Abstract
Advances in retooling microorganisms have enabled bioproduction of ‘drop-in’ biofuels, fuels that are compatible with existing spark-ignition, compression-ignition, and gas-turbine engines. As the majority of petroleum consumption in the United States consists of gasoline (47%), diesel fuel and heating oil (21%), and jet fuel (8%), ‘drop-in’ biofuels that replace these petrochemical sources are particularly attractive. In this review, we discuss the application of aldehyde decarbonylases to produce gasoline substitutes from fatty acid products, a recently crystallized reductase that could hydrogenate jet fuel precursors from terpene synthases, and the exquisite control of polyketide synthases to produce biofuels with desired physical properties (e.g., lower freezing points). With our increased understanding of biosynthetic logic of metabolic pathways, we discuss the unique advantages of fatty acid, terpene, and polyketide synthases for the production of bio-based gasoline, diesel and jet fuel.
In 2014, the levels of anthropogenic greenhouse gases (carbon dioxide, methane, and nitrous oxide) reached their highest levels in at least 800 000 years [1,2]. Transportation vehicles contributed approximately 14% of all global greenhouse gas emissions in 2010, with 95% of that derived from petroleum based fuels [3]. If fuels produced from renewable sources such as biomass, landfill gas or atmospheric CO2 replaced petrochemically-derived fuels, it would reduce CO2 emissions through the ‘closed CO2 cycle’: CO2 that is burned through combustion is reused from the atmosphere to produce the biofuel [4]. While no current biofuel should not be considered purely carbon neutral after accounting for collateral emissions, the production of ‘drop-in’ biofuels, fuels that are compatible with existing spark-ignition, compression-ignition and gas-turbine engines, could greatly reduce greenhouse gas emissions [5,6].
Microbial fermentation is a particularly attractive means of producing renewable biofuels. Genetically engineered microbes can utilize feedstocks from non-agricultural sources (e.g., switchgrass) that does not compete with food crops for land mass to produce various fuels and commodity chemicals. In this review, we discuss the microbial production of biofuels derived from three different classes of biosynthetic pathways: fatty acid, isoprenoid, and polyketide. Short chain alcohols (e.g., isobutanol, 1-butanol) are an important class of drop-in fuels, and will continue to be in the future. As they are produced from different pathways and are well reviewed [7], we exclude their discussion here. We provide the maximum theoretical mass yield and highest reported titers for these select ‘drop-in’ biofuels (Table 1) and discuss the production pathways. Fatty acid biosynthesis is the most well-established pathway to produce biofuels, and we discuss the recent work to synthesize short and medium chain alkanes as constituents of gasoline and diesel. Isoprenoid hydrocarbons often contain branching and ring structures that have high energy content, low water miscibility and reduced premature ignition, rendering them attractive biofuel substitutes for diesel and even jet fuel. Polyketide synthases, although they have been explored less thoroughly for such applications, also have attractive biosynthetic logic to produce high performance biofuels.
Table 1.
Maximum possible theoretical yield is given for each compound based on the substrate glucose under anaerobic conditions. The highest reported titres for each type of compound is illustrated
| Pathway | Compounds | Mass yield % (g/ghexose) | Highest reported titre |
|---|---|---|---|
| Fatty acid synthesis | |||
| Alkanes (C13–C17) | 30.7–30.8% | 300 mg/L [11] | |
| Alkanes (C9–C14) | 30.5–30.7% | 580 mg/L [16] | |
| Propane | 29.4% | 32 mg/L [20••] | |
| Isoprene synthesis | |||
| Bisabolene | 32.4% | 5.2 g/L [33] | |
| Farnesene | 32.4% | 1.1 g/L [36•] | |
| Pinene | 32.4% | 32 mg/L [38] | |
| Limonene | 32.4% | 700 mg/L [46] | |
| Polyketide synthesis | |||
| Multi-methyl-branched esters | 27.2–27.6% | 98 mg/L [51] | |
| Pentadecane | 30.8% | 140 mg/L [52•] |
Biofuels derived from fatty acid biosynthetic pathways
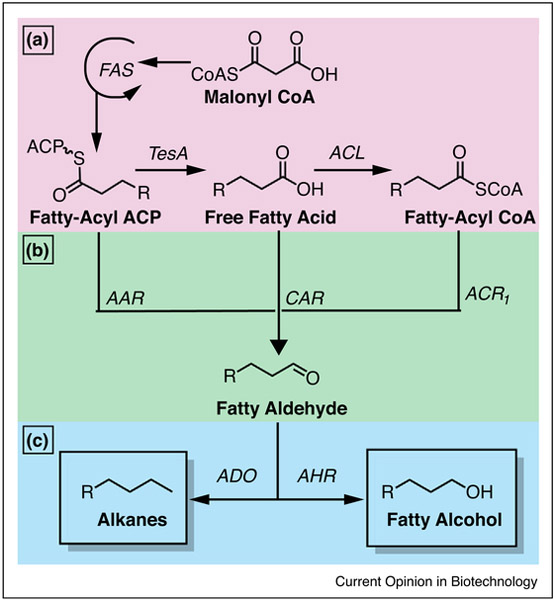
While the fatty acid pathway has been leveraged to produce alcohol, ketone, ester and olefin biofuel products [8–10], there is an exciting new avenue to synthesize short chain and medium chain alkanes that could be used in place of gasoline and diesel. Initiated by acetyl-CoA, fatty acid biosynthesis in Escherichia coli is performed by the fatty acid synthase complex (FAS) II that uses multiple, discrete enzymes to generate a saturated fatty acid (typically 14–18 carbons in length). Alkanes could be produced from the products of FAS (fatty-acyl carrier protein (ACP), free fatty acid, and fatty-acyl-CoA (Figure 1a) using a fatty aldehyde decarbonylase (ADO), first identified in the cyanobacterium Synechococcus elongatus, which converts fatty aldehydes to alkanes [11].
Figure 1.
Fatty acid synthesis of alkanes. (a) In the first step of fatty acid synthesis, acetyl-CoA and malonyl-CoA are transthioesterified to the fatty acid synthase (FAS), and the final product of each round of elongation is a fatty-acyl ACP. A thioesterase (TesA) can cleave the fatty-acyl ACP to generate a free fatty acid that reacts with an acyl-CoA ligase (ACL) to generate fatty-acyl CoA. (b) Fatty aldehydes can be generated from fatty-acyl ACP, free fatty acids, and fatty-acyl CoA through acyl carrier protein reductase (AAR), carboxylic acid reductase (CAR), and acyl CoA reductase (ACR1), respectively. (c) Fatty aldehydes can generate alkanes through aldehyde decarbonylase (ADO) and fatty alcohols through aldehyde reductase (AHR).
In a seminal report, Schirme et al. demonstrated that an acyl carrier protein reductase (AAR) could produce fatty aldehydes directly from fatty acyl-ACPs in E. coli (Figure 1b) [11]. With the heterologous expression of an improved ADO from Nostoc punctiforme, 300 mg/L of odd-numbered C13–C17 hydrocarbons were produced, 80% present extracellularly. A subsequent report demonstrated an AAR from Bacillus subtilis could produce even-numbered C14 and C16 when expressed with ADO [12]. While this method is appropriate for diesel fuel type molecules, the limitations in the chain-length profile of hydrocarbons synthesized precludes producing a substitute for gasoline, which is a blend of short-chain hydrocarbons (typically three to nine carbons) [13].
There are several advantages to producing alkanes from FFAs or acyl-CoA: fatty acids have been produced in higher abundance than fatty acyl-ACPs, production from fatty acids affords better control over chain length, and the pool of fatty acids can be manipulated [9,14,15]. A recent study described the in vivo production of a modified acyl-ACP thioesterase to produce short-chain fatty acids, which were then appended to CoA via an overexpressed fatty-acyl-CoA ligase (Figure 1b). The cells produced 580 mg/L of short chain alkanes from fatty-acyl-CoAs by the sequential reaction of Clostridium acetobutylicum fatty acyl-CoA reductase (ACR1) and Arabidopsis thaliana ADO [16]. As such, this strategy relied on expressing an acyl-ACP thioesterase to terminate FAS at the desired chain length. Howard and coworkers used a similar approach with heterologous thioesterases and branched FFAs in E. coli generating branched alkanes [17]. Eschewing heterologous thioesterases, Liu et al. engineered ACPs that prefer particular fatty acid chain lengths that can generate predictable alterations to the hydrocarbon cellular output [18]. While production of biofuels from fatty-acyl-CoA has enhanced control over the length of hydrocarbon chains produced, this pathway requires the additional processing step of CoA ligation compared to production from FFAs.
Recently, a more direct method to generate fatty aldehydes has used a carboxylic acid reductase (CAR) from Mycobacterium marinum to directly convert FFAs to fatty aldehydes (Figure 1b) [18]. Akhtar et al. demonstrated that this CAR can accept a broad range of substrate chain lengths. Furthermore, the CAR route is a more thermodynamically favored pathway than the cyanobacterial AAR route (−35.9 kJ/mol compared to −3.9 kJ/mol), and has much better in vitro kinetics than both the AAR and ACR1 pathways [19]. In a subsequent study, Kallio et al. used a thioesterase specific for butyryl-ACP to tune the FFA pool, which the CAR converted to generate short chain alkanes, producing 32 mg/L of propane in a shake flask fermentation [20••].
While biofuel production of alkanes is dependent on titers of FFAs, biofuel yields are much lower than the yields of FFAs due to factors including intermediate and product toxicity, specificity, and catalytic efficiency of the enzymatic machinery. Flux may also be inhibited by intracellular precursor supply, as FFAs are secreted by overexpressing strains [21]. The highest published titers of FFAs (8.6 g/L) have been achieved through modular optimization of fatty acid production followed by fed-batch fermentation [22]; however titers of biofuels generated from this process are much lower. As acyl-ACPs inhibit several enzymes of the endogenous Type II FAS in E. coli, a promising strategy could involve a parallel system of heterologous type I FAS systems [8,23•].
Currently the limiting factor in alkane production is ADO catalytic activity, an obstacle that is compounded by native aldehyde reductases (AHR) (Figure 1c) [10]. AHR seemingly competes with aldehyde decarbonylases for fatty aldehydes that result in the production of alcohols instead of alkanes (Figure 1) [24]. While removing competing AHR can increase flux to alkanes [25,26], recent work showed that moderate fatty alcohol production stimulated alkane biosynthetic flux, and when combined with other metabolic engineering improvements, 1.26 g/L in a fed-batch fermentation of alkanes was achieved [27••], the highest titer reported. As O2 and electron supply are co-substrates for the conversion of aldehydes to alkanes in this class of decarbonylases [28], an optimized electron transfer system can increase the catalytic rate of ADO [20••,27••]. While challenges remain in microbial production of alkanes through FASs, particularly in kinetic throughput, it is currently the most mature pathway for bioproduction of straight-chain hydrocarbons of short to medium chain length that compose gasoline and diesel.
Isoprenoid based biofuel production
The isoprenoid pathway, which elongates carbon skeleton chains through the five-carbon units by condensing the building blocks isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), has attracted great interest as a biofuel production platform. While bacteria can produce IPP through the methylerythitol-4-phosphate (MEP) pathway, a mevalonate-based (MVA) pathway has been reconstituted in E. coli, which greatly improves the flux to IPP (Figure 2a) [29,30]. Monoter-penes (C10), produced by the condensation of IPP and DMAPP, and sesquiterpenes (C15), produced from the condensation of two IPPs and one DMAPP, in particular have been identified as diesel and jet fuel substitutes. A terpene synthase converts the precursor to the final compound, which, following modification via hydrogenation or dimerization, is a suitable biofuel.
Figure 2.
Isoprenoid pathway for the production of biobased diesel and jet fuels. (a) Acetyl-CoA generates the building blocks for isoprenoid production through the mevalonate pathway. (b) Farenyl pyrophosphate synthase (FPPS) synthesizes feranyl pyrophosphate (FPP) from two IPP and one DMAPP. Farnesene and bisabolene are synthesized by their respective synthases from FPP. Hydrogenation of each molecule produces biodiesel fuel candidates. (c) Geranyl pyrophosphate synthase (GPPS), converts an IPP and DMAPP molecule to geranyl pyrophosphate (GPP). Limonene and pinene are synthesized by limonene and pinene synthase, respectively. Chemically dimerized pinene and hydrogenated limonene are biobased jet fuel candidates.
Bisabolene and farnesene are two desirable biodiesel precursors synthesized from farnesyl pyrophosphate (FPP) using bisabolene synthase (BS) and farnesene synthase (FS), respectively (Figure 2b). When chemically hydrogenated, bisabolane has similar fuel properties to D2 diesel fuel [31]. Bisabolene has been produced in E. coli and Saccharomyces cerevisiae at titers higher than 900 mg/L in shake flasks [32] and 5.2 g/L in a fermentation process [33]. Demonstrating an exploration of an alternative chassis organism that can use uncommon sugars as well as consume lignocellulosic biomass, our group engineered a strain of Streptomyces venezuelae to produce bisabolene at 10 mg/L, which is two orders of magnitude higher than the total terpene yield of wild-type S. venezuelae [34]. Similar to bisabolane, farnesane has better combustion properties than diesel with similar viscosity and density [31]. Farnesene, the dehydrogenated form of farnesane, is well-tolerated in S. cerevisiae, with yields of 170 mg/L in fed batch fermentations [35]. The kinetics of farnesene production were quantified using an in vitro reconstitution system with purified components, and using this information, Zhu et al. overexpressed genes encoding enzymes in the pathway to yield up to 1.1 g/L of farnesene in E. coli at the shake-flask scale [36•]. Farnesene has seen interest from industry as well, and recently Amyris announced a record low manufacturing cost of farnesene at $1.75 per liter [37].
While there has been much progress in the production of biodiesel, the development of biobased jet fuels to replace high-density, high-energy tactical fuels such as JP-10 and RJ-5 has lagged. Recently, it has been shown that the chemical dimerization of pinene results in a bicyclic terpene with comparable values for density and volumetric heating (0.94 g/mL and 39.5 MJ/L) as JP-10 [38]. Pinene titers, synthesized from GPP with a pinene synthase (Figure 2c), are an order of magnitude lower than those achieved for sesquiterpenes like bisabolene, most likely due to flux inhibition by its precursor GPP or toxicity of pinene to E. coli [39,40]. Addressing flux inhibition, a recent report found that a GPPS-PS protein fusion to relieve inhibition of GPPS by GPP produced 32 mg/L of pinene [38]. To reduce toxicity and increase pinene titer, general stress response proteins, heat-shock proteins, or efflux pumps could confer increased resistance. Indeed, a study found that the heterologous expression of YceI, a possible transporter protein belonging to the diverse and largely uncharacterized family of YceI genes, from Marinobacter aquaeolei increases resistance to pinene toxicity in E. coli [41].
The hydrogenated form of limonene, limonane, also has favorable properties for jet-biofuels [42,43]. Limonene is produced from the cyclization of GPP by limonene synthase (LS), which can be subsequently catalytically hydrogenated to limonane (Figure 2c). Early efforts to produce limonene suffered from low intracellular levels of its precursor geranyl pyrophosphate in E. coli [44]. Using the heterologous expression system of the MVA pathway consisting of GPP synthase, limonene synthase and a cytochrome P450, a recent study improved the yield of limonene to 400 mg/L from glucose in shake-flask cultures [43]. However, limonene toxicity due to the common oxidation product limonene hydroperoxide limits production [45], and a two-phase extractive fermentation can alleviate monoterpene toxicity [46]. Using a two liquid-phase fed-batch system, limonene titers reached 700 mg/L with glucose as the sole carbon source [46].
Limonene, bisabolene, and farnesene all require chemical hydrogenation after in vivo production. This capital cost could be eliminated through a hydrogenation enzyme, and recently, a report found that geranylgeranyl reductase (GGR) from Sulfolobus acidocaldarius catalyzes the hydrogenation of three out of four double bonds in GPP to produce a near saturated alkyl backbone. Natively produced as part of the organism’s unusual isoprenoid-based cellular membrane, the crystallized structure guided the selection of targeted mutations to increase the rate of hydrogenation [47••]. If GGR is further engineered to fully and rapidly hydrogenate GPP and FPP, GGR could be beneficial in producing final-state biofuel compounds in vivo.
Polyketide-based biofuel production
Polyketide synthases are molecular factories that produce an array of antibiotics, cancer therapeutics, and other medicinal compounds. While there are three types of PKSs, Type I PKSs are capable of full reductive processing to form saturated carbon skeletons that are particularly appealing as biofuels. Type I PKSs are also an attractive engineering target as the assembly line formation and rounds of elongation present an easily identifiable biosynthetic logic for the tailored production of specific hydrocarbons. A fully reducing module consists of the following domains: a ketosynthase (KS), an acyltransferase (AT), a dehydratase (DH), a methyltransferase (MT), an enoyl reductase (ER), a ketoreductase (KR), an acyl carrier protein (ACP), and a thioesterase (TE) (Figure 3). The versatility and tailoring of products by PKSs, along with reprogrammable domains, render PKSs a potentially important platform to produce biofuels with desired physical properties. One potential challenge that is presented when utilizing PKSs, however, is that they have been reported to be catalytically slower than fatty acid synthases. For example, FAS synthesis of palmitic acid is completed in less than a second, whereas the DEBS system requires approximately 2 min, at least in vitro [48,49]. However, the mechanistic basis of this difference in catalytic efficiency is unclear [49], and the measurements of PKS kinetics may be retarded by expression (followed by purification, and kinetic characterization) in E. coli, which may be a suboptimal folding environment for this particular class of enzymes [50]. Recent experiments in our lab have indicated that in other hosts, in vivo PKS kinetics may be closer to FAS kinetics than previously thought (results unpublished).
Figure 3.
Branching through polyketide synthase pathways. (a) An acyltransferase (AT, highlighted in red) selects for methylmalonyl-CoA and transfers it to a phosphopantetheine arm of the acyl carrier protein (ACP). A Claisen condensation reaction of methyl-malonate and the primer chain takes place at the ketosynthase (KS), resulting in a α-methyl group (red). The ACP then shuttles the resulting ketone through the processing domains that reduce (ketoreductase, KR) and dehydrate (dehydratase, DH) the β-ketone, where the enoyl reductase (ER) full reduces the keto group. (b) An alternative approach to generating a α-branched carbon (red) is through utilizing SAM-dependent methyl transferases (MT, highlighted in red).
In the first exploitation of PKSs for biofuel production, Menendez-Bravo et al. diverted the FFA pool to an iterative Type I PKS for the production of fatty alcohols and esters. By expressing an iterative Mycobacterium tuberculosis PKS that accepts methylmalonyl-CoA as a substrate, the group produced 98 mg/L of multi-methyl branched esters in E. coli [51]. In a more recent study, Liu et al. expressed the iterative Type I PKS from Streptomyces, SgcE, and its cognate thioesterase, SgcE10, in a two-plasmid system in E. coli. The group optimized the ratio of SgcE expression to SgcE10 expression, and in a fed-batch fermentation followed by chemical hydrogenation, the study reported a yield of 140 mg/L of pentadecane [52•]. While these iterative PKS pathways are technically simple and provided a starting point for engineering PKS pathways for biofuel production, modular Type I systems afford unique opportunities to precisely tailor the chemical structure. In a notable example of this versatility, our group recently engineered a chimeric Type I PKS with fully reductive processing domains into the first module of borreledin PKS, enabling the bio-based production of the commodity chemical, adipic acid [53•].
The high level of control provided by modular type I PKSs is especially useful to generate selectively branched biofuels, which is useful to lower the freezing point of fuels and is not easily done using FAS. Branching in PKSs occurs through the incorporation of methylmalonyl-CoA as a substrate or through S-adenosyl methionine (SAM)-dependent C-methyltransferases (Figure 3). Non-natural incorporation of AT domains can be pursued through AT domain exchanges, AT knockouts with complementation of a trans-AT domain, or AT-site directed mutagenesis (reviewed [54]). These methods often degraded the specificity of the AT for the alternative substrate, resulting in slower kinetics than the original AT domain. Recently, our group identified ‘hot spot’ AT boundaries that can be used to swap AT domains while maintaining protein stability and activity (indeed, in some cases, improving PKS kinetics) [55]. Methylation through SAM-dependent C-methyltransferases is an alternative approach that has not been extensively studied. Recent studies have helped to elucidate the reaction mechanism of methyltransferases, indicating that methylation can occur before or after Claisen condensation [56,57], and also that excised mono- and di-methyltransferases can function in vitro [57,58]. This exquisite control over product formation could generate customized products with desired combustion properties as biofuel substitutes.
Conclusions
The replacement of petroleum-based fuels with renewable fuel sources will only be accomplished through higher product yields and lower feedstock costs. As highlighted in this review, the production of biofuels (e.g., gasoline, diesel, and jet fuel) lags behind the production of substrates (e.g., fatty acids, isoprenoid precursors, malonyl-CoA). To transition to a ‘green’ transportation economy, this gap between substrate production and biofuel synthesis must be narrowed. While new pathways can be created through the discovery and repurposing of enzymatic functions (e.g., ADO in fatty acid synthesis for alkane production), commercial level production of biofuels will not be accomplished until we improve enzyme kinetics, toxicity tolerances, and metabolic flux. Improving enzyme kinetics usually involves directed evolution or bioprospecting for homologues with more appropriate substrate specificity. Directed evolution is frequently difficult because most fuel molecules require low throughput assays, limiting library size. Screening for fuel molecules in more tolerant microbes has better selection theoretically, but frequently evolving a more tolerant microbe to exogenously added fuel does not translate into a better production host when the pathway is inserted. Finally improving metabolic flux also suffers from the same low throughput assays, limiting library size. While these problems are tractable, solving all three concurrently is still difficult and expensive. Given these constraints, developing methodologies to make designs more rational and/or developing truly high throughput assays has the best potential to move the field forward toward the high titers generally needed to make processes attractive for industry to further investigate.
Our opinion is that the diverse fuel energy needs of the global economy may be best served by diversifying the metabolic pathways we choose to develop biofuel substitutes. While fatty acid synthases may produce the highest titers, the higher energy density of isoprenoids or customizability of polyketides may be the best choice to produce tailored biofuel products with the desired physical properties. Moreover, the community should leverage the versatility of these bioprocesses to search and identify ‘drop-in’ biofuels with superior performance.
Acknowledgements
This work was funded by the Joint BioEnergy Institute (JBEI), which is funded by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, under Contract DE-AC02-05CH11231, by the National Science Foundation under awards MCB-1442724 and NSF-GRFP DGE-1106400, and as part of the Co-Optimization of Fuels & Engines (Co-Optima) project sponsored by the U. S. Department of Energy (DOE) Office of Energy Efficiency and Renewable Energy (EERE), Bioenergy Technologies and Vehicle Technologies Offices.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.IPCC: Climate Change 2014 Synthesis Report Summary Chapter for Policymakers. IPCC; 2014. http://dx.doi.org/10.1017/CBO9781107415324. [Google Scholar]
- 2.Pacala S: Stabilization wedges: solving the climate problem for the next 50 years with current technologies. Science 2004, 305:968–972. [DOI] [PubMed] [Google Scholar]
- 3.Pachauri Rajendra K et al. : Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC; 2014. [Google Scholar]
- 4.Liao JC, Mi L, Pontrelli S, Luo S: Fuelling the future: microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol 2016, 14:288–304. [DOI] [PubMed] [Google Scholar]
- 5.Fargione J, Hill J, Tilman D, Polasky S, Hawthorne P: Land clearing and the biofuel carbon debt. Sci. J 2008, 319:1235–1237. [DOI] [PubMed] [Google Scholar]
- 6.d’Espaux L, Mendez-Perez D, Li R, Keasling JD: Synthetic biology for microbial production of lipid-based biofuels. Curr. Opin. Chem. Biol 2015, 29:58–65. [DOI] [PubMed] [Google Scholar]
- 7.Chen CT, Liao JC: Frontiers in microbial 1-butanol and isobutanol production. FEMS Microbiol. Lett 2016, 363. [DOI] [PubMed] [Google Scholar]
- 8.Coursolle D, Lian J, Shanklin J, Zhao H: Production of long chain alcohols and alkanes upon coexpression of an acyl-ACP reductase and aldehyde-deformylating oxygenase with a bacterial type-I fatty acid synthase in E. coli. Mol. Biosyst 2015, 11:2464–2472. [DOI] [PubMed] [Google Scholar]
- 9.Steen EJ, Kang Y, Bokinsky G, Hu Z, Schirmer A, McClure A, Del Cardayre SB, Keasling JD: Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 2010, 463:559–562. [DOI] [PubMed] [Google Scholar]
- 10.Mendez-Perez D, Begemann MB, Pfleger BF: Modular synthase-encoding gene involved in α-olefin biosynthesis in Synechococcus sp. strain PCC 7002. Appl. Environ. Microbiol 2011, 77:4264–4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schirmer A, Rude MA, Li X, Popova E, del Cardayre SB: Microbial biosynthesis of alkanes. Science 2010, 329:559–562. [DOI] [PubMed] [Google Scholar]
- 12.Harger M, Zheng L, Moon A, Ager C, An JH, Choe C, Lai Y, Mo B, Zong D, Smith MD et al. : Expanding the product profile of a microbial alkane biosynthetic pathway. ACS Synth. Biol 2012, 2:59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cline PV, Delfino JJ, Rao PSC: Partitioning of aromatic constituents into water from gasoline and other complex solvent mixtures. Environ. Sci. Technol 1991, 25:914–920. [Google Scholar]
- 14.Youngquist JT, Lennen RM, Ranatunga DR, Bothfeld WH, li WDM, Pfleger BF: Kinetic modeling of free fatty acid production in Escherichia coli based on continuous cultivation of a plasmid free strain. Biotechnol. Bioeng 2012, 109:1518–1527. [DOI] [PubMed] [Google Scholar]
- 15.Liu T, Vora H, Khosla C: Quantitative analysis and engineering of fatty acid biosynthesis in E. coli. Metab. Eng 2010, 12: 378–386. [DOI] [PubMed] [Google Scholar]
- 16.Choi YJ, Lee SY: Microbial production of short-chain alkanes. Nature 2013, 502:571–574. [DOI] [PubMed] [Google Scholar]
- 17.Howard TP, Middelhaufe S, Moore K, Edner C, Kolak DM, Taylor GN, Parker DA, Lee R, Smirnoff N, Aves SJ et al. : Synthesis of customized petroleum-replica fuel molecules by targeted modification of free fatty acid pools in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A 2013, 110:7636–7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Hicks WM, Silver PA, Way JC: Engineering acyl carrier protein to enhance production of shortened fatty acids. Biotechnol. Biofuels 2016, 9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akhtar MK, Turner NJ, Jones PR: Carboxylic acid reductase is a versatile enzyme for the conversion of fatty acids into fuels and chemical commodities. Proc. Natl. Acad. Sci. U. S. A 2013, 110:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kallio P, Pasztor A, Thiel K, Akhtar MK, Jones PR: An engineered pathway for the biosynthesis of renewable propane. Nat. Commun 2014, 5:4731. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In this work, the authors for the first time leverage ADO to produce a short-chain hydrocarbon, propane. This is accomplished through a heterologous thioesterase from Bacteriodis fragilis, and ADO’s limiting catalytic turnover is enhanced through a heterelogous ferredoxin/ferredoxin reductase pair that increases electron supply to ADO.
- 21.Liu H, Yu C, Feng D, Cheng T, Meng X, Liu W, Zou H, Xian M: Production of extracellular fatty acid using engineered Escherichia coli. Microb. Cell Fact 2012, 11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu P, Gu Q, Wang W, Wong L, Bower AGW, Collins CH, Koffas MAG: Modular optimization of multi-gene pathways for fatty acids production in E. coli. Nat. Commun 2013, 4:1409. [DOI] [PubMed] [Google Scholar]
- 23.Haushalter RW, Groff D, Deutsch S, The L, Chavkin TA, Brunner SF, Katz L, Keasling JD: Development of an orthogonal fatty acid biosynthesis system in E. coli for oleochemical production. Metab. Eng 2015, 30:1–6. [DOI] [PubMed] [Google Scholar]; • Heterologous Type I FAS’s from multiple species were expressed in E. coli, and the Corynebacterium glutamicum FAS was applied to produce fatty alcohols and methyl ketones. While the titer is lower than native FAS, further improvements could improve yield as this pathway directly produces acyl-CoAs without an acyl ligase.
- 24.Eser BE, Das D, Han J, Jones PR, Marsh ENG: Oxygen-independent alkane formation by non-heme iron-dependent cyanobacterial aldehyde decarbonylase: investigation of kinetics and requirement for an external electron donor. Biochemistry 2011, 50:10743–10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez GM, Atsumi S: Toward aldehyde and alkane production by removing aldehyde reductase activity in Escherichia coli. Metab. Eng 2014, 25:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheppard MJ, Kunjapur AM, Prather KLJ: Modular and selective biosynthesis of gasoline-range alkanes. Metab. Eng 2016, 33:28–40. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y, Xiao W-H, Zhang J-L, Xie Z-X, Ding M-Z, Yuan Y-J: Heterologous biosynthesis and manipulation of alkanes in Escherichia coli. Metab. Eng 2016, 38:19–28. [DOI] [PubMed] [Google Scholar]; •• Recent work that advances a pathway synergy between ADO and AHR, where increased titer of ADO is produced when AHR is moderately enhanced.
- 28.Li N, Chang WC, Warui DM, Booker SJ, Krebs C, Bollinger JM: Evidence for only oxygenative cleavage of aldehydes to alk(a/e)nes and formate by cyanobacterial aldehyde decarbonylases. Biochemistry 2012, 51:7908–7916. [DOI] [PubMed] [Google Scholar]
- 29.Martin VJJ, Pitera DJ, Withers ST, Newman JD, Keasling JD: Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol 2003, 21:796–802. [DOI] [PubMed] [Google Scholar]
- 30.Pitera DJ, Paddon CJ, Newman JD, Keasling JD: Balancing a heterologous mevalonate pathway for improved isoprenoid production in Escherichia coli. Metab. Eng 2007, 9:193–207. [DOI] [PubMed] [Google Scholar]
- 31.Millo F, Bensaid S, Fino D, Marcano SJC, Vlachos T, Debnath BK: Influence on the performance and emissions of an automotive Euro 5 diesel engine fueled with F30 from farnesane. Fuel 2014, 138:134–142. [Google Scholar]
- 32.Peralta-Yahya PP, Ouellet M, Chan R, Mukhopadhyay A, Keasling JD, Lee TS: Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun 2011, 2:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Özaydin B, Burd H, Lee TS, Keasling JD: Carotenoid-based phenotypic screen of the yeast deletion collection reveals new genes with roles in isoprenoid production. Metab. Eng 2013, 15:174–183. [DOI] [PubMed] [Google Scholar]
- 34.Phelan RM, Sekurova ON, Keasling JD, Zotchev SB: Engineering terpene biosynthesis in Streptomyces for production of the advanced biofuel precursor bisabolene. ACS Synth. Biol 2015, 4:393–399. [DOI] [PubMed] [Google Scholar]
- 35.Tippmann S, Scalcinati G, Siewers V, Nielsen J: Production of farnesene and santalene by Saccharomyces cerevisiae using fed-batch cultivations with RQ-controlled feed. Biotechnol. Bioeng 2016, 113:72–81. [DOI] [PubMed] [Google Scholar]
- 36.Zhu F, Zhong X, Hu M, Lu L, Deng Z, Liu T: In vitro reconstitution of mevalonate pathway and targeted engineering of farnesene overproduction in Escherichia coli. Biotechnol. Bioeng 2014, 111:1396–1405. [DOI] [PubMed] [Google Scholar]; • Through the full reconstituion of the mevalonate pathway, this group determined the isopentyl diphosphate isomerase (Idi) to be rate-limiting, and its overexpression resulted in a fivefold increase. The reconstitution method applied here is general, and could be very effective for the optimized production of other terpenoids.
- 37.Amyris: Amyris achieves record low cost farnesene production. 2015. URL: https://amyris.com/amyris-achieves-record-low-cost-farnesene-production/.
- 38.Harvey BG, Wright ME, Quintana RL: High-density renewable fuels based on the selective dimerization of pinenes. Energy Fuels 2010, 24:267–273. [Google Scholar]
- 39.Yang J, Nie Q, Ren M, Feng H, Jiang X, Zheng Y, Liu M, Zhang H, Xian M: Metabolic engineering of Escherichia coli for the biosynthesis of alpha-pinene. Biotechnol. Biofuels 2013, 6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarria S, Wong B, Martín H, Keasling J, Peralta-Yahya P: Microbial synthesis of pinene. ACS Synth. Biol 2014, 3:466–475. [DOI] [PubMed] [Google Scholar]
- 41.Tomko TA, Dunlop MJ: Engineering improved bio-jet fuel tolerance in Escherichia coli using a transgenic library from the hydrocarbon-degrader Marinobacter aquaeolei. Biotechnol. Biofuels 2015, 8:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryder JA: Jet fuel compositions and methods of making and using same. United States Pat. 2012, US8106247.
- 43.Alonso-Gutierrez J, Chan R, Batth TS, Adams PD, Keasling JD, Petzold CJ, Lee TS: Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng 2013, 19:33–41. [DOI] [PubMed] [Google Scholar]
- 44.Carter OA, Peters RJ, Croteau R: Monoterpene biosynthesis pathway construction in Escherichia coli. Phytochemistry 2003, 64:425–433. [DOI] [PubMed] [Google Scholar]
- 45.Chubukov V, Mingardon F, Schackwitz W, Baidoo EEK, Alonso-Gutierrez J, Hu Q, Lee TS, Keasling JD, Mukhopadhyay A: Acute limonene toxicity in Escherichia coli is caused by limonene hydroperoxide and alleviated by a point mutation in alkyl hydroperoxidase AhpC. Appl. Environ. Microbiol 2015, 81:4690–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brennan TCR, Turner CD, Krömer JO, Nielsen LK: Alleviating monoterpene toxicity using a two-phase extractive fermentation for the bioproduction of jet fuel mixtures in Saccharomyces cerevisiae. Biotechnol. Bioeng 2012, 109: 2513–2522. [DOI] [PubMed] [Google Scholar]
- 47.Kung Y, McAndrew RP, Xie X, Liu CC, Pereira JH, Adams PD, Keasling JD: Constructing tailored isoprenoid products by structure-guided modification of geranylgeranyl reductase. Structure 2014, 22:1028–1036. [DOI] [PubMed] [Google Scholar]; •• Here, the authors show the crystallized structure of a geranylgeranyl reductase (GGR) that hydrogenates carbon–carbon double bonds as part of its cell membrane synthesis pathway. Targeted mutations increased hydrogenation kinetics, and the authors speculate that GGR could be especially useful for isoprenoid-based biofuels.
- 48.Pieper R, Ebert-Khosla S, Cane D, Khosla C: Erythromycin biosynthesis: kinetic studies on a fully active modular polyketide synthase using natural and unnatural substrates. Biochemistry 1996, 35:2054–2060. [DOI] [PubMed] [Google Scholar]
- 49.Smith S, Tsai S-C: The type I fatty acid and polyketide synthases: a tale of two megasynthases. Nat. Prod. Rep 2007, 24:1041–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garg A, Xie X, Keatinge-Clay A, Khosla C, Cane DE: Elucidation of the cryptic epimerase activity of redox-inactive ketoreductase domains from modular polyketide synthases by tandem equilibrium isotope exchange. J. Am. Chem. Soc 2014, 136:10190–10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menendez-Bravo S, Comba S, Sabatini M, Arabolaza A, Gramajo H: Expanding the chemical diversity of natural esters by engineering a polyketide-derived pathway into Escherichia coli. Metab. Eng 2014, 24:97–106. [DOI] [PubMed] [Google Scholar]
- 52.Liu Q, Wu K, Cheng Y, Lu L, Xiao E, Zhang Y, Deng Z, Liu T: Engineering an iterative polyketide pathway in Escherichia coli results in single-form alkene and alkane overproduction. Metab. Eng 2015, 28:82–90. [DOI] [PubMed] [Google Scholar]; • Through a reconsitution of the iterative PKS SgcE, the authors determined optimized balance of the PKS with its thioesterase by refactoring its promoters.
- 53.Hagen A, Poust S, Rond de T, Fortman JL, Katz L, Petzold CJ, Keasling JD: Engineering a polyketide synthase for in vitro production of adipic acid. ACS Synth. Biol 2016, 5:21–27. [DOI] [PubMed] [Google Scholar]; • In this work, the authors swap in the full reducing domains of foreign PKS’s into the borreledin PKS to produce adipic acid. Importantly, the authors show that by removal of the thioesterase, the “Ppant ejection assay” can be used to identify and relieve bottlenecks.
- 54.Dunn BJ, Khosla C: Engineering the acyltransferase substrate specificity of assembly line polyketide synthases. J. R. Soc. Interface 2013, 10:20130297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuzawa S, Deng K, Wang G, Baidoo EEK, Northen TR, Adams PD, Katz L, Keasling JD: Comprehensive in vitro analysis of acyltransferase domain exchanges in modular polyketide synthases and its application for short-chain ketone production. ACS Synth. Biol 2016, 6(1):139–147 http://dx.doi.org/10.1021/acssynbio.6b00176. [DOI] [PubMed] [Google Scholar]
- 56.Poust S, Phelan RM, Deng K, Katz L, Petzold CJ, Keasling JD: Divergent mechanistic routes for the formation of gem-dimethyl groups in the biosynthesis of complex polyketides. Angew. Chem. Int. Ed. Engl 2015, 54:2370–2373. [DOI] [PubMed] [Google Scholar]
- 57.Wagner DT, Stevens DC, Mehaffey MR, Manion HR, Taylor RE, Brodbelt JS, Keatinge-Clay AT: α-Methylation follows condensation in the gephyronic acid modular polyketide synthase. Chem. Commun 2016, 52(57):8822–8825 http://dx.doi.org/10.1039/C6CC04418B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens DC, Wagner DT, Manion HR, Alexander BK, Keatinge-Clay AT: Methyltransferases excised from trans-AT polyketide synthases operate on N-acetylcysteamine-bound substrates. J. Antibiot 2016, 69(7):567–570 http://dx.doi.org/10.1038/ja.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]