Abstract
Background
Understanding health beliefs and how they influence willingness will enable the development of targeted curricula that maximize public engagement in Alzheimer’s disease (AD) risk reduction behaviors.
Methods
Literature on behavioral theory and community input was used to develop and validate a health beliefs survey about AD risk reduction among 428 community-dwelling adults. Principal component analysis was performed to assess internal consistency. Linear regression was performed to identify key predictors of Willingness to engage in AD risk reduction behaviors.
Results
The measure as well as the individual scales (Benefits, Barriers, Severity, Susceptibility and Social Norm) were found to be internally consistent. Overall, as Benefits and Barriers scores increased, Willingness scores also increased. Those without prior AD experience or family history had lower willingness scores. Finally, we observed an interaction between age and norms, suggesting that social factors related to AD prevention may differentially affect people of different ages.
Conclusions
The Alzheimer Prevention Beliefs Measure provides assessment of several health belief factors related to AD prevention. Age, Family History, Logistical Barriers and total Benefits are significant determinants of willingness to engage in AD risk reduction behaviors, such as seeing a doctor or making a lifestyle change.
Keywords: beliefs, health promotion, measurement
Introduction
Educational interventions aimed at raising public awareness have the potential to reduce the global burden of Alzheimer's Disease (AD).1 AD poses a substantial threat to public health worldwide, with a 2010 prevalence of approximately 4.7 million older adults affected in the United States (US), a number expected to triple by 2050.2 AD begins with a progressive, prodromal period (‘Preclinical AD’), which occurs at least 10 years prior to the emergence of cognitive decline.3 During this time, amyloidosis and other potentially reversible risk factors converge to influence the timing, nature and progression of the disease.4 For example, during midlife, even the presence of a mild metabolic disorder such as insulin resistance or benign vitamin B12 deficiency has been shown to increase the risk of brain degeneration in later life.5,6 Better detection of these and other reversible risk factors, at critical stages of the lifespan, is paramount in persons with genetic risk for late-onset AD.7 In fact, several national public health initiatives are targeting ‘brain health’, based on the scientific evidence showing the importance of reducing modifiable risk factors such as cardiovascular disease and diabetes.8 Of particular importance are on-going clinical trials, such as the Anti-Amyloid Treatment in Asymptomatic Alzheimer's study (‘A4 trial’). Preliminary Phase 3 findings suggest that treatment with the investigational drug solanezumab, an antiamyloid-beta monoclonal antibody, is safe and effective in lowering amyloid burden if delivered prior to the onset of later protein changes of AD.9
Despite the availability of interventions proven to reduce risk during midlife, and even with the widespread concern of this disease, public engagement in AD risk reduction remains low,10 especially for underrepresented minorities and those with learning disability (LD) or low health literacy. As a result, individuals carrying genetic or socioeconomic risk for AD continue throughout life with unaddressed risk, while Alzheimer's research studies routinely fail to meet recruitment goals.
Gender and race-based differences in pre-existing beliefs about AD are particular important.11–15 However, prior studies exploring the relationship between pre-existing health beliefs and willingness to engage in AD risk reduction have yielded mixed results, with few measures incorporating behavioral theory.10–16 Prior studies have not assessed health literacy levels or presence of LD. As a result, the health beliefs that most influence willingness, in specific, underrepresented groups, remain to be identified. This is a critical research gap because the future of AD prevention depends upon early detection and treatment of risk factors in minimally symptomatic, susceptible adults.
In this study, our objectives were (i) to develop and validate a self-report questionnaire of AD health beliefs using a community-based, participatory approach; (ii) to describe AD health beliefs in an urban-based sample with varying levels of health literacy; and (iii) to examine whether self-reported beliefs are associated with self-reported willingness. We hypothesized that demographic factors such as gender, race and level of health literacy would influence health beliefs, and that health beliefs would be directly related to willingness to engage in AD risk reduction behaviors such as making a lifestyle change, seeing a doctor, taking a medication or participating in an AD prevention trial. These hypotheses were based on the Health Belief Model (HBM) and other behavioral theory models, which posit that health beliefs such as personal susceptibility, perceived severity of the disease, perceived benefits or barriers, and social norms are a key mediator of willingness to engage in a wide variety of health-related behaviors.17,18
A better understanding of health beliefs and how they influence willingness will enable the development of targeted curricula that maximize public engagement in AD risk reduction behaviors during midlife.10 In 2014, The U.S. Department of Health and Human Services (HHS) updated ‘The National Plan to Address Alzheimer's Disease’, addressing the need to develop measures of quality care to ensure that people with Alzheimer's receive culturally competent, high-quality services. This objective can be achieved through the development of validated survey questions that can be used at the national, state and local levels to track awareness and perceptions about cognitive health, thereby improving access to clinical services and research trials and potentially influencing patient outcomes.19
Methods
Questionnaire development
The questionnaire was informed by: (i) a systematic review of the literature on behavioral theory; and (ii) extended discussion with content experts including two board-certified neurologists, a stroke prevention educator, a survey developer, a nurse educator, a social worker and a caregiver of dementia patients. The purpose of this process was to ensure content validity of the new measure. The measure included both original items and items adapted from prior validated scales, including the Fear of Alzheimer's Disease Scale20 and the Motivation to Change Lifestyle and Health Behaviors for Dementia Risk Reduction Scale.21 Semi-structured interviews were then conducted with a representative sample of persons for whom the measure would be used. The purpose of that process was to maximize face and construct validity. The questionnaire was revised based on the feedback of these interviews.
Primary predictor—health beliefs
To assess the primary predictor, Health Beliefs, we used a 5-point Likert scale (Strongly disagree, value of 1; Strongly agree, value of 5) using a set of questions specifically addressing the following domains; Perceived Severity, Perceived Susceptibility, Perceived Benefits, Perceived Barriers and Social Norms. Beliefs can be favorably or unfavorably associated with action.22,23 In our study, we propose that someone who thinks AD is severe, knows they have high risk, recognizes the benefits of acting and expects few barriers to action would be expected to have high willingness to prevent AD. Therefore a high total score on the beliefs questions was considered an indication that a person's health beliefs were ‘favorable’ to engagement.
Primary outcome measure—willingness to engage in AD risk reduction
To assess the primary outcome, Willingness, individuals were asked to rate their willingness to engage in four specific behaviors related to AD prevention (e.g. seeing a doctor, registering for an online course, signing up to be notified about AD prevention studies and determining personal risk). These items were presented using a Stages of Change scale,24 ranging from pre-contemplation (‘won't do’, value of 1) to maintenance (‘already did’, value of 5). Participants then rated themselves using a 5-point Likert scale (‘Very unlikely’, value of 1; ‘Very likely’, value of 5) regarding the likelihood of engaging in eight behaviors varying in degree of action (e.g. taking an online survey, talking to a nurse, seeing a primary care doctor, seeing a neurologist, memory testing, getting a blood test, getting a brain scan and getting a genetic blood test). A high total score on the Stages of Change question was considered an indication of high total Willingness. Stages of change models have been used in a wide variety of conditions ranging from tobacco cessation to cancer prevention.22–24
Age, gender, ethnicity and educational attainment were obtained by self-report. Health literacy was measured using the Brief Health Literacy Screening measure.25 To assess for childhood LD, we included a battery of nine questions about prior evaluation, prior diagnosis, perceived diagnosis, childhood learning difficulty and family history of LD. The sum of affirmative answers was calculated, and individuals answering affirmatively to at least 3/9 questions were classified as having Possible LD. We were limited to these questions because self-report measures to assess childhood LD have not been developed and validated in a memory loss prevention population. Personal Experience with AD was measured using three questions about family history, caregiving status, and working in a medical profession. Individuals endorsing at least one of these factors were classified as having some Prior Experience with AD. The Alzheimer's Disease Knowledge Scale (ADKS), a comprehensive measure of AD knowledge with good psychometric properties, was used to assess basic knowledge of AD.26
Study population and procedure
Participants were recruited from a variety of community-based sources to maximize heterogeneity of sample. Programs included health fairs and educational talks and community events including soup kitchens, food pantries, adult day cares, churches and social services organizations. Events were sponsored by the Weill Cornell Medical College Clinical and Translational Science Center (CTSC) and the Heart to Heart Community Outreach Program Partnership, a free diabetes/heart disease screening and intervention program. Events were held primarily in minority and underserved communities of New York City. All Questionnaires were delivered in person, were paper and pencil, and returned immediately to study team. Participants were offered a $10 voucher for completing the survey. This study was approved by the Weill Cornell Institutional Review Board.
Statistical analysis
Internal consistency of the predefined constructs (Willingness, Benefits, Barriers, Susceptibility, Severity, and Norms) was assessed using Cronbach's alpha. Each construct consisted of 4–14 items. For each construct, the ‘Cronbach's alpha if item is deleted’ was also calculated. If the overall Cronbach's alpha was <0.7, and deletion of a single item resulted in a Cronbach's alpha >0.7, then the item was deleted.
After using this criterion to determine which items should remain in the predefined constructs, factor analysis using the principal axis factoring extraction method and direct oblimin with Kaiser Normalization rotation method was applied on each construct. The direct oblimin rotation allowed for the factors to be correlated. Factors with eigenvalues >1 and factor loading of 0.50 or greater were retained. Once we performed factor analysis on the predefined constructs, and removed items as necessary, we repeated factor analysis again on the entire set of items. We also performed further analyses to assess concurrent validity and generalizability.
Once reliable factors were identified, we performed descriptive comparisons of belief factor scores across subgroups. Summary statistics were provided as counts and percentages for categorical variables and as means, standard deviations (SD), ranges and medians for continuous variables. In additional to the summary statistics, analysis of each potential factor was completed one at a time in univariate analysis. Finally, using all statistically significant predictors, along with all interactions, a multivariable, regression model was built. The model included four fixed covariates: age, gender, college education and ethnicity. P-values < 0.05 were considered to be statistically significant. Statistical analysis was performed using SAS V9.4.27
Results
A total of 428 community-dwelling adults completed the survey. Most of the surveys were completed at community health fairs (n = 387, 90.4%), with the remainder completed after a live presentation (n = 20), or during a clinic visit for an affected family member (n = 30). The sample was relatively young, with a mean age of 42 years (SD = 16.63), range of 18–94. A large proportion of the group were women (72%) and self-reported as Non-Hispanic/Latino (n = 246, 57.5%). The sample also comprised a significant representation of persons with low education (n = 220, 51.4% high school or less), who were first-generation immigrants (n = 145, 33.9% foreign-born), low health literacy (n = 94, 22%) and a self-reported history of LD (n = 19, 4.4%). A considerable number of participants reported that they had both prior experience as a caregiver (n = 382, 89.3%) and a familial history of AD (n = 361, 84.3%). See Table 1 for the demographic characteristics.
Table 1.
Univariate analysis, willingness score, demographics and health beliefs
| Characteristic | Statistic/category | N = 428 | P-value |
|---|---|---|---|
| WILLINGNESS_SCORE | N | 428 | |
| Mean ± SD | 43.0 ± 8.57 | — | |
| Range | 12.0–60.0 | ||
| Median | 44 | ||
| AGE | N | 421 | |
| Mean ± SD | 42.8 ± 16.63 | 0.31 | |
| Range | 18.0–94.0 | ||
| Median | 43 | ||
| GENDER | Female | 308 (72.0%) | 0.95 |
| Male | 109 (25.5%) | ||
| Missing values | 11 (2.6%) | ||
| RACE | Black | 192 (44.9%) | 0.87 |
| White | 97 (22.7%) | ||
| Other | 79 (18.5%) | ||
| Missing values | 60 (14.0%) | ||
| ETHNICITY | Hispanic or Latino | 98 (22.9%) | 0.21 |
| Not Hispanic or Latino | 246 (57.5%) | ||
| Missing values | 84 (19.6%) | ||
| EDUCATIONAL_ATTAINMENT | Graduate degree | 81 (18.9%) | 0.04 |
| Bachelor's degree | 122 (28.5%) | ||
| High school degree | 168 (39.3%) | ||
| Less than high school | 52 (12.1%) | ||
| Missing values | 5 (1.2%) | ||
| COLLEGE | No | 220 (51.4%) | 0.08 |
| Yes | 203 (47.4%) | ||
| Missing values | 5 (1.2%) | ||
| COUNTRY_OF_BIRTH | Other country | 145 (33.9%) | 0.44 |
| USA | 280 (65.4%) | ||
| Missing values | 3 (0.7%) | ||
| COHORT | Community | 11 (2.6%) | 0.64 |
| Live present | 387 (90.4%) | ||
| Office | 30 (7.0%) | ||
| PRIOREXPERIENCE_CAREGIVER | No | 382 (89.3%) | 0.006 |
| Yes | 41 (9.6%) | ||
| Missing values | 5 (1.2%) | ||
| PRIOREXPERIENCE_FAMHISTORY | No | 361 (84.3%) | <0.001 |
| Yes | 63 (14.7%) | ||
| Missing values | 4 (0.9%) | ||
| LOW HEALTH LITERACY | No | 323 (75.5%) | 0.74 |
| Yes | 94 (22.0%) | ||
| Missing values | 11 (2.6%) | ||
| LEARNING DISABILITY | No | 409 (95.6%) | 0.47 |
| Yes | 19 (4.4%) | ||
| SUSCEPTIBILITY_SYMPTOMS | N | 424 | 0.27 |
| Mean ± SD | 17.0 ± 7.15 | ||
| Range | 8.0–40.0 | ||
| Median | 16 | ||
| BENEFITS_EMPOWERMENT | N | 428 | <0.001 |
| Mean ± SD | 24.8 ± 4.26 | ||
| Range | 6.0–30.0 | ||
| Median | 24 | ||
| BARRIERS_LOGISTICS | N | 422 | <0.001 |
| Mean ± SD | 29.3 ± 6.68 | ||
| Range | 8.0–40.0 | ||
| Median | 30 | ||
| BARRIERS_STIGMA | N | 428 | 0.08 |
| Mean ± SD | 20.5 ± 5.47 | ||
| Range | 2.0–30.0 | ||
| Median | 20 | ||
| PERCEIVED_SEVERITY | N | 422 | 0.007 |
| Mean ± SD | 12.6 ± 3.45 | ||
| Range | 4.0–20.0 | ||
| Median | 13 | ||
| BENEFITS_GENERAL | N | 422 | <0.001 |
| Mean ± SD | 15.8 ± 3.27 | ||
| Range | 4.0–20.0 | ||
| Median | 16 | ||
| BENEFITS_EARLYDETECTION | N | 428 | <0.001 |
| Mean ± SD | 11.5 ± 2.44 | ||
| Range | 3.0–15.0 | ||
| Median | 12 | ||
| SUSCEPTIBILITY_HEALTHVIGILANCE | N | 422 | 0.26 |
| Mean ± SD | 6.7 ± 2.28 | ||
| Range | 3.0–15.0 | ||
| Median | 6 | ||
| SUSCEPTIBILITY_CONCERN | N | 424 | <0.001 |
| Mean ± SD | 11.0 ± 2.77 | ||
| Range | 3.0–15.0 | ||
| Median | 11 | ||
| SUSCEPTIBILITY_SCORE | N | 424 | 0.58 |
| Mean ± SD | 23.7 ± 8.30 | ||
| Range | 8.0–55.0 | ||
| Median | 23 | ||
| SEVERITY_SCORE | N | 422 | 0.05 |
| Mean ± SD | 14.6 ± 3.71 | ||
| Range | 5.0–25.0 | ||
| Median | 15 | ||
| NORMS_SCORE | N | 424 | 0.003 |
| Mean ± SD | 9.9 ± 3.75 | ||
| Range | 1.0–20.0 | ||
| Median | 11 | ||
| BENEFITS_SCORE | N | 428 | <0.001 |
| Mean ± SD | 51.8 ± 8.20 | ||
| Range | 13.0–65.0 | ||
| Median | 52 | ||
| BARRIERS_SCORE | N | 428 | <0.001 |
| Mean ± SD | 49.4 ± 10.52 | ||
| Range | 10.0–70.0 | ||
| Median | 49 | ||
| TOTAL_ADKS | N | 426 | 0.37 |
| Mean ± SD | 19.8 ± 4.52 | ||
| Range | 8.0–30.0 | ||
| Median | 20 | ||
| TOTAL_BELIEF_SCORE | N | 428 | <0.001 |
| Mean ± SD | 148.9 ± 17.94 | ||
| Range | 49.0–193.0 | ||
| Median | 150 |
Internal consistency, construct validity and generalizability
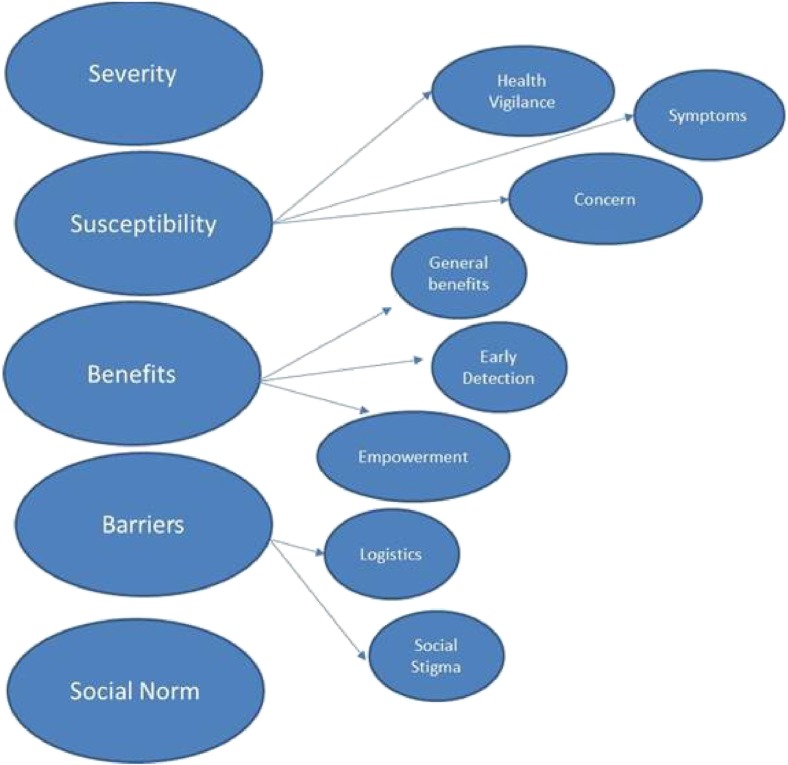
Overall, each predefined factor structure (Willingness, Benefits, Barriers, Susceptibility, Severity and Social Norm) was found to be internally consistent. Two items were removed (both from the Perceived Severity construct) in order to increase the internal consistency of that particular scale. For four of the factors, several additional sub-constructs were identified, yielding a total of 10 Health Belief factors, shown in Fig. 1. Perceived susceptibility consisted of Health Vigilance, Concern and Symptom sub-constructs. Perceived benefits consisted of General, Early Detection and Empowerment sub-constructs. Perceived barriers were either related to Social Stigma or Logistics. Social Norm and Severity did not show evidence of sub-constructs. The setting in which the measure was taken (in a clinic by a caregiver, at a presentation or at a community health fair) did not influence any of these results.
Fig. 1.
Sub-factors of health beliefs regarding AD risk reduction.
Health beliefs and willingness
The total Willingness score for the whole sample ranged from 12 to 60, (M = 43, SD = 8.6). About 30–40% reported ‘Ready, will do’ to make an appointment with a doctor to discuss ways to prevent AD, register for an online course to learn about AD prevention, sign up to be notified about trials or know their personal AD risk. Table 2 shows a summary of willingness and likelihood of engaging in behaviors and tests to reduce AD risk.
Table 2.
Final multivariable model
| Factor | Parameter estimate | Standard error | Test statistic | P-value |
|---|---|---|---|---|
| Intercept | −1.40564 | 3.99047 | −0.35 | 0.7249 |
| Age | 0.18223 | 0.06825 | 2.67 | 0.0080 |
| Gender | 0.18389 | 0.89150 | 0.21 | 0.8367 |
| College education or higher | 0.18330 | 0.79135 | 0.23 | 0.8170 |
| Ethnicity | 1.07113 | 0.89920 | 1.19 | 0.2344 |
| Prior experience with family history of Alzheimer's | 3.64468 | 1.12439 | 3.24 | 0.0013 |
| Barriers logistics | 0.23827 | 0.06280 | 3.79 | 0.0002 |
| Total norms score | 1.01189 | 0.28578 | 3.54 | 0.0005 |
| Total benefits score | 0.51970 | 0.05334 | 9.74 | <0.0001 |
| Age × norms interaction | −0.01952 | 0.00647 | −3.02 | 0.0028 |
In univariate analyses, the following factors were significantly related to the total Willingness score: Educational attainment, Prior experience as caregiver, Prior experience with family history, Benefits (empowerment), Barriers (logistics), Perceived severity, Benefits (general), Benefits (early detection), Susceptibility (concern), Norms, Benefits (total), Barriers (total), Beliefs (total). Willingness increased as education increased. Those with prior experience as a caregiver and those with family history of AD had higher Willingness than those without. The remaining univariate statistically significant factors (continuous variables) increased as Willingness increased (Tables 3 and 4).
Table 3.
Summary of initial factor analysis and reliability measure scores
| Factor name | # of items | # of items removed | # of items with λ > 1 | Cumulative variance explained (%) | Initial Cronbach's alpha | Final Cronbach's alpha |
|---|---|---|---|---|---|---|
| Willingness | 12 | 0 | 2 | 51.7 | 0.885 | 0.885 |
| Benefits | 13 | 0 | 3 | 69.5 | 0.913 | 0.913 |
| Barriers | 14 | 0 | 2 | 52.0 | 0.874 | 0.874 |
| Susceptibility | 14 | 0 | 3 | 66.8 | 0.849 | 0.849 |
| Severity | 6 | 2 | 1 | 46.9 | 0.665 | 0.774 |
| Norms | 4 | 0 | 1 | 62.6 | 0.865 | 0.865 |
λ = Eigenvalue.
Table 4.
Pattern matrix for final included items
| Factor | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| To what extent do you agree with these statements? | ||||||||||
| AD is one of the worst diseases someone could have | 0.039 | −0.025 | 0.078 | −0.013 | 0.011 | 0.755 | −0.034 | 0.053 | −0.004 | 0.048 |
| I would rather have a painful physical illness (e.g. cancer or AIDS) than AD | −0.001 | −0.030 | −0.036 | 0.075 | −0.001 | 0.566 | 0.052 | −0.035 | −0.044 | −0.024 |
| The thought of AD scares me | −0.041 | 0.113 | −0.010 | −0.072 | 0.009 | 0.698 | −0.031 | −0.021 | 0.010 | 0.028 |
| AD is more serious than other diseases | 0.030 | −0.002 | −0.071 | 0.013 | −0.054 | 0.654 | −0.034 | 0.047 | −0.006 | −0.015 |
| In my opinion, compared to other people my age: | ||||||||||
| My chances of developing AD are higher | −0.036 | −0.019 | 0.037 | 0.008 | −0.047 | 0.106 | 0.038 | 0.034 | −0.518 | 0.183 |
| My overall body health is worse | −0.051 | 0.055 | −0.129 | −0.035 | 0.026 | −0.040 | −0.084 | −0.046 | −0.700 | −0.160 |
| My overall brain health is worse | −0.021 | −0.028 | 0.003 | 0.024 | −0.011 | 0.014 | 0.015 | −0.012 | −0.897 | −0.019 |
| Next are some questions about how healthy you think your brain is. Over the last seven days: | ||||||||||
| I have had trouble forming thoughts | −0.844 | −0.029 | 0.027 | 0.020 | −0.049 | −0.044 | −0.028 | 0.086 | −0.014 | −0.014 |
| My thinking has been slow | −0.853 | −0.075 | −0.031 | 0.030 | −0.024 | −0.005 | −0.053 | 0.040 | −0.011 | 0.002 |
| I have had trouble concentrating | −0.801 | 0.070 | −0.023 | −0.055 | 0.000 | 0.074 | −0.034 | −0.089 | 0.008 | −0.026 |
| I have had to work really hard to pay attention or I would make a mistake | −0.877 | −0.001 | 0.019 | −0.020 | 0.043 | 0.063 | 0.013 | −0.052 | −0.031 | −0.015 |
| My brain was not working as well as usual | −0.872 | 0.020 | −0.016 | 0.025 | 0.013 | −0.005 | 0.021 | −0.005 | 0.043 | 0.033 |
| I have worked harder to keep track of what I am doing | −0.800 | 0.004 | −0.032 | −0.057 | −0.016 | −0.005 | −0.013 | −0.041 | 0.005 | 0.041 |
| I have had trouble shifting back and forth between different activities that require thinking | −0.849 | 0.057 | −0.016 | 0.023 | −0.029 | −0.071 | 0.047 | 0.003 | −0.030 | −0.008 |
| Problems with memory interfered with my daily life | −0.825 | −0.061 | 0.024 | 0.037 | 0.014 | −0.050 | 0.048 | 0.057 | −0.037 | 0.024 |
| How important is your brain health? | ||||||||||
| I have to pay attention to my brain health | −0.030 | 0.104 | −0.052 | 0.041 | 0.008 | 0.014 | −0.133 | −0.005 | 0.116 | 0.699 |
| I am concerned about my brain health | −0.033 | 0.059 | −0.044 | 0.025 | −0.016 | 0.016 | −0.101 | 0.020 | −0.131 | 0.674 |
| I often think about my brain health | −0.024 | 0.059 | 0.030 | 0.031 | 0.049 | 0.049 | −0.129 | −0.006 | 0.023 | 0.658 |
| Seeing a doctor to discuss brain health would help me to: | ||||||||||
| Know my personal risk for developing AD | 0.007 | 0.079 | −0.003 | −0.069 | 0.062 | 0.057 | −0.096 | 0.631 | 0.039 | 0.037 |
| Detect AD before I ever get symptoms | 0.024 | −0.006 | −0.004 | −0.029 | −0.006 | 0.011 | −0.035 | 0.957 | 0.050 | −0.052 |
| Reduce my chances of developing AD | −0.012 | 0.092 | 0.020 | 0.000 | 0.008 | −0.013 | −0.006 | 0.603 | −0.045 | −0.012 |
| Knowing my personal risk for AD would help me to: | ||||||||||
| Set my personal affairs in order | 0.052 | 0.744 | 0.017 | 0.020 | −0.058 | −0.049 | −0.013 | 0.121 | −0.021 | 0.010 |
| Make plans for long-term care | 0.020 | 0.864 | 0.004 | −0.019 | −0.018 | −0.052 | −0.028 | 0.009 | −0.076 | 0.044 |
| Prepare my family | −0.005 | 0.808 | −0.006 | 0.035 | 0.033 | −0.028 | −0.110 | 0.001 | −0.016 | −0.023 |
| Do important things sooner | −0.040 | 0.883 | 0.025 | 0.056 | 0.043 | 0.028 | 0.030 | −0.011 | 0.011 | −0.011 |
| Identify things I can change about my behavior | −0.016 | 0.752 | −0.010 | 0.007 | −0.001 | 0.058 | −0.018 | 0.012 | 0.023 | 0.048 |
| Identify things I can change about my health | 0.018 | 0.804 | 0.017 | −0.029 | −0.018 | 0.089 | 0.076 | 0.081 | 0.071 | 0.048 |
| Overall how beneficial is: | ||||||||||
| Seeing a doctor to discuss brain health | −0.055 | −0.004 | −0.015 | 0.045 | 0.091 | 0.063 | −0.677 | 0.120 | 0.009 | 0.105 |
| Learning about AD | −0.004 | 0.006 | −0.011 | 0.014 | −0.022 | 0.020 | −0.890 | 0.003 | 0.054 | 0.024 |
| Signing up to be notified about AD prevention studies | −0.014 | −0.005 | 0.100 | 0.058 | −0.064 | 0.000 | −0.741 | 0.025 | −0.089 | 0.119 |
| Knowing my personal risk for future AD | 0.054 | 0.055 | −0.032 | −0.026 | 0.011 | −0.017 | −0.845 | 0.042 | −0.019 | −0.002 |
| I do not want to know my personal risk for AD because: | ||||||||||
| It would cause me more worry than good | 0.088 | 0.021 | −0.013 | 0.028 | 0.600 | −0.019 | 0.018 | 0.062 | 0.018 | 0.105 |
| My health insurance might change | 0.006 | −0.048 | 0.090 | −0.091 | 0.657 | 0.019 | 0.031 | −0.052 | 0.026 | 0.090 |
| My friends or family might treat me differently | −0.006 | 0.047 | −0.024 | 0.039 | 0.872 | −0.004 | 0.034 | 0.026 | −0.001 | −0.066 |
| My employer might treat me differently | −0.042 | −0.004 | 0.030 | −0.045 | 0.805 | 0.014 | 0.031 | −0.003 | −0.043 | −0.057 |
| My feelings about myself might change | −0.012 | −0.073 | −0.027 | 0.080 | 0.756 | −0.046 | −0.031 | 0.070 | 0.009 | −0.027 |
| AD risk should be kept a secret | 0.083 | 0.111 | 0.045 | −0.110 | 0.458 | −0.012 | −0.186 | −0.115 | 0.052 | −0.020 |
| What are some practical barriers to doing things to prevent AD? | ||||||||||
| I do not have access to medical care when I need it | 0.003 | −0.041 | 0.720 | 0.011 | 0.033 | 0.093 | −0.048 | −0.064 | 0.034 | −0.117 |
| My doctor would not know about brain health | 0.031 | −0.032 | 0.683 | 0.039 | 0.034 | 0.021 | 0.004 | 0.022 | −0.009 | −0.057 |
| I do not know which doctor to see about my brain health | 0.044 | −0.008 | 0.730 | 0.030 | −0.044 | −0.082 | 0.027 | 0.005 | −0.012 | −0.062 |
| The test would be inconvenient | 0.063 | 0.123 | 0.673 | −0.040 | −0.002 | −0.025 | 0.013 | 0.002 | −0.021 | 0.048 |
| I do not have enough time | −0.005 | 0.047 | 0.673 | 0.051 | 0.076 | −0.100 | 0.018 | 0.012 | −0.048 | 0.149 |
| I do not have enough money | 0.000 | −0.067 | 0.764 | 0.058 | 0.063 | −0.001 | −0.006 | 0.099 | −0.005 | 0.039 |
| It is hard to get information about AD | 0.017 | 0.030 | 0.725 | −0.095 | −0.075 | 0.034 | −0.020 | −0.048 | 0.099 | 0.054 |
| It is hard to understand information about AD | −0.055 | 0.029 | 0.714 | −0.066 | 0.004 | −0.031 | −0.020 | 0.001 | 0.060 | −0.040 |
| How many of your friends are doing the following: | ||||||||||
| Seeing doctors to discuss brain health? | 0.011 | −0.070 | −0.023 | 0.634 | −0.044 | −0.068 | 0.057 | 0.059 | −0.062 | 0.118 |
| Taking supplements/vitamin to improve brain health? | −0.040 | 0.051 | −0.021 | 0.886 | 0.018 | 0.052 | −0.025 | −0.056 | 0.053 | −0.070 |
| Making lifestyle changes (diet, sleep, exercise) specifically to improve brain health? | 0.002 | 0.064 | 0.069 | 0.823 | 0.006 | 0.041 | −0.071 | −0.027 | −0.008 | −0.059 |
| Signing up for brain games programs such as Lumosity®? | 0.027 | 0.016 | −0.021 | 0.784 | −0.004 | 0.003 | −0.013 | −0.046 | 0.022 | 0.055 |
In multivariable analyses, after adjusting for age, gender, education, and ethnicity, the following factors were identified as independent factors for inclusion in the final model: Prior experience with family history, Barriers (logistics), Benefits (total) and Interaction between Norms score and Age. The other univariate significant factors fell out of the final model as they did not provide enough independent information to warrant their inclusion. Table 5 shows the final model.
Table 5.
Results from overall sample on The Willingness to Prevent AD Scale
| Will not do (%) | Have not considered (%) | Considered but have not decided (%) | Ready, will do (%) | Already did (%) | |
|---|---|---|---|---|---|
| If these options were available to you, would you do any of the following things? | |||||
| Make an appointment with a doctor to discuss ways to prevent AD | 5 | 31 | 22 | 37 | 4 |
| Register for an online course to learn about AD | 8 | 35 | 19 | 30 | 8 |
| Sign up to be notified about AD prevention research studies | 8 | 33 | 19 | 36 | 4 |
| Know my personal risk for AD | 4 | 27 | 22 | 40 | 7 |
| What would you be willing to do to measure your brain health? | |||||
| Take an online survey about health and lifestyle | 5 | 5 | 17 | 46 | 27 |
| See a nurse | 5 | 15 | 20 | 38 | 23 |
| See a primary care doctor | 4 | 7 | 11 | 41 | 37 |
| See a neurologist / memory expert | 4 | 10 | 17 | 37 | 32 |
| Take tests on memory and thinking | 4 | 4 | 12 | 44 | 36 |
| Get a blood test | 3 | 5 | 12 | 42 | 38 |
| Get a brain scan | 5 | 12 | 18 | 34 | 31 |
| Get a genetic test (a blood test that looks at your DNA) | 5 | 8 | 14 | 40 | 33 |
Discussion
Main finding of the study
In this study, we used the literature on behavioral theory, in addition to community input, to develop and validate a health beliefs survey about AD risk reduction which could enable assessment of key health beliefs known to influence health behavior. The measure, as a whole, as well as the individual scales (Benefits, Barriers, Severity, Susceptibility and Social Norm), were found to be internally consistent. In addition, the data demonstrated additional, more specific aspects of general health beliefs. Overall, as the Benefits and Barriers scores increased, the Willingness score also increased. Those without prior experience or family history had lower willingness scores than those with prior experience or family history. Finally, we observed an interaction between age and norms, suggesting that social factors related to AD prevention may differentially affect people of different ages.
What is already known on this topic
In general, our results were consistent with prior studies showing that age, prior experience with AD, and Perceived Benefits are key factors when it comes to willingness to undergo susceptibility testing.10,28,29 In the Risk Evaluation and Education for AD study, 47/196 participants (24%) who were systematically contacted through AD research registries subsequently underwent susceptibility testing. This number may be inflated due to the fact that the sampling frame consisted of people who were part of AD research registries and were therefore more likely to be interested in undergoing testing.15 We did not have direct data about behavioral decisions, but approximately 40% of our sample reported willingness to know their own risk. These numbers corroborate the idea that a significant number of individuals are already interested in susceptibility testing, and also demonstrate the significant amount of work left to be done by educators in creating curricula to convey the benefits of susceptibility testing.
Curiously, we found no race or ethnicity-related differences in health beliefs, despite several prior studies demonstrating these differences. Researchers have found African Americans to be significantly more likely to associate memory loss with normal aging, less knowledgeable about genetics and AD risk, and different from non-Hispanic Whites with respect to beliefs about causes and effectiveness of various options for risk reduction or treatment.12–14,15 Dementia symptoms are also likely to be considered a normal part of the aging process among Asian American immigrants.13 Our study raises the important question whether prior race-related differences in health beliefs were actually attributable to health literacy levels, which were not measured robustly in those studies.
Limitations of the study
Our study had several limitations. Participants in this study were recruited from a convenience sample of community-dwelling adults and therefore the group is not necessarily representative of the overall U.S. population. We did not measure test-retest reliability. Importantly, our question items were not delivered in random order, so our data could have been subject to the Halo effect, whereby subjects’ overall impression of the construct influenced most of the responses. Also, since we delivered different numbers of questions for each construct (based on the availability of good questions in the literature), our total Beliefs score weights certain factors higher than others. Regarding LD, the study may have some classification bias because validated measures for diagnosing LD in the adult are not validated for these types of populations. Finally, the ADKS scale we used to measure AD knowledge does not directly address prevention-specific facts which have may have more implications with respect to perceived control.
Key strengths of this study were the incorporation of behavioral theory into the measure, the involvement of the community in drafting the questionnaire, the diversity of the sample population, the greater attention to health literacy levels, and also the inclusion of a Willingness scale that takes into account a comprehensive approach to AD risk reduction. The population was relatively young (mean age 42), which is a strength because risk reduction for AD must begin in midlife or sooner.
Future research is required to examine the test-retest reliability of this measure, to incorporate better assessment of AD prevention knowledge, and to further explore how health literacy and LD influence beliefs and willingness. This is particularly important because the tool could ultimately be used to measure the effectiveness of educational interventions to increase engagement for underrepresented groups.
What this study adds
It is well established that an individual's health behaviors are influenced by multiple factors, including personal beliefs and the perceived value or benefit of the behavior.30,31 To our knowledge, this is one of the first studies that used Behavioral Theory to construct a self-report measure for the assessment of AD risk reduction beliefs. The Alzheimer Prevention Beliefs Measure provides an assessment of several health belief factors related to AD prevention. Age, Family History (or lack thereof), Logistical Barriers and total Benefits are significant determinants of willingness to engage in AD risk reduction behaviors, such as seeing a doctor, making a lifestyle change or participating in prevention research trials.
Development of theory-based measures of AD beliefs could enable more accurate public health surveillance of AD prevention attitudes. This could lead to the development of targeted, hypothesis-driven educational messaging aimed at reducing barriers to earlier diagnosis and prevention, especially for underrepresented groups. A tool such as this may also play a role in better defining strategies to increase recruitment into Alzheimer's prevention research trials.32
Acknowledgements
The authors would like to acknowledge the CTSC grant UL1 TR000457-06 for the support of this research study.
References
- 1. Anderson LA, Egge R. Expanding efforts to address Alzheimer's disease: the healthy brain initiative. Alzheimers Dement 2014;10(5):S453–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hebert LE, Weuve J, Scherr PA et al. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 2013;80(19):1778–83. Epub 2013/02/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amieva H, Le Goff M, Millet X et al. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol 2008;64(5):492–8. [DOI] [PubMed] [Google Scholar]
- 4. Seifan A, Schelke M, Obeng-Aduasare Y et al. Early life epidemiology of Alzheimer's disease-a critical review. Neuroepidemiology 2015;45(4):237–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Willette AA, Bendlin BB, Starks EJ et al. Association of insulin resistance with cerebral glucose uptake in late middle–aged adults at risk for Alzheimer disease. JAMA Neurol 2015;72(9):1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agnew-Blais JC, Wassertheil-Smoller S, Kang JH et al. Folate, vitamin B-6, and vitamin B-12 intake and mild cognitive impairment and probable dementia in the women's health initiative memory study. J Acad Nutr Diet 2015;115(2):231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perna L, Mons U, Rujescu D et al. Apolipoprotein E e4 and cognitive function: a modifiable association results from two independent cohort studies. Dement Geriatr Cogn Disord 2015;41(1–2):35–45. [DOI] [PubMed] [Google Scholar]
- 8.Alzheimer's Association. Brain Health. 2016http://www.alz.org/we_can_help_brain_health_maintain_your_brain.asp (24 May 2016, date last accessed).
- 9. Siemers ER, Sundell KL, Carlson C et al. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement 2016;12(2):110–20. [DOI] [PubMed] [Google Scholar]
- 10. Roberts J, McLaughlin SJ, Connell CM. Public beliefs and knowledge about risk and protective factors for Alzheimer's disease. Alzheimers Dement 2014;10(0):S381–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rovner BW, Casten RJ, Harris LF. Cultural diversity and views on Alzheimer's disease in older African Americans. Alzheimer Dis Assoc Disord 2013;27(2):133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hipps YG, Roberts JS, Farrer LA et al. Differences between African Americans and Whites in their attitudes toward genetic testing for Alzheimer's disease. Genet Test 2003;7(1):39–44. [DOI] [PubMed] [Google Scholar]
- 13. Braun KL, Browne CV. Perceptions of dementia, caregiving, and help seeking among Asian and Pacific Islander Americans. Health Soc Work 1998;23(4):262–74. [DOI] [PubMed] [Google Scholar]
- 14. Connell CM, Roberts JS, McLaughlin SJ et al. Racial differences in knowledge and beliefs about Alzheimer disease. Alzheimer Dis Assoc Disord 2009;23(2):110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akinleye I, Roberts J, Royal CDM et al. Differences between African American and White Research Volunteers in their attitudes, beliefs and knowledge regarding genetic testing for Alzheimer's disease. J Genet Couns 2011;20(6):650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim S, Sargent-Cox KA, Anstey KJ. A qualitative study of older and middle-aged adults’ perception and attitudes towards dementia and dementia risk reduction. J Adv Nurs 2015;71(7):1694–703. [DOI] [PubMed] [Google Scholar]
- 17. Becker MH. The Health Belief Model and Personal Health Behavior, (Vol. 2), Thorofare, NJ: Slack, 1974. [Google Scholar]
- 18. Menon G, Raghubir P, Agrawal N. Health risk perceptions and consumer psychology In: Haugtvedt CP, Herr P. (eds). Handbook Consumer Psychology. Mahwah, NJ: Lawrence Erlbaum and Associates, 2006. [Google Scholar]
- 19. United States Department of Health Human Services National Plan to Address Alzheimer's Disease: 2013 Update. Washington, DC: US Department of Health and Human Services, http://aspe hhs gov/daltcp/napa/NatlPlan shtml Accessed. 2013;4(3):2014. [Google Scholar]
- 20. French SL, Floyd M, Wilkins S et al. The fear of Alzheimer's disease scale: a new measure designed to assess anticipatory dementia in older adults. Int J Geriatr Psychiatry 2012;27(5):521–8. [DOI] [PubMed] [Google Scholar]
- 21. Kim S, Sargent-Cox K, Cherbuin N et al. Development of the motivation to change lifestyle and health behaviours for dementia risk reduction scale. Dement Geriatr Cogn Dis Extra 2014;4(2):172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Godin G, Kok G. The theory of planned behavior: a review of its applications to health-related behaviors. Am J Health Promot 1996;11(2):87–98. [DOI] [PubMed] [Google Scholar]
- 23. Ajzen I, Cote NG. Attitudes and the prediction of behavior. In: Crano W, Prislin R. (eds). Attitudes and Attitude Change, New York: Taylor and Francis, 2008. [Google Scholar]
- 24. Norcross JC, Prochaska JO. Using the stages of change. Harv Ment Health Lett 2002;18(11):5–7. [PubMed] [Google Scholar]
- 25. Chew LD, Griffin JM, Partin MR et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med 2008;23(5):561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carpenter BD, Balsis S, Otilingam PG et al. The Alzheimer's disease knowledge scale: development and psychometric properties. Gerontologist 2009;49(2):236–47. doi:10.1093/geront/gnp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. SAS Institute The SAS system for Windows. 9.4 ed. Cary, NC 2013.
- 28. Cutler SJ, Hodgson LG. To test or not to test: interest in genetic testing for Alzheimer's disease among middle-aged adults. Am J Alzheimers Dis Other Demen 2003;18(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roberts J. Anticipating response to predictive genetic testing for Alzheimer's disease: a survey of first-degree relatives. Gerontologist 2000;40(1):43–52. [DOI] [PubMed] [Google Scholar]
- 30. Institute of Medicine Health and Behavior: The Interplay of Biological, Behavioral, and Societal Influences. Washington, DC: National Academies Press, 2001. [PubMed] [Google Scholar]
- 31. Jayanti RK, Burns AC. The antecedents of preventive health care behavior: an empirical study. J Acad Market Sci 1998;26(1):6–15. [Google Scholar]
- 32. Grill JD, Galvin JE. Facilitating alzheimer's disease research recruitment. Alzheimer Dis Assoc Disord 2014;28(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]