Abstract
Objective
As individuals age, they monitor how well they are aging by comparing themselves with their peers. We examined whether such social comparisons contribute to change in one’s subjective age over time and whether they mediate the relationship of health and cognitive functioning with subjective age.
Method
A total of 3,427 participants from the Midlife in the United States study were assessed on subjective age on two occasions 10 years apart. Measures of cognition and health were taken at the second wave along with social comparison measures for health and memory.
Results
The results showed that social comparisons of memory and health mediated the relationship between memory performance and level of subjective age as well as the relationship between functional health and subjective age change.
Discussion
The results suggest that those who have better functioning in aging-relevant domains have a more favorable view of themselves compared with others, which in turn predicted a younger subjective age and smaller increases in subjective age over 10 years. When social comparisons were considered, those who had better health and memory and more favorable comparative assessments did not feel that they had aged as much as those who had lower functioning and assessments.
Keywords: Aging, Cognition, Health, Subjective age
As individuals age, they expect to experience some degree of decline in their physical health and cognitive functioning. The manner in which individuals actually age, however, varies widely from person to person. People often appraise their own aging process, perhaps making determinations based on factors such as their physical health (see Settersten & Hagestad, 2015). This appraisal process contributes to building a subjective view on how well they are aging.
Subjective age, or how old one feels, typically does not correspond to chronological age. In fact, most adults over the age of 30 report a subjective age lower than their chronological age (Rubin & Berntsen, 2006). An individual’s subjective age takes into consideration factors such as life satisfaction, prevalence of physical and cognitive symptoms of aging, and their own personal beliefs and fears of the aging process (Montepare & Lachman, 1989). Subjective age has also been correlated with several important health outcomes. In older adults, a younger subjective age has been associated with better health and well-being (Stephan, Caudroit, & Chalabaev, 2011; Westerhof et al., 2014) as well as decreased mortality risk (Kotter-Grühn, Kleinspehn-Ammerlahn, Gerstorf, & Smith, 2009). Given this evidence, it would seem that subjective age is an important indicator for how well an individual is aging. While much of the past work on subjective age has focused on subjective age as a predictor variable (Stephan, Caudroit, Jaconelli, & Terracciano, 2014; Westerhof, et al. 2014), there has been limited attention to factors that might predict changes in subjective age over time (Spuling, Miche, Wurm, & Wahl, 2013). Given the important health outcomes that are predicted by subjective age, it is important to understand which factors might lead to changes in an individual’s subjective age. The primary goal of the present study is to investigate the factors that influence how subjective age changes over a 10-year period.
Links Between Subjective Age and Cognition
Although physical health has been tied to both subjective age (Barak & Stern, 1986; Hubley & Russell, 2009) and cognitive functioning (Albert et al., 1995; van Boxtel et al., 1998), only a few studies have examined the relationship between subjective age and cognitive performance. In one such study, which used data from the Midlife in the United States (MIDUS) longitudinal study, participants who reported a younger subjective age performed better on two cognitive factors 10 years later (Stephan et al., 2014). In that study, subjective age was found to predict cognitive performance above and beyond chronological age, as well as the participants’ health, measured by chronic disease burden. Furthermore, they conclude that the relationship between subjective age and episodic memory 10 years later was partially mediated by body mass index (BMI), but not by physical activity, and the relationship between executive function and subjective age was partially mediated by physical activity, but not BMI. However, that study did not examine change in subjective age or the contemporaneous relationships of cognition and subjective age. Thus, it is unclear to what extent subjective age exerts distal and/or concurrent influences.
Subjective age has also been shown to predict better memory performance as well as a slower decline in performance (Stephan, Sutin, Caudroit, & Terracciano, 2015). Although some studies have demonstrated that lower subjective age predicts better cognitive performance, not all work on subjective age supports the connection between subjective age and cognition. One such study failed to find a relationship between subjective age and cognitive functioning in a sample of very old Swedish adults (Infurna, Gerstorf, Robertson, Berg, & Zarit, 2010). This may be due to overall low cognitive performance for the very old sample (84 to 90 years old). It is also possible that the question that was used to measure subjective age (“Do you feel old?”; Yes, No, and Partly) could not capture relatively small variations in subjective age. Additionally, studies that do demonstrate a relationship between cognition and subjective age have only used subjective age from one time point prior to testing (Stephan et al., 2014, 2015), thus limiting any considerations of whether it is cognition that leads to changes in subjective age.
Health and Subjective Age
Past research has used self-reported health (e.g., Ward, 2013) or chronic health problems (or disease burden) as a proxy variable for overall health (e.g., Stephan et al., 2014). Self-reported health ratings are useful in gauging participants’ perception of their own health but provide little information regarding their actual physical performance. Similarly, chronic problems are often a suitable and reliable predictor of health, but it may not be the optimal measure of health in older participants, particularly for research concerning subjective age. First, many chronic problems, such as blood pressure and high cholesterol, typically cannot be seen or consciously experienced by the participant; these individuals may know they have high cholesterol if their doctor has told them, but it rarely impacts their daily lives except for taking daily medications. Second, some such conditions that are defined as chronic (e.g., cancer) are not necessarily a sign of aging. Finally, by definition, some chronic problems have persisted over some time. This may include conditions that participants were diagnosed with years, or even decades, earlier (e.g., allergies, skin conditions, and digestive problems). Such chronic conditions are not likely to be directly tied to assessing age-specific declines. In contrast, functional health may be a more relevant aspect of health with regard to determining one’s subjective age.
Functional health measures how an individual’s overall health impacts his/her daily activities. Functional health provides a measure that is both observable to participants and to others and captures how conditions may be influencing their physical activity and their daily lives. Functional health has previously been linked to positive self-perceptions of aging. Individuals 50 and older who showed more positive self-perceptions of aging reported higher levels of functional health than those with less positive perceptions. Further, this study suggests that functional health did not lead to changes in self-perceptions of aging (Levy, Slade, & Kasl, 2002). Although these results suggest that individuals with a more positive outlook on aging maintained a higher level of functional health, it is unclear whether these individuals would also report a younger subjective age as a result.
Because participants can directly observe and experience their functional health, it is likely to play a role in judging subjective age. There is some past work that suggests functional health and subjective age are related (Infurna et al., 2010). However, as noted previously, the measure of subjective age has some limitations, suggesting the need for further investigation. Moreover, the link between subjective age and functional health has not been supported by all prior work. In one study, evidence for a link between subjective age and functional health was found cross-sectionally, but not longitudinally (Spuling et al., 2013). However, the authors cited as a limitation the fact that their participants were of above average health and functional status that may have limited their ability to find a relationship due to a restricted range. We believe that functional health would also have implications for assessing subjective age. Other work (Stephan, Sutin, & Terracciano, 2015a) has investigated a link between subjective age and both observable experiences (grip strength, expiratory flow, and waist circumference) and unobservable measures (blood pressure and telomere length). These unobservable measures were not related to subjective age. In contrast, the observable measures were found to be related to subjective age; lower grip strength, lower expiratory flow, and a larger waist circumference were all associated with older subjective age. These studies suggest that subjective age is influenced by overt indicators of aging, such as those measured by functional health.
Subjective Age Change Over Time
The vast majority of the existing literature on subjective age has been cross-sectional in nature, however, a few studies have examined the change in subjective age over time. One such study, which also used data from the MIDUS, reported modest changes in subjective age over a 10-year period (Ward, 2013). This study investigated how self-reported health (on a scale from 1 to 10) influenced subjective age change. Participants who reported health improvements during that time also reported a younger subjective age at Wave 2 compared with Wave 1. Participants who reported no health changes over that time also reported a slightly younger subjective age, whereas participants who reported health declines showed a slight increase in subjective age. Additionally, changes in subjective age have been found to be related to changes in personality (Stephan, Sutin, & Terracciano, 2015b). Participants who reported the largest increases in subjective age demonstrated declines in extraversion, openness, agreeableness, and conscientiousness. Participants with more stable subjective age scores showed greater decreases in neuroticism. Both of these studies show that changes in health and psychological variables are tied to changes in subjective age. These studies demonstrate the importance of further research on sources of change in subjective age over time.
The Possible Role of Social Comparisons
Little is known about possible mechanisms by which cognitive performance or health could influence subjective age. There is some evidence that individuals monitor their abilities when estimating their subjective age. In fact, participating in cognitive testing has been shown to influence subjective age in older adults (Hughes, Geraci, & De Forrest, 2013). Across four studies, older adults reported feeling subjectively older after taking, or just expecting to take, a memory test, when compared with younger adults or older adults taking a vocabulary test. However, it should be noted that in these studies, individual differences in cognitive performance were not directly related to differences in the amount of increase in subjective age.
Adults often compare their own aging process with that of their peers or with the older generation preceding them (Staudinger, 2015). These comparative observations form the expectations for their own aging process. If participants view themselves as being better than their same-aged peers in various age-related realms (i.e., health and memory), participants may view themselves as younger than their actual age. Past research has demonstrated that favorable social comparisons help preserve mental health in older adults (Heidrich & Ryff, 1993a, 1993b). However these ideas have rarely been applied to the research on subjective aging. Thus far, the best evidence to support the theory that older adults use social comparisons to determine their own subjective age comes from a study by Stephan, Chalabaev, Kotter-Grühn, and Jaconelli (2013). In this study, older adults reported how old they generally felt prior to a handgrip test. Following the handgrip test, half of the participants were told they performed better than most of their same-aged peers; the other half were given no feedback. Following this feedback, participants performed another handgrip task and then reported how old they felt at that time. Participants who received positive feedback reported a younger subjective age than their baseline, whereas participants who received no feedback showed no change. These results support the theory that older adults use social comparisons to construct subjective age. However, several questions remain. First, do older adults need explicit feedback, or do they inherently make these kinds of social comparisons? Second, do social comparisons mediate the relationship of cognitive performance and health with changes in subjective age?
The Current Study
Previous research by Stephan and colleagues (2014) found a relationship between subjective age and factor scores for episodic memory and executive function 10 years later. They concluded that subjective age predicts later cognitive performance. However, in that study, only Wave 1 subjective age was considered in relation to cognitive functioning at Wave 2. Thus, the relationship they found between earlier subjective age and subsequent cognitive functioning does not take into account the extent of stability and change in subjective age nor the concurrent relationship. The present study considers subjective age from both Wave 1 and Wave 2 of the MIDUS data set and examines to what extent functional health and cognitive functioning predict changes in subjective age. Results from prior work support the theory that cognitive function (Hughes et al., 2013; Stephan et al., 2014, 2015) and physical performance (Infurna et al., 2010; Stephan et al., 2013, 2015a) are independently related to subjective age. However, it is unlikely that individuals only consider one dimension when comparing themselves to their peers. Therefore, we propose a model considering both of these factors simultaneously.
Much of the previous research has demonstrated that subjective age is tied to functional health and cognition. However, typically these studies have not addressed how health and cognitive factors are related to changes in subjective age. Additionally, little work has focused on the underlying mechanisms that drive this connection. Subjective age has been observed to change as people age (Ward, 2013), but these changes vary from person to person. It is unclear what leads some individuals to show greater increases or decreases in subjective age over time. The present study examined possible mechanisms that may link cognition and health with intra-individual changes in subjective age.
It is important to note that while the present paper examines health and cognitive functioning as antecedents of subjective age, we acknowledge that previous work has also found support for the alternative direction, that is, subjective age is associated with subsequent changes in health (Westerhof et al. 2014) and cognition (Stephan et al., 2015). Nevertheless, given the evidence that experience with physical tasks (Stephan et al., 2013) and memory tests (Hughes et al., 2013) can at least momentarily affect subjective age, we believe it is plausible that there is an ongoing reciprocal relationship between subjective age and functional performance. The primary goal of the current study is to identify factors that predict change in subjective age over a 10-year span. We predicted that individuals with higher cognitive performance and functional health would show a younger subjective age and a smaller increase in subjective age over time than individuals with lower cognitive performance and worse functional health. After examining the direct effects of health and cognitive functioning on subjective age, we also considered the mediating role of social comparisons. We expected that the relationship of health and cognition to subjective age, as well as change in subjective age, would be mediated by social comparisons. Individuals judge their own abilities and functioning in comparison with the abilities of their age peers; thus, those who see their own health and/or cognition as worse than others of their age are expected to report feeling older to a greater extent relative to their actual age and compared with their initial level of subjective age.
Method
Participants
Participants were from the first (1994–1995) and second waves of MIDUS (2004–2006), a national probability sample of noninstitutionalized adults from the 48 contiguous states. They were selected using random digit dialing (RDD) of telephone numbers with age and gender information about the household composition, with an overall response rate of 70% for the telephone interview (Brim et al., 2004). The study also included siblings (N = 949) of the main respondents, randomly selected from the RDD sample, as well as a subpopulation of twins (N = 1,913) obtained after screening a representative national sample of approximately 50,000 households. At Wave 2, the longitudinal retention rate, adjusted for mortality, was 75% (N = 4,955; Radler & Ryff, 2010). For the present analyses, we only considered participants with complete data that resulted in a sample of 3,427 participants. The participants included in the analyses ranged from 32 to 84 years old (M = 55.98, SD = 12.19).
As reported in previous studies (Lachman, Agrigoroaei, Tun, & Weaver, 2014), individuals in the sample who participated in Wave 2 showed some differences on Wave 1 variables, compared with those who dropped out of the study. At Wave 1, participants who remained in the study were more educated (14.18 years of education vs. 13.35 years), t(7085) = −13.33, p < .01; more likely to be female participants (55.09% female vs. 49.56%), χ2(1) = 22.53, p < .01; reported higher levels of functional health (86.73 vs. 81.59), t(6229) = −9.00, p < .01; and were older (47.07 years vs. 45.76 years), t(7039) = −4.22, p < .01 than participants who dropped out. There was no difference for subjective age at Wave 1, t(6157) < 1.
Control Variables
Age, gender, chronic health problems, and education measures that have been found to be highly correlated with subjective age, functional health, and cognitive performance served as control variables. Chronological age was determined by subtracting participants’ date of birth from the date the phone portion of the study was completed. Participants reported their highest level of educational attainment that was recoded into years of education.
Independent Variables
Episodic memory
In MIDUS, seven cognitive dimensions were tested using the Brief Test of Adult Cognition by Telephone (BTACT; Lachman & Tun, 2008; Lachman et al., 2014; Tun & Lachman, 2008). This included two measures of episodic memory (immediate and delayed free recall of 15 words). Following exploratory and confirmatory factor analysis (Lachman, Agrigoroaei, Murphy, & Tun, 2010), an episodic memory factor was computed using the immediate and delayed word recall. The episodic memory factor score was computed as a standardized mean of the z-scored measures loading on the factor. Memory measures were only available at Wave 2 of the study.
Executive function
The remaining five cognitive tests in the BTACT were working memory span (backward digit span—the highest span achieved in repeating strings of digits in reverse order), verbal fluency (the number of words produced from the category of animals in 60s), inductive reasoning (completing a pattern in a series of 5 numbers), processing speed (the number of digits produced by counting backward from 100 in 30s), and attention switching and inhibitory control (the Stop and Go Switch Task; Tun & Lachman, 2008). As with episodic memory, an executive function composite score was computed following exploratory and confirmatory factor analysis (Lachman et al., 2010). The executive function factor score was computed as a standardized mean of the z-scored measures loading on the factor. Executive function measures were only available at Wave 2 of the study.
Functional health
In keeping with past research (Ware & Sherbourne, 1992), functional health was measured using a 10-item self-reported questionnaire. At Wave 2, participants rated whether their health limited them in doing 10 different activities (e.g., limited bathing or dressing, climbing stairs, and carrying groceries) using a 4-point scale (1 = a lot, 4 = not at all). A mean score was computed by averaging scores for each of the 10 activities and coded so that higher scores indicated higher levels of functional health.
Mediating Variables
Social comparisons
Comparative memory and comparative health were both phrased the same way: “Compared to other people your age, how would you rate your: (health/memory).” Participants responded on a 5-point Likert-type scale from 1 (Excellent) to 5 (Poor). These scores were then reverse coded, such that a higher score indicates a more favorable social comparison. A high comparative memory score indicated that a participant feels his or her memory was better than same-aged peers. Likewise, a high comparative health score indicated that a participant feels he or she was in better health than same-aged peers. Both of these measures were assessed at Wave 2. For the mediational models, a social comparison composite score was calculated by averaging the two measures.
Dependent Variable
Subjective age
Subjective age was measured at both waves by asking participants: “Many people feel older or younger than they actually are. What age do you feel most of the time?” Participants responded by providing a number in years to estimate the age they felt. Wave 1 subjective age was entered as a control variable in our models in order to examine residual change over the 8 to 10 years. We used raw subjective age and included chronological age as a control variable, rather than a subjective age difference score (subjective minus chronological age) or discrepancy score (subjective minus chronological age, divided by chronological age) that others have used in previous research. Because of these differences in how subjective age has been analyzed in past work, we performed sensitivity analyses to test our hypotheses using other operational definitions of subjective age: subjective age difference scores, discrepancy scores, subjective age change between MIDUS Wave 1 and Wave 2, as well as subjective age change as a proportion (Wave 2 minus Wave 1, divided by time passed). Given that the results were consistent regardless of the method of representing subjective age, we only present the results using the raw subjective age, controlling for chronological age.
Statistical Analysis
The primary goal of the present study was to investigate social comparison as a possible mechanism linking health and cognitive functioning with changes in subjective age. For the mediation analyses, we only included participants with complete data that resulted in 3,427 participants. We conducted this mediation analysis using MEDIATE for SPSS (Hayes & Preacher, 2014). All three independent variables (episodic memory, executive function, and functional health) were entered into the model simultaneously. Age, gender, education, and chronic health problems were entered into the analysis as control variables. Model 1 tested whether social comparisons of memory and health mediated the relationship between health and cognitive function with the level of subjective age. In a second model, subjective age from Wave 1 of MIDUS was added as an additional control variable to examine residualized change. Model 2 tested whether social comparisons mediated the relationship between functional health and cognitive function with changes in subjective age.
As our sample also included siblings of the main respondents and a subpopulation of twins, all analyses were reexamined as multilevel models, with family ID as a random effect predicting the intercept to account for potential nonindependence in the data set, in accordance with past work (Lachman & Agrigoroaei, 2010; Human et al., 2013). Parameter estimates obtained from these analyses accounting for this data clustering were consistent with the results from the mediation analyses. All results presented reflect those obtained from the MEDIATE analyses.
We conducted a sensitivity analysis to determine whether extreme values would impact the results of the present analyses. Values were considered outliers if they exceeded the mean by 3 SD or more in either direction. These extreme values were replaced with a value equal to 3 SD above or below the mean, and the mediation analyses were repeated. All results were the same and the results reported below represent the original values.
Results
Table 1 presents descriptive statistics for all variables in the study. For correlations between all variables, see Table 2. As expected, a repeated measures analysis of variance (ANOVA) demonstrated that participants felt older at Wave 2 (46.31 years) than they felt at Wave 1 (39.33), F(1, 3300) = 1890.96, p < .01, MSE = 79113.20, η2 = .36. This was expected, as participants were indeed older. However, the increase in subjective age (M = 6.92, SD = 9.15) was on average less than the actual age increase; the average amount of time that passed between waves was 8.91 years (SD = 0.63 years). At Wave 1, participants on average felt 7.73 years younger than their actual age. By Wave 2, participants felt 9.72 years younger than their actual age, which was found to be a significant change using a repeated measures ANOVA, F(1, 3300) = 156.08, p < .01, MSE = 6524.23, η2 = .05.
Table 1.
Descriptive Statistics
| Variables | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|
| Chronological age Wave 2 | 32.00 | 84.00 | 55.98 | 12.19 |
| Subjective age Wave 1 | 0.00 | 120.00 | 39.33 | 11.65 |
| Subjective age Wave 2 | 3.00 | 150.00 | 46.31 | 13.43 |
| Years of education | 6.00 | 20.00 | 14.18 | 2.58 |
| Chronic health problems | 0.00 | 25.00 | 2.41 | 2.34 |
| Episodic memory | −3.11 | 3.79 | 0.00 | 1.00 |
| Executive function | −5.00 | 3.48 | 0.00 | 1.00 |
| Functional health | 0.00 | 100.00 | 79.27 | 25.54 |
| Comparative health | 1.00 | 5.00 | 3.68 | 0.94 |
| Comparative memory | 1.00 | 5.00 | 3.54 | 0.89 |
| Social comparison composite | 1.00 | 5.00 | 3.60 | 0.81 |
| Subjective age difference Wave 1 | −58.00 | 73.00 | −7.73 | 9.03 |
| Subjective age difference Wave 2 | −61.00 | 67.00 | −9.72 | 9.80 |
| Subjective age change | −53.00 | 83.00 | 6.92 | 9.15 |
Notes. N = 3,427. Subjective age difference is defined as the difference between subjective age and chronological age (subjective − chronological), where a negative value represents a younger subjective age and a positive value represents an older subjective age. Subjective age change is defined as the difference between Wave 2 and Wave 1 subjective age.
Table 2.
Correlations for all variables
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Chronological age | — | ||||||||||
| 2. Subjective age (Wave 1) | .71* | — | |||||||||
| 3. Subjective age (Wave 2) | .71* | .74* | — | ||||||||
| 4. Years of education | −.10* | −.10* | −.12* | — | |||||||
| 5. Chronic health problems | .30* | .32* | .32* | −.13* | — | ||||||
| 6. Episodic memory | −.33* | −.28* | −.28* | .19* | −.15* | — | |||||
| 7. Executive function | −.42* | −.34* | −.36* | .40* | −.25* | .42* | — | ||||
| 8. Functional health | −.34* | −.37* | −.39* | .22* | −.52* | .18* | .31* | — | |||
| 9. Comparative memory | −.01 | −.14* | −.18* | .18* | −.26* | .11* | .20* | .28* | — | ||
| 10. Comparative health | .00 | −.16* | −.20* | .21* | −.38* | .11* | .20* | .49* | .54* | — | |
| 11. Social comparison composite | −.01 | −.17* | −.22* | .22* | −.37* | .13* | .22* | .44* | .87* | .88* | — |
Note. Listwise N = 3,295.
*p < .05 indicates a significant correlation.
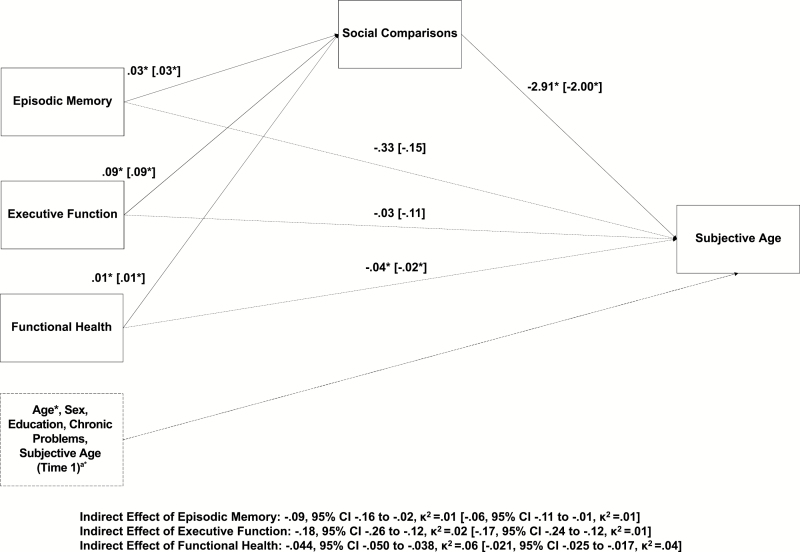
For the mediational models, coefficients and 95% confidence intervals (CIs) are provided. For the mediational effect, kappa squared (κ2) is provided as a measure of effect size, as recommended by Preacher and Kelley (2011). Model 1 tested whether episodic memory, executive function, and functional health were related to concurrent subjective age and whether this relationship would be mediated by social comparisons (see Figure 1). The total effects model that does not consider the effect of the mediator demonstrated that episodic memory (−.42, p < .01) and functional health (−.08, p < .01) were significantly related to subjective age; executive function was not (−.28, p = .16). While controlling for age, sex, chronic health problems, and education, episodic memory was related to social comparisons (.03, p < .05), indicating that higher memory scores were related to higher social comparisons, as expected. Executive function was also positively related to social comparisons (.09, p < .01), as was functional health (.01, p < .01). Higher social comparison scores were also related to lower subjective age (−2.91, p < .01). The mediation analysis demonstrated that social comparisons mediated the relationship between episodic memory and subjective age (indirect effect: −.09, 95% CI: −.16 to −.02, κ2 =.01); the direct path between episodic memory and subjective age was not significant (−.33, p = .07). The analysis also supported mediation for the effect of functional health on subjective age (indirect effect: −.044, 95% CI: −.050 to −.038, κ2 = .06). The direct effect between functional health and subjective age was significant (−.04, p < .01). The indirect effect between executive function and subjective age was also significant (−.25, 95% CI: −.34 to −.18, κ2 = .02) and the direct effect was not significant (−.03, p = .88); however, as the total effect for executive function was not significant, this cannot be interpreted as evidence for mediation.
Figure 1.
Mediation models for subjective age (Model 1) and subjective age change (Model 2). Model 1: the relationship of episodic memory, executive functioning, and functional health on concurrent subjective age, mediated by social comparisons. *p < .05. Age, sex, chronic health problems, and education were entered as covariates. Model 2: the relationship of episodic memory, executive functioning, and functional health on change in subjective age, mediated by social comparisons. Model 2 parameters are presented in square brackets []. Note that Time 1 subjective age was included as an additional covariate in Model 2 to examine predictors of change in subjective age. In both models, direct paths from the independent variables to the dependent variables are presented with dotted lines. Covariates are presented with a dashed line.
Model 2 tested whether social comparisons mediated the relationship of episodic memory, executive function, and functional health on change in subjective age (see Figure 1). To accomplish this, we added Wave 1 subjective age as a control variable, in addition to age, sex, chronic health problems, and education. The total effects model only found a significant relationship between functional health and subjective age change (−.05, p < .01). Neither episodic memory (−.21, p = .21) nor executive function (−.28, p = .12) was related to subjective age change when including functional health in the model. The mediation results demonstrated that episodic memory was related to social comparisons (.03, p < .05), as were executive function (−.09, p < .01) and functional health (.01, p < .01). Better social comparisons were in turn related to subjective age change (−2.00, p < .01). Regarding the mediation effects, social comparisons mediated the relationship between functional health and subjective age change (indirect effect: −.021, 95% CI: −.025 to −.017, κ2 = .04); the direct effect between functional health and subjective age change was also significant (−.02, p < .01). The indirect effects of episodic memory (−.06, 95% CI: −.11 to −.02, κ2 = .01) and executive function (−.17, 95% CI: −.24 to −.12, κ2 = .01) were both significant, however, this cannot be interpreted as evidence of mediation as the total effects were not significant.
Discussion
The goals of the present study were to examine whether cognitive performance and functional health were related to concurrent subjective age and 8- to 10-year changes in subjective age and whether these relationships were mediated by social comparisons with same-age peers. As expected, both functional health and memory performance predicted the level of subjective age. However, contrary to predictions, executive function did not independently predict the level of subjective age when both functional health and episodic memory were included in the model. This adds to prior work (Stephan et al., 2014) that found a relationship between both episodic memory and executive function and subjective age without functional health in the models.
We also found that functional health was related to changes in subjective age. Social comparisons again partially mediated this relationship. Better functional health was related to more favorable social comparison scores, which in turn was related to younger subjective age. Participants with more favorable comparisons (i.e., participants who rated themselves as “good” or “excellent” in their health and memory compared with their age peers) demonstrated a larger increase in their subjective age discrepancy (chronological age minus subjective age), that is, they felt younger to a greater extent over time than those with less favorable comparisons. This result was obtained using ANOVA, with social comparisons as a between-subjects variable (high vs. low comparisons; F(1, 3299) = 12.40, p < .001). Between Wave 1 and Wave 2, participants with more favorable comparative memory went from 9.29 years younger than their actual age to 11.85 years younger, a difference of 2.55 years. Furthermore, those with less favorable comparative memory scores (i.e., participants who rated themselves as poor to average) only reported a difference of 1.44 years, reporting feeling 6.21 years younger at Wave 1 and 7.64 years younger at Wave 2.
These results suggest a viable underlying mechanism that links functioning in aging-relevant domains, such as memory and health, to subjective age. Past work has linked subjective age to cognitive (Stephan et al., 2014, 2015) as well as physical performance (Stephan et al., 2013), but these studies have suggested subjective age influences cognition and health. Although some past work has suggested mechanisms such as personality traits or depressive symptoms to explain the link between subjective age and health outcomes (see Kotter-Grühn, Kornadt, & Stephan, 2015), to our knowledge none have looked specifically at the role of social comparisons. Subjective age has been called a protective mechanism, and it has been suggested that individuals with lower subjective age may be living in a biologically “younger” body. The present results add to this literature by suggesting that one’s self-assessment of functioning relative to same-age peers contributes to judgments of one’s subjective age. Some previous work is consistent with this hypothesis. For example, in a study by Hughes, Geraci, and De Forrest (2013) participants exposed to a memory test reported feeling older than before the memory test. However, participants did not feel older after taking a vocabulary test. These results suggest that because memory decline is associated more closely with aging than vocabulary ability, participants viewed the exposure to a memory test as a measuring stick of their age. We suggest that individuals think about their own aging-related changes and performance relative to same-age peers when determining their own subjective age.
The age range of the present study is wider than some of the past work on subjective age. We included the sample’s whole range of available ages because we believe that subjective age is tied to performance measures throughout the life span. Although the youngest participants in the sample had fewer functional limitations than the older participants, there was variability across the age spectrum. This is likely tied in part to the wide range in socioeconomic status that is tied to differences in health across the age spectrum. Moreover, when comparing the results for those aged under 60 years versus above 60 years, the findings were consistent.
The present results demonstrated that health and cognitive performance are related to subjective age change over time. Nevertheless, other work has found that subjective age predicts cognitive performance and health. Little is known, however, about possible mechanisms linking subjective age to changes in health and cognition, and there is still a call for more research on this topic (Kotter-Grühn et al., 2015). Given the observational nature of the current study, we are limited from making direct conclusions as to the causal relationship or directionality of the variables. The study is also limited by the fact that cognitive measures were not given at Wave 1, and therefore, we cannot examine how cognitive change over 10 years is related to subjective age change.
Another consideration is change in functional health over time. Although functional health at Wave 1 was measured, the Wave 2 measure was used in the present analyses to be consistent with the timing of cognitive measures that were only given during Wave 2. The availability of several variables only at Wave 2, including the social comparison variables used as a mediator, is one limitation of the present study. We were able to test a model using Wave 1 functional health, and the results did not change. Additionally, we found that change in subjective age was correlated with change in functional health. With only two occasions, however, we cannot clearly determine directionality. With future waves of data we will be able to test a reciprocal or cross-lagged model to examine the ongoing processes involving the antecedents and consequences of subjective age.
The present results are noteworthy, as they demonstrate a mechanism by which performance and functioning influences subjective age. This supplements findings from past work that has shown that subjective age predicts performance and functioning. These results provide initial support for the hypothesis that there is an ongoing reciprocal process whereby health and cognition affect changes in subjective age and in turn subjective age affects health and cognition. We have also added evidence for social comparisons as one potential mechanism underlying the relationship between subjective age and health. Future longitudinal and experimental work will be necessary to determine how changes in performance influence changes in subjective age.
Funding
This work was supported by the National Institute on Aging grant PO1 AG20166 and the NRSA Institutional Training grant 5T32AG000204.
References
- Albert M. S. Jones K. Savage C. R. Berkman L. Seeman T. Blazer D., & Rowe J. W (1995). Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychology and Aging, 10, 578–589. doi:10.1037/0882-7974.10.4.578 [DOI] [PubMed] [Google Scholar]
- Barak B., & Stern B (1986). Subjective age correlates: A research note. The Gerontologist, 26, 571–578. doi:10.1093/geront/26.5.571 [DOI] [PubMed] [Google Scholar]
- Brim O. G. Ryff C. D., & Kessler R. C (2004). How healthy are we? A national study of well-being at midlife. Illinois: University of Chicago Press. [Google Scholar]
- Hayes A. F., & Preacher K. J (2014). Statistical mediation analysis with a multicategorical independent variable. British Journal of Mathematical and Statistical Psychology, 67, 451–470. doi:10.1111/bmsp.12028 [DOI] [PubMed] [Google Scholar]
- Heidrich S. M., & Ryff C. D (1993. a). Physical and mental health in later life: The self-system as mediator. Psychology and Aging, 8, 327–338. doi:10.1037/0882-7974.8.3.327 [DOI] [PubMed] [Google Scholar]
- Heidrich S. M., & Ryff C. D (1993. b). The role of social comparisons processes in the psychological adaptation of elderly adults. Journal of Gerontology, 48, 127–136. doi:10.1093/geronj/48.3.P127 [DOI] [PubMed] [Google Scholar]
- Hubley A. M., Russell L. B. (2009). Predictions of subjective age, desired age, and age satisfaction in older adults: Do some health dimensions contribute more than others?International Journal of Behavioral Development, 33, 12–21. doi:10.1177/0165025408099486 [Google Scholar]
- Hughes M. L. Geraci L., & De Forrest R. L (2013). Aging 5 years in 5 minutes: The effect of taking a memory test on older adults’ subjective age. Psychological Science, 24, 2481–2488. doi:10.1177/0956797613494853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human L. J. Biesanz J. C. Miller G. E. Chen E. Lachman M. E., & Seeman T. E (2013). Is change bad? Personality change is associated with poorer psychological health and greater metabolic syndrome in midlife. Journal of Personality, 81, 249–260. doi:10.1111/jopy.12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter-Grühn D. Kleinspehn-Ammerlahn A. Gerstorf D., & Smith J (2009). Self-perceptions of aging predict mortality and change with approaching death: 16-Year longitudinal results from the Berlin Aging Study. Psychology and Aging, 24, 654–667. doi:10.1037/a0016510 [DOI] [PubMed] [Google Scholar]
- Kotter-Grühn D. Kornadt A. E., & Stephan Y (2015). Looking beyond chronological age: Current knowledge and future directions in the study of subjective age. Gerontology, 62, 86–93. doi:10.1159/000438671 [DOI] [PubMed] [Google Scholar]
- Infurna F. J. Gerstorf D. Robertson S. Berg S., & Zarit S. H (2010). The nature and cross-domain correlates of subjective age in the oldest old: Evidence from the OCTO Study. Psychology and Aging, 25, 470–476. doi:10.1037/a0017979 [DOI] [PubMed] [Google Scholar]
- Lachman M. E., Agrigoroaei S. (2010). Promoting functional health in midlife and old age: Long-term protective effects of control beliefs, social support, and physical exercise. PloS ONE, 5. doi:10.1371/journal.pone.0013297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman M. E. Agrigoroaei S. Murphy C., & Tun P. A (2010). Frequent cognitive activity compensates for education differences in episodic memory. The American Journal of Geriatric Psychiatry, 18, 4–10. doi:10.1097/JGP.0b013e3181ab8b62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman M. E. Agrigoroaei S. Tun P. A., & Weaver S. L (2014). Monitoring cognitive functioning: Psychometric properties of the Brief Test of Adult Cognition by Telephone. Assessment, 21, 404–417. doi:10.1177/1073191113508807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman M. E., & Tun P. A (2008). Cognitive testing in large-scale surveys: Assessment by telephone. In Hofer S. M., Alwin D. F. (Eds.), Handbook of cognitive aging: Interdisciplinary perspectives (pp. 506–523). Los Angeles, CA: Sage Publications. [Google Scholar]
- Levy B. R. Slade M. D., & Kasl S. V (2002). Longitudinal benefit of positive self-perceptions of aging on functional health. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57, 409–417. doi:10.1093/geronb/57.5.P409 [DOI] [PubMed] [Google Scholar]
- Montepare J. M., & Lachman M. E (1989). “You’re only as old as you feel”: self-perceptions of age, fears of aging, and life satisfaction from adolescence to old age. Psychology and Aging, 4, 73–78. doi:10.1037/0882-7974.4.1.73 [DOI] [PubMed] [Google Scholar]
- Preacher K. J., & Kelley K (2011). Effect size measures for mediation models: Quantitative strategies for communicating indirect effects. Psychological Methods, 16, 93–115. doi:10.1037/a0022658 [DOI] [PubMed] [Google Scholar]
- Radler B. T., & Ryff C. D (2010). Who participates? Accounting for longitudinal retention in the MIDUS national study of health and well-being. Journal of Aging and Health, 22, 307–331. doi:10.1177/0898264309358617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. C., & Berntsen D (2006). People over forty feel 20% younger than their age: Subjective age across the lifespan. Psychonomic Bulletin & Review, 13, 776–780. doi:10.3758/BF03193996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settersten R. A. Jr., & Hagestad G. O (2015). Subjective aging and new complexities of the life course. Annual Review of Gerontology and Geriatrics, 35, 29–53. doi:10.1891/0198-8794.35.29 [Google Scholar]
- Spuling S. M. Miche M. Wurm S., & Wahl H.-W (2013). Exploring the causal interplay of subjective age and health dimensions in the second half of life. Zeitschrift für Gesundheitspsychologie, 21, 5–15. doi:10.1026/0943-8149/a000084 [Google Scholar]
- Staudinger U. M. (2015). Images of aging: Outside and inside perspectives. Annual Review of Gerontology and Geriatrics, 35, 187–209. doi:10.1891/0198-8794.35.29 [Google Scholar]
- Stephan Y. Caudroit J., & Chalabaev A (2011). Subjective health and memory self-efficacy as mediators in the relation between subjective age and life satisfaction among older adults. Aging & Mental Health, 15, 428–436. doi:10.1080/13607863.2010.536138 [DOI] [PubMed] [Google Scholar]
- Stephan Y. Caudroit J. Jaconelli A., & Terracciano A (2014). Subjective age and cognitive functioning: A 10-year prospective study. The American Journal of Geriatric Psychiatry, 22, 1180–1187. doi:10.1016/j.jagp.2013.03.007 [DOI] [PubMed] [Google Scholar]
- Stephan Y. Chalabaev A. Kotter-Grühn D., & Jaconelli A (2013). “Feeling younger, being stronger”: an experimental study of subjective age and physical functioning among older adults. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 68, 1–7. doi:10.1093/geronb/gbs037 [DOI] [PubMed] [Google Scholar]
- Stephan Y., Sutin A. R., Caudroit J., Terracciano A. (2015). Subjective age and changes in memory in older adults. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, doi:10.1093/geronb/gbv010 [DOI] [PubMed] [Google Scholar]
- Stephan Y. Sutin A. R., & Terracciano A (2015. a). How old do you feel? The role of age discrimination and biological aging in subjective age. PloS ONE, 10, e0119293. doi:10.1371/journal.pone.0119293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan Y. Sutin A. R., & Terracciano A (2015. b). Subjective age and personality development: A 10-year study. Journal of Personality, 83, 142–154. doi:10.1111/jopy.12090 [DOI] [PubMed] [Google Scholar]
- Tun P. A., & Lachman M. E (2008). Age differences in reaction time and attention in a national telephone sample of adults: Education, sex, and task complexity matter. Developmental Psychology, 44, 1421–1429. doi:10.1037/a0012845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel M. P. Buntinx F. Houx P. J. Metsemakers J. F. Knottnerus A., & Jolles J (1998). The relation between morbidity and cognitive performance in a normal aging population. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 53, M147–M154. doi:10.1093/gerona/53A.2.M147 [DOI] [PubMed] [Google Scholar]
- Ward R. A. (2013). Change in perceived age in middle and later life. International Journal of Aging and Human Development, 76, 251–267. doi:10.02190/AG.76.3.e [DOI] [PubMed] [Google Scholar]
- Ware J. E., & Sherbourne C. D (1992). The MOS 36-item short-form health survey (SF-36): Conceptual framework and item selection. Medical Care, 30, 473–483. doi:10.1097/000056 50-199206000-00002 [PubMed] [Google Scholar]
- Westerhof G. J. Miche M. Brothers A. F. Barrett A. E. Diehl M. Montepare J. M., & Wurm S (2014). The influence of subjective aging on health and longevity: A meta-analysis of longitudinal data. Psychology and Aging, 29, 793–802. doi:10.1037/a0038016 [DOI] [PubMed] [Google Scholar]