Abstract
Our understanding of muscle glycosylation to date has derived from studies in mouse models and a limited number of human lectin histochemistry studies. As various therapeutic approaches aimed at treating patients with muscular dystrophies are being translated from rodent models to human, it is critical to better understand human muscle glycosylation and relevant disease-specific differences between healthy and dystrophic muscle. Here, we report the first quantitative characterization of human muscle glycosylation, and identify differentiation- and disease-specific differences in human muscle glycosylation. Utilizing a panel of 13 lectins with varying glycan specificities, we surveyed lectin binding to primary and immortalized myoblasts and myotubes from healthy and dystrophic sources. Following differentiation of primary and immortalized healthy human muscle cells, we observed increased binding of Narcissus pseudonarcissus agglutinin (NPA), PNA, MAA-II and WFA to myotubes compared to myoblasts. Following differentiation of immortalized healthy and dystrophic human muscle cells, we observed disease-specific differences in binding of NPA, Jac and Tricosanthes japonica agglutinin-I (TJA-I) to differentiated myotubes. We also observed differentiation- and disease-specific differences in binding of NPA, Jac, PNA, TJA-I and WFA to glycoprotein receptors in muscle cells. Additionally, Jac, PNA and WFA precipitated functionally glycosylated α-DG, that bound laminin, while NPA and TJA-I did not. Lectin histochemistry of healthy and dystrophic human muscle sections identified disease-specific differences in binding of O-glycan and sialic acid-specific lectins between healthy and dystrophic muscle. These results indicate that specific and discrete changes in glycosylation occur following differentiation, and identify specific lectins as potential biomarkers sensitive to changes in healthy human muscle glycosylation.
Keywords: disease-specific, human, lectin, muscle glycosylation, muscular dystrophy, skeletal muscle
Introduction
Myofiber attachment to the extracellular matrix (ECM) is necessary for proper muscle function. The basement membrane plays a critical role in the transfer of contractile forces by facilitating force transmission both longitudinally and laterally. Loss of connection between myofibers and the ECM causes a drop in both the absolute and specific force generated by a muscle (Holmberg and Durbeej 2013). The major adhesion complexes present at the muscle cell surface, or sarcolemma, are the dystrophin-glycoprotein complex (DGC), the homologous utrophin-glycoprotein complex (UGC) expressed at the neuromuscular and myotendinous junctions (NMJ/MTJ), and the α7β1 integrin heterodimer (Marshall et al. 2013). All three complexes independently bind laminins in the ECM and intracellular actin cytoskeleton to provide myofiber-ECM attachment (Marshall et al. 2013). Loss of various components of each adhesion complex cause various muscular dystrophies. For example, loss of dystrophin expression in Duchenne Muscular Dystrophy (DMD) prevents the entire DGC from being present at the sarcolemma (K. P. Campbell and Kahl 1989; Ibraghimov-Beskrovnaya et al. 1992; Ervasti and Campbell 1993). Similarly, loss of any of the sarcoglycans (SGs) from the DGC causes various limb-girdle muscular dystrophies (LGMDs) (Mizuno et al. 1994; Roberds et al. 1994; Bönnemann et al. 1995; Noguchi et al. 1995) and loss of α7-integrin is known to cause a congenital myopathy (Mayer et al. 1997; Hayashi et al. 1998).
In addition to the presence of adhesion complexes at the sarcolemma, proper glycosylation of component glycoproteins in the complexes is necessary for myofiber-ECM binding. Improper glycosylation of alpha-dystroglycan (α-DG) in the DGC results in loss of laminin binding. Specifically, addition of the glucuronic acid-xylose disaccharide repeat known as matriglycan is requisite for laminin binding as are the underlying glycan structures to which matriglycan is added (Yoshida-Moriguchi et al. 2010; Hara et al. 2011; Inamori et al. 2012; Briggs et al. 2016). To date, mutations in 24 genes necessary for α-DG glycosylation have been identified. Mutations in these 24 genes cause loss-of-function, disrupt α-DG glycosylation, and contribute to a wide spectrum of congenital α-DG disorders known as dystroglycanopathies (Yoshida-Moriguchi and Campbell 2015).
Various therapeutic approaches for muscular dystrophies are being explored, including restoring dystrophin expression (e.g., exon skipping or gene editing), modulating immune responses, potential stem cell therapies and different strategies to upregulate utrophin, to compensate for the loss of dystrophin (Guiraud et al. 2015; Mendell et al. 2016; Ricotti et al. 2016; Young et al. 2016; Campbell et al. 2017; Lim et al. 2017; Reinig et al. 2017). One method for upregulating utrophin utilization currently being studied focuses on manipulating the glycosylation status of α-DG. In rodent muscle, differential glycosylation of α-DG correlates with cellular localization and protein complex association. For example, α-DG expressed ubiquitously throughout the sarcolemma binds the sialic acid and GlcNAc-specific lectin wheat germ agglutinin (WGA) (K. P. Campbell and Kahl 1989; Nguyen et al. 2002; Marshall et al. 2012). In contrast, α-DG associated with the UGC is restricted to the NMJ and MTJ in rodent muscle and binds the GalNAc-specific lectin Wisteria floribunda agglutinin (WFA) (Nguyen et al. 2002; Xia et al. 2002; Marshall et al. 2012). Thus, various groups have sought to increase utrophin utilization, to increase UCG-mediated adhesion at the sarcolemma in the absence of dystrophin, by manipulating this differential glycosylation of α-DG.
Current approaches to alter α-DG glycosylation in order to increase utrophin utilization still face challenges. Overexpression of the GalNAc-transferase B4Galnt2 increases WFA binding to α-DG and reduces the dystrophic pathology in several dystrophic mouse models by increasing abundance of laminin and various UGC components (Nguyen et al. 2002; Xu et al. 2007b, 2009; Thomas et al. 2016). However, the exact mechanism by which B4Galnt2 overexpression reduces muscular dystrophy pathology in mice remains poorly defined. Additionally, potential off-target sites of GalNAc addition resulting from B4Galnt2 overexpression have yet to be determined. Our group previously identified lobeline, a small molecule antagonist of the acetylcholine receptor (AChR), in a high throughput screen for molecules which upregulate WFA binding to murine muscle cells (Cabrera et al. 2012). While lobeline increased WFA binding to both healthy and dystrophic primary murine muscle cells, we observed no increase in WFA binding following treatment of either healthy or dystrophic human inducible, directly reprogrammable myotubes (iDRMs) generated from control and dystrophic fibroblasts (Cabrera et al. 2012).
In 1982, Sanes et al. first demonstrated that the lectin Dolichos biflorus agglutinin (DBA) selectively and specifically binds the NMJ and not the extra-synaptic sarcolemma of rodent muscle (Sanes and Cheney 1982). In a follow-up study, additional GalNAc-specific lectins (VVA, SBA, HPA) were shown to bind the rodent NMJ specifically while lectins with other glycan specificities (e.g., ConA, WGA, RCA, PHA-L) bound the sarcolemma indiscriminately, demonstrating that the rodent NMJ is uniquely modified with terminal GalNAc structures (Scott et al. 1988). However, while VVA binding to rodent muscle is highly specific for the NMJ, VVA binding to human muscle is not NMJ-specific (Scott et al. 1988), indicating that terminal GalNAc residues may not be restricted to the NMJ in human muscle as observed in rodent muscle. Furthermore, GalNAc-specific lectins such as WFA may be poor biomarkers for disease-specific differences or for the UGC in human skeletal muscle. Beyond our current understanding of the process by which matriglycan is added to α-DG, human muscle glycosylation remains poorly characterized. Additionally, disease-specific differences in human muscle glycosylation have not been evaluated beyond a small collection of immunohistochemistry studies in the 1980s, and to date no glycan structure, present on α-DG or any other glycoprotein, which correlates with the UGC has been identified in human muscle. In the present study, we have characterized the glycophenotype of human skeletal muscle cells (HSMCs) during differentiation, and identified differences between healthy and dystrophic muscle. These findings reinforce and extend prior observations regarding differences between rodent and human muscle cell glycosylation and may identify glycan structures that will be useful biomarkers to evaluate novel therapies for muscular dystrophies.
Results
To quantify changes in glycosylation following differentiation of human myoblasts into fused, multinucleated myotubes, we performed lectin-binding assays on fixed primary and immortalized, male, healthy, HSMCs (Supplementary data, Table 1) utilizing a panel of 13 lectins with a wide variety of specificities (Table I) (McMorran et al. 2016). To quantify changes in N-glycosylation, Concanavalin A (ConA) and Narcissus pseudonarcissus agglutinin (NPA) were included for oligomannose specificity, while Phaseolus vulgaris leucoagglutinin (PHA-L) was included for complex N-glycan specificity (Cummings and Kornfeld 1982; Kaku et al. 1990; Mega et al. 1992). Four lectins specific for various O-glycan structures were included: Peanut agglutinin (PNA), Jacalin (Jac), Amaranthus caudatus agglutinin (ACA) and Ricinus communis agglutinin-I (RCA-120) (Drysdale et al. 1968; Lotan et al. 1975; Rinderle et al. 1989; Wu et al. 2003). Four sialic acid binding lectins were also included: Sambucus nigra agglutinin (SNA), Tricosanthes japonica agglutinin-I (TJA-I), Maackia amurensis agglutinin-II (MAA-II) and WGA (Greenaway and LeVine 1973; Shibuya et al. 1987; Yamashita et al. 1992; Konami et al. 1994). WFA and DBA both bind glycans terminated with GalNAc residues (Uchida et al. 1978; Etzler et al. 1981) and were included due to use of the lectins historically in identifying the rodent neuromuscular junction (Sanes and Cheney 1982; Scott et al. 1988; Nguyen et al. 2002; Marshall et al. 2012).
Table I.
Description of lectins
| Lectin | Nominal specificity | References |
|---|---|---|
| ConA | High Mannose | Mega et al. (1992) |
| NPA | High Mannose | Kaku et al. (1990) |
| PHA-L | Complex N-glycans | Cummings and Kornfeld (1982) |
| PNA | Asialo O-glycans | Lotan et al. (1975) |
| Jac | Sialylated O-glycans | Wu et al. (2003) |
| ACA | LacNAc, tolerates sialylation | Rinderle et al. (1989) |
| RCA-120 | LacNAc | Drysdale et al. (1968) |
| SNA | 2,6-linked sialic acid | Shibuya et al. (1987) |
| TJA-I | 2,6-linked sialic acid | Yamashita et al. (1992) |
| MAA-II | 2,3-linked sialic acid | Konami et al. (1994) |
| WGA | Sialic acid, GlcNAc | Greenaway and LeVine (1973) |
| WFA | Terminal GalNAc | Uchida et al. (1978) |
| DBA | Terminal GalNAc | Etzler et al. (1981) |
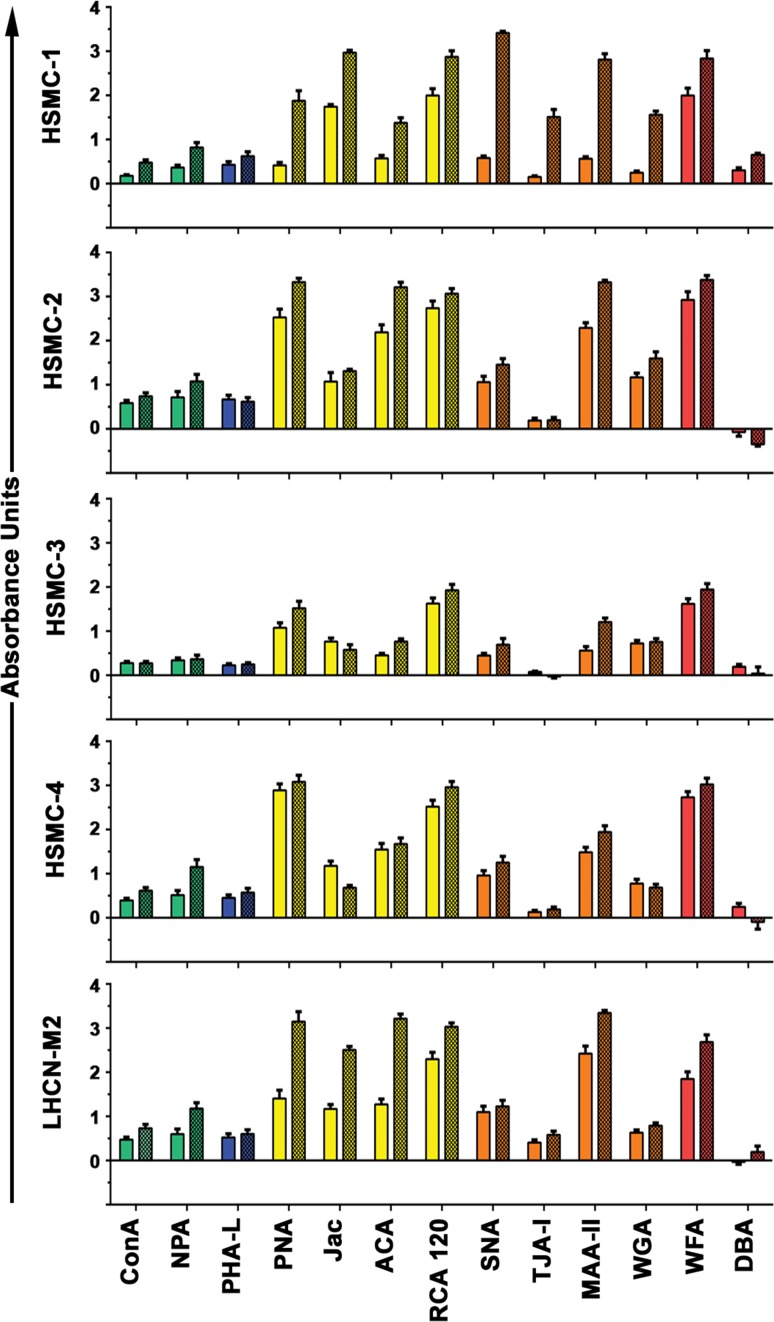
While reactivity of healthy human myoblasts with the various lectins at day 0 (d0) varied considerably, lectin binding to the immortalized human myoblasts generally recapitulated binding patterns seen in the four primary muscle cells. Following five days of differentiation (d5), distinct increases in binding of three lectins (PNA, MAA-II, WFA) were observed across all cell sources while no change was observed in the binding of PHA-L and DBA (Figure 1). In addition, NPA binding increased in three of the four primary cell lines. This indicates that the increases observed in binding of some lectins was not the result of a global increase in glycosylation following differentiation, but rather, changes in discrete and specific types of glycosylation. For example, focusing on lectins that recognize N-glycans, ConA and NPA binding increased following differentiation, while no change in binding of PHA-L was observed, underlying the specificity of these changes. Following differentiation we observed increased binding of lectins that recognize various O-glycans (PNA, Jac, ACA and RCA-120). Specifically, binding of PNA, ACA and RCA-120 to some degree across all four primary cell lines while binding of Jac was more variable. Similarly, differentiated healthy myotubes bound substantially more sialic acid-specific lectins (MAA-II, SNA and TJA-I) following differentiation. Interestingly, no significant reactivity of any cell line at either d0 or d5 with DBA was observed. In total, these results demonstrate that discrete and specific changes in muscle cell glycosylation occur following differentiation and that immortalized human myoblasts recapitulate changes observed in primary human muscle cells.
Fig. 1.
Changes in cell surface glycosylation following differentiation of primary and immortalized healthy human myotubes. Healthy primary (HSMC-1, -2, -3, -4) and immortalized (LHCN-M2) human myoblasts (d0, solid bars) and myotubes (d5, hatched bars) were characterized with a panel of 13 lectins. Increased binding of four O-glycan-specific lectins as well as NPA and MAA-II (high-mannose N-glycan and sialic acid-specific, respectively) was observed for both immortalized healthy human muscle cells and four primary healthy human muscle cell preparations. This figure is available in black and white in print and in color at Glycobiology online.
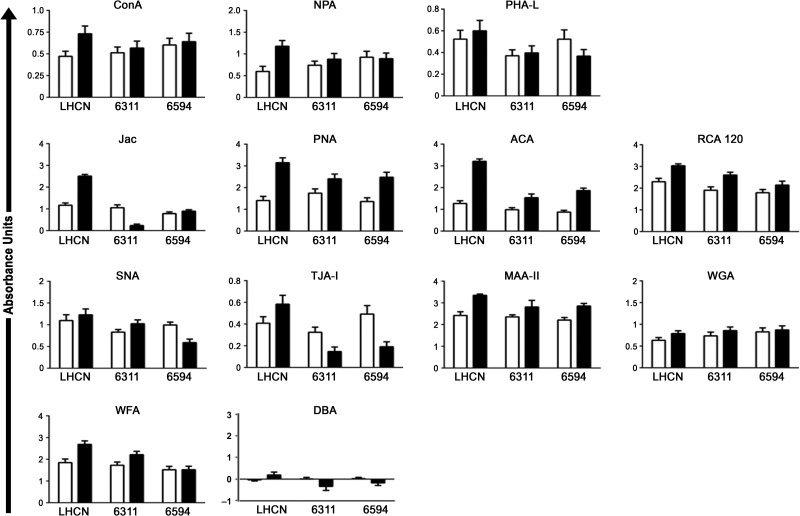
To identify disease-specific differences in human myoblast glycosylation, we performed lectin-binding assays on the immortalized healthy myoblasts and compared this with two immortalized dystrophic lines (Supplementary data, Table 1). We compared binding of the 13 lectin panel at d0 and d5 across the three cell lines and found disease-specific changes in binding of four lectins following differentiation: NPA, Jac, TJA-I and WFA (Figure 2). Binding of NPA, specific for high mannose N-glycans, to healthy cells increased substantially following differentiation while almost no change was observed in NPA binding to dystrophic cells. These changes did not result from a general increase in total N-glycosylation, as no difference was observed in binding of the complex N-glycan-specific lectin PHA-L to dystrophic myotubes compared to healthy controls. Similarly, binding of Jac to healthy muscle cells increased following differentiation while binding to dystrophic myotubes either decreased or showed no change. Again this was a specific difference, as other lectins which recognize O-glycan structures (PNA, ACA and RCA-120) showed increases following differentiation independent of disease-state. TJA-I binding also changed in a disease-specific manner; TJA binding to healthy muscle cells increased while dystrophic cells showed a reduction in binding following differentiation. Binding of WFA to healthy muscle cells increased following differentiation, while minimal or no change was observed in binding to dystrophic myotubes. No cell lines surveyed bound DBA regardless of differentiation- or disease-status.
Fig. 2.
Disease-specific changes in lectin binding to HSMCs. Healthy (LHCN) immortalized human myoblasts (d0, open bars) and myotubes (d5, black bars) were compared to immortalized muscle cells from two patients with DMD (6311 and 6594). NPA, Jac and TJA-I binding to healthy myotubes increased on d5 compared to day 0, while binding of these lectins to dystrophic myotubes did not change or decreased. Increased binding of PHA-L, PNA, ACA, RCA-120, MAA-II, and WGA to d5 myotubes were observed regardless of disease-status. Human muscle cells did not bind DBA regardless of differentiation- or disease-status.
The results demonstrated that distinct, disease-specific changes in glycosylation occur following in vitro human myoblast differentiation and that NPA, Jac and TJA-I may represent potential lectin biomarkers sensitive to changes in healthy human muscle glycosylation. Moreover, as we have previously demonstrated, specific lectin binding to different cells reveals more than just nominal sugar specificity (McMorran et al. 2016); for example, while binding of NPA and ConA increased following differentiation, the magnitude of the change was not identical. SNA and TJA-I both nominally recognize α2,6-linked sialic acid, however, we observed differences in the degree of binding of these two lectins. Similarly, WFA and DBA nominally bind GalNAc but our previous work demonstrated that binding of these lectins to murine muscle cells differed substantially (McMorran et al. 2016).
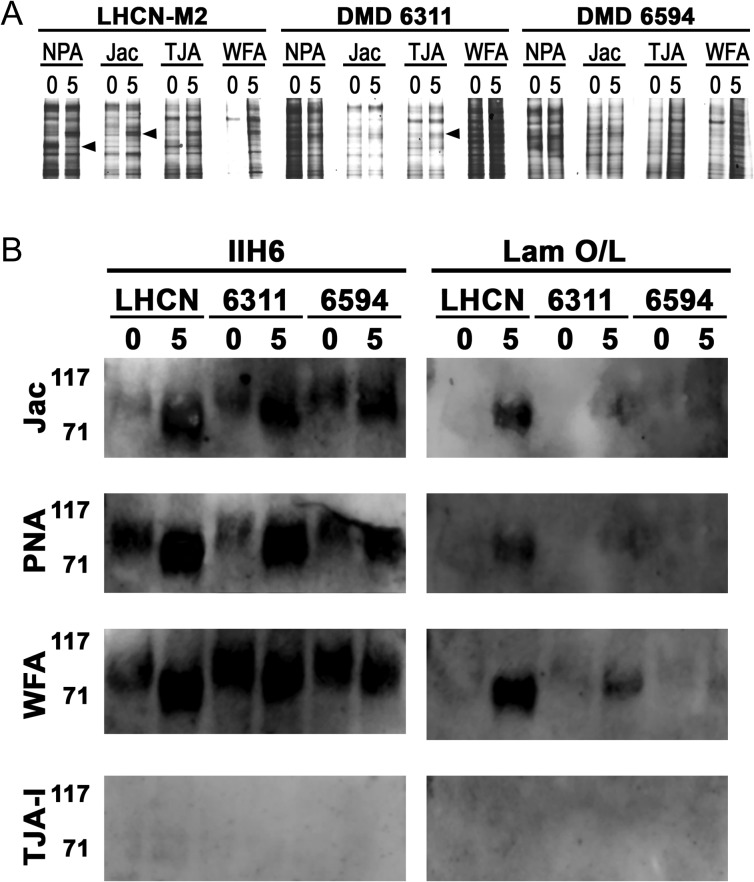
To determine if the lectins recognized distinct glycoprotein receptors, we next performed lectin precipitations from lysates of d0 confluent myoblasts and d5 differentiated myotubes. As shown in Figure 3A, NPA, Jac, TJA-I and WFA all precipitated a variety of glycoproteins. Differentiation-specific differences in the glycoproteins precipitated from healthy cells were observed across all four lectins (Figure 3A, arrowheads); most notably, WFA precipitated significantly more glycoproteins from healthy muscle cells at d5 compared to the few bands observed at d0. As reactivity of α-DG with various lectins has been utilized to distinguish between DGC- and UGC-associated α-DG in rodent muscle (K. P. Campbell and Kahl 1989; Nguyen et al. 2002; Marshall et al. 2012), we asked if any lectins were able to precipitate α-DG, detected by the monoclonal antibody IIH6, and if the α-DG was capable of binding laminin. Jac, PNA and WFA all precipitated α-DG at d0 and d5 while TJA-I and NPA did not (Figure 3B, data not shown). Of note, these three lectins all precipitated more α-DG at d5 compared to d0, detected by IIH6. We next asked if α-DG precipitated by each of the lectins was functionally O-mannosylated and could bind laminin. Interestingly, laminin was not bound by α-DG precipitated by any of the lectins at d0 regardless of disease-status. Furthermore, α-DG precipitated from healthy myotubes by Jac, PNA and WFA bound substantially more laminin compared to α-DG precipitated from dystrophic myotubes, indicating a greater extent of functional O-mannosylation in healthy mature myotubes. (Figure 3B, right panels).
Fig. 3.
Different lectins precipitate different glycoproteins from healthy and dystrophic human myotubes. NPA, Jac, TJA-1, WFA and PNA were added to lysates of healthy (LHCN) or dystrophic (DMD 6311 (6311)/DMD 6594 (6594)) d0 myoblasts and d5 myotubes. Precipitates were separated by SDS-PAGE and detected by SYPRO® Ruby Protein gel stain (A). Parallel blots were probed for matriglycan with mAb IIH6 (B, left panels) and functional O-mannosylation by laminin binding (B, right panels). TJA-I and NPA (data not shown) precipitates both showed no binding of IIH6 and laminin.
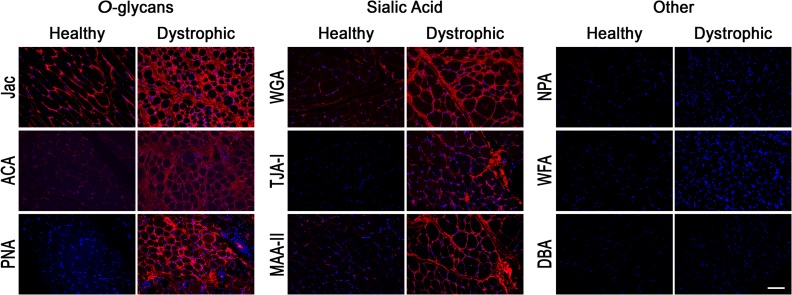
As we noted disease-specific differences in binding of NPA, TJA-I and Jac to differentiated myotubes in vitro, we asked which of the lectins bound to innervated human skeletal muscle in a disease-specific manner. We obtained de-identified, anonymized skeletal muscle biopsies from control and DMD patients through the UCLA Translational Pathology Core Laboratory. Biotinylated lectins were added to sections of paraffin-embedded, formalin-fixed healthy and dystrophic human skeletal muscle, and bound lectins were detected with Texas Red conjugated streptavidin and imaged. In healthy muscle, we observed differential binding of the three lectins with O-glycan specificities; Jac bound healthy muscle ubiquitously throughout the sarcolemma, while ACA bound in a very punctate fashion and PNA did not bind regardless of lectin concentration (Figure 4, data not shown). Interestingly, we observed increased binding of all three lectins to dystrophic muscle from DMD patients compared to healthy controls. Most surprisingly, we observed abundant and ubiquitous binding of PNA to the sarcolemma of muscle from a DMD patient, compared to healthy control muscle where we detected no PNA binding. Of the three sialic acid-specific lectins assayed, WGA bound the healthy sarcolemma ubiquitously while MAA-II staining was punctate and TJA-I staining was negative. Binding of all three lectins to dystrophic muscle sections was increased compared to healthy controls, with the most striking increase in binding of TJA-I. Binding of NPA, WFA and DBA to section of fixed muscle were negative regardless of disease-status, although we did detect NPA and WFA, but not DBA, binding to dystrophic muscle that was frozen prior to sectioning, rather than formalin-fixed (Supplementary data Figure 1). In total, these results indicate disease-specific differences of distinct glycan types, primarily O-glycans detected by PNA and sialic acids detected by TJA-I, in healthy vs. dystrophic human skeletal muscle.
Fig. 4.
Disease-specific differences in O-glycan and sialic acid-specific lectin binding to human skeletal muscle. Serial transverse 5 μm sections of anonymized, formalin-fixed, paraffin-embedded, healthy and dystrophic human skeletal muscle were rehydrated and stained with biotinylated lectins: Jac, ACA, PNA, WGA, TJA-I, MAA-II, NPA, WFA and DBA. Bound lectins were detected with Texas Red-Avidin D (red) and sections were counterstained with DAPI. “Other” lectin nominal specificities: NPA- high mannose N-glycans, WFA- GalNAc, DBA- GalNAc. Scale bar is 100 μm. This figure is available in black and white in print and in color at Glycobiology online.
Discussion
While significant progress in the past decade has elucidated the specific glycan structures on α-DG bound by laminin, our understanding of muscle glycosylation outside of α-DG matriglycan remains limited, and has focused on rodent muscle (Nguyen et al. 2002; Gundry et al. 2009; Yoshida-Moriguchi and Campbell 2015; Go et al. 2017). As previous studies of human skeletal muscle have been limited to qualitative lectin histochemistry (Dunn et al. 1982; Capaldi et al. 1984b, 1984a, 1985b, 1985a; Voit et al. 1988), we sought to perform the first quantitative characterization of human skeletal muscle glycosylation utilizing primary and immortalized cells from healthy and dystrophic patients. Utilizing a panel of 13 lectins, we identified differentiation- and disease-specific changes in muscle cell glycosylation in these primary cell lines. Following differentiation of both primary and immortalized myotubes, binding of NPA, PNA, MAA-II and WFA increased (Figure 1). Given the specificities of these lectins, glycoproteins at the healthy myotube surface bear more high mannose N-glycans, asialo O-glycans and structures terminated with either α2,3-linked sialic acid or GalNAc residues, compared to undifferentiated myoblasts (Table II). These lectins may represent appropriate lectin markers of differentiation and may correlate with temporally regulated expression of unique glycoprotein receptors, glycosyltransferases or availability of sugar donor substrates.
Table II.
Summary of lectin binding
| Lectin | Healthy Mt/Mb | Dys Mt/Mb | IIH6 binding | Laminin binding |
|---|---|---|---|---|
| ConA | + | Nc | Np | Np |
| NPA | + | Nc | − | − |
| Jac | ++ | −/Nc | + | + |
| PNA | ++ | + | + | + |
| ACA | ++ | + | Np | Np |
| RCA-120 | + | + | Np | Np |
| TJA-I | + | −− | − | − |
| MAA-II | + | + | Np | Np |
| WFA | + | Nc | + | + |
| DBA | Nb | Nb | Np | Np |
Nc- no change, Nb- no binding, Np-not performed. ++ very strong, + uniformly detectable, − weak and non-uniform, −− negative.
In addition to differentiation-specific differences in lectin binding, we were also able to identify disease-specific differences in lectin reactivity. Binding of NPA, Jac and TJA-I to healthy myotubes increased after differentiation, while no change or a reduction in binding to dystrophic myotubes was observed. Healthy myotubes therefore express glycoproteins bearing abundant high mannose N-glycans as detected by NPA, sialylated O-glycans as detected by Jac and glycans terminated with α2,6-linked sialic acid as detected by TJA-I. In contrast, there was little difference in abundance of these glycans between dystrophic myoblasts and myotubes, with no change in NPA reactive high mannose N-glycans or sialylated O-glycans and fewer α2,6-linked sialic acid terminated glycans on differentiated dystrophic cells (Table II). It is important to note that these are two clonal lines derived from two different patients and that additional cell sources must be analyzed to validate these findings across patients with DMD.
What drives these disease-specific differences in lectin reactivity? The differentiated, multinucleated healthy myotubes we observed at d5 expressed dystrophin and the DGC, as detected by immunoblotting, while dystrophic myotubes did not, as expected (data not shown). If NPA, Jac and TJA-I recognize glycans present on glycoproteins composing the DGC, then dystrophic myotubes lacking dystrophin and the DGC would not present these glycans at the sarcolemma, resulting in the disease-specific differences in lectin reactivity we observe. Alternatively, NPA, Jac and TJA-I could bind other sarcolemmal glycoproteins impacted indirectly by the loss of the DGC. For example, loss of the DGC component protein sarcospan has been shown to impair glycosylation of α-DG with WFA-reactive structures in mice (Marshall et al. 2012). Therefore, loss of the DGC may alter intracellular signaling and drive a shift in glycosyltransferase expression affecting the glycan structures present on sarcolemmal glycoproteins, which would alter lectin reactivity.
Manipulating skeletal muscle glycosylation by altering glycosyltransferase expression is one potential therapeutic approach for DMD. In rodent muscle, the NMJ is highly enriched for glycans terminated with WFA-reactive GalNAc residues which correlate with localization of the UGC (Marshall et al. 2012; McMorran et al. 2016). While both utrophin and WFA reactivity are restricted to the NMJ in healthy murine muscle, utrophin and WFA reactivity both spread ubiquitously throughout the sarcolemma of dystrophic mouse muscle (Marshall et al. 2012; McMorran et al. 2016). Expression of the GalNAc-transferase B4Galnt2 was restricted to the NMJ of healthy mice and therefore proposed to modify UGC-associated α-DG, rationalizing the finding that B4Galnt2 overexpression rescues the dystrophic phenotype in a variety of muscular dystrophy mouse models (Nguyen et al. 2002; Xia et al. 2002; Xu et al. 2007b, 2009; Thomas et al. 2016). While expression of B4Galnt2 has been observed in murine muscle (Xia et al. 2002), we did not observe expression in murine C2C12 myoblasts or myotubes previously (McMorran et al. 2016), or in immortalized human muscle cells, regardless of differentiation- or disease-status (McMorran, Nairn, Moremen and Baum, unpublished data). Additionally, our current data demonstrate that an increase in WFA binding to dystrophic human muscle is not the sole disease-specific difference in glycosylation: NPA, PNA and TJA-I ubiquitously bound the sarcolemma of dystrophic human skeletal muscle with no detectable binding to healthy human skeletal muscle (Figure 4, Supplementary data, Figure 1). The exact mechanism by which B4Galnt2 overexpression rescues the dystrophic phenotype in mice is not currently understood (Xu et al. 2007a; Yoon et al. 2009; Thomas et al. 2016). Furthermore, disease-specific differences in human muscle glycosylation remain poorly understood. Therefore, significant translational hurdles remain for development of B4Galnt2 overexpression as a potential DMD therapy.
The four lectins we assayed precipitated distinct sets of glycoproteins from healthy and dystrophic human myotubes (Figure 3, Table II). However, not all lectins bound α-DG and not all α-DG that was precipitated by these lectins was sufficiently modified with matriglycan to bind laminin. Our prior work demonstrated that inhibition of complex N-glycan formation via treatment with a mannosidase inhibitor reduced laminin binding to endogenous α-DG, without a concomitant reduction in IIH6 reactivity (Cabrera et al. 2012), although both IIH6 and laminin binding are dependent on the activity of LARGE (Inamori et al. 2012). Our results extend the observation that IIH6 and laminin binding are not exactly equivalent, as Jac, PNA and WFA all precipitated α-DG from d0 myoblasts that bound IIH6 but did not bind laminin (Figure 3B). It is also clear that there is altered glycosylation of cell surface glycoproteins other than α-DG on myotubes compared to myoblasts; while NPA and TJA-I binding increased following muscle cell differentiation in a disease-specific manner (Figure 2), neither lectin precipitated α-DG detected by IIH6 or laminin binding (Figure 3).
Iwata et al. found an approximately 5-fold increase in binding of PNA to dystrophic muscle across multiple muscular dystrophy rodent models including J2N-k hamsters (δ-sarcoglycan−/− model of LGMD2F), mdx mice (DMD) and Dy/dy mice (Lama2−/− model of merosin-deficient muscular dystrophy). In this study, the authors observed increased binding of PNA to muscle sections from a single patient with Becker muscular dystrophy and a single patient with limb-girdle muscular dystrophy, compared to healthy human skeletal muscle controls (Iwata et al. 2013). We also saw a robust increase in PNA binding to skeletal muscle from DMD patients, compared to healthy controls which did not bind PNA (Figure 4). Iwata et al. reported very little change in ACA binding to dystrophic tissue, while we observed an increase in ACA binding to multiple dystrophic tissue samples compared to healthy controls. As Iwata et al. observed an increase in PNA binding with relatively no change in binding of ACA, they concluded that cell surface content of sialic acid is generally reduced in dystrophic muscle, regardless of genotypic or phenotypic differences in dystrophic diseases. However, we observed no such reduction in sialic acid content on muscle derived from patients with DMD as detected by lectin binding; in contrast, binding of three sialic acid-specific lectins (WGA, TJA-I and MAA-II) was increased substantially for dystrophic muscle compared to healthy controls (Figure 4). Collectively, lectin reactivity of healthy and dystrophic tissue samples also underscore species-specific differences in glycosylation of innervated muscle. Increased binding of WFA and PNA represent the only two disease-specific differences in lectin binding to dystrophic mouse tissue (Marshall et al. 2012; Iwata et al. 2013). We observed increased WFA staining of frozen but not formalin fixed paraffin embedded dystrophic human tissue. Increased binding of Jac and lectins specific for sialic acid (WGA, TJA-I and MAA-II) represent novel disease-specific differences in innervated muscle glycosylation not previously reported in human or mouse muscle.
Here we report differentiation-specific differences in binding of lectins specific for high mannose N-glycans (NPA), O-glycans (PNA), sialic acid residues (MAA-II) and GalNAc terminated structures (WFA) (Figure 1). Increases in binding of NPA and MAA-II following differentiation represent species-specific differences in glycosylation, as we previously found no increase in ConA (also specific for high mannose N-glycans) or MAA-II (or any other sialic acid-specific lectins) binding following differentiation of murine C2C12 cells (McMorran et al. 2016). These species-specific differences highlight the need to use human muscle cell sources in vitro when studying glycosylation. C2C12 cells have long been the preferred in vitro model due to ease of use and lack of appropriate alternative cell sources, and their use has provided insight into murine integrin and glycolipid glycosylation (Gundry et al. 2009; Go et al. 2017). Our results demonstrate that immortalized human myotubes recapitulate changes observed in primary human myotubes and therefore provide an appropriate alternative for in vitro study of human muscle glycobiology. Moreover, identification of lectin biomarkers to identify and follow disease-specific differences in muscle glycosylation and to directly interrogate the glycan products on the cell surface may allow facile and cost-effective analysis of the effects of novel therapeutics on human muscle cells. As lectins bind glycan structures independent of glycoprotein backbone, unlike glycan-specific antibodies, lectins may be sensitive biomarkers for changes in glycosylation agnostic to expression of specific proteins.
Our current findings represent the first quantitative characterization of human muscle glycosylation, and highlight differentiation- and disease-specific differences in lectin reactivity to HSMCs and tissue. Disease-specific differences in TJA-I binding were observed on in vitro myotube cultures as well as ex vivo tissue sections. Future research should focus on understanding the mechanisms driving this difference, as well as those observed in binding of O-glycan and sialic acid-specific lectins. Furthering our understanding of human muscle glycosylation specifically will allow for the selection of an appropriate lectin biomarker for the healthy human muscle “glycophenotype” which could aid in the discovery of novel therapeutics (Cabrera et al. 2012), and provide a sensitive endpoint measure for other therapeutics currently under development for muscular dystrophy.
Materials and Methods
Cells
Immortalized healthy human myoblasts LHCN-M2 (Zhu et al. 2007) were a generous gift from Woodring Wright Lab (UT Southwestern, Dallas, TX) and immortalized dystrophic human myoblasts DMD 6311 and DMD 6594 (Mamchaoui et al. 2011) were a kind gift from Vincent Mouly Lab (UPMC Université Paris, Paris, FR). Four distinct lots of primary HSMCs were purchased and randomly numbered 1–4 (HSMC1-4) (PromoCell GmbH, Heidelberg, Germany). Immortalized and primary human muscle cells were grown in skeletal muscle cell growth medium (Promocell GmbH, Heidelburg, Germany) plus ciprofloxacin (10 μg/mL) on 0.1% porcine gelatin and passaged when approximately 60% confluent.
Reagents
The following biotinylated lectins and proteins were purchased (Vector Laboratories, Burlingame, CA): Concanavalin A (ConA), Narcissus psuedonarcissus agglutinin (NPA), PHA-L, peanut agglutinin (PNA), jacalin agglutinin (Jac), RCA-120, ACA, WGA, SNA, Maackia amurensis lectin II (MAA-II), WFA, DBA and bovine serum albumin (BSA). TJA-I was purchased (Medicago, Uppsala, Sweden) and biotinylated using EZ-Link™ Sulfo-NHS-Biotin kit (21335) (ThermoFisher Scientific, Waltham, MA) according to manufacturer’s instruction and stored in 10 mM HEPES, 0.15 M NaCl, pH 7.5, 0.1 mM Ca2+ at −20°C.
Antibodies against matriglycan (IIH6C4) (EMD Millipore, Billerica, MA) and laminin (L939) (Sigma-Aldrich, St. Louis, MO) and secondary goat anti-mouse IgG+M (H+L)-HRP and donkey anti-rabbit IgG (H+L)-HRP (Jackson ImmunoResearch, West Grove, PA) antibodies were purchased. The following reagents were purchased: streptavidin conjugated horseradish peroxidase (SA-HRP; Jackson ImmunoResearch), EHS Laminin (L2020), protease inhibitor cocktail (PIC; P8340) (Sigma-Aldrich), SYPRO® Ruby protein gel stain (S12000), 1-Step Ultra TMB-ELISA Substrate solution (34029), SuperSignal™ West Femto Maximum Sensitivity Substrate (34094, ThermoFisher Scientific), VECTASHIELD™ Antifade Mounting Medium with DAPI (H-1500), Texas-Red conjugated Avidin D (A-2006), streptavidin-agarose (SA-5010), and Avidin/Biotin blocking kit (SP-2001) (Vector Laboratories).
Lectin binding assays
Lectin binding assays were performed in 96-well plates as described previously with the following modification (McMorran et al. 2016). Of note, 5 × 103 healthy or dystrophic, immortalized or primary human myoblasts were plated per well coated with 0.1% porcine gelatin. Cells were grown until approximately 80–85% confluent and differentiated for 5 days. Cells were collected at the time of differentiation (D0) or 5 days following differentiation and fixed overnight at 4°C in 2% paraformaldehyde in phosphate-buffered saline (PBS). Non-specific binding to fixed cells was blocked by incubating in 1% BSA in PBS for 1 h at room temperature. Optimal lectin concentrations were determined for the LCHN-M2 cell line by performing dose-response curves for each lectin and determining the mid-point of the linear range for each (data not shown). Lectin specificities have been previously described (Table I). Triplicate wells were then incubated in the following lectins overnight at 4°C: ConA (5 ng/mL), NPA (2.5 μg/mL), PHA-L (10 ng/mL), PNA (100 ng/mL), Jac (5 μg/mL), RCA-120 (10 ng/mL), ACA (50 ng/mL), WGA (5 ng/mL), SNA (2.5 μg/mL), TJA-I (2.5 μg/mL), MAA-II (100 ng/mL), WFA (250 ng/ml) and DBA (5 μg/mL). Control cells were incubated with equivalent concentrations of biotinylated BSA, as a negative control Following overnight incubation, cells were rinsed with 1% BSA/0.1% Tween-20/1× PBS solution and incubated with SA-HRP (50 g/mL). Bound lectins were detected using 1-Step Ultra TMB-ELISA Substrate solution per manufacturer’s instructions with a colorimetric spectrophotometer (Bio-Rad Benchmark Plus) at 450 nm. Values are specific lectin binding after subtraction of background binding of biotinylated BSA at the appropriate concentrations.
Lectin precipitations
For enrichment of lectin receptors, 1 × 106 healthy or dystrophic myoblasts were plated per 10 cm dish coated with 0.1% porcine gelatin, and collected once confluent (day 0) and following 5 days of differentiation (day 5). At appropriate time points, cells were washed with ice-cold PBS and scraped in ice-cold radioimmunoprecipitation assay buffer (25 mM Tris • HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) containing protease inhibitor cocktail. Lysates were rotated at 4°C for 1 h and clarified by centrifugation at 13,300 rpm for 20 min at 4°C. Protein concentration was determined by Pierce™ BCA Protein Assay (ThermoFisher Scientific). Lysates (150 μg) were added to 25 μg biotinylated lectin and 20 μL streptavidin-agarose overnight at 4°C. Following lectin precipitation, beads were washed three times with cold lysis buffer plus protease inhibitor cocktail and denatured in NuPAGE sample buffer and reducing agent (ThermoFisher Scientific). Proteins were then separated on 3–8% BisTris polyacrylamide gels for staining with SYPRO® Ruby protein gel stain as described previously in (McMorran et al. 2016).
Laminin overlay
Laminin overlays were performed on separated lectin receptors as described previously (Cabrera et al. 2012). Briefly, lectin receptors precipitated per above were separated on 3–8% BisTris gels, transferred to nitrocellulose and blocked in 5% non-fat dry milk in laminin-binding buffer. Blots were then sequentially incubated in EHS laminin, rabbit anti-laminin (1:1000), and donkey anti-rabbit HRP (1:3000). Bound antibody was detected by enhance chemiluminesence on a LI-COR Odyssey Fc.
Tissue staining
Deidenitified, anonymized, 5 mm, paraffin-embedded or frozen human muscle tissue sections were provided by the Translational Pathology Core Laboratory at UCLA (David Geffen School of Medicine, UCLA). Paraffin-embedded tissue sections were deparaffinized with xylene, ethanol and rehydrated with 1× PBS. Sections were stained as described previously (McMorran et al. 2016). Briefly, non-specific binding to tissue sections was blocked with 1% BSA/1× PBS and Avidin/Biotin blocking kit according to manufacturer’s instructions. Serial sections were incubated overnight at 4°C in biotinylated lectins: ConA (5 μg/mL), NPA (10 μg/mL), PHA-L (10 μg/mL), PNA (10 μg/mL), Jac (10 μg/mL), RCA-120 (10 μg/mL), ACA (5 μg/mL), SNA (10 μg/mL), TJA-I (10 μg/mL), MAA-II (5 μg/mL), WGA (10 μg/mL), WFA (25 μg/mL) and DBA (25 μg/mL). Bound antibodies and lectins were detected via fluorescein-avidin, mounted with VECTASHIELD™ to prevent photobleaching and visualized on an Olympus BX51 fluorescence microscope and Olympus DP2-BSW software (Olympus America Inc., Center Valley, PA).
Supplementary Material
Acknowledgements
We thank Mabel Pang and Katrin Schaefer (Baum Lab) for helpful discussion, Negar Khanlou for assistance with muscle tissue sample acquisition, and Rachelle Crosbie-Watson for assistance with fluorescence microscopy. We thank the UCLA Translational Pathology Core Laboratory for their preparation of de-identified human skeletal muscle tissue and the UCLA Center for Duchenne Muscular Tissue Repository for immortalized healthy and dystrophic human cell lines.
Abbreviations
ACA, Amaranthus caudatus agglutinin; AChR, acetylcholine receptor; α-DG, alpha-dystroglycan; B4Galnt2, beta-1,4-N-acetylgalactosaminyltransferase 2; BSA, bovine serum albumin; ConA, Concanavalin A; DBA, Dolichos biflorus; DGC, dystrophin-glycoprotein complex; DMD, Duchenne muscular dystrophy; ECM, extracellular matrix; iDRM, inducible, directly reprogrammable myotube; LGMD, limb-girdle muscular dystrophy; Jac, jacalin; MAA-II, Maackia amurensis agglutinin-II; MTJ, myotendous junction; NMJ, neuromuscular junction; NPA, Narcissus pseudonarcissus agglutinin; PHA-L, Phaseolus vulgaris leucoagglutinin; PNA, peanut agglutinin; RCA-120, Ricinus communis agglutinin-I; SG, sarcoglycan; SNA, Sambucus nigra agglutinin; TJA-I, Tricosanthes japonica agglutinin-I; WGA, wheat germ agglutinin; WFA, Wisteria floribunda agglutinin.
Supplementary data
Funding
Muscular Dystrophy Association RG 254647 (to L.G.B.), NIH P30 AR057230 (to the UCLA Center for Duchenne Muscular Dystrophy) and the UCLA Center for Duchenne Muscular Dystrophy Fellowship (to B.J.M.).
Conflict of interest statement
The authors declare no conflict of interest.
References
- Briggs DC, Yoshida-Moriguchi T, Zheng T, Venzke D, Anderson ME, Strazzuli A, Moracci M, Yu L, Hohenester E, Campbell KP.. 2016. Structural basis of laminin binding to the LARGE glycans on dystroglycan. Nat Chem Biol. 12(10):810–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bönnemann CG, Modi R, Noguchi S, Mizuno Y, Yoshida M, Gussoni E, McNally EM, Duggan DJ, Angelini C, Hoffman EP.. 1995. Beta-sarcoglycan (A3b) mutations cause autosomal recessive muscular dystrophy with loss of the sarcoglycan complex. Nat Genet. 11(3):266–273. [DOI] [PubMed] [Google Scholar]
- Cabrera PV, Pang M, Marshall JL, Kung R, Nelson SF, Stalnaker SH, Wells L, Crosbie-Watson RH, Baum LG.. 2012. High throughput screening for compounds that alter muscle cell glycosylation identifies new role for N-glycans in regulating sarcolemmal protein abundance and laminin binding. J Biol Chem. 287(27):22759–22770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C, McMillan HJ, Mah JK, Tarnopolsky M, Selby K, McClure T, Wilson DM, Sherman ML, Escolar D, Attie KM.. 2017. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: Results of a randomized, placebo-controlled clinical trial. Muscle Nerve. 55(4):458–464. [DOI] [PubMed] [Google Scholar]
- Campbell KP, Kahl SD. 1989. Association of dystrophin and an integral membrane glycoprotein. Nature. 338(6212):259–262. [DOI] [PubMed] [Google Scholar]
- Capaldi MJ, Dunn MJ, Sewry CA, Dubowitz V.. 1984. a. Binding of Ricinus communis I lectin to the muscle cell plasma membrane in diseased muscle. J Neurol Sci. 64(3):315–324. [DOI] [PubMed] [Google Scholar]
- Capaldi MJ, Dunn MJ, Sewry CA, Dubowitz V.. 1984. b Altered binding of Ricinus communis I lectin by muscle membranes in Duchenne muscular dystrophy. J Neurol Sci. 63(1):129–141. [DOI] [PubMed] [Google Scholar]
- Capaldi MJ, Dunn MJ, Sewry CA, Dubowitz V.. 1985. a. Lectin blotting of human muscle. Identification of a high molecular weight glycoprotein which is absent or altered in Duchenne muscular dystrophy. J Neurol Sci. 68(2–3):225–231. [DOI] [PubMed] [Google Scholar]
- Capaldi MJ, Dunn MJ, Sewry CA, Dubowitz V.. 1985. b. Lectin binding in human skeletal muscle: a comparison of 15 different lectins. Histochem J. 17(1):81–92. [DOI] [PubMed] [Google Scholar]
- Cummings RD, Kornfeld S. 1982. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 257(19):11230–11234. [PubMed] [Google Scholar]
- Drysdale RG, Herrick PR, Franks D. 1968. The specificity of the haemagglutinin of the Castor bean, Ricinus communis. Vox Sang. 15(3):194–202. [DOI] [PubMed] [Google Scholar]
- Dunn MJ, Sewry CA, Dubowitz V. 1982. Cytochemical studies of lectin binding by diseased human muscle. J Neurol Sci. 55(2):147–159. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. 1993. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 122(4):809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzler ME, Gupta S, Borrebaeck C. 1981. Carbohydrate binding properties of th Dolichos biflorus lectin and its subunits. J Biol Chem. 256(5):2367–2370. [PubMed] [Google Scholar]
- Go S, Veillon L, Ciampa MG, Mauri L, Sato C, Kitajima K, Prinetti A, Sonnino S, Inokuchi JI.. 2017. Altered expression of ganglioside GM3 molecular species and a potential regulatory role during myoblast differentiation. J Biol Chem. 292(17):7040–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway PJ, LeVine D. 1973. Binding of N-acetyl-neuraminic acid by wheat-germ agglutinin. Nat New Biol. 241(110):191–192. [DOI] [PubMed] [Google Scholar]
- Guiraud S, Squire SE, Edwards B, Chen H, Burns DT, Shah N, Babbs A, Davies SG, Wynne GM, Russell AJ et al. 2015. Second-generation compound for the modulation of utrophin in the therapy of DMD. Hum Mol Genet. 24(15):4212–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundry RL, Raginski K, Tarasova Y, Tchernyshyov I, Bausch-Fluck D, Elliott ST, Boheler KR, Van Eyk JE, Wollscheid B.. 2009. The mouse C2C12 myoblast cell surface N-linked glycoproteome: identification, glycosite occupancy, and membrane orientation. Mol Cell Proteomics. 8(11):2555–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Kanagawa M, Kunz S, Yoshida-Moriguchi T, Satz JS, Kobayashi YM, Zhu Z, Burden SJ, Oldstone MB, Campbell KP.. 2011. Like-acetylglucosaminyltransferase (LARGE)-dependent modification of dystroglycan at Thr-317/319 is required for laminin binding and arenavirus infection. Proc Natl Acad Sci USA. 108(42):17426–17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi YK, Chou FL, Engvall E, Ogawa M, Matsuda C, Hirabayashi S, Yokochi K, Ziober BL, Kramer RH, Kaufman SJ et al. 1998. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat Genet. 19(1):94–97. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Durbeej M. 2013. Laminin-211 in skeletal muscle function. Cell Adh Migr. 7(1):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP.. 1992. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 355(6362):696–702. [DOI] [PubMed] [Google Scholar]
- Inamori K, Yoshida-Moriguchi T, Hara Y, Anderson ME, Yu L, Campbell KP.. 2012. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science. 335(6064):93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, Suzuki O, Wakabayashi S. 2013. Decreased surface sialic acid content is a sensitive indicator of muscle damage. Muscle Nerve. 47(3):372–378. [DOI] [PubMed] [Google Scholar]
- Kaku H, Van Damme EJ, Peumans WJ, Goldstein IJ.. 1990. Carbohydrate-binding specificity of the daffodil (Narcissus pseudonarcissus) and amaryllis (Hippeastrum hybr.) bulb lectins. Arch Biochem Biophys. 279(2):298–304. [DOI] [PubMed] [Google Scholar]
- Konami Y, Yamamoto K, Osawa T, Irimura T.. 1994. Strong affinity of Maackia amurensis hemagglutinin (MAH) for sialic acid-containing Ser/Thr-linked carbohydrate chains of N-terminal octapeptides from human glycophorin A. FEBS Lett. 342(3):334–338. [DOI] [PubMed] [Google Scholar]
- Lim KR, Maruyama R, Yokota T. 2017. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des Devel Ther. 11:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R, Skutelsky E, Danon D, Sharon N.. 1975. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 250(21):8518–8523. [PubMed] [Google Scholar]
- Mamchaoui K, Trollet C, Bigot A, Negroni E, Chaouch S, Wolff A, Kandalla PK, Marie S, Di Santo J St Guily JL et al. 2011. Immortalized pathological human myoblasts: Towards a universal tool for the study of neuromuscular disorders. Skelet Muscle. 1:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JL, Kwok Y, McMorran BJ, Baum LG, Crosbie-Watson RH.. 2013. The potential of sarcospan in adhesion complex replacement therapeutics for the treatment of muscular dystrophy. FEBS J. 280(17):4210–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JL, Holmberg J, Chou E, Ocampo AC, Oh J, Lee J, Peter AK, Martin PT, Crosbie-Watson RH.. 2012. Sarcospan-dependent Akt activation is required for utrophin expression and muscle regeneration. J Cell Biol. 197(7):1009–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Saher G, Fässler R, Bonnemann A, Echtermeyer F, von der Mark N, Misoge N, Pöschl E, von der Mark K.. 1997. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 17(3):318–323. [DOI] [PubMed] [Google Scholar]
- McMorran BJ, McCarthy FE, Gibbs EM, Pang M, Marshall JL, Nairn AV, Moremen KW, Crosbie-Watson RH, Baum LG.. 2016. Differentiation-related glycan epitopes identify discrete domains of the muscle glycocalyx. Glycobiology. 26(10):1120–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega T, Oku H, Hase S. 1992. Characterization of carbohydrate-binding specificity of concanavalin A by competitive binding of pyridylamino sugar chains. J Biochem. 111(3):396–400. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Goemans N, Lowes LP, Alfano LN, Berry K, Shao J, Kave EM, Mercuri E, Eteplirsen Study Group and Telethon Foundation DMD Italian Network . 2016. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann Neurol. 79(2):257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Noguchi S, Yamamoto H, Yoshida M, Suzuki A, Hagiwara Y, Havashi YK, Arahata K, Nonaka I, Hira S et al. 1994. Selective defect of sarcoglycan complex in severe childhood autosomal recessive muscular dystrophy muscle. Biochem Biophys Res Commun. 203(2):979–983. [DOI] [PubMed] [Google Scholar]
- Nguyen HH, Javasinha V, Xia B, Hoyte K, Martin PT.. 2002. Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice. Proc Natl Acad Sci USA. 99(8):5616–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S, McNally EM, Ben Othmane K, Hagiwara Y, Mizuno Y, Yoshida M, Yamamoto H, Bönnemann CG, Gussoni E, Denton PH et al. 1995. Mutations in the dystrophin-associated protein gamma-sarcoglycan in chromosome 13 muscular dystrophy. Science. 270(5237):819–822. [DOI] [PubMed] [Google Scholar]
- Reinig AM, Mirzaei S, Berlau DJ. 2017. Advances in the treatment of duchenne muscular dystrophy: new and emerging pharmacotherapies. Pharmacotherapy. 37(4):492–499. [DOI] [PubMed] [Google Scholar]
- Ricotti V, Spinty S, Roper H, Hughes I, Tejura B, Robinson N, Layton G, Davies K, Muntoni F, Tinsley J.. 2016. Safety, tolerability, and pharmacokinetics of SMT C1100, a 2-Arylbenzoxazole utrophin modulator, following single- and multiple-dose administration to pediatric patients with duchenne muscular dystrophy. PLoS One. 11(4):e0152840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderle SJ, Goldstein IJ, Matta KL, Ratcliffe RM.. 1989. Isolation and characterization of amaranthin, a lectin present in the seeds of Amaranthus caudatus, that recognizes the T- (or cryptic T)-antigen. J Biol Chem. 264(27):16123–16131. [PubMed] [Google Scholar]
- Roberds SL, Leturcq F, Allamand V, Piccolo F, Jeanpierre M, Anderson RD, Lim LE, Lee JC, Tomé FM, Romero NB. 1994. Missense mutations in the adhalin gene linked to autosomal recessive muscular dystrophy. Cell. 78(4):625–633. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Cheney JM. 1982. Lectin binding reveals a synapse-specific carbohydrate in skeletal muscle. Nature. 300(5893):646–647. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Bacou F, Sanes JR. 1988. A synapse-specific carbohydrate at the neuromuscular junction: association with both acetylcholinesterase and a glycolipid. J Neurosci. 8(3):932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ.. 1987. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2–6)Gal/GalNAc sequence. J Biol Chem. 262(4):1596–1601. [PubMed] [Google Scholar]
- Thomas PJ, Xu R, Martin PT. 2016. B4GALNT2 (GALGT2) Gene Therapy Reduces Skeletal Muscle Pathology in the FKRP P448L Mouse Model of Limb Girdle Muscular Dystrophy 2I. Am J Pathol. 186(9):2429–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Yamaizumi M, Mekada E, Okada Y, Tsuda M, Kurokawa T, Sugino Y.. 1978. Reconstitution of hybrid toxin from Fragment A of diphtheria toxin and a subunit of Wistaria floribunda lectin. J Biol Chem. 253(18):6307–6310. [PubMed] [Google Scholar]
- Voit T, Sewry CA, Dunn MJ, Dubowitz V.. 1988. Binding of Ricinus communis I lectin to developing dystrophic muscle in human fetus. J Neurol Sci. 84(2–3):301–314. [DOI] [PubMed] [Google Scholar]
- Wu AM, Wu JH, Lin LH, Lin SH, Liu JH.. 2003. Binding profile of Artocarpus integrifolia agglutinin (Jacalin). Life Sci. 72(20):2285–2302. [DOI] [PubMed] [Google Scholar]
- Xia B, Hoyte K, Kammescheidt A, Deernick T, Ellisman M, Martin PT.. 2002. Overexpression of the CT GalNAc transferase in skeletal muscle alters myofiber growth, neuromuscular structure, and laminin expression. Dev Biol. 242(1):58–73. [DOI] [PubMed] [Google Scholar]
- Xu R, Camboni M, Martin PT. 2007. a. Postnatal overexpression of the CT GalNAc transferase inhibits muscular dystrophy in mdx mice without altering muscle growth or neuromuscular development: evidence for a utrophin-independent mechanism. Neuromuscul Disord. 17(3):209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, DeVries S, Camboni M, Martin PT.. 2009. Overexpression of Galgt2 reduces dystrophic pathology in the skeletal muscles of alpha sarcoglycan-deficient mice. Am J Pathol. 175(1):235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Chandrasekharan K, Yoon JH, Camboni M, Martin PT.. 2007. b. Overexpression of the cytotoxic T cell (CT) carbohydrate inhibits muscular dystrophy in the dyW mouse model of congenital muscular dystrophy 1A. Am J Pathol. 171(1):181–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Umetsu K, Suzuki T, Ohkura T.. 1992. Purification and characterization of a Neu5Ac alpha 2-->6Gal beta 1-->4GlcNAc and HSO3(-)-->6Gal beta 1-->GlcNAc specific lectin in tuberous roots of Trichosanthes japonica. Biochemistry. 31(46):11647–11650. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Chandreasekharan K, Xu R, Glass M, Singhal M, Martin PT.. 2009. The synaptic CT carbohydrate modulates binding and expression of extracellular matrix proteins in skeletal muscle: Partial dependence on utrophin. Mol Cell Neurosci. 41(4):448–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Campbell KP. 2015. Matriglycan: A novel polysaccharide that links dystroglycan to the basement membrane. Glycobiology. 25(7):702–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L, Campbell KP.. 2010. O-mannosyl phosphorylation of alpha-dystroglycan is required for laminin binding. Science. 327(5961):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CS, Hicks MR, Ermolova NV, Nakano H, Jan M, Younesi S, Karumbayaram S, Kumagai-Cresse C, Wang D, Zack JA et al. 2016. A single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell. 18(4):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CH, Mouly V, Cooper RN, Mamchoui K, Bigot A, Shay JW, Di Santo JP, Butter-Browne GS, Wright WE.. 2007. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: Consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell. 6(4):515–523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.