Abstract
Purpose
To determine whether functional connectivity (FC) mapping of nucleus basalis of Meynert (NBM) cholinergic network (hereafter, NBM FC) could provide a biomarker of central cholinergic deficits with predictive potential for response to cholinesterase inhibitor (ChEI) treatment.
Materials and Methods
The Alzheimer’s Disease Neuroimaging Initiative (ADNI) was approved by the institutional review boards of all participating sites. All participants and their representatives gave written informed consent prior to data collection. NBM FC was examined in 33 healthy control participants, 102 patients with mild cognitive impairment (MCI), and 33 patients with AD by using resting-state functional MRI data from the ADNI database. NBM FC was compared between groups before and after 6 months of ChEI treatment in MCI. Associations between baseline NBM FC and baseline cognitive performance as well as cognitive outcomes after treatment were investigated.
Results
Compared with the healthy control group, NBM FC was decreased in patients with untreated MCI and increased in patients with AD treated with ChEI (corrected P ˂ .05). Global cognition (Alzheimer’s Disease Assessment Scale-Cognitive subscale score) was associated with NBM FC (r = −0.349; P ˂ .001). NBM FC was higher 6 months after ChEI compared with before ChEI in treated MCI (corrected P ˂ .05), but did not change at 6 months in patients with untreated MCI (corrected P ˂ .05). Baseline NBM FC in MCI strongly predicted cognitive outcomes 6 months after ChEI (R2 = 0.458; P = .001).
Conclusion
Functional dissociation of the nucleus basalis of Meynert from a cortical network may explain the cognitive deficits in dementia and allow for the selection of individuals who are more likely to respond to cholinesterase inhibitors at early disease stages.
Published under a CC BY-NC-ND 4.0 license.
See also the editorial by Chiang in this issue.
Introduction
Cholinergic deficits are a hallmark of Alzheimer disease (AD). The link between cholinergic deficits and cognitive impairment is well established (1) and cholinesterase inhibitors (ChEIs) are the mainstay symptomatic pharmacotherapy, with moderate effectiveness in established AD (2). However, only 18%–48% patients undergoing treatment show clinically significant improvement, and about 7% of patients develop adverse effects severe enough to stop the treatment (3). Additionally, no clinical effect has been established in patients with mild cognitive impairment (MCI) (4).
Individual diagnosis of central cholinergic deficits could be beneficial to address the lack of treatment response in MCI and the variable treatment response in established AD. Conceivably, increasing the cholinergic tone in patients without a cholinergic deficit is unlikely to lead to cognitive improvement, and conversely may make patients particularly prone to adverse effects (5). Several markers of cholinergic deficits were previously proposed (6–9). However, none of these markers gained widespread use because they are too expensive or invasive, lack robustness, or are not widely available. It would be advantageous to identify a simple and fast MRI test to reliably map cholinergic deficits as part of the routine MRI work-up (10). Additionally, it has been suggested that high-dose ChEI may be more efficacious for AD (11), increasing the need for a robust cholinergic biomarker to assist selection of candidates for potentially more aggressive ChEI treatment.
The nucleus basalis of Meynert (NBM) provides the primary source of cholinergic inputs to the cerebral cortex and its widespread projections form the NBM cholinergic network (12). Resting-state functional connectivity (FC) mapping of NBM cholinergic network (hereafter, NBM FC) has been demonstrated in healthy people (13), suggesting that it is possible to use NBM FC to delineate cholinergic deficits in AD and MCI. NBM FC has a number of key advantages for potential clinical use because of its noninvasive nature and expected high specificity to map cholinergic pathways, given that over 90% of NBM neurons are cholinergic (12). Taken together, NBM FC may probe the central cholinergic dysfunction and underlying neuronal degeneration so that we may be able to identify those people most likely to benefit from treatment with ChEI.
We hypothesized that reductions of NBM FC are a feature of MCI and AD; underlie cognitive impairment across the cognitively normal, MCI, and dementia spectrum not treated with ChEI; can be partially reversed with ChEI; and predict treatment response to ChEI in MCI and AD. We then undertook a series of posthoc tests to address the role of concurrent use of anticholinergic drugs, presence of cerebrospinal fluid (CSF) biomarkers of amyloid pathology, apolipoprotein E (APOE) ε4 genotype, and disease progression.
Materials and Methods
Participants
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (available at https://adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, PET, other biologic markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. Written informed consent was obtained from all individuals.
Data were selected based on the availability of resting-state functional MRI data and Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) at both screening and 6 months after initiation of ChEI. As described in the ADNI visit schedule, the maximum interval between baseline and screening visits was prescribed as 28 days. A total of 175 participants (34 healthy control participants, 59 patients with early MCI, 48 patients with late MCI, and 34 patients with AD) were included in this study (details of diagnostic criteria in Appendix E1 [online]). Demographic and clinical information was used to screen for existing pathologies, dementia diagnosis, and medication history (14). All participants completed a series of cognitive assessments and we chose ADAS-Cog to reflect cognitive function. ADAS-Cog comprises a comprehensive assessment of cognitive functions including memory and executive function that are linked with cholinergic function (15) and is commonly used as a primary outcome neuropsychological measure for AD clinical trials.
Information of dosing schedules for ChEI, memantine, and medications with potential anticholinergic effect was manually extracted (details in Appendix E1 [online]). At the screening visit, five patients with early MCI (8.5%), three patients with late MCI (6.3%), and all patients with AD were already taking ChEI. Five healthy control participants (14.7%), nine patients with early MCI (15.2%), four patients with late MCI (8.3%), and five patients with AD (14.7%) were taking anticholinergic drugs. One patient with late MCI (2.1%) and two patients with AD (5.9%) were taking memantine at the screening visit. Eight patients with early MCI (13.6%) and 13 patients with late MCI (30.2%) were prescribed ChEI after the screening visit.
To explore interactions between NBM FC and genotype or amyloid pathology as potential confounding factors (16,17), we manually extracted the status of APOE ε4 genotype and presence of CSF amyloid-β 42 from http://www.ADNI.org. Participants were designated as APOE ε4 carriers if they had one or two copies of allele 4, and as noncarriers if they had no allele 4 in their genotype. CSF amyloid-β 42, rather than amyloid PET, was chosen to reflect abnormal amyloid accumulation, given that it has been recently shown that CSF amyloid-β 42 becomes abnormal in the earliest stages of AD before amyloid PET starts (18). The cutoffs for abnormal CSF amyloid-β 42 used in this study were as follows: normal CSF amyloid-β 42 (participants with negative CSF Aβ 42 status) greater than 201.6 ng/L, and abnormal amyloid-β 42 (participants with positive CSF Aβ 42 status) less than 182.4 ng/L (18). Thirteen participants did not have APOE data.
Resting-State Functional MRI Acquisition and Quality Assessment Protocol
All participants were imaged with a 3.0-T Philips MR unit at multiple sites with the same ADNI 3.0-T imaging protocol. Participants were instructed to keep their eyes open during imaging. The data had been acquired by using a standard echo-planar imaging functional MRI protocol (repetition time msec/echo time msec, 3000/30; flip angle, 80°; number of sections, 48; section thickness, 3.3 mm; 140 volumes; spatial resolution, 3 × 3 × 3 mm3; matrix, 64 × 64).
To account for possible artifacts resulting from micromotion, a rigorous protocol of data quality assessment was applied, modified from a multicenter functional MRI protocol (19), resulting in a final data set of 168 participants (33 healthy control participants, 59 patients with early MCI, 43 patients with late MCI, and 33 patients with AD). Good-quality functional MRI images 6 months after ChEI initiation were available in 12 patients with late MCI and in six patients with early MCI. Table 1 summarizes demographic, clinical, APOE ε4 status, Aβ-42 status, and cognitive information of the included participants.
Table 1:
Demographics and Clinical and Cognitive Information
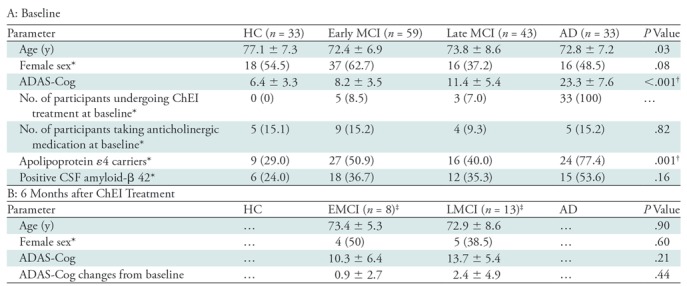
Note.—Unless otherwise specified, data are means ± standard deviations. AD = Alzheimer disease, ADAS-Cog = Alzheimer’s Disease Assessment Scale-Cognitive subscale, ChEI = cholinesterase inhibitor, CSF = cerebrospinal fluid, HC = healthy control participants, MCI = mild cognitive impairment.
*Data are numbers, with percentages in parentheses.
†Significant level at P ˂ .05.
‡In total, eight patients with early MCI and 13 patients with late MCI had ADAS-Cog score 6 months after undergoing ChEI treatment. Among these patients, six patients with early MCI and 12 patients with late MCI patients had good-quality resting-state functional MR images at 6 months after undergoing ChEI treatment.
Seed-based FC Analysis
Seed-based FC analysis, which identifies the pattern of brain areas displaying correlated time series with respect to a predefined region, was carried out with software (FMRI Expert Analysis Tool [FEAT], version 6.0) in FSL (version 5.0.10; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FEAT) (20) by manually creating a mask (16 voxels, 581 mm3) in the NBM on functional MRI data sets for each participant. The approach of seed-based analysis and the method of testing the robustness of manual seed region selection are provided in Appendix E2 (online). Standard statistical Z transformation was applied to the correlation coefficient of the time series between NBM and the rest of the brain in FSL (15); thus, a normal distributed score was computed to index NBM FC.
Statistical Analysis
One-way analysis of variance and χ2 test in SPSS (version 21; SPSS, Chicago, Ill) were used to compare demographics and cognitive performance between healthy control participants, patients with early MCI, patients with late MCI, and patients with AD. Statistical significance was set at P ˂ .05.
All main statistical tests and secondary posthoc tests were voxel based and corrected for multiple tests as implemented in FSL. Inference on first-level analysis was based on Z statistical images of each individual thresholded at Z greater than 2.3 and a corrected cluster significance threshold of P ˂ .05. Z statistical images of voxel-based high-level analyses were estimated based on Z greater than 1.96, and a familywise error–corrected cluster significance threshold of P ˂ .05. Age and mean relative displacement, which is the net amount of motion between consecutive functional volumes (21), were treated as further between-participant covariates of no interest in all higher-level analyses and was carried out by using FMRIB’s Local Analysis of Mixed Effects (FLAME) (20) in FSL. Additional outlier de-weighting in FEAT was used to automatically detect and account for outlier data points for each voxel.
Posthoc Shapiro-Wilk tests in SPSS were undertaken on Z scores extracted from results of the main tests for the four hypotheses (described next) to test for normality.
To test hypothesis 1—that NBM FC is lower in patients with MCI and patients with AD compared with healthy control participants with normal cognitive function—we undertook a voxel-based F test to compare the NBM FC maps between groups (healthy control participants, patients with MCI, and patients with AD) by using multiple test correction within FEAT in FSL. Posthoc pairwise t tests (healthy control participants vs patients with MCI and healthy control participants vs patients with AD) were then used to determine the direction of the effect if the F test was significant. Significant level of the t tests was at corrected P ˂ .025. Posthoc tests were performed in cohorts not taking anticholinergic drugs, and to explore the effects of APOE and Aβ status.
To investigate hypothesis 2—that NBM FC correlates with cognitive performance but in the absence of an a priori hypothesis of the specific regions affected within the NBM network—we undertook voxel-based correlation analysis of NBM FC maps with ADAS-Cog in the study population not taking ChEI. Posthoc tests included correlation analysis in the subgroup of participants not taking anticholinergic drugs to prevent possible medication-induced confounding effects.
To study hypothesis 3—that ChEI would increase NBM FC without an a priori hypothesis of where these effects would be—we again used voxel-based change analysis of NBM FC maps comparing before ChEI treatment and 6 months after ChEI initiation in 18 patients with MCI (voxel-based paired t test FEAT in FSL corrected for multiple comparisons). In view of the naturalistic study design, we sought to control for possible disease progression effects that might confound our change analysis. Hence, we assessed possible serial disease progression effects of NBM FC maps over an interval of 6 months in a matched (for age, sex, and disease severity) group of 18 patients with MCI (six with early MCI and 12 with late MCI) who did not undergo ChEI treatment in the interval by using voxel-based paired t test in FEAT as posthoc test.
Last, we studied the hypothesis that low NBM FC would predict the cognitive response to ChEI. We used voxel-wise correlation analysis of baseline NBM FC maps with changes of ADAS-Cog 6 months after initiating ChEI. Again, because of the naturalistic study design, we assessed whether NBM FC at baseline might also predict cognitive change in an untreated cohort (posthoc test).
Results
A total of 168 participants (mean age ± standard deviation, 73.8 years ± 7.7; 87 women [51.8%] and 81 men [49.2]) were included. Figure 1 shows the flowchart of the participant selection. Healthy control participants were significantly older than were patients with MCI and patients with AD (P = .03) (Table 1). The manual method to define NBM FC maps was considered robust (Appendix E2 [online]).
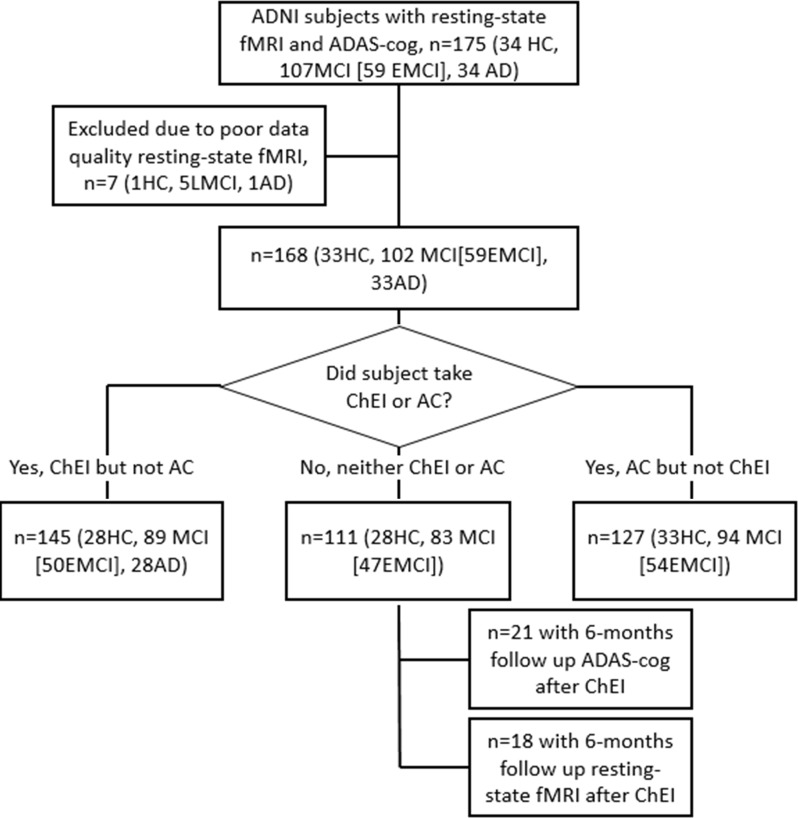
Figure 1:
Flowchart shows participant selection. AC = anticholinergic treatment, AD = Alzheimer disease, ADAS-Cog = Alzheimer’s Disease Assessment Scale-Cognitive subscale, ADNI = Alzheimer’s Disease Neuroimaging Initiative, ChEI = cholinesterase inhibitor, EMCI = early mild cognitive impairment, HC = healthy control participants, LMCI = late mild cognitive impairment, MCI = mild cognitive impairment.
NBM FC Pattern in Healthy Aging and Abnormalities in MCI and AD
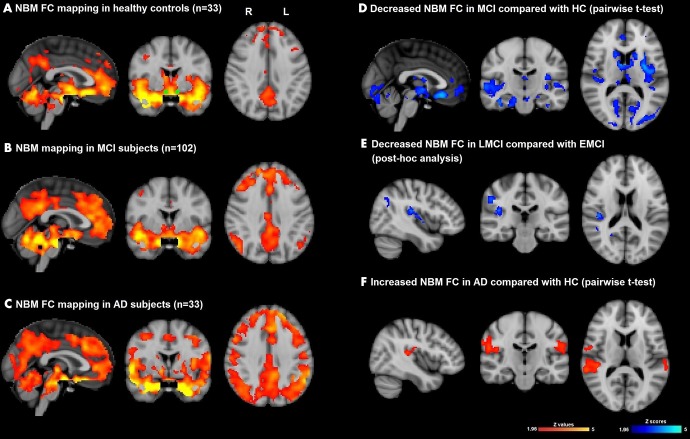
In healthy control participants, the NBM was functionally connected with the anterior cingulate cortex, bilateral hippocampi, caudate, medial frontal gyri, insular cortex, superior temporal gyri, and occipital lobes (Fig 2, A). Patients with MCI (Fig 2, B) and patients with AD (Fig 2, C) showed qualitatively similar NBM FC patterns but additional NBM FC was seen in AD with the bilateral occipital lobes, bilateral medial temporal gyri, and posterior cingulate cortex. F test showed a significant between-group difference of NBM FC between healthy control participants, patients with MCI, and patients with AD (Fig E1 [online]). By using further pairwise t tests, we found that patients with MCI at baseline had decreased NBM FC in the anterior cingulate cortex, bilateral hippocampus, bilateral caudate, bilateral insular cortex, bilateral superior and medial temporal gyri, and bilateral occipital lobes, compared with healthy control participants (Fig 2, D; Table 2). Also, group comparison confirmed significantly increased NBM FC with the right hippocampus, left middle and inferior frontal gyri, and bilateral superior temporal gyrus in patients with AD compared with healthy control participants (Fig 2, F; Table 2) when age and mean relative displacement were controlled.
Figure 2:
Images show functional connectivity (FC) mapping of nucleus basalis of Meynert (NBM) cholinergic network (hereafter, NBM FC) (whole cohort) in, A, healthy aging (healthy control participants [HC], n = 33; corrected P ˂ .05) and also shows seed mask (green), B, in patients with mild cognitive impairment (MCI) (n = 102; corrected P ˂ .05), and, C, in patients with Alzheimer disease (AD) (n = 33; corrected P ˂ .05). Significance was set at P ˂ .05. D, Difference NBM FC map between MCI and HC (blue-light blue indicates reductions in MCI; pairwise t test, corrected P ˂ .025). E, Difference NBM FC between late MCI (LMCI) and early MCI (EMCI) (blue-light blue indicates reductions in LMCI; posthoc pairwise t test, P ˂ .05). F, Difference NBM FC between AD and HC (red-yellow indicates increased FC in AD; pairwise t test, corrected P ˂ .025). All results were masked by gray matter masks obtained from Montreal Neurological Institute 152 standard-space T1-weighted average structural template image. All t test analyses were controlled for age and mean relative displacement.
Table 2:
Brain Regions Where NBM FC Are Associated with Cognition and Cholinesterase Inhibitory Effects
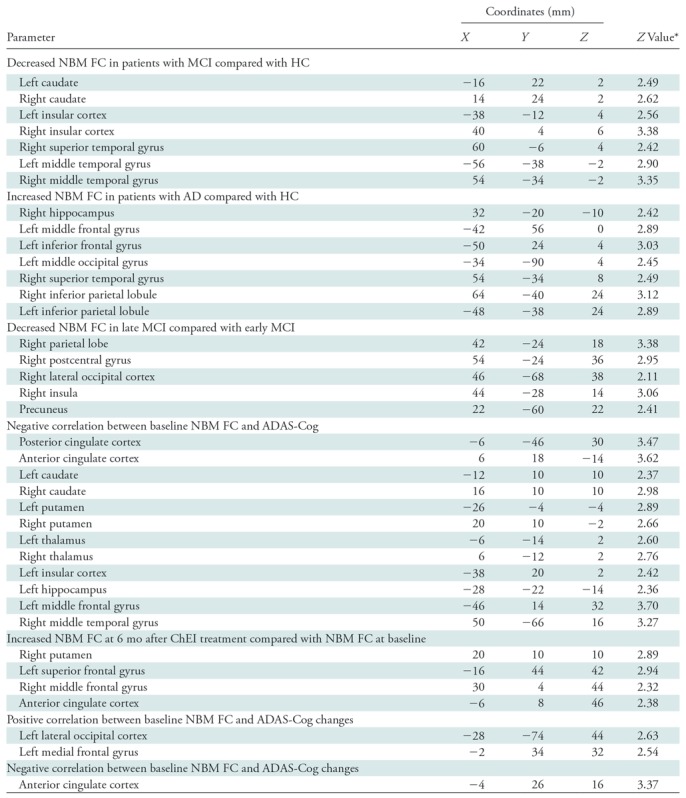
Note.—AD = Alzheimer disease, ADAS-Cog = Alzheimer’s Disease Assessment Scale-Cognitive subscale, ChEI = cholinesterase inhibitor, HC = healthy control participants, FC = functional connectivity, MCI = mild cognitive impairment, NBM = nucleus basalis of Meynert.
*All statistical images were thresholded at a familywise error–corrected threshold of P ˂ .05.
Effects of Age, Anticholinergic Drugs, Disease Progression, Genotype, and Amyloid Pathology on NBM FC
We ran several posthoc analyses to explore possible moderator factors. In healthy control participants and patients with MCI without ChEI or anticholinergic drugs, there was no significant correlation between age and Z score of clusters showing increased NBM FC in AD compared with healthy control participants (P = .74).
We repeated the between-group comparison after exclusion of participants who were taking anticholinergic drugs, which revealed similar results as in the whole cohort (Fig E2 [online]).
To further explore the effects of disease progression without confounding effects from ChEI or anticholinergic drugs, we also compared patients with untreated early MCI and untreated late MCI. The results revealed a decreased NBM FC with the right parietal lobe, right postcentral gyrus, right lateral occipital cortex, right insula, and precuneus in late MCI compared with early MCI (age and mean relative displacement–controlled, familywise error–corrected P ˂ .05) (Fig 2, E; Table 2).
To explore the effect of genotype and amyloid pathology on NBM FC without confounding effects from ChEI or anticholinergic drugs, binary logistic regression analyses between the status of APOE ε4, amyloid-β 42 status, and Z scores of clusters showing difference of NBM FC (healthy control participants vs patients with AD) were conducted. No significant correlation was found between the Z score of clusters showing increased NBM FC in AD and the status of APOE ε4 (P = .26) or amyloid-β 42 status (P = .27).
NBM FC and Cognitive Impairment
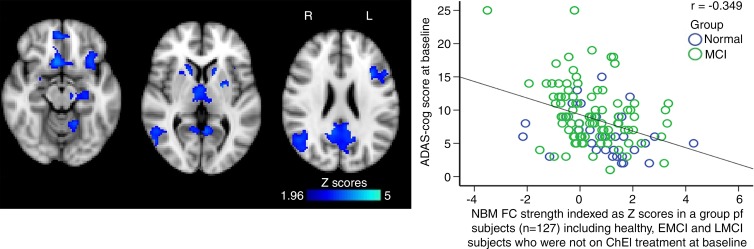
A significant but modest negative correlation (r = −0.349; P ˂ .001) (Fig 3, Table 2) was found between baseline ADAS-Cog and NBM FC with the posterior cingulate cortex, anterior cingulate cortex, bilateral caudate, bilateral putamen, bilateral thalamus, left insular cortex, left hippocampus, left middle frontal gyrus, and right middle temporal gyrus in the group of participants not taking ChEI at baseline (n = 127 [33 healthy control participants and 54 patients with MCI]). This correlation was largely unchanged when 16 participants who were taking anticholinergic drugs were excluded (n = 111; r = −0.351; P ˂ .001) (Fig E3 [online]).
Figure 3:
Images show functional connectivity (FC) mapping of nucleus basalis of Meynert (NBM) cholinergic network (hereafter, NBM FC) and cognition. Negative correlation map between baseline Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) and NBM FC located in significant clusters (blue-light blue) in group of participants including healthy control participants (HC), patients with early mild cognitive impairment (EMCI), and patients with late mild cognitive impairment (LMCI) who were not undergoing cholinesterase inhibitor (ChEI) treatment (n = 127). All results were masked by gray matter masks obtained from Montreal Neurological Institute 152 standard-space T1-weighted average structural template image. Scatterplot shows negative correlation between baseline ADAS-Cog and FC between NBM and significant clusters. All analyses were controlled for age and mean relative displacement. Significance was set at corrected P ˂ .05. MCI = mild cognitive impairment.
To control for a possible general cognitive effect exerted by loss of global FC, and specifically impaired FC in the posterior cingulate cortex, we investigated whether posterior cingulate cortex or primary visual cortex FC was associated with ADAS-Cog. No significant correlation between cognitive performance and posterior cingulate cortex or primary visual cortex FC was identified, supporting a weak but specific association between NBM FC and cognition.
NBM FC Changes after Initiation of ChEI
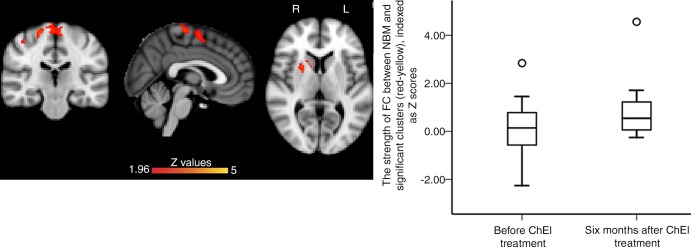
A total of 18 patients with MCI (mean age, 72.2 years ± 7.3; seven women [38.9%] and 11 men [61%]; six with early MCI and twelve with late MCI) underwent functional MRI before and 6 months after starting ChEI. Compared with pretreatment, the 6-month follow-up images showed significantly increased FC between NBM and the right putamen, right caudate, left superior frontal gyrus, right middle frontal gyrus, and anterior cingulate cortex (corrected P = .001) (Fig 4, Table 2). Because of the naturalistic ADNI cohort design, the observed serial changes may be related to disease progression rather than to ChEI treatment. To control for such potentially confounding effects, we compared the baseline NBM FC in 18 patients with untreated MCI (mean age, 72.8 years ± 5.1; seven women [38.9%] and 11 men [61%]; six with early MCI and 12 with late MCI) with their 6-month follow-up NBM FC, which did not reveal significant changes.
Figure 4:
Images show serial changes in functional connectivity (FC) mapping of nucleus basalis of Meynert (NBM) cholinergic network (hereafter, NBM FC) between baseline and 6 months after undergoing cholinesterase inhibitor (ChEI) treatment in 18 patients with mild cognitive impairment (MCI). NBM FC significantly increased from baseline to 6 months after ChEI in right anterior striatum and prefrontal cortex (red-yellow). Box plot shows distribution of strength of FC between NBM and significant clusters (red-yellow) before and 6 months after ChEI, indexed as Z score. All results were masked by gray matter masks obtained from Montreal Neurological Institute 152 standard-space T1-weighted average structural template image. Significance level was at familywise error–corrected P ˂ .05 (corrected for multiple comparisons) and corrected for age and mean displacement.
NBM FC at Baseline to Predict Cognitive Outcome after ChEI
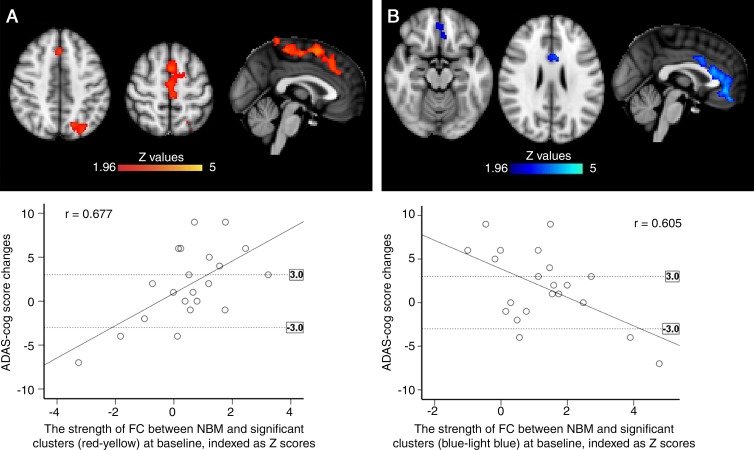
We then assessed the potential of baseline NBM FC to predict the cognitive outcome after 6 months of ChEI. NBM FC in both the lateral cholinergic network (left lateral occipital cortex) and the medial cholinergic network (left medial frontal gyrus) was strongly and positively correlated with changes in ADAS-Cog scores over 6 months of ChEI (R2, 0.458; P = .001) (Fig 5, A; Table 2) in line with the hypothesis that lower FC may predict increased benefits from ChEI. Unexpectedly, we also found a similarly strong anticorrelation between NBM FC in the medial cholinergic network (anterior cingulate cortex) and ADAS-Cog changes 6 months after starting ChEI (R2 = 0.366; P = .006) (Fig 5, B; Table 2). To further control for possible confounding predictor effects associated with disease progression but unrelated to ChEI, we studied the association between NBM FC at baseline and ADAS-Cog changes 6 months after baseline in 21 patients with MCI who did not take ChEI in the interval, which did not reveal significant associations. Additionally, to investigate whether APOE ε4 or amyloid-β 42 status had an effect on the predictive power of NBM FC on treatment response, the association between the Z score of clustering showing positive correlation with ADAS-Cog changes (Fig 5, A) and APOE ε4 and amyloid-β 42 status was explored, but no significant results were revealed (APOE ε4, P = .94; amyloid-β 42 status, P = .25).
Figure 5:
Images show correlation between baseline functional connectivity (FC) mapping of nucleus basalis of Meynert (NBM) cholinergic network (hereafter, NBM FC) and changes of cognitive performance assessed by Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) over 6 months of treatment with cholinesterase inhibitor. A, Positive correlation between ADAS-Cog change (posttreatment minus pretreatment; positive difference represents worsening cognition) and baseline NBM FC (red) in patients with mild cognitive impairment (MCI). Scatterplot illustrates positive correlation for significant clusters. B, Negative correlation between changes of ADAS-Cog and NBM FC (blue) at baseline in patients with MCI. Scatterplot shows negative correlation in significant clusters. Dotted lines in scatterplots indicate three-point change (improve/decline) on ADAS-Cog, which is considered as clinically relevant. All analyses were controlled for age and mean relative displacement. All results were masked by gray matter masks obtained from Montreal Neurological Institute 152 standard-space T1-weighted average structural template image. Significance level was at corrected P ˂ .05.
Discussion
Our study investigated the potential of basal forebrain FC as a biomarker of cholinergic dysfunction in MCI and early AD by using the publicly available ADNI data sets. Seeding FC maps in the NBM allowed us to identify established cortical and subcortical networks with known cholinergic innervation by the NBM. Four main findings demonstrated diagnostic, prognostic, and predictive biomarker properties. First, NBM FC was decreased in patients with untreated MCI, but was increased in patients with early AD who were taking ChEI compared with the control group. Second, global cognitive performance in healthy participants and patients with untreated MCI was correlated with NBM FC predominantly in the default mode network. Third, the NBM FC deficit was partially responsive to indirect cholinomimetic treatment in patients with MCI, showing significant increases 6 months after ChEI initiation, but not in an untreated parallel group. Fourth, baseline NBM FC strongly predicted cognitive outcomes in patients with MCI 6 months after starting ChEI, but not in an untreated parallel group.
The pattern of cortical and subcortical NBM FC, including hubs of the default mode and the salience networks and the bilateral caudate nuclei, is largely consistent with the anatomic identification of the medial and lateral cholinergic pathways (22) and a previous report in healthy younger people (13). Specifically, the medial cholinergic pathway matches the FC between NBM and medial prefrontal cortices, anterior cingulate cortex, and subcortical regions. The capsular division of the lateral cholinergic pathway is consistent with the FC between NBM and bilateral hippocampi, as is the perisylvian division with the FC between NBM and bilateral insula. Different from Li et al (13), we did not find areas that were anticorrelated with NBM, which can be explained by the fact that we avoided to regress out global signal changes, thereby minimizing spurious negative cross-correlations between brain regions (23).
Compared with healthy control participants, patients with MCI showed decreased NBM FC within both the medial and lateral cholinergic system. This finding is well in line with the early neurodegeneration of the NBM and several PET studies showing a widespread reduction of acetylcholine activities in MCI (25,26). The results are also in broad accordance with two recent NBM FC studies in MCI (27) and MCI in Parkinson disease (28) showing reduced NBM FC associated with cognitive deficits. However, we found a more extended pattern of NBM FC deficit in MCI compared with Li et al (27), which may be related to our postprocessing method and the medication effects. Further decrease of NBM FC in cases of late MCI versus early MCI supports the progressive nature of cholinergic dysfunction.
In a large group of healthy control participants and patients with MCI who were not taking ChEI, we showed a significant but modest correlation between cognitive performance and NBM FC within the medial and lateral cholinergic system. Findings were not affected by excluding participants who were taking anticholinergic drugs. The regions within the NBM FC network showing associations with cognitive performance partly overlap with the default mode network such as the posterior cingulate cortex and the frontal, temporal, and parietal cortices. The pattern identified and the direction of association provide further support for the cholinergic hypothesis of dementia (2) in line with previous studies showing reduced acetylcholine activities in multiple brain regions by using PET imaging techniques (29,30).
Interestingly, we did not find a further decrease of NBM FC in AD, but we observed an increased NBM FC in patients with AD compared with healthy control participants. This finding—at first surprising—may be related to a treatment effect, as the ADNI repository only holds data from patients with AD already receiving treatment with ChEI. In line with pharmacologic imaging studies showing increased activation after cholinergic challenge (31,32), ChEI is expected to increase the cholinergic tone and the NBM FC. However, this interpretation remains speculative without comparison with NBM FC maps of patients with untreated AD.
To further characterize a possible treatment effect, we studied serial NBM FC changes between baseline and 6-month posttreatment data in a subgroup of patients with MCI. This showed significantly increased NBM FC within the right putamen, right caudate, left superior frontal gyrus, and right middle frontal gyrus compared with pretreatment, suggesting at least partial restoration of cholinergic modulation of neural activities. This result concords well with the expected treatment effects of ChEI to restore synaptic activities within the cholinergic pathways and enhanced brain activity as shown in pharmacologic functional MRI studies (33). Taken together, our results of increased FC between NBM and left superior frontal gyrus and right middle frontal gyrus at rest after ChEI treatment may explain increased task responsiveness shown previously (33). However, in our study, we cannot directly allocate the observed NBM FC change to a pharmacologic effect of ChEI because of the naturalistic design. Nevertheless, in a matched cohort of patients with untreated MCI, we did not find a serial effect of NBM FC over 6 months supporting our interpretation that the observed NBM FC changes after ChEI reflect cholinomimetic effects rather than the disease process.
It would be clinically useful to identify patients who will benefit most from ChEI to improve the treatment effectiveness in established AD, and to enable stratification of patients with MCI for future treatment trials. Although clinical effectiveness of ChEI has not been proven in patients with MCI, it is conceivable that only those patients with MCI with reduced NBM FC may benefit more from ChEI. We observed a pattern of reduced NBM FC at baseline that was associated with better cognitive outcomes at 6 months after taking ChEI, mainly located in the left lateral occipital cortex (lateral cholinergic pathway) and the left middle frontal gyrus (medial cholinergic pathway), supporting the notion that imaging metrics have the potential to predict the cognitive response to ChEI in MCI and AD (34,35). By contrast, baseline NBM FC was not associated with cognitive outcome in those patients with MCI who did not take ChEI in the 6-month interval, making a confounding predictive effect of cognitive decline from disease progression less likely. The predictive potential is large as the overall effect was strong, with several clusters explaining up to 48% of the variance in cognitive outcome. Interestingly, we also observed a pattern of negative correlation between baseline NBM FC, mainly located in the anterior cingulate cortex, and cognitive change 6 months after taking ChEI. The direction of this association may be explained by the known high degree of regional heterogeneity of cholinergic receptors and possible disease stage effects (36).
Clinical translational potential of NBM FC is particularly high because task-free resting-state functional MRI can be easily incorporated into the current routine diagnostic work-up of patients with memory complaints. If the prediction of treatment response can be confirmed in prospective studies, then NBM FC could broaden the clinical remit of MRI for work-up of memory impairment beyond the current diagnostic support to assist treatment stratification.
Our study was limited by the design of the ADNI study. All the participants with AD had already started to take ChEI when they were recruited. Thus, we could not investigate the effects of ChEI on NBM FC in patients with AD. Because of the observational nature of the ADNI study, our findings on ChEI effects on NBM FC and the prediction of cognitive outcomes by NBM FC could not be placebo controlled. However, serial data were available in matched patients with untreated MCI, allowing us to make a serial effect from disease progression unlikely. Because of the small cohort of patients undergoing ChEI, for the comparison of NBM FC before and after 6 months of ChEI treatment, we chose a less rigorous multiple test correction at the voxel level alone. Larger, prospective studies are needed to confirm the reported treatment changes of NBM FC and the predictive value of NBM FC at baseline. Another potential limitation was the robustness of the seed-based analysis because of the small size and ill-defined borders of the NBM at functional MRI, making manual delineation challenging. We addressed this by showing the robustness of the manual drawing method against small shifts of the seeds by using an in-house Matlab (version R2016a; Mathworks, Natick, Mass) code. This step could be improved by semiautomatic seed selection.
In summary, our study shows that NBM FC metrics obtained from a brief task-free functional MRI at 3.0 T has promising prognostic and predictive biomarker properties for central cholinergic dysfunction. If confirmed in prospective studies, then NBM FC could assist treatment stratification and facilitate ongoing drug development for optimized restoration of cholinergic functions in people living with or at risk for dementia.
Summary
Nucleus basalis of Meynert functional connectivity metrics obtained from resting-state functional MRI have promising prognostic and predictive biomarker properties for central cholinergic dysfunction.
Implications for Patient Care
■ The findings suggest that nucleus basalis of Meynert functional connectivity metrics obtained from resting-state functional MRI have promising biomarker properties for measuring central cholinergic dysfunction and predicting treatment response to cholinesterase inhibition.
■ If confirmed in future studies, then nucleus basalis of Meynert functional connectivity may assist treatment stratification and facilitate ongoing drug development for optimized restoration of cholinergic functions in people living with or at risk for dementia.
APPENDIX
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgments
The authors thank Dr Christopher Tench for the support of statistical analysis.
Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health [NIH] Grant U01 AG024904) and Department of Defense ADNI (award number W81XWH-12–2-0012). The ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation, Araclon Biotech, BioClinica. Biogen, Bristol-Myers Squibb, CereSpir, Cogstate, Eisai, Elan Pharmaceuticals, Eli Lilly, EuroImmun, F. Hoffmann-La Roche and its affiliated company Genentech, Fujirebio, GE Healthcare, IXICO, Janssen Alzheimer Immunotherapy Research & Development, Johnson & Johnson Pharmaceutical Research & Development, Lumosity, Lundbeck, Merck, Meso Scale Diagnostics, NeuroRx Research, Neurotrack Technologies, Novartis Pharmaceuticals, Pfizer, Piramal Imaging, Servier, Takeda Pharmaceutical Company, and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (https://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. The resting-state network data were made available by the WU-Minn Human Connectome Project (1U54MH091657), funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research.
Supported by Alzheimer's Disease Neuroimaging Initiative (U01 AG024904, W81XWH-12-2-0012). D.M. supported by Nottingham NIHR Biomedical Research Centre. J.P.T. supported by Newcastle NIHR Biomedical Research Centre. Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health [NIH] Grant U01 AG024904) and Department of Defense ADNI (award number W81XWH-12–2-0012
Disclosures of Conflicts of Interest: D.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. X.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. M.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. J.P.T. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. D.P.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is employed by University of Nottingham; has grants/grants pending with Arthritis Research UK, Michael J. Fox Foundation for Parkinson’s Research, NIHR Nottingham Biomedical Research Centre, and Parkinson’s UK. Other relationships: disclosed no relevant relationships.
Abbreviations:
- AD
- Alzheimer disease
- ADAS-Cog
- Alzheimer’s Disease Assessment Scale-Cognitive subscale
- ADNI
- Alzheimer’s Disease Neuroimaging Initiative
- ChEI
- cholinesterase inhibitor
- CSF
- cerebrospinal fluid
- FC
- functional connectivity
- MCI
- mild cognitive impairment
- NBM
- nucleus basalis of Meynert
References
- 1.Richter N, Allendorf I, Onur OA, et al. The integrity of the cholinergic system determines memory performance in healthy elderly. Neuroimage 2014;100:481–488. [DOI] [PubMed] [Google Scholar]
- 2.Craig LA, Hong NS, McDonald RJ. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci Biobehav Rev 2011;35(6):1397–1409. [DOI] [PubMed] [Google Scholar]
- 3.Lanctôt KL, Herrmann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 2003;169(6):557–564. [PMC free article] [PubMed] [Google Scholar]
- 4.Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev 2012;(9):CD009132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gauthier S. Cholinergic adverse effects of cholinesterase inhibitors in Alzheimer’s disease: epidemiology and management. Drugs Aging 2001;18(11):853–862. [DOI] [PubMed] [Google Scholar]
- 6.Fotiou DF, Stergiou V, Tsiptsios D, Lithari C, Nakou M, Karlovasitou A. Cholinergic deficiency in Alzheimer’s and Parkinson’s disease: evaluation with pupillometry. Int J Psychophysiol 2009;73(2):143–149. [DOI] [PubMed] [Google Scholar]
- 7.Bittner DM, Wieseler I, Wilhelm H, Riepe MW, Müller NG. Repetitive pupil light reflex: potential marker in Alzheimer’s disease? J Alzheimers Dis 2014;42(4):1469–1477. [DOI] [PubMed] [Google Scholar]
- 8.Naicker P, Anoopkumar-Dukie S, Grant GD, Neumann DL, Kavanagh JJ. Central cholinergic pathway involvement in the regulation of pupil diameter, blink rate and cognitive function. Neuroscience 2016;334:180–190. [DOI] [PubMed] [Google Scholar]
- 9.Bohnen NI, Frey KA. Imaging of cholinergic and monoaminergic neurochemical changes in neurodegenerative disorders. Mol Imaging Biol 2007;9(4):243–257. [DOI] [PubMed] [Google Scholar]
- 10.APA Work Group on Alzheimer’s Disease and Other Dementias , Rabins PV, Blacker D, et al. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Second edition. Am J Psychiatry 2007;164(12 Suppl):5–56. [PubMed] [Google Scholar]
- 11.Cummings JL, Isaacson RS, Schmitt FA, Velting DM. A practical algorithm for managing Alzheimer’s disease: what, when, and why? Ann Clin Transl Neurol 2015;2(3):307–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol 1983;214(2):170–197. [DOI] [PubMed] [Google Scholar]
- 13.Li CS, Ide JS, Zhang S, Hu S, Chao HH, Zaborszky L. Resting state functional connectivity of the basal nucleus of Meynert in humans: in comparison to the ventral striatum and the effects of age. Neuroimage 2014;97:321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alzheimer’s Disease Neuroimaging Initiative 2. ADNI2: defining Alzheimer's disease procedures manual. https://adni.loni.usc.edu/wp-content/uploads/2008/07/adni2-procedures-manual.pdf. Accessed December 16, 2016.
- 15.Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology 2011;36(1):52–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ 2000;321(7274):1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aerssens J, Raeymaekers P, Lilienfeld S, Geerts H, Konings F, Parys W. APOE genotype: no influence on galantamine treatment efficacy nor on rate of decline in Alzheimer’s disease. Dement Geriatr Cogn Disord 2001;12(2):69–77. [DOI] [PubMed] [Google Scholar]
- 18.Palmqvist S, Mattsson N, Hansson O; Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid analysis detects cerebral amyloid-β accumulation earlier than positron emission tomography. Brain 2016;139(Pt 4):1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman L, Glover GH. Report on a multicenter fMRI quality assurance protocol. J Magn Reson Imaging 2006;23(6):827–839. [DOI] [PubMed] [Google Scholar]
- 20.Jezzard P, Matthews PM, Smith SM. Functional MRI: an introduction to methods. Oxford, England: Oxford University Press, 2001. [Google Scholar]
- 21.Hlinka J, Alexakis C, Hardman JG, Siddiqui Q, Auer DP. Is sedation-induced BOLD fMRI low-frequency fluctuation increase mediated by increased motion? MAGMA 2010;23(5-6):367–374. [DOI] [PubMed] [Google Scholar]
- 22.Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain 1998;121(Pt 12):2249–2257. [DOI] [PubMed] [Google Scholar]
- 23.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 2009;44(3):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cottam WJ, Auer DP. Group differences in default mode network connectivity not just anti-correlation depend on choice of nuisance regressor model [abstr]. In: Proceedings of the Twenty-Fifth Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2017. [Google Scholar]
- 25.Marcone A, Garibotto V, Moresco RM, et al. [11C]-MP4A PET cholinergic measurements in amnestic mild cognitive impairment, probable Alzheimer’s disease, and dementia with Lewy bodies: a Bayesian method and voxel-based analysis. J Alzheimers Dis 2012;31(2):387–399. [DOI] [PubMed] [Google Scholar]
- 26.Rinne JO, Kaasinen V, Järvenpää T, et al. Brain acetylcholinesterase activity in mild cognitive impairment and early Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2003;74(1):113–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Jia X, Qi Z, et al. Altered functional connectivity of the basal nucleus of Meynert in mild cognitive impairment: a resting-state fMRI Study. Front Aging Neurosci 2017;9:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim I, Shin NY, Bak Yunjin, Hyu Lee P, Lee SK, Mee Lim S. Early-onset mild cognitive impairment in Parkinson’s disease: altered corticopetal cholinergic network. Sci Rep 2017;7(1):2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhl DE, Koeppe RA, Minoshima S, et al. In vivo mapping of cerebral acetylcholinesterase activity in aging and Alzheimer’s disease. Neurology 1999;52(4):691–699. [DOI] [PubMed] [Google Scholar]
- 30.Herholz K, Weisenbach S, Zündorf G, et al. In vivo study of acetylcholine esterase in basal forebrain, amygdala, and cortex in mild to moderate Alzheimer disease. Neuroimage 2004;21(1):136–143. [DOI] [PubMed] [Google Scholar]
- 31.Goekoop R, Scheltens P, Barkhof F, Rombouts SA. Cholinergic challenge in Alzheimer patients and mild cognitive impairment differentially affects hippocampal activation: a pharmacological fMRI study. Brain 2006;129(Pt 1):141–157. [DOI] [PubMed] [Google Scholar]
- 32.Rombouts SA, Barkhof F, Van Meel CS, Scheltens P. Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2002;73(6):665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saykin AJ, Wishart HA, Rabin LA, et al. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain 2004;127(Pt 7):1574–1583. [DOI] [PubMed] [Google Scholar]
- 34.Kilimann I, Grothe M, Heinsen H, et al. Subregional basal forebrain atrophy in Alzheimer’s disease: a multicenter study. J Alzheimers Dis 2014;40(3):687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Antuono PG, Xie C, et al. Changes in regional cerebral blood flow and functional connectivity in the cholinergic pathway associated with cognitive performance in subjects with mild Alzheimer’s disease after 12-week donepezil treatment. Neuroimage 2012;60(2):1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinotoh H, Namba H, Fukushi K, et al. Brain acetylcholinesterase activity in Alzheimer disease measured by positron emission tomography. Alzheimer Dis Assoc Disord 2000;14(Suppl 1):S114–S118. [DOI] [PubMed] [Google Scholar]
- 37.Aging brain care: Anticholinergic Cognitive Burden scale—2012 update. http://www.miltonkeynesccg.nhs.uk/resources/uploads/ACB_scale_-_legal_size.pdf. Accessed June 2017.
- 38.Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016;73(6):721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ 2006;332(7539):455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.