsp1 were accurate, this would qualify its expression as a sensitive indicator of EMT activation. However, given the variable manifestations of the EMT program in various tissues2–4,14, there is no reason to believe that a marker of EMT activation in renal tubular cells will serve as a useful indicator in unrelated tissues, in this case the mammary epithelium. In fact, there is abundant evidence that Fsp1 is not an integral component of all EMT programs17, as Fsp1-knockout mice are capable of undergoing all stages of developmental EMT and are viable and fertile18. Moreover, in our own studies, while we could indeed detect Fsp1 expression in the bulk population of mesenchymal carcinoma cells by reverse-transcription PCR3, when we employed immunofluorescence staining to investigate the expression of Fsp1, we found that it was only expressed in a very small fraction of carcinoma cells that had activated versions of the EMT program as indicated by the expression of the Snail and Zeb1 EMT transcription factors, known master regulators of EMT programs (Fig. 1b, c and Extended Data Fig. 1). Hence, we think that the authors’ conclusion1 that EMT did not occur in the metastatic cells that failed to undergo Fsp1-Cre-mediated GFP activation cannot be sustained.
Fischer et al.1 also used a second transgene, Vim-creER, in which expression of the tamoxifen-activated CreER recombinase is driven by a Vim promoter. The authors administered tamoxifen three times a week to activate the CreER recombinase following formation of primary tumours. However, this genetic tracing approach also fails to meet the two criteria we proposed above. First, vimentin is expressed weakly or undetectably in carcinoma cells that have, by a number of other criteria, indeed activated versions of the EMT program in the MMTV-PyMT model (Fig. 1b, c and Extended Data Fig. 1). Second, the recombination efficiency of the Vim-CreER tracing system is far from complete. As is evident in extended data fig. 3 of Fischer et al.1, a large fraction of tumour-associated stromal cells that express vimentin at high levels remained GFP-negative even after weeks of tamoxifen administration. Hence, once again the absence of GFP-labelled cells in the metastases could not be used to conclude that versions of the EMT program had not been expressed in metastasis-initiating cells.
Fischer et al.1 also undertook to suppress the EMT program in primary carcinoma cells through forced expression of miR-200, which can directly inhibit expression of the Zeb1 EMT transcription factor. The authors observed that forced miR-200 expression failed to suppress metastasis and concluded once again that activation of an EMT program was not involved in the metastatic dissemination of primary carcinoma cells. However, in addition to suppressing Zeb1, miR-200 is known to have EMT-independent functions in promoting metastatic colonization. miR-200 can directly suppress expression of Sec23, thereby dampening the secretion of metastasis-suppressing proteins such as TINAGL119. In fact, a previous report has demonstrated that the metastasis-promoting function of miR-200 is able to counterbalance its potential anti-metastatic effects19. Furthermore, in the paper by Fischer et al.1, it was apparent that the observed EMT suppression by miR-200 was very incomplete, resulting in only a <10% reduction of N-cadherin and an approximate twofold reduction of Twist EMT transcription factor expression; this indicated that various versions of the EMT program could still operate under this experimental condition.
Given the potential pitfalls in the experimental design and observations, we conclude that the Fischer et al.1 report was insufficient to rule out the contribution of EMT to metastasis. Moreover, in the same PyMT mouse model of breast cancer formation, Cre-mediated genetic deletion of Snail in tumour cells almost completely abolished lung metastasis, reducing it from an average of200 lung metastases to less than 10 lesions14. Furthermore, PCR analysis of the genomic DNA extracted from the remaining metastatic lesions failed to detect the Cre-recombined Snail allele, indicating that these remaining metastases probably arose due to incomplete deletion of the Snail gene in primary tumour cells14. These results were confirmed recently by Ni et al.20; together these two studies provide direct genetic evidence of a critical role of the EMT transcription factor Snail in breast cancer metastasis. Thus, we believe that the notion that the observed metastatic dissemination of mammary tumours does indeed depend on EMT programs continues to be a viable mechanism to explain metastatic dissemination.
Methods
Mammary tumours from Snai1YFP/+;MMTV-PyMT5 animals were fixed in 10% formalin and paraffin sectioned. Tumour sections were deparaffinized and antigen retrieval performed with Nuclear Decloaker (Biocare Medical). Sections were blocked with 1% normal donkey serum in PBST (PBS with 0.3% Triton X-100), incubated with primary antibody at 4 °C overnight, washed with PBS, incubated with secondary antibodies (Biotium) and DAPI, washed with PBS, and mounted in ProLong Gold antifade reagent (Invitrogen P36930). For anti-Zeb1 immunofluorescence, the staining was amplified with the TSA Plus System (Perkin Elmer) following the manufacturer’s instructions. Stained sections were imaged using the Zeiss LSM700 confocal microscope and analysed with Zen software.
Extended Data
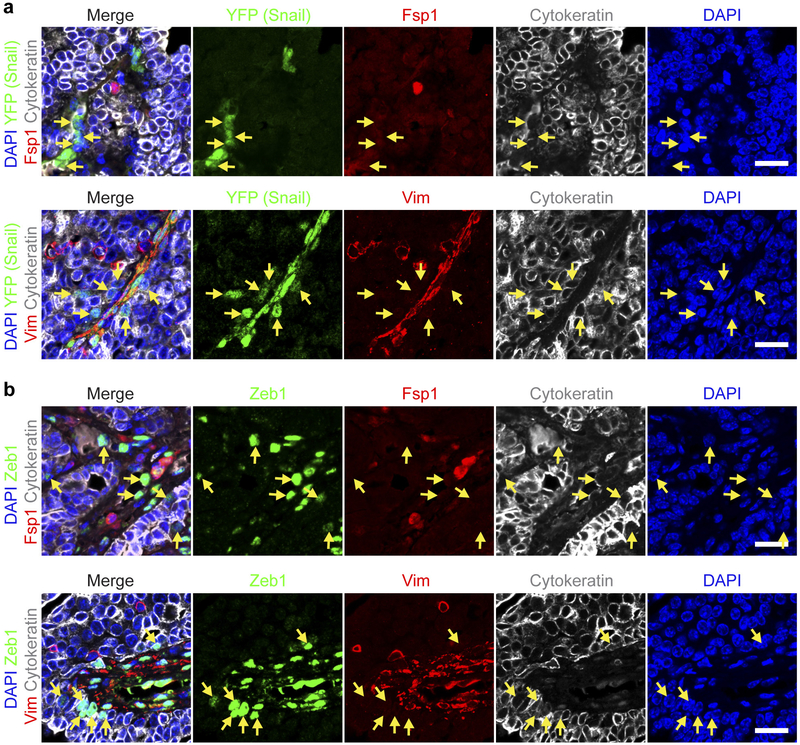
Extended Data Figure 1 |. Individual channels of the stained images shown in Fig. 1b, c.
a-d, DAPI nuclear staining is shown in blue. Anti-cytokeratin staining is shown in grey. Snail-expressing cells are marked by anti-YFP staining in green (a, b). Anti-Zeb1 staining is shown in green (c, d). Anti-Fspl (a, c) and anti-Vim (b, d) staining are shown in red. Arrows indicate cytokeratin-positive tumour cells that express either the Snail (a, b) or the Zeb1 (c, d) EMT transcription factor but, at the same time, lack evident Fsp1 (a, c) or Vim (b, d) expression. Scale bars, 20 μm.
Footnotes
Data availability. All data are available from the corresponding author upon reasonable request.
Competing Financial Interests R.A.W. is listed as an inventor on three patent applications filed by the Whitehead Institute for Biomedical Research relating to the formation of cancer stem cells through activation of an EMT program. US patent application number US 14/441,697 describes a method to use PKCα/FRA1 pathway inhibitors to target carcinoma cells that are identified by their increased expression of EMT-related genes. US patent application number US 15/307,657 describes a method to induce cancer stem cells to undergo EMT rendering them amenable to conventional treatments. US patent application number US 14/065,311 describes methods for identifying compounds and compositions that target cancer stem cells, including cancer stem cells that have undergone EMT R.A.W. is also listed as an inventor on two related patents that have been granted. US patent number US9212347 describes methods for preparing progenitor cells by inducing EMT. US patent number US9308238 describes methods and compounds for modulating and inducing EMT. R.A.W. is also a cofounder of Verastem Inc.
References
- 1.Fischer KR et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527, 472–476 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan T Z. et al. Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol. Med 6, 1279–1293 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye X et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 525, 256–260 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieto MA, Huang RY, Jackson RA & Thiery JP EMT: 2016. Cell 166, 21–45 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Cano A et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Batlle E et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2, 84–89 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Comijn J et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell 7, 1267–1278 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Bolós V et al. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. CellSci 116, 499–511 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Yang J et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Yu M et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grande MT et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med 21, 989–997 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Ocaña OH et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 22, 709–724 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Tsai JH, Donaher JL, Murphy DA, Chau S & Yang J Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22, 725–736 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran HD et al. Transient SNAIL1 expression is necessary for metastatic competence in breast cancer. Cancer Res. 74, 6330–6340 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beerling E et al. Plasticity between epithelial and mesenchymal states unlinks EMT from metastasis-enhancing stem cell capacity. Cell Reports 14, 2281–2288 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okada H, Danoff TM, Kalluri R & Neilson EG Early role of Fsp1 in epithelial-mesenchymal transformation. Am. J. Physiol 273, F563–F574 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Osterreicher CH et al. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc. Natl Acad. Sci. USA 108, 308–313 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue C, Plieth D, Venkov C, Xu C & Neilson EG The gatekeeper effect of epithelial-mesenchymal transition regulates the frequency of breast cancer metastasis. Cancer Res. 63, 3386–3394 (2003). [PubMed] [Google Scholar]
- 19.Korpal M et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med 17, 1101–1108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni T et al. Snaill-dependent p53 repression regulates expansion and activity of tumour-initiating cells in breast cancer. Nat. Cell Biol. 18, 1221–1232 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]