Abstract
Objective
To study time trends in the incidence of persistent cognitive decline (PCD), and whether an increase or decrease is explained by changes in well-known risk factors of dementia.
Method
Data from the Longitudinal Aging Study Amsterdam over a period of 20 years were used. Subsamples of 65–88 year-olds were selected at 7 waves, with numbers ranging from 1,800 to 1,165. Within-person change in cognitive functioning was used to determine PCD. In logistic generalized estimating equations (GEE), time (0, 3, 6, 9, 13, and 16 years) was the main predictor of 3-year PCD incidence. Explanatory variables were lagged one wave before incident PCD and included in separate models.
Results
PCD incidence was 2.5% at first, and 3.4% at last follow-up. GEE showed a positive time trend for PCD incidence [Exp(B)time = 1.042; p < .001]. None of the explanatory variables significantly changed the strength of the regression coefficient of linear time. Higher age, lower education, diabetes mellitus, smoking, lower body-mass index, and lower level of physical activity were associated with higher incidence of PCD.
Conclusion
An increase in PCD incidence over time was found. Although well-known risk factors were associated with incidence per se, they did not explain the increase in incidence of PCD.
Keywords: Dementia, Incidence, Older adults, Risk factors
Studying trends in rates of cognitive decline and dementia in nationally representative surveys provides a basis for estimates of future demands for care, which is needed for policy making and planning health and welfare resources in dementia care (Alzheimer’ s Disease International, 2015; Prince et al., 2013). Changes in rates of cognitive decline and dementia may be expected because of the rising prevalence of some of its risk factors and an increasing number of persons at risk (Matthews et al., 2013; Prince, Ali, Guerchet, Prina, Albanese & Wu, 2016). On the other hand, higher education (Meng et al., 2012), better treatment for cardiovascular diseases (CVD) and diabetes especially in high-income countries might lead to a decrease in dementia rates (Matthews et al., 2013).
Studies directed on trends in prevalence rates showed decreasing rates of dementia in the United Kingdom (Mathews et al., 2013), Germany (Doblhammer, Fink, & Fritze, 2015), and Spain (Lobo et al., 2007), increasing rates in Canada (Kosteniuk et al., 2016), Western Europe (Prince et al., 2013), China (Chan et al., 2013) and Japan (Dodge et al., 2012), and stable rates in Sweden (Wiberg, Waern, Billstedt, Ostling, & Skoog, 2013), and the United States (Hall et al., 2009; Rocca et al., 2011).
Secular trends in the incidence of dementia are less well studied but most results seem to indicate a decrease or stable incidence rate. The incidence rates of dementia in subsequent birth cohorts in the United States (Satizabal et al., 2016), Canada (Kosteniuk et al., 2016), and the United Kingdom (Matthews et al., 2016) decreased, whereas in France (Grasset et al., 2015) the incidence rates of dementia diagnosis remained stable. In the Netherlands a non-significant decrease in incidence rates was reported (Schrijvers et al., 2012), while another study based on the clinical records of general practitioners found an increase in incidence of dementia in the Netherlands (Van Bussel et al., 2017).
In most studies, dementia was defined according to strict criteria, which excluded persons with Mild Cognitive Impairment (MCI), whereas many persons with MCI fulfill the strict dementia criteria in the subsequent years. Before a formal diagnosis of dementia can be made, there is a period of more than average cognitive decline over a long period of time. To measure these cognitive changes long follow-up assessments are needed. The Longitudinal Aging Study Amsterdam (LASA), a nationally representative sample with 20-year follow-up measures of cognitive functioning in the Netherlands has such data. This enables us to study the incidence of significant and persistent cognitive decline, thus covering both MCI and dementia.
In the present study, we aimed to examine whether the incidence of persistent cognitive decline (PCD, for definition see Method section) changed between 1995 and 2012 taking into account age and sex. Subsequently, we studied whether an increase or decrease in the time trend can be explained by its known risk factors education, CVD, diabetes mellitus, high blood pressure, anti-hypertensive medication, life-style, and depressive symptoms.
Method
Sample
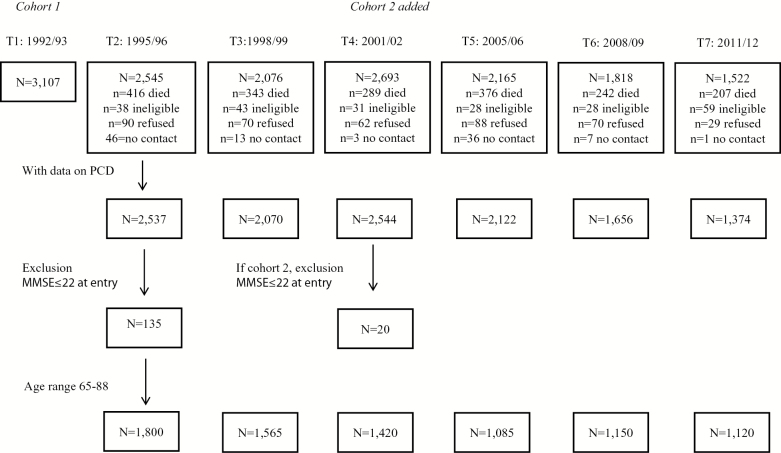
Data were used from LASA, an ongoing prospective population based-study, focusing on predictors and change in cognitive, emotional, physical, and social functioning in later life. Details on the procedures on sampling, data collection, and nonresponse have been described in Huisman and colleagues (2011). In short, a random sample of older men and women, aged 55–85 stratified by age and sex according to the expected 5-year mortality, was drawn from the population registries of 11 municipalities in three geographic areas across the Netherlands. The first measurement wave took place in 1992/1993 and included 3,107 participants. Follow-up assessments were conducted approximately every 3 years. For the present study, data were used from eight waves covering 23 years of follow-up (T1: 1992/1993; T2: 1995/1996; T3: 1998/1999; T4: 2001/2002; T5: 2005/2006; T6: 2008/2009; T7: 2011/2012; and T8: 2015/2016). As the composition of the PCD variable required at least two waves of cognitive functioning, incidence of PCD and time trend analyses were available at T2, T3, T4, T5, T6, and T7. At each wave respondents aged 65–88 were included in the study sample, as this was the age range available in all waves. To exclude possible prevalent dementia cases at baseline, persons with an MMSE of 22 or less (mean MMSE in persons with low education—1.5 SD) at entry were excluded from the study sample. See Figure 1 for a flow chart of the study sample. Interviews were conducted in the homes of the respondents by specially trained and intensively supervised interviewers. The study was approved by the Ethical Review Board of the VU University Medical Center. Written informed consent was obtained from all respondents.
Figure 1.
Flow chart of study sample. Note. PCD = persistent cognitive decline; MMSE = Mini-Mental State Examination.
Persistent Cognitive Decline
The Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975) was administered during all waves. Participants who were not able or refused to participate in the complete follow-up interview were asked to participate in a telephone interview, which included an abbreviated version of the MMSE. This version included the following items: year; day of the week; month; two streets in the neighborhood; address; repeating three words; the highest score on either subtracting (100–7) or spelling backwards; remembering three words (Van den Kommer et al., 2008). For participants who were not able or refused to complete the telephone interview, an abbreviated version of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE, de Jonghe, Schmand, Ooms, & Ribbe, 1997) was administered. This short version has been shown to measure cognitive change in everyday activities of older adults and may be used as an efficient rating scale for clinical assessment of dementia (de Jonghe et al., 1997). Clinically significant cognitive decline was defined by a minimum score of 28 (i.e., the maximum score of 5 on at least four areas of the IQCODE, and a score of 4 on the remaining two areas) (Van den Kommer et al., 2008).
PCD was based on as (a) a significant decline of at least 2 Standard Deviations (SD) below the mean decline on the MMSE since the last wave (measured between wave (W) and W+1) or a score of ≥28 points on the IQCODE and (b) continued cognitive decline up to the next wave (confirmed at W+2, thus coded at W+1 as having PCD), that is, decline of at least 1 SD below the sample mean decline on the MMSE or a score of ≥28 points on the IQCODE at the next wave (W+2). Furthermore, if applicable the interviewers recorded reasons for loss-to-follow-up, in which “dementia” was one of the response categories. In addition, other relevant data recorded by the interviewers, information from General Practitioners (GP) concerning dementia diagnosis by GP or specialist, data on psychogeriatric nursing home admittance and data concerning cause of death (ICD codes of dementia) were used to obtain information about a PCD diagnosis.
Respondents who showed clinically significant decline over 3 years of follow-up on the MMSE or IQCODE, but with a undetermined trajectory due to missing data at follow-up (W+2), or insufficient continued decline (i.e., not fully confirmed at W+2), were characterized as having “PCD” in case of: (a) an MMSE score of 18 or lower at the last available wave (W+1 or W+2); (b) records or relevant information from the interviewers indicating dementia as the reason for loss-to-follow-up; (c) GP records of a dementia diagnosis; (d) records of psychogeriatric nursing home admittance; and (e) loss-to-follow-up due to death with dementia documented as the cause of death.
Covariates
Potential confounders and explanatory variables were chosen on the basis of previous literature on predictors of cognitive decline and dementia (Van der Flier et al., 2005, Van den Kommer, Comijs, Dik, Jonker, & Deeg, 2008, Qiu, Xu, & Fratiglioni, 2010; Middleton & Yaffe, 2010). Sex and age were included as confounding variables. Level of education measured in years was included as a time-invariant (fixed) continuous explanatory variable. CVD, diabetes mellitus, high blood pressure, use of anti-hypertensive medication, alcohol use, smoking status, physical activity, body mass index (BMI), and depressive symptoms were all included as time-variant explanatory variables measured at T1, T2, T3, T4, T5, and T6, that is, one wave prior to PCD was determined. Diabetes mellitus and CVD (angina pectoris, myocardial infarct, congestive heart failure, cardiac arrhythmia, peripheral arterial disease, and cerebrovascular accident) were measured using self-report and medication use. High blood pressure defined as ≥140/90 and use of anti-hypertensive medication were included as dichotomous variables. Alcohol use was assessed by asking for the number of alcoholic units per week over the past year, and for the number of days per week on which alcohol was consumed, and was thereafter classified as “no,” middle,” and “high” consumption according to the Netherlands Economic Institute index (Reinhard and Rood-Bakker, 1998). Smoking status was assessed by self-report and dichotomized into “smokers” and “nonsmokers.” Former smokers were classified as smokers if they stopped smoking less than 15 years ago, whereas former smokers who stopped smoking for 15 years or more were classified as nonsmokers since mortality in former smokers approaches the level of never smokers after a smoking cessation time of 10–20 years (Kawachi et al., 1993, Paganini-Hill et al., 1994). Physical activity was assessed by means of the LASA physical activity questionnaire (LAPAQ; Stel et al., 2004). Frequency and duration of indoor and outdoor physical activities were measured and converted to total time spent on physical activities in min/day. Physical activity was included as a continuous variable. BMI was calculated as weight (kg)/height (m)2, and was included as a continuous variable. Depressive symptoms were assessed with the Center for Epidemiologic Studies Depression Scale (CES-D), a widely used 20-item self-report scale designed to measure depressive symptoms in the community (Radloff, 1977). The total score was dichotomized according to the generally applied cut-off score of ≥16, that has been validated for both men and women to identify persons with clinically relevant depressive symptoms (Beekman et al., 1997).
Data Analysis
To prevent selection bias and loss of information due to missing values on the covariates, multiple imputation was performed. Imputations were generated using the multivariate imputation by chained equations (MICE) procedure as implemented in SPSS. This is a flexible imputation method that uses a series of regression imputation models based on the information of other variables to estimate and substitute the missing values. We included all covariates and the outcome measure in the imputation model for optimal estimation of missing values. Seventy datasets were generated, on the basis of the percentage of missing values in the total sample. Imputed data on the outcome measure and data imputed on covariates for already deceased participants were not used in the analyses. The pooled results of the imputed datasets are presented.
To make sure that changes in incidence of PCD reflected time trends, and not the general ageing of the population incidence rates for PCD at T2–T7 were age- and gender-weighted according to the composition of the sample at T5, which closely resembled that of the general Dutch population. Probability weights were computed by dividing 5-year age and gender strata proportions at T2, T3, T4, T6, and T7 by proportions of the same strata at T5.
Time trend analyses were performed using generalized estimating equations (GEE). At the subsequent waves, subsets (65–88 years old) from the total LASA sample were included that were partly overlapping; therefore repeated measurements within subjects are taken into account by including an unstructured correlation structure. GEE analyses were not weighted since all models were adjusted for age and gender. A time variable was created to represent the increase in study years (0, 3, 6, 9, 13, and 16 years of follow-up). First, the predictive value of time in years for PCD as the outcome measure was tested for significance (at p < .05). Second, a quadratic term of time was added to test the presence of a nonlinear time trend. If significant (p < .10), the quadratic term was retained in the model. Third, the potential explanatory variables (lagged, measured one wave earlier) were entered into the model one by one to examine whether these could explain a time trend in incidence of PCD.
Two series of sensitivity analyses were performed, in which respondents showing significant cognitive decline but; (a) with an undetermined trajectory due to loss-to-follow-up, or (b) with less than 1 SD below the mean decline on the MMSE, stability or improvement but with an MMSE ≤18 at subsequent waves, or (c) for whom no additional sources (i.e., recorded reason for loss-to-follow-up, GP records, recorded cause of death, recorded living situation) indicated dementia, were (a) recoded from “PCD” to “no PCD,” or (b) excluded from the analyses.
Results
The characteristics of the sample at the six waves before PCD assessment are presented in Table 1. PCD incidence at T2 (1995/1996) was 2.45%, weighted by age and sex of the Dutch population (Table 2). The incidence appears to increase over the subsequent 10 years to 4.19% at T4 (2005/2006), and to decline in the subsequent 6 years to 3.36%. The results from the sensitivity analyses are also presented in Table 2. In these analyses respondents showing clinically significant cognitive decline (>2 SD) between two waves, but the trajectory of further cognitive decline in the subsequent years is inconsistent or uncertain due to missing information are coded as “no PCD” (sensitivity analyses 1) or are excluded (sensitivity analyses 2). Obviously, incidence rates are overall lower, but show a similar trend over time.
Table 1.
Characteristics (Lagged One Wave) of the Study Sample Aged 65–88 at T1–T6 and With Data on PCD and MMSE ≤22 at Study entry
| Characteristics (pooled) | T1 (1992/1993), n = 1,800 | T2 (1995/1996), n = 1,565 | T3 (1998/1999), n = 1,420 | T4 (2001/2002), n = 1,085 | T5 (2005/2006), n = 1,150 | T6 (2008/2009), n = 1,120 |
|---|---|---|---|---|---|---|
| Education, in years, mean (SD) | 8.81 (3.30) | 8.98 (3.24) | 9.15 (3.26) | 9.33 (3.26) | 9.96 (3.40) | 10.27 (3.41) |
| Cardiovascular disease, yes, % (n) | 26.33 (474) | 25.07 (392.4) | 25.56 (363.0) | 28.40 (308.1) | 28.83 (331.5) | 32.23 (361) |
| Diabetes mellitus, yes, % (n) | 5.93 (106.8) | 6.28 (98.3) | 7.60 (107.9) | 9.58 (103.9) | 11.77 (135.3) | 12.41 (139) |
| High blood pressure, yes, % (n) | 27.69 (498.5) | 68.33 (1069.4) | 68.68 (975.3) | 59.14 (641.7) | 61.86 (711.4) | 50.58 (566.5) |
| Use of anti-hypertensive, yes, % (n) | 26.64 (479.5) | 30.23 (473.1) | 34.89 (495.4) | 39.76 (431.4) | 47.14 (542.1) | 48.25 (540.4) |
| Alcohol use | ||||||
| No | 21.59 (388.7) | 23.11 (361.6) | 18.68 (265.2) | 18.06 (196) | 16.33 (187.8) | 16.04 (179.7) |
| Middle | 70.52 (1269.4) | 65.81 (1030) | 70.74 (1004.5) | 71.64 (777.3) | 71.51 (822.4) | 72.07 (807.2) |
| High | 7.88 (141.8) | 11.08 (173.4) | 10.58 (150.3) | 10.29 (111.7) | 12.16 (139.8) | 11.88 (133.1) |
| Smoking status, smoker, % (n) | 38.18 (687.2) | 32.68 (511.5) | 26.52 (376.6) | 28.53 (309.5) | 22.48 (258.5) | 26.48 (296.6) |
| Body mass index mean (SD) | 26.51 (24.17–29.31) | 26.62 (24.17–29.19) | 26.90 (24.45–29.49) | 26.76 (24.56–29.76) | 27.05 (24.78–30.02) | 26.97 (24.66–30.10) |
| Physical activity, min/day, median (interquartile range) | 140.96 (79.87–218.43) | 142.13 (86.97–210.64) | 140.63 (82.60–210.98) | 139.35 (81.91–208.76) | 132.92 (83.70–199.22) | 135.40 (83.59–198.09) |
| Clinically relevant depressive symptoms, yes, % (n) | 13.47 (242.4) | 13.42 (210.0) | 15.10 (214.4) | 13.96 (151.5) | 14.29 (164.3) | 10.47 (117.3) |
Note. PCD = persistent cognitive decline; MMSE = Mini-Mental Status Examination; SD = standard deviation.
Table 2.
Incidence of PCD at Six Time Points Weighted According to Age and Sex in the Dutch Population (2005/2006)
| Wave | Sensitivity analyses | ||
|---|---|---|---|
| Weighted % | Weighted % a | Weighted % b | |
| T2: 1995/1996 | 2.45 | 1.13 | 1.15 |
| T3: 1998/1999 | 3.19 | 1.76 | 1.78 |
| T4: 2001/2002 | 3.46 | 1.82 | 1.85 |
| T5: 2005/2006 | 4.191 | 3.182 | 3.223 |
| T6: 2008/2009 | 3.00 | 1.98 | 2.00 |
| T7: 2011/2012 | 3.36 | 2.37 | 2.39 |
Note: PCD = persistent cognitive decline.
aSensitivity analysis recoding persons with undetermined trajectory to “no PCD.”
bSensitivity analysis excluding persons with undetermined trajectory from the analyses.
c4-Year incidence, (derived) 3-year incidence [(4.19/4) × 3 = 3.14].
d4-Year incidence, (derived) 3-year incidence [(3.18/4) × 3 = 2.39].
e4-Year incidence, (derived) 3-year incidence [(3.22/4) × 3 = 2.49].
Logistic GEE (Table 3) shows that time is positively associated with incident PCD in the models that were adjusted for age and sex [Exp(B)time = 1.035, 95% confidence interval (CI): 1.015–1.055; p = .001]. The quadratic term of time was nonsignificant (p > .10), and was therefore not retained in the model. Adding the explanatory variables in the consecutive models did not substantially change the strength of the time trend. In sum, we found evidence for a significant positive time trend in PCD incidence between 1995 and 2012, in age-and-sex adjusted models. Changes in level of education, CVD, diabetes mellitus, high blood pressure, anti-hypertensive medication, life-style, and depressive symptoms did not explain this increase in PCD incidence rate.
Table 3.
Time Trend in Incident PCD over a Period of 16 Years
| Main analyses | Exp(B) | p-Value |
|---|---|---|
| Model 1: corrected for age, sex | ||
| Time | 1.035 | .001 |
| Model 2: additionally adjusted for education | ||
| Time | 1.037 | <.001 |
| Model 3: additionally adjusted for CVD, diabetes | ||
| Time | 1.033 | .002 |
| Model 4: additionally adjusted for high blood pressure and use of anti-hypertensive medication | ||
| Time | 1.036 | 0.001 |
| Model 5: additionally adjusted for life-style | ||
| Time | 1.042 | <0.001 |
| Model 6: additionally adjusted for depressive symptoms | ||
| Time | 1.042 | <.001 |
| Age | 1.166 | <.001 |
| Sex (male) | 0.841 | .231 |
| Education | 0.948 | .013 |
| No cardiovascular disease | 0.954 | .759 |
| No diabetes mellitus | 0.430 | <.001 |
| No high blood pressure | 1.015 | .909 |
| No anti-hypertensives | 1.143 | .385 |
| No alcohol use (vs high) | 1.376 | .258 |
| Middle alcohol use (vs high) | 1.129 | .643 |
| No smoking | 0.702 | .017 |
| Physical activity | 0.998 | .027 |
| BMI | 0.939 | .001 |
| No clinically relevant depressive symptoms | 0.834 | .287 |
| Sensitivity analyses | Exp(B) | p-Value |
| Model 6a: additionally adjusted for depressive symptoms (n = 3,031/8,460) | ||
| Time | 1.069 | <.001 |
| Model 6b: additionally adjusted for depressive symptoms (n = 3,001/8,335) | ||
| Time | 1.069 | <.001 |
Note: Pooled data are shown. PCD = persistent cognitive decline; CVD = cardiovascular disease; n = 3,031/8,455 observations.
aSensitivity analysis recoding persons with undetermined trajectory to “no PCD.”
bSensitivity analysis excluding persons with undetermined trajectory from the analyses.
The fully adjusted models showed that respondents with higher age, lower education, diabetes mellitus, who smoked, with lower BMI, and who were less physically active were more likely to be classified with PCD after 3 years. Time trend in PCD incidence remained significant despite taking into account these explanatory factors [Exp(B)time=1.042, 95% CI: 1.020–1.065; p < .001].
The first set of sensitivity analyses in which respondents who showed clinically significant cognitive decline but with an undetermined trajectory were recoded from “PCD” to “no PCD,” showed an increase in effect, with a significant positive time trend for incident dementia in the fully adjusted model [Exp(B)time = 1.069, 95% CI: 1.042–1.097; p < .001]. The confidence interval of this effect, however, largely overlaps with the confidence interval of the time trend found in the main analyses, indicating that the positive time effect in these sensitivity analyses was not significantly higher.
The second set of sensitivity analyses in which respondents who showed clinically significant decline but an undetermined trajectory were excluded from the analyses, showed similar results to the first set of sensitivity analyses with a significant positive time trend for incident dementia [Exp(B)time = 1.069, 95% CI: 1.042–1.097; p < .001] in the fully adjusted model.
Discussion
We found an increase in incidence rates of PCD over a 16-year follow-up period. In 1995/1996, the incidence rate was 2.45 and in 2011/2012, it was 3.36. Per 3–4 years the incidence rate of PCD showed relative increases by about 5% between 1995/1996 and 2005/2006 and then dropped again but remained at a higher level than in 1995/1996. The relatively high incidence rate in 2005/2006 may have been caused by the 4-year follow-up period since 2001/2002, whereas all other incidence rates concerned 3-year periods. Nevertheless, the increase in incidence clearly took place in the first 10 years of our study period. The time trend we found could not be explained by changes in sociodemographic characteristics, CVD, diabetes, high blood pressure, use of hypertensive medication, life-style, and depressive symptoms as present 3 years before PCD. During the study period another cohort (55–65 years old) was added. As this could have influenced our findings we did an additional sensitivity analyses (results not shown). These showed that adding cohort to the model did not affect the strength of the time trend. Also, cohort was not significantly associated with incidence of PCD.
The increase in incidence rates we found is not in line with most previous studies on trends in incidence rates of dementia in Western countries, which mainly showed stable or decreasing incidence rates. In the Netherlands previously a nonsignificant decrease (Schrijvers et al., 2012) and an increase of dementia incidence (Van Bussel et al., 2017) was shown. An increase of rates of PCD might have been expected because of the rising incidence of its risk factors, such as higher life expectancy, diabetes, obesity, physical inactivity, and higher alcohol consumption (Matthews et al., 2013, Prince et al., 2016). Most of these risk factors were indeed associated with PCD incidence per se, but did not explain the increase of PCD incidence rates over time. We are not sure how to explain the increase in incidence of PCD. This is the first study evaluating incidence of PCD, while others evaluated dementia. The difference in definition of these outcomes may also partly be responsible for the deviating findings. Also, the apparent increase may be related to risk factors other than the ones mentioned above.
In most of the previous studies dementia was defined according to strict criteria, and excluded MCI cases. We focused on PCD rather than a formal diagnosis of dementia, thus including MCI and dementia cases, while at the same time circumventing possible changes over time in diagnostic procedures. This could have influenced the prevalence rates, but not the incidence rates. By applying PCD criteria the same persons can be identified as when using strict dementia criteria, but in an earlier phase. Our incidence rates are indeed largely in line with the estimated incidence rates of dementia in Europe (Alzheimer’s Disease International, 2015).
Furthermore, in the present population-based study we were not able to diagnose or specify underlying causes of the persistent decline in cognitive functioning, using for instance the revised NIA-AA criteria for Alzheimer’s disease in which biomarkers are part of the diagnostic criteria or NINDS-AIREN criteria for classification of vascular dementia. However, despite the unknown etiology of the decline the mere finding of an increase in incidence rates is an important finding in terms of projections of future health care needs, costs and planning, and requires future studies as such.
In the Diagnostic Statistical Manual (DSM)-5 a new category, neurocognitive disorders (NCD), is introduced (American Psychiatric Association, 2013). This category replaces former MCI and dementia diagnoses in the DSM-IV. The introduction of NCD stresses that more than average cognitive decline and dementia are a continuum. Our definition of PCD is comparable with the newly introduced NCD criteria. We were able to determine significant and persistent cognitive decline within a person over a period of 6 years, which is a prerequisite for diagnosing NCD. However, we were not able to distinguish between mild and major NCD, since we had no adequate information on limitations in activities of daily life.
In the present study, we use longitudinal data from LASA, which has a rich set of variables covering physical, mental, and social functioning over more than 20 years of follow-up. The chance that we by mistake classified persons as having PCD (false positives) is small, because we determined the persistence of the decline over a period of 6 years, including three observations. We used as much information as possible from the proxy or general practitioner of persons who dropped out of the study due to severe cognitive problems to limit the consequences of drop-out for our results. There are, however, no indications that the drop-out rate is different throughout the years, so this can not explain the time trend that we found.
We might have missed some early dementia cases. In our study, persons were considered as having clinically significant cognitive decline over 3 years when they declined at least 2 SD below the mean decline on the MMSE since the last wave. This might be too strict; there may have been people with early phase dementia with a somewhat slower decline that we missed. However, when we would have lowered our criteria to for instance 1.5 SD, we would have introduced a higher risk for including persons without dementia as cases (false positives).
In our study, the persons with severe dementia and also the severe mentally or physically ill were less likely to sustain a full interview. As these frail persons were the ones we were especially interested in we asked them to participate in a short interview or telephone interview or we asked them permission to ask a proxy about their functioning. This way we were able to get important information about their cognitive functioning. Also by looking into contact information forms, GP information and information about admission to a dementia care unit of a nursing home, we were able to get information about possible dementia, which we included in our PCD diagnoses. Nevertheless, we did not have this information from all respondents who dropped out of the study. Therefore, our incidence rates might be an underestimation of the true PCD incidence. Also, persons who deceased before significant cognitive decline could have been measured may have been become incident cases before death. However, the change in incidence rates is most likely not influenced by this information bias, because the same procedures were followed during the full study period. Thus, our conclusion regarding an increase of the incidence rates is not affected.
Even though we cannot fully explain our findings of increasing incidence of PCD over time, it is important to note our findings are largely in line with the projections made by ADI (Alzheimer’s Disease International, 2015). This is important to realize and it shows that—even when taking into account the beneficial effects of a healthier lifestyle and improved treatment of cardiovascular risk factors—there is a great need to further study which factors account for the increase of PCD. The increase of the aging population may lead to an exponential growth of the number of people with PCD in the near future, which will be a major challenge for social-care systems and health care costs.
In conclusion, we found evidence for an increase in PCD incidence over time, taking into account age and sex. Although socio-demographic and health characteristics were associated with incidence per se, they did not explain the increase in incidence of PCD. Further studies are necessary to explain the increase of PCD incidence.
Funding
This work was supported by the following funding source, NIA P30 AG012846, to the University of Michigan. The Longitudinal Aging Study Amsterdam is largely supported by the Netherlands Ministry of Health Welfare and Sports, Directorate of Long-Term Care.
Acknowledgement
Author contributions: H. C. Comijs, together with T. N. van den Kommer and D. J. H. Deeg planned the study, and applied for funding. H. C. Comijs and T. N. van den Kommer composed the outcome variable. T. N. van den Kommer performed statistical analyses and D. J. H. Deeg supervised applied methodology. All authors contributed to the writing and revision of the manuscript.
Conflict of Interest
All authors have no conflicts of interest.
References
- American Psychiatric Association.(2013). Diagnostic and statistical manual of mental disorders. 5th ed Washington, DC: Author. [Google Scholar]
- Alzheimer’ s Disease International (2015). World Alzheimer report 2015. The global impact of dementia. An analysis of prevalence, incidence, cost and trends. London: Author. [Google Scholar]
- Beekman A. T., Deeg D. J., Van Limbeek J., Braam A. W., De Vries M. Z., & Van Tilburg W (1997). Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): Results from a community-based sample of older subjects in The Netherlands. Psychological Medicine, 27, 231–235. [DOI] [PubMed] [Google Scholar]
- Chan K. Y., Wang W., Wu J. J., Liu L., Theodoratou E., Car J., … Rudan I; Global Health Epidemiology Reference Group (GHERG) (2013). Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet (London, England), 381, 2016–2023. doi:10.1016/S0140-6736(13)60221-4 [DOI] [PubMed] [Google Scholar]
- de Jonghe J. F., Schmand B., Ooms M. E., & Ribbe M. W (1997). Abbreviated form of the Informant Questionnaire on cognitive decline in the elderly. Tijdschrift voor Gerontologie en Geriatrie, 28, 224–229. [PubMed] [Google Scholar]
- Doblhammer G., Fink A., & Fritze T (2015). Short-term trends in dementia prevalence in Germany between the years 2007 and 2009. Alzheimers & Dementia, 11, 291–299. doi:10.1016/j.jalz.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Dodge H. H., Buracchio T. J., Fisher G. G., Kiyohara Y., Meguro K., Tanizaki Y., & Kaye J. A (2012). Trends in the prevalence of dementia in Japan. International Journal of Alzheimer’s Disease, 2012, 956354. doi:10.1155/2012/956354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., & McHugh P. R (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Grasset L., Brayne C., Joly P., Jacqmin-Gadda H., Peres K., Foubert-Samier A., … Helmer C (2015). Trends in dementia incidence: Evolution over a 10-year period in France. Alzheimer & Dementia, 12, 272–280. doi:10.1016/j.jalz.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Hall K. S., Gao S., Baiyewu O., Lane K. A., Gureje O., Shen J., … Hendrie H. C (2009). Prevalence rates for dementia and Alzheimer’s disease in African Americans: 1992 versus 2001. Alzheimer’s & Dementia, 5, 227–233. doi:10.1016/j.jalz.2009.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman M., Poppelaars J., van der Horst M., Beekman A. T., Brug J., van Tilburg T. G., & Deeg D. J (2011). Cohort profile: The Longitudinal Aging Study Amsterdam. International Journal of Epidemiology, 40, 868–876. doi:10.1093/ije/dyq219 [DOI] [PubMed] [Google Scholar]
- Kawachi I., Colditz G. A., Stampfer M. J., Willett W. C., Manson J. E., Rosner B., … Speizer F. E (1993). Smoking cessation in relation to total mortality rates in women. A prospective cohort study. Annals of Internal Medicine, 119, 992–1000. doi:10.7326/0003-4819-119-10-199311150-00005 [DOI] [PubMed] [Google Scholar]
- Kosteniuk J. G., Morgan D. G., O’Connell M. E., Kirk A., Crossley M., Teare G. F., … Quail J. M (2016). Simultaneous temporal trends in dementia incidence and prevalence, 2005–2013: A population-based retrospective cohort study in Saskatchewan, Canada. International Psychogeriatrics, 28, 1643–1658. doi:10.1017/S1041610216000818 [DOI] [PubMed] [Google Scholar]
- Lobo A., Saz P., Marcos G., Dia J. L., De-la-Camara C., Ventura T., … Aznar S; ZARADEMP Workgroup (2007). Prevalence of dementia in a southern European population in two different time periods: The ZARADEMP Project. Acta Psychiatrica Scandinavica, 116, 299–307. doi:10.1111/j.1600-0447.2007.01006.x [DOI] [PubMed] [Google Scholar]
- Matthews F. E., Arthur A., Barnes L. E., Bond J., Jagger C., Robinson L., & Brayne C (2013). A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: Results of the Cognitive Function and Ageing Study I and II. The Lancet, 26, 1405–1412. doi:10.1016/S0140-6736(13)61570–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews F. E., Stephan B. C., Robinson L., Jagger C., Barnes L. E., Arthur A., & Brayne C; Cognitive Function and Ageing Studies (CFAS) Collaboration (2016). A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nature Communications, 7, 11398. doi:10.1038/ncomms11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., & D’Arcy C (2012). Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One, 7, e38268. doi:10.1371/journal.pone.0038268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton L. E., & Yaffe K (2010). Targets for the prevention of dementia. Journal of Alzheimer’s Disease, 20, 915–924. doi:10.3233/JAD-2010-091657 [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A., & Hsu G (1994). Smoking and mortality among residents of a California retirement community. American Journal of Public Health, 84, 992–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M., Ali G. C., Guerchet M., Prina A. M., Albanese E., & Wu Y. T (2016). Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer’s Research & Therapy, 8, 23. doi:10.1186/s13195-016-0188-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., & Ferri C. P (2013). The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s & Dementia, 9, 63–75.e2. doi:10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Qiu C., Xu W., & Fratiglioni L (2010). Vascular and psychosocial factors in Alzheimer’s disease: Epidemiological evidence toward intervention. Journal of Alzheimer’s disease, 20, 689–697. doi:10.3233/JAD-2010-091663 [DOI] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. [Google Scholar]
- Reinhard O., & Rood-Bakker D (1998). Alcoholgebruik in beeld. Standaardmeetlat [Alcohol use in image. Standards]. Rotterdam: Nederlands Economisch Instituut. [Google Scholar]
- Rocca, W.A., Petersen, R.C., Knopman, D.C., Hebert, L.E., Evans, D.A., Hall, K.S., ... White, L.R.(2011). Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimer’s & Dementia, 7, 80–93. doi:10.1016/j.jalz.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satizabal C. L., Beiser A. S., Chouraki V., Chêne G., Dufouil C., & Seshadri S (2016). Incidence of dementia over three decades in the Framingham Heart Study. The New England Journal of Medicine, 11, 523–532. doi:10.1056/NEJMoa1504327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers E. M., Verhaaren B. F., Koudstaal P. J., Hofman A., Ikram M. A., & Breteler M. M (2012). Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology, 78, 1456–1463. doi:10.1212/WNL.0b013e3182553be6 [DOI] [PubMed] [Google Scholar]
- Stel V. S., Smit J. H., Pluijm S. M., Visser M., Deeg D. J., & Lips P (2004). Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. Journal of Clinical Epidemiology, 57, 252–258. doi:10.1016/j.jclinepi.2003.07.008 [DOI] [PubMed] [Google Scholar]
- Van der Flier W. M., & Scheltens P (2005). Epidemiology and risk factors of dementia. Journal of Neurology, Neurosurgery, and Psychiatry, 76(Suppl. 5):v2–v7. doi:10.1136/jnnp.2005.082867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bussel E. F., Richard E., Arts D. L., Nooyens A. C., Coloma P. M., de Waal M. W., … Moll van Charante E. P (2017). Dementia incidence trend over 1992–2014 in the Netherlands: Analysis of primary care data. PLoS Medicine, 14, e1002235. doi:10.1371/journal.pmed.1002235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Kommer T.N., Comijs H.C., Dik M.G., Jonker C., & Deeg D.J.H (2008). Development of classification models for early identification of persons at risk for persistent cognitive decline. Journal of Neurology, 255, 1486–1494. doi:10.1007/s00415-008-0942-3 [DOI] [PubMed] [Google Scholar]
- Wiberg P., Waern M., Billstedt E., Ostling S., & Skoog I (2013). Secular trends in the prevalence of dementia and depression in Swedish septuagenarians 1976–2006. Psychological Medicine, 43, 2627–2634. doi:10.1017/S0033291713000299 [DOI] [PubMed] [Google Scholar]