Abstract
Background
The number of dementia patients will increase over the next decades. However, we lack information on the geographic distribution of these patients. We aimed to describe the variation of dementia prevalence and to then compare the observed to expected prevalence.
Methods
This study is based on a 20% sample of Medicare beneficiaries in 2008. The crude dementia prevalence was calculated and age/sex standardized to the US population for states. We used the World Alzheimer Report 2009 prevalence to compare estimates.
Results
4.8 million persons were included. The adjusted prevalence is 8.24%, varying from 5.96 to 9.55% across states. The diagnosed prevalence is lower than the expected in most states. Overall, we estimate over 100 000 undiagnosed dementia patients in Medicare.
Conclusions
The high state variation suggests that the number of diagnosed dementia cases does not fall evenly across all states and hence may require different levels of state-level planning.
Keywords: chronic disease, diagnosis, health services
Introduction
Dementia is a disease with major impact on patients and their caregivers as well as the health system itself; it is related to disability as well as to increased mortality.1–4 According to the 2005 Delphi consensus study, ∼24 million persons are suffering from dementia worldwide. For North America, the authors estimated 3.4 million affected persons in the year 2001, with a steep future increase to between 9 and over 11 million by 2040, depending on the estimates.5–8 Globally, the prevalence of dementia has been shown to vary across countries likely due in part to differences in demographics, education and genetics, but also likely due to different approaches to screening and diagnosis.9–12 Even though the disease poses major challenges for patients and the health system in the near future, there are no nationwide studies on the regional variation in prevalence of dementia across the USA.
Existing prevalence studies in the USA are typically based on in-person assessments for cohorts selected from urban areas7,13–15 combined with demographic data to estimate prevalence for the larger population16,17 (see also Refs 18,19). For example, a state estimation published by Hebert et al.12 in 2004 was based on one of the larger urban studies, the Chicago Health and Aging Project, combined with US Census data.20,21 The only nationally representative sample is the Aging, Demographics, and Memory study (ADAMS), a subsample of the Health and Retirement Study; however, the low-region-specific sample size in that study does not allow a prevalence calculation by state.22 An up-to-date estimate of the dementia prevalence is crucial for health services planning to ensure that the needed services can be provided for patients—not only on a national level but also for states.
Yet, we lack the comprehensive epidemiological data on which to make accurate estimates for all the regions and diverse populations that make up the USA. Due to the prohibitive expense and complexity of obtaining that primary data, we need to rely on other sources, such as diagnostic information in medical records. The Medicare claims data are one source for diagnosed dementia and several studies have reported on its good specificity and fair sensitivity.23–26 As a result, we know that claims data alone likely underestimate the true prevalence by missing earlier disease. Moreover, there may be differences in disease ascertainment related to differences in recognition of symptoms and willingness to document the disease based on patient cultural or physician practice norms. Previous studies have shown that clinician and patient factors (such as, problematic communication between the physician and the patients or stigmatization of the disease) contribute to whether dementia is recognized and documented by the physician.27–30 In addition, different state policies, such as funding for elder services and outreach into the community, could influence whether a person will be identified with dementia. If large gaps in expected numbers of dementia cases compared with actual cases are identified, many elements of disease ascertainment could potentially be modifiable.
The aim of this study is to describe the degree of regional variation of diagnosed dementia prevalence using Medicare claims data for the entire USA and to compare those rates with expected rates based on epidemiologic estimates applied to US Census data. By state, we determine whether there are regions in which under-documentation of dementia may be present.
Methods
This study is based on a 20% random sample of Medicare beneficiaries in the year 2008.
Medicare is the US governmental health insurance for people over 65 as well as those under 65 with certain disabilities. Generally, all US citizens or permanent residents over 65 who paid into social security are eligible for Medicare. In 2008, 47.9 million people were enrolled in Medicare. For this sample, those <65 years (7.8 million) were excluded, as well as beneficiaries who were not in Fee-for-Service Medicare (16.6 million) (see https://www.ccwdata.org/web/guest/medicare-data; https://www.cms.gov/Medicare/Medicare-General-Information/MedicareGenInfo/index.html). The sample is created by Centers for Medicare and Medicaid Services based on the ninth digit of the unique identifier used by Medicare. Included beneficiaries had to be enrolled in Part A and B the entire year and be 65 years and older. Beneficiaries were identified as dementia patients if they had one claim with the diagnosis of dementia using the ICD-9 codes included in the Chronic Condition Warehouse dementia definition. Included diagnosis codes (ICD-9) were as follows: 331.0, 331.1, 331.11, 331.19, 331.2, 331.7, 331.82, 290.0, 290.1, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.40, 290.41, 290.42, 290.43, 291.2, 294.0, 294.1, 294.10 and 294.11 (see http://www.ccwdata.org/cs/groups/public/documents/document/ccw_conditioncategories2011.pdf).
Observed prevalence
The observed claims-based prevalence is calculated nationally, by demographic categories using beneficiary characteristics found in the Medicare denominator file, and for each state. These crude rates for the single states were then standardized to the US population size, age and sex distribution (as of 201031).
Expected prevalence
For comparison with epidemiological estimates, we used the most up-to-date prevalence estimates for the USA which are from the World Alzheimer Report 200932 (see also Ref. 33). This report shows the results of a meta-analysis based on published data across regions worldwide. We used the North American estimates that included the USA and Canada; however, the age- and sex-specific prevalence of dementia was based on US studies only. The report included published studies on the prevalence of dementia for those aged 60 and older. Only studies with some age stratification could be included; the meta-analysis shows the prevalence for dementia by age groups and gender. Due to the limited number of studies and sample sizes in those studies, other factors (such as race/ethnicity, socio-economic background) could not been taken into account. For the USA, six studies were used for the meta-analysis, one of which based on a nationally representative sample.32 The national prevalence rates given in the World Alzheimer Report use different age groupings than those in our Medicare cohort. We therefore reweighted the World Alzheimer Report's prevalence rates by applying the age groups for men and women in the US 2010 Census population and calculated the national expected prevalence based on those epidemiologic estimates.31
To examine the expected number of dementia patients for each state, we used the age- and sex-specific prevalence of the World Alzheimer Report 2009 and applied it to each state's Medicare fee-for-service population. We estimate the crude number of cases for the entire population by inflating the observed cases in the 20% sample by 5. We then calculated the percentage difference between observed and expected number of dementia patients.
All data analyses were performed using the Statistical Analysis Systems SAS (Version 9.2); the map of the regional distribution was made with ESRI ArcGIS 10.0. The prevalence results and the percent difference between observed and expected are mapped in quintile categories.
Results
A total of 4.8 million persons aged 65 and older were included in this study. The overall observed prevalence of dementia in the Medicare sample was 8.5% (Table 1). It increased from 2.8% for the persons aged 65–74 to 24.9% in the group aged 85 and older. The prevalence for women is higher than for men (9.94 versus 6.39). The prevalence is also noticeably higher in blacks and Hispanics compared with whites and other ethnicities.
Table 1.
Prevalence of dementia in fee-for-service Medicare (2008) compared with the prevalence rates given in the World Alzheimer Report 2009
|
Prevalence of dementia
| ||
|---|---|---|
| US prevalence in FFS Medicare population | US prevalence in World Alzheimer Report 2009a | |
| Overall | 8.46 | 8.37 |
| Age groups | ||
| 65–74 | 2.76 | 2.54 |
| 75–84 | 10.48 | 8.76 |
| 85 and older | 24.88 | 30.50 |
| Gender | ||
| Women | 9.94 | 9.78 |
| Men | 6.39 | 7.41 |
| Ethnicity | ||
| Black | 11.26 | Does not apply |
| White | 8.24 | Does not apply |
| Hispanic | 12.25 | Does not apply |
| Other | 6.47 | Does not apply |
FFS, fee-for-service.
aRe-grouped according to the US Census population 2010 to match the Medicare age groups.
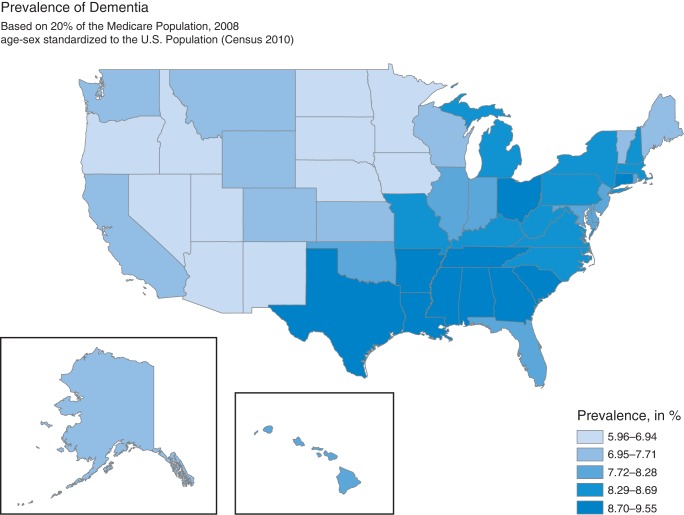
To control for regional variation due to different demographic structures in the states, we age- and sex-adjusted our study sample to the US population (based on the Census 2010).31 State prevalence rates are highly variable ranging from 6.0 to 9.6% (Fig. 1). The highest prevalence rates are found in the South and East (highest prevalence in Texas, Mississippi and Louisiana), whereas the lowest prevalence is found in the Central North and West (lowest prevalence in South Dakota, Arizona and Minnesota).
Fig. 1.
State prevalence of Medicare FFS population, age/sex adjusted to the USA: census population.
The national Medicare rates are similar to the expected prevalence rates found in primary data studies. The World Alzheimer Report reports an overall prevalence of 6.5%; this however includes all persons aged 60 and older. After our re-calculation of their results using Census data to match the age range in our study, the national prevalence for persons aged 65 and older in the USA would be 8.4% (7.4% for men and 9.8% for women, respectively) compared with the Medicare rate of 8.5% (Table 1). This national rate, however, masks significant variation in the difference between observed and expected prevalence across the states.
We find that some states have higher and others lower than the epidemiologic estimates would suggest (Table 2). Table 2 shows the actual number of diagnosed dementia patients and the number we would expect based on the state population and the prevalence estimated of the World Alzheimer Report. While in some states, the number of diagnosed patients exceeds the estimated number, the prevalence is underestimated in the majority of states; in California alone, we would expect over 21 000 more persons with a dementia diagnosis. The percent difference between the observed and estimated expected per state is shown in Table 2. The highest discrepancies between the observed diagnosed prevalence and the estimated prevalence are seen in South Dakota and Arizona, where the number of diagnosed patients is under 70% of the expected prevalence. On the high side, the diagnosed prevalence exceeds the expected by over 10% in Texas and Mississippi. Based on the observed prevalence by states and the expected prevalence in this cohort, we estimate that over 100 000 people with dementia are not diagnosed with the disease across the USA in fee-for-service Medicare.
Table 2.
Standardized state prevalence and observed and estimated number of dementia patients by state
| State | Adjusted prevalence in the Medicare cohort | Observed number of Medicare beneficiaries diagnosed with dementia | Estimated number of dementia cases in the Medicare sample | Number of persons potentially not diagnosed | % Difference |
|---|---|---|---|---|---|
| AK | 7.28 | 2425 | 2897 | 472 | −16.29 |
| AL | 9.30 | 39 565 | 36 120 | −3445 | 9.54 |
| AR | 9.06 | 26 870 | 25 396 | −1474 | 5.81 |
| AZ | 6.01 | 21 315 | 30 842 | 9527 | −30.89 |
| CA | 7.61 | 143 975 | 165 321 | 21 346 | −12.91 |
| CO | 7.49 | 21 260 | 24 726 | 3466 | −14.02 |
| CT | 9.20 | 36 480 | 34 859 | −1621 | 4.65 |
| DC | 8.10 | 16 730 | 17 958 | 1228 | −6.84 |
| DE | 7.72 | 5925 | 6603 | 678 | −10.26 |
| FL | 8.19 | 151 265 | 160 682 | 9417 | −5.86 |
| GA | 8.94 | 55 495 | 52 856 | −2639 | 4.99 |
| HI | 8.12 | 6485 | 6957 | 472 | −6.79 |
| IA | 6.90 | 24 685 | 31 295 | 6610 | −21.12 |
| ID | 6.19 | 4560 | 6425 | 1865 | −29.03 |
| IL | 8.04 | 81 095 | 87 811 | 6716 | −7.65 |
| IN | 7.92 | 50 515 | 55 031 | 4516 | −8.21 |
| KS | 7.45 | 15 590 | 18 262 | 2672 | −14.63 |
| KY | 8.69 | 33 195 | 32 516 | −679 | 2.09 |
| LA | 9.33 | 32 490 | 29 643 | −2847 | 9.60 |
| MA | 8.32 | 49 555 | 52 340 | 2785 | −5.32 |
| MD | 7.90 | 29 160 | 31 903 | 2743 | −8.60 |
| ME | 7.71 | 14 010 | 15 619 | 1609 | −10.30 |
| MI | 8.50 | 73 340 | 75 169 | 1829 | −2.43 |
| MN | 6.11 | 22 435 | 31 873 | 9438 | −29.61 |
| MO | 8.43 | 63 860 | 65 673 | 1813 | −2.76 |
| MS | 9.51 | 21 040 | 18 733 | −2307 | 12.31 |
| MT | 7.09 | 8280 | 10 219 | 1939 | −18.97 |
| NC | 8.58 | 71 060 | 70 794 | −266 | 0.38 |
| ND | 6.52 | 8080 | 10 919 | 2839 | −26.00 |
| NE | 6.57 | 13 750 | 18 236 | 4486 | −24.60 |
| NH | 8.30 | 11 950 | 12 465 | 515 | −4.13 |
| NJ | 8.22 | 71 320 | 76 074 | 4754 | −6.25 |
| NM | 6.94 | 7410 | 9288 | 1878 | −20.22 |
| NV | 6.70 | 9460 | 12 193 | 2733 | −22.41 |
| NY | 8.67 | 127 135 | 129 273 | 2138 | −1.65 |
| OH | 8.93 | 86 765 | 84 091 | −2674 | 3.18 |
| OK | 8.28 | 25 640 | 26 483 | 843 | −3.18 |
| OR | 6.68 | 17 210 | 22 622 | 5412 | −23.92 |
| PA | 8.29 | 100 690 | 106 601 | 5911 | −5.55 |
| RI | 8.26 | 7850 | 8373 | 523 | −6.24 |
| SC | 8.89 | 31 045 | 29 943 | −1102 | 3.68 |
| SD | 5.96 | 7760 | 11 389 | 3629 | −31.87 |
| TN | 9.30 | 60 070 | 54 988 | −5082 | 9.24 |
| TX | 9.55 | 154 585 | 138 729 | −15 856 | 11.43 |
| UT | 6.83 | 10 785 | 13 655 | 2870 | −21.02 |
| VA | 8.49 | 54 095 | 54 832 | 737 | −1.34 |
| VT | 7.37 | 5170 | 6088 | 918 | −15.07 |
| WA | 7.11 | 35 685 | 43 911 | 8226 | −18.73 |
| WI | 7.49 | 39 360 | 46 205 | 6845 | −14.81 |
| WV | 8.68 | 13 700 | 13 313 | −387 | 2.91 |
| WY | 7.71 | 1695 | 1911 | 216 | −11.30 |
| US | 8.24 | 2 023 870 | 2 130 103 | 106 233 | −4.99 |
Discussion
Main finding of this study
Our results show that while the national rate of diagnosed of dementia is similar to epidemiologic estimates, the prevalence rates vary widely across states, even after adjusting for age and sex differences. Some areas, especially in the southeast, show somewhat higher prevalence rates than expected, while states in the Midwest and West show markedly lower prevalence relative to expected. The difference between observed and expected prevalence rates translate nationally in ∼100 000 people with dementia who may be unrecognized.
What is already known on this topic
The World Alzheimer Report 2009 includes the most recent available epidemiologic studies on nationwide dementia prevalence. However, it does so as a meta-analysis combining results of studies derived from a variety of settings, most of them urban samples. The estimates hence have the limitations of the underlying study data. Reviews and meta-analyses that combined the results of prevalence estimations32,34 have concluded that the different prevalence results across studies are largely explained by the difference in definitions of dementia used.35 Yet, the consistency of prevalence found internationally (i.e. to the prevalence in Western Europe, where a broader database could be included) lends credence to the validity of the World Alzheimer Report estimates, and they currently are our most agreed upon estimate. Our observed national prevalence based on claims evidence of diagnosis in the USA aligns with what would be expected based on the estimated rate.
What this study adds
While the national rate aligns with estimates, state-specific rates vary significantly from the estimated sex and age-adjusted prevalence. There are three main potential explanations for the differences between observed and expected state prevalence rates. First, it is possible although unlikely that the ‘real’ prevalence varies between the states in such a way to generate national rates that align with estimates. Second, the epidemiological estimates could be inaccurate for any given state due to differences in the socio-demographic make up of the population of each region. And third, there may be regional differences, in clinical practice or in attitudes towards dementia, that lead to variation in disease recognition and documentation.
The epidemiological estimates used to calculate expected prevalence account for gender and age; age being the main risk factor for dementia.36 A limitation of those estimates is that important factors that influence the incidence of dementia, such as educational background and genetics, are not taken into account.22,36–41 From a small area regional perspective, differences between communities in population educational attainment could influence expected rates. The genetic variations in small populations could also influence the incidence, but we do not believe for regions as large as states that this factor would be a large component.
The higher than expected prevalence found in some southern states may be explained by incomplete adjustment for population characteristics. In the south, there are higher rates of low income, low education and also a higher concentration of African Americans. The correlation between dementia and race/ethnicity is very uncertain. Some studies report a higher prevalence for Hispanics and African American older adults compared with Caucasians; however, those differences were not significant if controlled for factors like education or gender.22,42 The differences in prevalence between ethnicities are probably a complex combination of genetics, cardiovascular risk factors, education and socio-economic status, as well as cultural perceptions about aging that influence both the recognition as well as the treatment of dementia.43–45
While inaccuracy of the epidemiologic estimates plausibly can explain higher than expected prevalence rates of dementia in some regions, it is difficult to argue that the areas with very large under-ascertainment could be the result of failing to adjust for genetics or education. A more likely explanation of under-ascertainment is that older adults with dementia symptoms in those areas are either not recognized or not diagnosed. Part of the explanation could be that claims data are less likely to capture early-stage dementia cases.23,24 There is not a clear reason, however, why the low sensitivity of claims would be different in one state compared with another. Alternatively, there are likely other differences that lead to regional differences in diagnosed dementia: differences in efforts to educate the community about the disease, in access to providers who are confident in making the diagnosis, medical practice norms toward identification and availability of resources that influence the desirability of a dementia diagnosis label.46
There is not any evidence in the existing literature that can help unpack these potential root causes of regional differences in under-diagnosis. We do know that patients and caregivers feel a sense of stigmatization or taboo regarding the diagnosis.30 Patients may need to understand whether there is any added value to their overall care by obtaining a diagnosis. Some potential advantages are the opportunity to do advanced care planning or to access medications or support services that target people with dementia.47 Some states may offer more support services, which make the value of a diagnosis to the individual higher.
Communication issues between the physician and the patients and his/her caregivers have also been implicated in delayed or lacking diagnosis.30 Like patients, physicians may not historically have seen benefit in applying the label,48 although that may be changing with the introduction of medications onto the market. Providers may recognize the disease but not document it out of similar concerns as patients or out of discomfort with diagnostic criteria or management strategies. In addition, they may not be screening patients for dementia. While some argue that early identification could help patients and their families, it is not yet universally accepted in the USA.47,49–51 Individuals might benefit from an early diagnosis if embedded in an advanced care management plan, but there are also concerns about harms.52 A review of potential harms of screening did not identify enough studies to demonstrate harms but suggested a positive screen might have an implications on long-term care insurability and may lead to depression or anxiety.47 Given the absence of disease modifying therapies for early disease, the US Preventive Services Task Force determination on screening for dementia was that it is unknown whether the benefits outweigh the costs.51 At the same time, cognitive screening has been shown to be acceptable to the general public,53–56 and it is required as part of the Medicare Annual Wellness Visit as of 2012.57 The mixed messages about the value of screening likely translate into inconsistent practices in clinician offices across the USA.
Limitations of this study
The observed prevalence is based on claims data that are subject to misclassification of diagnosis. Prior studies demonstrate that while specificity of claims is very good, sensitivity especially for early-stage disease is only good.23,24 As a result, the under-ascertainment we detect may preferentially miss early-stage dementia patients. And as noted above, the sources for calculating expected prevalence only provide adjustment factors for gender and age when other population factors may also be influencing results. In addition, our regional analysis is also not adjusted for the state variation in mortality. One could argue that with higher life expectancy the survival with dementia might also be longer, influencing the prevalence. However, comparing our results with the age-adjusted death rates by states, the regions with the highest observed prevalence rates are those with higher mortality rates.58 Overall, mortality is therefore not likely to explain the variation in dementia or differences in observed to expected prevalence.
Conclusion
As the baby boom generation advances in age, the number of dementia patients will increase dramatically over the next decades. From a policy perspective, there is great value in understanding the true scope and burden of the dementia in the population and particular in each state. Many health policy decisions concerning dementia patients are made on a state level (such as Medicaid policies around nursing home use). As the demographic shift occurs, states must adjust their strategies to provide access to long-term care, develop the workforce and fund these services largely through Medicaid programs. So care planning has to be done now to address the needs of this future generation of older adults. This study shows that the number of cases may not fall evenly across all US states and hence may require different levels of state-level planning. In addition, there is a potentially significant number of people with dementia who remain unrecognized in the Medicare system. Greater understanding of how to bring people with dementia to attention of health-care providers and how resources can be optimally planned to the growing population with dementia are important areas for future research, and it also needs clinical as well as policy attention.
Funding
Support for this research was provided by The Commonwealth Fund. The views presented here are those of the authors and should not be attributed to The Commonwealth Fund or its directors, officers, or staff.
Acknowledgements
D.K. is a German Harkness Fellow for Health Policy by the Commonwealth Fund.
References
- 1. Agüero-Torres H, Fratiglioni L, Guo Z, et al. Mortality from dementia in advanced age: a 5-year follow-up study of incident dementia cases. J Clin Epidemiol 1999;52(8):737–43. [DOI] [PubMed] [Google Scholar]
- 2. Agüero-Torres H, Fratiglioni L, Guo Z, et al. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health 1998;88(10):1452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koller D, Kaduszkiewicz H, van den Bussche H, et al. Survival in patients with incident dementia compared with a control group: a five-year follow-up. Int psychogeriatrics 2012;24(9):1522–30. [DOI] [PubMed] [Google Scholar]
- 4. Helmer C, Joly P, Letenneur L, et al. Mortality with dementia: results from a French prospective community-based cohort. Am J Epidemiol 2001;154(7):642–8. [DOI] [PubMed] [Google Scholar]
- 5. Ferri CP, Prince MJ, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366(9503):2112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sloane PD, Zimmerman S, Suchindran C, et al. The public health impact of Alzheimer's disease, 2000–2050: potential implication of treatment advances. Annu Rev Public Health 2002;23:213–31. [DOI] [PubMed] [Google Scholar]
- 7. Evans D. Estimated prevalence of Alzheimer's disease in the United States. Milbank Q 1990;68(2):267–89. [PubMed] [Google Scholar]
- 8. Hebert LE, Weuve J, Scherr Pa, et al. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013;80(19):1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Llibre Rodriguez JJ, Ferri CP, Acosta D, et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet 2008;372(9637):464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fratiglioni L, De Ronchi D, Agüero-Torres H. Worldwide prevalence and incidence of dementia. Drugs Aging 1999;15(5):365–75. [DOI] [PubMed] [Google Scholar]
- 11. Russ TC, Batty GD, Hearnshaw GF, et al. Geographical variation in dementia: systematic review with meta-analysis. Int J Epidemiol 2012;41(4):1012–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hebert LE, Scherr PA, Bienias JL, et al. State-specific projections through 2025 of Alzheimer disease prevalence. Neurology 2004;62(9):1645. [DOI] [PubMed] [Google Scholar]
- 13. Bienias JL, Beckett La, Bennett DA, et al. Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dis 2003;5(5):349–55. [DOI] [PubMed] [Google Scholar]
- 14. Kokmen E, Beard C, Offord K, et al. Prevalence of medically diagnosed dementia in a defined United States population Rochester, Minnesota, January 1, 1975. Neurology 1989;39(6):773–6. [DOI] [PubMed] [Google Scholar]
- 15. Bachman DL, Wolf PA, Linn R, et al. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology 1992;42(1):115–9. [DOI] [PubMed] [Google Scholar]
- 16. Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population. Arch Neurol 2003;60(8):1119–22. [DOI] [PubMed] [Google Scholar]
- 17. Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health 1998;88(9):1337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brookmeyer R, Evans D, Hebert L. National estimates of the prevalence of Alzheimer's disease in the United States. Alzheimer's Dement 2011;7(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rocca W, Petersen R, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer's disease, dementia, and cognitive impairment in the United States. Alzheimer’s Dement 2011;7(1):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alzheimer's Association. 2012 Alzheimer's disease facts and figures. 2009. http://www.alz.org/national/documents/report_alzfactsfigures2009.pdf (7 January 2014, date last accessed). [DOI] [PubMed]
- 21. Thies W, Bleiler L. 2011 Alzheimer's disease facts and figures. Alzheimers Dement 2011;7(2):208–44. [DOI] [PubMed] [Google Scholar]
- 22. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007;29(1–2):125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor DH, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer's disease. J Clin Epidemiol 2002;55(9):929–37. [DOI] [PubMed] [Google Scholar]
- 24. Pressley J. Dementia in community-dwelling elderly patients: a comparison of survey data, medicare claims, cognitive screening, reported symptoms, and activity limitations. J Clin Epidemiol 2003;56(9):896–905. [DOI] [PubMed] [Google Scholar]
- 25. Taylor DH, Østbye T, Langa KM, et al. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis 2009;17(4):807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ostbye T, Taylor DH, Clipp EC, et al. Identification of dementia: agreement among National Survey Data, medicare claims, and death certificates. Health Serv Res 2008;43(1 Pt 1):313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaduszkiewicz H, Bachmann C. Telling “‘the truth’” in dementia—Do attitude and approach of general practitioners and specialists differ? Patient Educ Couns 2008;70:220–6. [DOI] [PubMed] [Google Scholar]
- 28. Valcour VG, Masaki KH, Curb JD, et al. The detection of dementia in the primary care setting. Arch Intern Med 2000;160(19):2964–8. [DOI] [PubMed] [Google Scholar]
- 29. Mitchell AJ, Meader N, Pentzek M. Clinical recognition of dementia and cognitive impairment in primary care: a meta-analysis of physician accuracy. Acta Psychiatr Scand 2011;124(3):165–83. [DOI] [PubMed] [Google Scholar]
- 30. Batsch NL, Mittelman MS. Overcoming the stigma of dementia World Alzheimer Report 2012. 2012. http://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf (7 January 2014, date last accessed).
- 31. Howden L, Meyer J. Age and sex composition: 2010. 2010 Census Briefs, US Dep Commer 2011. http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf (7 January 2014, date last accessed).
- 32. Prince MJ, Jackson J. World Alzheimer Report 2009. 2009. http://www.alz.co.uk/research/files/WorldAlzheimerReport.pdf (7 January 2014, date last accessed).
- 33. Prince MJ, Bryce R, Albanese E, et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9(1):63–75.e2. [DOI] [PubMed] [Google Scholar]
- 34. General US, Office A. Alzheimer's disease: estimates of prevalence in the United States. 1998:GEO/HEHS-98-16 http://www.gao.gov/archive/1998/he98016.pdf (7 January 2014, date last accessed).
- 35. Wilson R, Weir D, Leurgans S. Sources of variability in estimates of the prevalence of Alzheimer's disease in the United States. Alzheimer’s Dement 2011;7(1):74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Daviglus ML, Plassman BL, Pirzada A, et al. Risk factors and preventive interventions for Alzheimer disease: state of the science. Arch Neurol 2011;68(9):1185–90. [DOI] [PubMed] [Google Scholar]
- 37. Caamaño-Isorna F, Corral M, Montes-Martínez A, et al. Education and dementia: a meta-analytic study. Neuroepidemiology 2006;26(4):226–32. [DOI] [PubMed] [Google Scholar]
- 38. Ott A, Breteler MMB, van Harskamp F, et al. Prevalence of Alzheimer's disease and vascular dementia: association with education. The Rotterdam study. BMJ 1995;310(6985):970–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karp A, Kåreholt I, Qiu C, et al. Relation of education and occupation-based socioeconomic status to incident Alzheimer's disease. Am J Epidemiol 2004;159(2):175–83. [DOI] [PubMed] [Google Scholar]
- 40. Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer's disease. Arch Med Res 2012;43(8):600–8. [DOI] [PubMed] [Google Scholar]
- 41. Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med 2013;369(24):2275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 2004;52(2):195–204. [DOI] [PubMed] [Google Scholar]
- 43. Chin A, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer's disease. Alzheimer Dis Assoc Disord 2011;25(3):187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alzheimer's Association 2014 Alzheimer's disease facts and figures. Alzheimer’s Dement 2014;10(2):e47–92. [DOI] [PubMed] [Google Scholar]
- 45. Weiner MF. Perspective on race and ethnicity in Alzheimer's disease research. Alzheimers Dement 2008;4(4):233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bradford A, Kunik ME, Schulz P, et al. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord 2009;23(4):306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boustani MA, Peterson B, Harris R, et al. 1. Screening for Dementia [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US), 2003. [PubMed] [Google Scholar]
- 48. Drickamer MA, Lachs MS. Should patients with Alzheimer's disease be told their diagnosis? N Engl J Med 1992;326(14):947–51. [DOI] [PubMed] [Google Scholar]
- 49. Rice DP, Fillit HM, Max W, et al. Prevalence, costs, and treatment of Alzheimer's disease and related dementia: a managed care perspective. Am J Manag Care 2001;7(8):809–20. [PubMed] [Google Scholar]
- 50. Prince MJ, Bryce R, Ferri CP. World Alzheimer Report 2011: the benefits of early diagnosis and intervention. 2011. http://www.alz.co.uk/research/WorldAlzheimerReport2011.pdf (7 January 2014, date last accessed).
- 51. Boustani MA, Peterson B, Hanson L, et al. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2003;138(11):927–37. [DOI] [PubMed] [Google Scholar]
- 52. Wimo A, Jönsson L, Bond J, et al. The worldwide economic impact of dementia 2010. Alzheimers Dement 2013;9(1):1–11. [DOI] [PubMed] [Google Scholar]
- 53. Smebye KL, Kirkevold M, Engedal K. How do persons with dementia participate in decision making related to health and daily care? A multi-case study. BMC Health Serv Res 2012;12(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Holsinger T, Boustani MA, Abbot D, et al. Acceptability of dementia screening in primary care patients. Int J Geriatr Psychiatry 2011;26(4):373–9. [DOI] [PubMed] [Google Scholar]
- 55. Luck T, Luppa M, Sieber J, et al. Attitudes of the German general population toward early diagnosis of dementia - results of a representative telephone survey. PLoS ONE 2012;7(11):e50792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brayne C, Fox C, Boustani MA. Dementia screening in primary care. Is it time? JAMA 2007;298(20):2409–12. [DOI] [PubMed] [Google Scholar]
- 57. Cordell CB, Borson S, Boustani MA, et al. Alzheimer's Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimer‘s Dement 2013;9(2):141–50. [DOI] [PubMed] [Google Scholar]
- 58. Miniño AM. Death in the United States, 2011. NCHS Data Brief 2013;(115):1–8. [PubMed] [Google Scholar]