Abstract
Contralateral delay activity (CDA) has long been argued to track the number of items stored in visual working memory (WM). Recently, however, Berggren and Eimer [Berggren, N., & Eimer, M. Does contralateral delay activity reflect working memory storage or the current focus of spatial attention within visual working memory? Journal of Cognitive Neuroscience, 28, 2003–2020, 2016] proposed the alternative hypothesis that the CDA tracks the current focus of spatial attention instead of WM storage. This hypothesis was based on the finding that, when two successive arrays of memoranda were placed in opposite hemifields, CDA amplitude was primarily determined by the position and number of items in the second display, not the total memory load across both displays. Here, we considered the alternative interpretation that participants dropped the first array from WM when they encoded the second array because the format of the probe display was spatially incompatible with the initial sample display. In this case, even if the CDA indexes active storage rather than spatial attention, CDA activity would be determined by the second array. We tested this idea by directly manipulating the spatial compatibility of sample and probe displays. With spatially incompatible displays, we replicated Berggren and Eimer’s findings. However, with spatially compatible displays, we found clear evidence that CDA activity tracked the full storage load across both arrays, in line with a WM storage account of CDA activity. We propose that expectations of display compatibility influenced whether participants viewed the arrays as parts of a single extended event or two independent episodes. Thus, these findings raise interesting new questions about how event boundaries may shape the interplay between passive and active representations of task-relevant information.
INTRODUCTION
Working memory (WM) enables individuals to actively maintain information in mind to support a wide variety of cognitive processes. The close link of WM capacity to many cognitive functions and fluid intelligence (Unsworth, Fukuda, Awh, & Vogel, 2014; McVay & Kane, 2012; Fukuda, Vogel, Mayr, & Awh, 2010; Unsworth & Engle, 2007) motivates the effort to understand its neural underpinnings and to develop methods to track the contents of this online memory system. In this context, contralateral delay activity (CDA), a sustained negative-going deflection in the ERP of the EEG contralateral to memorized items, has shown great promise as a neural index of WM storage (Kuo, Stokes, & Nobre, 2012; Vogel, McCollough, & Machizawa, 2005; Vogel & Machizawa, 2004; Vogel, Woodman, & Luck, 2001; for a review, see Luria, Balaban, Awh, & Vogel, 2016). CDA amplitude scales with the number of items stored in WM (Balaban & Luria, 2015) and predicts individual differences in WM capacity (Unsworth et al., 2014; Vogel & Machizawa, 2004). Thus, the predominant view in the literature is that the CDA indexes the number of items that are held in memory.
More recently, an alternative notion of the CDA, the attentional activation account, has been suggested (Berggren & Eimer, 2016). The authors argue that the CDA indexes the lingering focus of spatial attention at the location of the last items that were encoded into WM. This notion is consistent with a large body of CDA studies because the positions of the memoranda in a typical CDA procedure are actively attended when they are encoded. Moreover, the attentional activation account provides a plausible account of why CDA amplitude is higher in multiple-object tracking tasks compared with simple change detection tasks (Drew, Horowitz, Wolfe, & Vogel, 2012; Drew & Vogel, 2008). Multiple-object tracking tasks require constant deployment of attention to targets as they move about the display. Thus, the increased CDA amplitude in these tasks can potentially be explained with a continuous update of the locus of attention that contributes to CDA amplitude.
Berggren and Eimer (2016) tested the attentional activation account with a sequential encoding version of the change detection task. In typical CDA studies, participants are cued to remember items on one side of a bilateral display, and the CDA manifests as a sustained negativity contralateral to the memorized items. In contrast, Berggren and Eimer’s study presented memoranda in two successive memory arrays (M1 and M2) that were sometimes on opposite sides of the display. Previous studies showed that, when memoranda from M1 and M2 appeared on the same side, CDA amplitude tracked the total storage load (Ikkai, McCollough, & Vogel, 2010; Vogel et al., 2005). In other words, adding m items to the n items that were already maintained resulted in a CDA amplitude that corresponded to a load of m + n items. Berggren and Eimer (2016), however, presented M2 on the opposite side of M1 in half the trials. This allowed them to track CDA amplitude when attention had to be moved to the opposite hemifield when M2 was presented. They found that each memory display elicited a CDA and that the size and polarity of the CDA was primarily determined by the number of items and hemifield of the M2 items. That is, when M2 was presented on the opposite side of M1, the polarity of the CDA switched, and CDA amplitude now reflected the number of items encoded from the second display. For example, if M1 showed one item in the left hemifield and then M2 three items in the right hemifield, a relatively small CDA initially emerged in posterior right electrode sites and then a relatively large CDA in posterior left electrode sites. The authors argued that these findings supported the attentional activation account, because CDA amplitude was determined not by the total number of memoranda across M1 and M2, but instead by the number (and positions) of the items presented in the most recent encoding period.
Although Berggren and Eimer’s (2016) findings fall in line with the attentional activation account, we propose that these findings can also be accommodated by the standard view that the CDA reflects active storage in WM. Specifically, we hypothesized that participants allowed the M1 items to drop from WM when M2 was presented and then retrieved M1 representations from a passive memory state at the end of the trial. There are multiple ways in which such a strategy might be implemented. For example, it has long been recognized that long-term memory (LTM) can contribute to performance in short-term retention tasks (Shelton, Elliott, Matthews, Hill, & Gouvier, 2010; Atkinson & Shiffrin, 1971), especially when there is no necessity to maintain all items in an active state. Alternatively, it has also been proposed that short-term retention can be supported by so-called “activity-silent” representations (Rose et al., 2016; Stokes, 2015) that do not generate persistent neural activity but which may be distinct from those in LTM. Regardless of the specific mechanism at play, the temporary absence of CDA activity may not undermine the hypothesis that the CDA indexes active storage when passive storage processes can also support task performance. In the present work, we present an alternative explanation of Berggren and Eimer’s (2016) findings and then provide direct evidence that the CDA indexes active storage rather than the current focus of spatial attention.
Our alternative account of Berggren and Eimer’s (2016) findings is that participants deprioritized M1 items in WM and viewed the M2 items as part of a distinct encoding episode. We believe this may have happened because of a task design in which the probe displays were spatially incompatible with the format of the M1 and M2 sample displays. When M1 and M2 were presented in opposite hemifields in the Berggren and Eimer (2016) procedure, the ensuing probe display was presented in the center of the screen with M1 and M2 items interleaved amongst each other (see Figure 1). This design required participants to detect changes across displays that were spatially translated and then combined in a manner that participants may have had difficulty visualizing. Our hypothesis is that this challenge may have motivated participants to encode M1 and M2 as separate episodes, such that M1 items were dropped from active storage during the encoding of the M2 items. This would explain why the CDA in the second retention interval, after both M1 and M2 have been shown, was mainly driven by the number of items in M2. In fact, it was found that when probes are presented in a different spatial configuration as in the memory display, performance can drop dramatically compared with probes being presented at the same location in memory and probe displays (Jiang, Olson, & Chun, 2000).
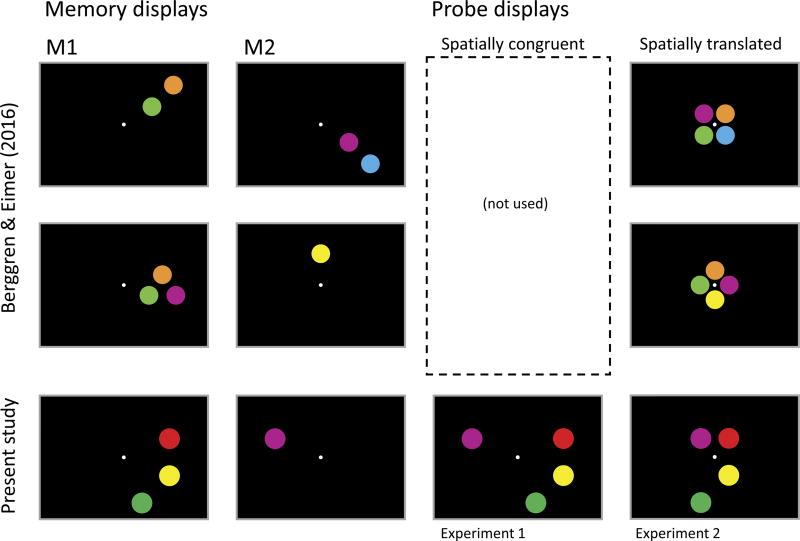
Figure 1.
Illustration of congruent and incongruent probe displays in sequential encoding change detection tasks. (A) M1 and M2 show memory arrays from memory displays used in Berggren and Eimer (2016; note that distractors are not shown here to clarify the point about the arrangement of targets). (B) Probe displays can be spatially congruent, that is, they are a 1:1 combination of M1 and M2 without spatial translation, similar to the ones used in this study (left) or they can be arranged in a square or diamond pattern close to the fixation, as used by Berggren and Eimer (right). Note that the latter manifests a spatial translation from memory to probe displays.
To test our account of Berggren and Eimer’s findings, we directly compared CDA activity using two different probe displays. Here, we will show that a modest change to the probe display used in the Berggren and Eimer (2016) study can motivate simultaneous storage of the contents of M1 and M2, thereby producing a CDA that reflects the total storage load across the two sequentially presented displays. The spatially congruent displays in Experiment 1 encouraged participants to integrate—and thus concurrently store–the items across the M1 and M2 displays by holding constant the position of each item across the memory and probe displays. In contrast, the interleaved displays in Experiment 2 were modeled after those in the Berggren and Eimer (2016) study, such that all items in the probe display were presented spatially translated with items corresponding to M1 and M2 interleaved (see Figure 1B, right). In the spatially congruent conditions of Experiment 1, we predicted that CDA activity should reflect the total number of items stored. Thus, in Experiment 1, when M1 and M2 were on the same side of space, the CDA should track the total number of items stored from both displays. At the same time, when M1 and M2 were on opposite sides in Experiment 1, we predicted that the CDA would track the difference in the number of items stored from each side, yielding a sustained negativity contralateral to the larger array. In contrast, with the interleaved displays of Experiment 2, we expected to replicate the findings of Berggren and Eimer (2017), such that CDA activity was primarily determined by the number of items presented in the most recent display. As the results will show, these predictions were borne out by the data, providing a reaffirmation of the hypothesis that CDA activity tracks the total contents of WM rather than the current focus of spatial attention.
EXPERIMENT 1
Methods
Participants
Twenty volunteers naive to the objective of the experiment participated for payment (~15 USD per hr). Participants were aged 19–32 years (M = 24.9, SD = 3.6) and reported normal or corrected-to-normal visual acuity as well as normal color vision. Six participants were female, and three were left-handed. The experiment was conducted with the written understanding and consent of each participant. Three additional participants were excluded from analysis because of (1) technical issues or (2) lack of task compliance.
Apparatus
Participants were seated in a comfortable chair in a dimly lit, electrically shielded and sound-attenuated chamber. Participants put their head in a chinrest at a distance of 65 cm from the screen. They responded with button presses on a standard keyboard that was placed in front of them. Stimulus presentation and response collection were controlled by a Windows PC using PsychToolBox 3 routines in MATLAB (Version 8.6.0). All stimuli were presented on an LCD-TN screen (BenQ XL2430-B).
Stimuli
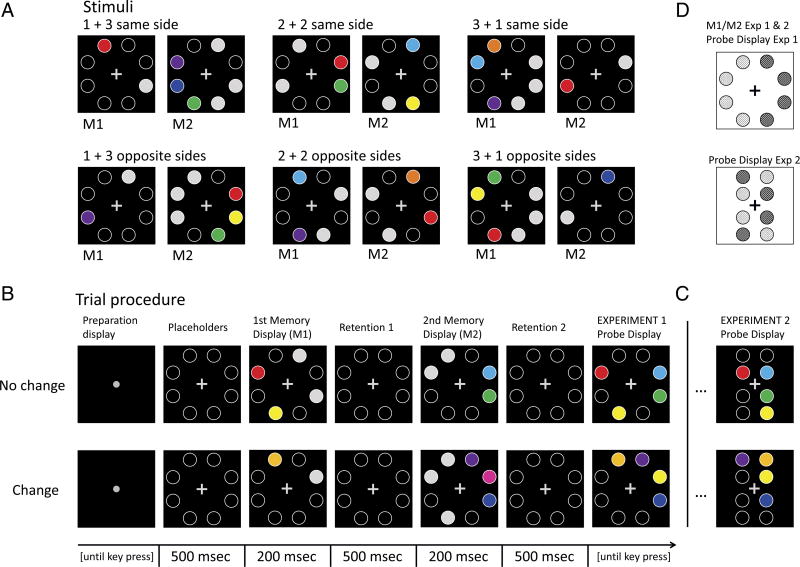
All stimuli were presented on a black background. In each trial, participants saw four memory items presented in two sequential displays (see Figure 2A). Both memory displays (M1 and M2) showed eight white empty circles (1.5° diameter, 2 pixels line width, RGB: 255-255-255) as placeholders, four in each hemifield, presented on an imaginary circle (3° eccentricity), around a central fixation cross (see Figure 2A). Both M1 and M2 had one, two, or three of the placeholders filled with a distinct color, serving as memory items. There was a total of nine possible colors: red (RGB: 255-0-0), pink (255-0-255), purple (128-0-255), blue (0-0-255), cyan (0-255-255), light green (0-255-0), dark green (0-130-60), yellow (255-255-0), orange (255-128-0), with an average luminance of 65 cd/m2. The total number of memory items in M1 and M2 combined was always four: When M1 had one memory item, M2 had three; when M1 had two items, M2 had two as well; and when M1 had three, M2 had one. Within each memory display, all memory items were presented on either the left or right hemifield (equiprobably). To counterbalance the physical input on both hemifields, the same number of placeholders were filled with gray on the opposite side of the memory items in both M1 and M2. The gray was matched in luminance to the average of the nine possible colors (RGB: 148-148-148, 65 cd/m2). Note that the RGB values were matched to be equiluminant on the specific hardware used in the present experiments and may differ in other laboratories depending on the screen or the graphic card used. The position of the gray circles was random and unrelated to the position of the colored circles. In half the trials, M1 and M2 showed memory items on the same side; in the other half of the trials, memory items were presented on opposite sides. The probe display was a spatially congruent combination of M1 and M2; it showed placeholders filled with colors at the same location as in M1 and M2 (but not the gray circles). Half the trials were “same” trials, that is, the same placeholders were filled with the same colors at the same locations as in M1 and M2. The other half of the trials were “change” trials, that is, the same placeholders were filled in the probe display as in combination of M1 and M2, but one item had a different color, namely one of the five colors that were not used in M1 or M2. In change trials, each of the four items was equally likely to change color, regardless of whether it was presented in M1 or M2. There were six conditions: 3 memory load conditions (1 + 3 vs. 2 + 2 vs. 3 + 1) × 2 side conditions (same side vs. opposite sides).
Figure 2.
(A) Stimuli from the six Load Change × Side Switch conditions. The total WM load was always four items in all conditions. Memory items were successively added in M1 and M2 (1 + 3, 2 + 2, 3 + 1), and M1 and M2 items were presented in the same hemifield (top row) or in opposite hemifields (bottom). (B) Trial procedure for Experiment 1 and 2. Participants had to remember colored circles from two displays, M1 and M2, and ignore gray circles. All displays were identical in Experiments 1 and 2, except for the probe display. The probe display showed the combined memory items from M1 and M2 in a spatially congruent manner (Experiment 1) or (C) with interspersed positions (Experiment 2). Participants had to indicate whether all colors did not change from M1/M2 to the probe display (no change trials, top row) or whether one item changed (change trials, bottom row). (D) Spatial arrangement of probe displays in Experiments 1 and 2 in relation to memory displays M1 and M2. In Experiment 1, the probe display had the same spatial layout as M1 and M2, that is, no item position changed (top). In Experiment 2, the probe display comprising interspersed half circles from M1 and M2. This means that probes in M1 or M2 that appeared on the left (dotted circles, top) reappeared in the center and equally likely in the left or right hemifield (dotted circles, bottom).
Procedure
Before each trial, a “ready” display was presented that only showed a central gray fixation dot (0.29° visual angle in length, RGB: 151-151-151, 70 cd/m2 luminance; see Figure 2B). The ready display indicated for the participant to fixate the center and prepare for the upcoming trial. After participants pressed spacebar to start the trial, a display with a gray central fixation cross (0.29° diameter, RGB: 151-151-151, 70 cd/m2) and empty placeholders was shown. Subsequently, M1 was shown for 200 msec and was followed by a retention interval of 500 msec during which only a fixation cross and empty placeholders were presented. After that, M2 was shown for 200 msec and followed by a second retention interval of 500 msec with empty placeholders only. The trial concluded with the probe display that was shown until participants responded. Participants were instructed to remember all colors from M1 and M2 at their respective location and report if the colors shown in the probe display were identical to the combination of M1 and M2. If they were the same, participants were to press a key labeled “same,” and if they were different, they were to press a key labeled “different”. Accuracy was emphasized and there was no time limit to respond, but participants were encouraged to respond promptly. After response was given, an intertrial interval of 1000 msec with a black background only followed before the next ready display indicated the start of a new trial. Participants were given the opportunity to practice the task before the experiment with direct performance feedback after each trial until they reached a good performance level. Practice typically lasted 1–2 min.
Gaze position was tracked at a sampling rate of 1000 Hz for both eyes with an EyeLink 1000+ eye tracker (SR Research Ltd.). A direct gaze feedback violation procedure was applied from 250 msec after the trial start until the onset of the probe display, that is, for 1650 msec. If participants’ gaze was not within 1.5° of the center of the fixation cross during that time or if they blinked, the trial was aborted and a message “eye movement” (or “blink”) was presented on the screen before a ready screen indicated the restart of the trial. The remaining trials were shuffled so as to put the aborted trial in a random position within the sequence and make its reappearance unpredictable. The entire experiment consisted of at least 960 trials, separated into 20 blocks of 48 trials without gaze violation. Any detected gaze violation extended the experiment by one trial. Feedback about their performance (percent correct) was provided to participants after each block.
EEG Recording
EEG was recorded with Ag–AgCl active electrodes (Brain-Products actiCAP) from 32 scalp sites (according to the International 10/20 System: FP1/2, F7/8, F3/4, Fz, FC5/6, FC1/2, C3/4, Cz, TP9/10, CP5/6, CP1/2, P7/8, P3/4, PO7/8, PO3/4, Pz, O1/2, Oz). Horizontal and vertical EOGs were recorded with passive electrodes bipolarly of ~1 cm from the outer canthi of the eyes and from above and below the observers’ right eye, respectively. Fpz served as the ground electrode, and all electrodes were referenced to TP10 and re-referenced offline to the average of all electrodes. Impedances for active electrodes were kept below 10 kΩ. Sampling rate was 1000 Hz, with a high cutoff filter of 125 Hz and a low cutoff filter of 0.01 Hz (half power cutoff, 24 dB roll-off).
Data Analysis
Behavioral data
Accuracy for the probe response was calculated separately for Load Change (1 + 3 vs. 2 + 2 vs. 3 + 1) and Side Switch (same side vs. opposite sides) and forwarded to a two-way ANOVA for repeated measures.
EEG data
EEG was averaged offline over a 1600-msec epoch, including a 200-msec prestimulus baseline, with epochs time-locked to M1 onset. Trials with incorrect responses and eye-related artifacts from 0 to 1400 msec were excluded from the analysis (Experiment 1: 8.9%, SD = 8.6%; Experiment 2: 5.2%, SD = 5.2%). Eye-related artifacts were identified when eye-tracking data indicated gaze drift (>2° from fixation) or saccades (difference in gaze position between first and second half of 50-msec time window > 1°; time window moving in 5-msec steps). In addition, segments were excluded from further analysis on an individual-channel basis when the absolute voltage exceeded 80 µV (excluding less than 4% of trials in both Experiments 1 and 2 for both PO7 and PO8).
Mean contralateral and ipsilateral activity in the ERP was calculated for each participant for the electrode pool PO7/8, separately for each Load Change condition, and each Side Switch condition, resulting in 12 waveforms. The CDA was determined as the mean lateralized amplitude (difference of contra minus ipsi) for an epoch of 200–700 msec (during Retention Interval 1) and for a second epoch of 900–1400 msec (during Retention Interval 2). The CDA data were analyzed with a three-way ANOVA with the factors Epoch (first vs. second retention interval), Load Change (1 + 3, 2 + 2, 3 + 1), and Side Switch (same side, opposite sides).
Results
Accuracy
Accuracy was modulated by Load Change (M1+3 = 82.4%, M2+2 = 79.3%, M3 + 1 = 80.0%), F(2, 38) = 9.37, p < .001, η2 = .33, but not by Side Switch (Msame = 80.0%, Msame = 81.2%), p = .076, and there was no interaction, p = .099. Follow-up t tests revealed that Load Change 1 + 3 yielded a higher accuracy than Load Change 2 + 2 (p = .002) or Load Change 3 + 1 (p < .001), whereas Load Change 2 + 2 and Load Change 3 + 1 were equally accurate (p = .364). For change trials, the accuracy was similar for items presented in M2 (M = 70%) and items presented in M1 (M = 69%), t(19) = 0.54, p = .594.
Contralateral Delay Activity
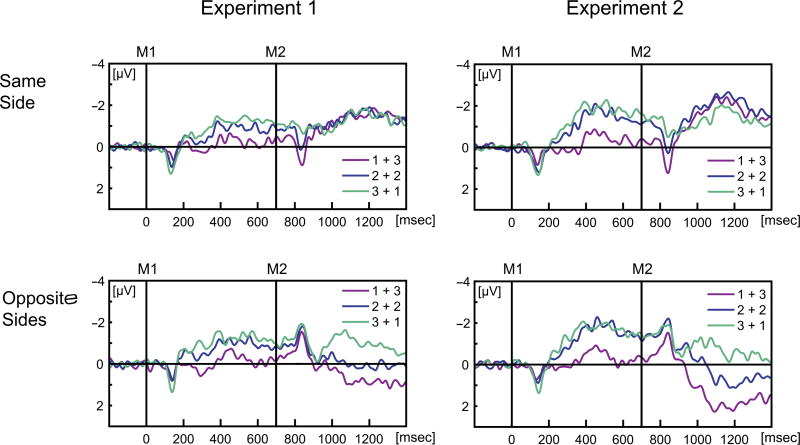
The CDA results were in line with the prediction that M1 and M2 items were stored concurrently (see Figure 3, left column). In the first retention interval (300–700 msec), CDA amplitude increased with the number of items presented in M1 (MLoad1 = −0.17 µV, MLoad2 = −0.81 µV, MLoad3 = −1.11 µV), F(2, 38) = 16.29, p < .001, η2 = .46 (linear trend, p < .001). There was no interaction of Load Change and Side Switch (p = .845). In the second retention interval, CDA amplitude varied, depending on whether items in M1 and M2 were presented on the same or on opposite sides as revealed by a two-way interaction of Load Change and Side Switch, F(2, 38) = 10.75, p = .001, η2 = .36. In line with the notion that the CDA reflects the active maintenance of information in WM, CDA amplitude did not vary as a function of Load Change in the second retention interval when M1 and M2 were presented on the same side (i.e., CDA was independent of the load increment: M1+3 = −1.36 µV, M2+2 = −1.32 µV, M3+1 = −1.30 µV), F(2, 38) = 0.07, p = .934, η2 < .01. Further in line with our expectation that the CDA tracks the difference in the number of items stored from each side, when M1 and M2 were presented on opposite sides, CDA amplitude was manifest as a sustained negativity contralateral to the larger array, main effect of Load Change, F(2, 38) = 17.97, p < .001, η2 = .49. More precisely, CDA switched polarity when the load in M2 was larger than in M1 (M1+3 = 0.74 µV), whereas the CDA stayed negative when the load in M2 was smaller than in M1 (M3+1 = −0.90 µV). Finally, the CDA went down to zero when the load in M1 and M2 were identical (M2+2 = 0.03 µV). Direct comparisons of CDA amplitudes for different conditions can be found in Table 1. A three-way interaction of Epoch, Load Change, and Side Switch, F(2, 38) = 11.12, p < .001, η2 = .37, confirmed that the differential change of the CDA from M1 to M2 varied as a function of whether memory items were presented on the same or on different sides.
Figure 3.
Grand-averaged difference waves (contra–ipsi) for electrode pool of PO7 and PO8. Results for Experiments 1 and 2 are shown in the left column and right column, respectively. M1 and M2 denote the onset of the first memory display (0 msec) and the second memory display (700 msec). In both Experiments 1 and 2, there were 2 × 3 conditions. Rows show the Side Change conditions separately: The top row shows trials in which both memory displays M1 and M2 appeared on the same side (M1 and M2 both in left or both in right hemifield), and the bottom row shows trials in which memory displays appeared on opposite sides (M1 in left and M2 in right hemifield or vice versa). The colors in each panel code the Load Change condition: Pink lines show trials in which M1 shows one item and M2 shows three items; blue lines show trials in which M1 and M2 show two items each; green lines show trials in which M1 shows three items and M2 shows one item. Waveforms are filtered with a 30-Hz low pass filter for display purposes.
Table 1.
Direct Comparisons of CDA Amplitudes between Conditions (Upper Part for Comparisons between Epochs, Lower Part for Comparisons between Load Changes)
| Experiment 1 | Experiment 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Epoch | Side | Load Change | Last Load | ΔM | t | p | ΔM | t | p |
| Comparison First and Second Epoch | |||||||||
| Epoch 1 | Same vs. opposite | 1 + 3 | 1 | −0.03 | −0.36 | .726 | 0.01 | 0.05 | .962 |
| 2 + 2 | 2 | 0.06 | 0.46 | .653 | 0.14 | 1.07 | .297 | ||
| 3 + 1 | 3 | −0.01 | −0.05 | .959 | −0.08 | −0.51 | .613 | ||
| Epoch 2 | Same vs. opposite | 1 + 3 | 3 | −2.10 | −4.30 | .000 | −3.32 | −7.90 | .000 |
| 2 + 2 | 2 | −1.35 | −3.61 | .002 | −2.47 | −6.58 | .000 | ||
| 3 + 1 | 1 | −0.39 | −1.61 | .113 | −0.76 | −2.97 | .008 | ||
| Comparison Load Change | |||||||||
| Epoch 1 | Same | 1 + 3 vs. 2 + 2 | 1 vs. 2 | 0.59 | 3.50 | .002 | 1.02 | 6.09 | .000 |
| 1 + 3 vs. 3 + 1 | 1 vs. 3 | 0.93 | 4.38 | .000 | 1.33 | 6.44 | .000 | ||
| 2 + 2 vs. 3 + 1 | 2 vs. 3 | 0.33 | 1.80 | .088 | 0.31 | 1.88 | .076 | ||
| Epoch 2 | Same | 1 + 3 vs. 2 + 2 | 3 vs. 2 | −0.04 | −0.21 | .834 | 0.22 | 1.32 | .203 |
| 1 + 3 vs. 3 + 1 | 3 vs. 1 | −0.06 | −0.37 | .714 | −0.35 | −1.65 | .115 | ||
| 2 + 2 vs. 3 + 1 | 2 vs. 1 | 0.03 | −0.15 | .884 | −0.58 | −2.17 | .043 | ||
| Epoch 1 | Opposite | 1 + 3 vs. 2 + 2 | 1 vs. 2 | 0.69 | 7.08 | .000 | 1.16 | 5.95 | .000 |
| 1 + 3 vs. 3 + 1 | 1 vs. 3 | 0.96 | 4.56 | .000 | 1.24 | 6.04 | .000 | ||
| 2 + 2 vs. 3 + 1 | 2 vs. 3 | 0.27 | 1.22 | .239 | 0.08 | 0.48 | .638 | ||
| Epoch 2 | Opposite | 1 + 3 vs. 2 + 2 | 3 vs. 2 | 0.71 | 3.86 | .001 | 1.08 | 4.45 | .000 |
| 1 + 3 vs. 3 + 1 | 3 vs. 1 | 1.64 | 4.69 | .000 | 2.21 | 6.43 | .000 | ||
| 2 + 2 vs. 3 + 1 | 2 vs. 1 | 0.93 | 3.53 | .002 | 1.13 | 3.70 | .002 | ||
The left three columns show the labels for the comparisons. The fourth column shows the number of items in M2 (“last load”) for better comprehensibility. The columns on the right-hand side show the numerical difference in CDA amplitude for the comparison at hand (ΔM) and the t value and significance level (t, p) of each comparison for Experiments 1 and 2. Data with bold emphasis indicate comparisons that are significant on the 5% level (two-tailed, uncorrected for multiple comparisons).
To sum up, the results of Experiment 1 supported a WM storage account of CDA activity. When M1 and M2 were presented in the same hemifield, CDA amplitude reflected the total number of items stored rather than the number of items in the second display. When M1 and M2 were presented in different hemifields, CDA amplitude tracked the difference in the number of items stored from each side, such that a sustained negativity was observed contralateral to the side that had more memoranda. When M1 and M2 contained the same number of items, the CDA was eliminated. Thus, it appears that the spatially congruent probe displays encouraged simultaneous storage of M1 and M2 items, thereby yielding CDA activity that was determined by the contents of both displays rather than M2 alone, as predicted by the attentional activation account. Experiment 2 provides converging evidence for this conclusion by demonstrating that interleaved probe displays (similar to those in the Bergrenn and Eimer study) produce a very different result.
EXPERIMENT 2
Methods
Participants
Twenty volunteers naive to the objective of the experiment participated for payment (~15 USD per hr). Participants were aged 19–27 years (M = 22.4, SD = 2.6) and reported normal or corrected-to-normal visual acuity as well as normal color vision. Nine participants were female, and two were left-handed. The experiment was conducted with the written understanding and consent of each participant.
Stimuli and Procedure
All stimuli and procedures were identical to Experiment 1, with one exception: The probe display was constructed by spatially translating the items from the memory displays into an interleaved arrangement in the center of the screen (see Figure 2C). Thus, two columns of four items were presented at the same laterality as the four more central items in the memory display (i.e., 1.5° visual angle; see Figure 1C). To familiarize participants with the task and make the memory–probe relation more comprehensible, they first practiced the task with the same probe display as in Experiment 1 (spatially congruent) until they reached a good performance level. Then participants practiced the same task with a moving probe display until they reached good performance levels. In the moving probe displays, participants saw the halves of the circle become increasingly interspersed until they reached the predefined position. Finally, participants practiced the task with an immediately interspersed probe display as in the later task, until they reached a good performance level. Throughout the entire practice, which lasted typically 1–3 min, direct performance feedback was provided after each trial.
Results
Accuracy
Accuracy was not affected by Load Change (M1+3 = 82.0%, M2+2 = 81.2%, M3+1 = 80.2%), p = .276, or Side Switch (Msame = 81.3%, Mopp = 81.0%), p = .622, and there was no interaction, p = .944. For change trials, the accuracy was higher for items presented in M2 (M = 92%) than items presented in M1 (M = 90%), t(19) = 2.90, p = .009.
Contralateral Delay Activity
The CDA results were in line with the prediction that mostly M2 items were actively maintained when the spatial mapping between memory and probe displays was not congruent (see Figure 3, right column). Like in Experiment 1, the CDA amplitude increased with the number of items presented in M1 in the first retention interval (M1+3 = −0.30 µV, M2+2 = −1.39 µV, M3+1 = −1.59 µV), F(2, 38) = 43.31, p < .001, η2 = .70 (linear trend p < .001). In the second retention interval, CDA amplitude varied depending on whether items in M1 and M2 were presented on the same or on opposite sides, as revealed by a two-way interaction of Load Change and Side Switch, F(2, 38) = 22.16, p < .001, η2 = .54. Unlike in Experiment 1, CDA amplitude in the second epoch varied as a function of Load Change when M1 and M2 were presented on the same side (M1+3 = −1.69 µV, M2+2 = −1.91 µV, M3+1 = −1.33 µV), F(2, 38) = 3.49, p = .041, η2 = .16. Thus, with interleaved probe displays, the CDA did not reflect the active maintenance of information from both M1 and M2. In fact results from trials in which M1 and M2 appeared on opposite sides indicated that the CDA amplitude was mainly (but not entirely) driven by the load from M2, F(2, 38) = 27.13, p < .001, η2 = .59. CDA amplitude showed a strong reversed polarity when three items were presented in M2 (M1+3 = 1.64 µV) and a small reversed polarity when two items were presented in M2 (M2+2 = 0.56 µV). When one item was presented in M2, a small CDA was found (M3+1 = −0.57 µV). If the CDA solely represented maintenance of information from M2, an inverse CDA should also be found for one item in M2, which was not the case. A model described in the following paragraph will address this and estimate the relative contribution of M1 and M2 to CDA amplitude. Direct comparisons of CDA amplitudes for different conditions can be found in Table 1. A three-way interaction of Epoch, Load Change, and Side Switch, F(2, 38) = 51.51, p < .001, η2 = .73, confirmed that the differential change of the CDA from M1 to M2 varied as a function of whether memory items were presented on the same or on different sides.
Modeling the Net CDA Amplitude
The WM storage account implies that the CDA amplitude is a function of the number of elements maintained in WM and their relative position. Adding items in WM should yield an increase in CDA amplitude proportional to the increase in WM load if all items are presented on the same side. For example, the CDA amplitude for three items should be roughly the same as the sum of the CDA amplitudes for one and two items. Because of the nature of the CDA as a lateralized component, adding items on the opposite hemifield as items currently held in WM should analogously decrease the CDA amplitude or even inverse the polarity of the CDA when more items from the second display are encoded into memory. For example, when one item from the left visual field is stored in WM and three items are added to the right visual field, the CDA should have the same size as for one item, and the polarity of the CDA should switch from the first to the second display. Several results from Experiment 2 suggest that the CDA is determined by M2 when the probe display is not spatially congruent. For example, the CDA amplitude for the 3 + 1 condition and the 2 + 2 do not add up to the same size in the same-side condition, and M2 elicits a relatively strong CDA contralateral to two items in the opposite-side condition. However, other results from Experiment 2 suggest that some information from M1 is reflected in the CDA. For example in the 3 + 1 condition, CDA polarity does not flip and remains negative contralateral to M1.
To estimate the relative contribution of M1 and M2 to the CDA amplitude in the second retention interval, we created a model based on mean CDA amplitude in the first and second retention interval (all following analyses were done using the same time windows as for the statistical analyses reported earlier, i.e., 300–700 and 1000–1400 msec). First, we calculated the mean CDA amplitude across participants (grand-averaged) and across Side Switch conditions in the first retention interval for one item (CDA1; from the 1 + 3 condition), two items (CDA2; 2 + 2), and three items (CDA3; 3 + 1). To address the potential contribution of both M1 and M2 to the CDA amplitude, the projected mean CDA amplitude in the second retention interval was then estimated by either summing (same side) or subtracting (opposite sides) the grand-averaged CDA amplitudes from M1 and M2. The projected grand-averaged CDA amplitudes were calculated as follows:
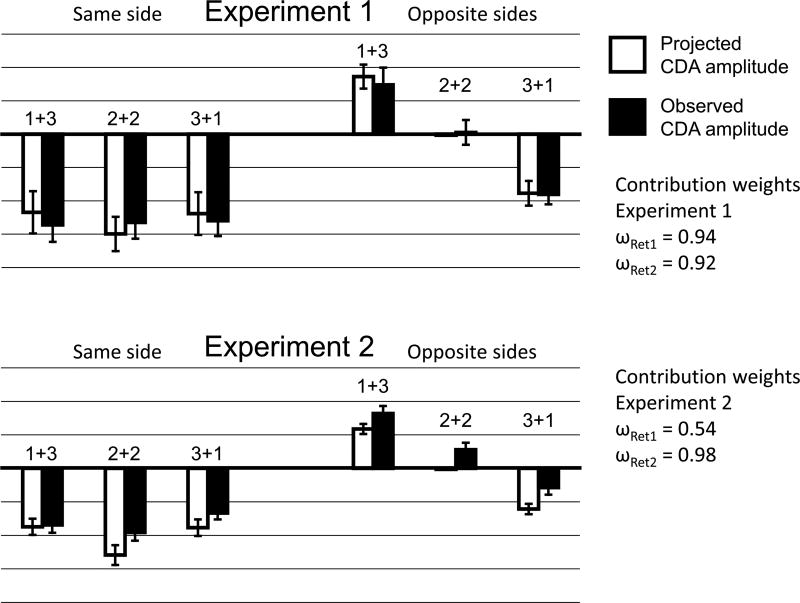
Weights ωRet1 and ωRet2 (weights for CDA from first/second retention interval) were identified that minimized the residual (res = squared difference) of the six projected and six actual grand-averaged CDA amplitudes in Retention Interval 2. For Experiment 1, weights were determined as ωRet1 = 0.94 and ωRet2 = 0.92, with an average residual of res = 0.016 µV (across the six Load Change × Side Switch conditions), suggesting an approximately identical contribution of M1 and M2 to the CDA amplitude. In fact a simplistic model with ωRet1 = 1 and ωRet2 = 1 yielded res = 0.022 µV and was thus only slightly worse. Direct comparisons (t tests projected vs. actual data, two-tailed, uncorrected for repeated measures) showed no differences, all ps ≥ .256 for the optimal model and all ps ≥ .083 for the simplistic model (see Figure 4). For Experiment 2, weights were determined as ωRet1 = 0.54 and ωRet2 = 0.98, with an average residual of res = 0.023 µV, suggesting that M2 contributed twice as much to the CDA as M1, albeit information from both displays seem to affect CDA amplitude. A simplistic model with ωRet1 = 1 and ωRet2 = 1 yielded a severely worse residual, res = 0.341 µV. Direct comparisons showed no differences, all ps ≥ .129 between the optimal model and the observed data, but clear differences for all projected and observed CDA amplitudes (all ps ≤ .05) except for SS1 + 3 (p = .313) for the simplistic model.
Figure 4.
Model for contributions of M1 and M2 to CDA amplitude after M2 (1000–1400 msec) for Experiment 1 (top) and Experiment 2 (bottom). The projected CDA amplitude (empty bars) was calculated as a weighted sum of the grand-averaged CDA during the first retention interval (300–700 msec), for example, the projected CDA for “3+1” in Experiment 2 is 0.54 × CDA amplitude for Load 3 during Retention Interval 1+ 0.98 × CDA amplitude for Load 1 during Retention Interval 1. The observed CDA amplitude (filled bars) is shown for comparison. The closer empty and filled bars, the better the model prediction for that condition. Error bars indicate standard errors of the mean. There was no significant difference between the projected and observed CDA amplitude for any condition.
The model shows that, in Experiment 1, when probes were spatially congruent with the memory displays, CDA amplitude in the second retention interval indexed the concurrent storage of items from both M1 and M2. In contrast, when the probes were interleaved in Experiment 2, CDA amplitude was well described by summing half of the CDA from M1 and the full CDA response to M2. We note that this empirical pattern mirrors that observed by Berggren and Eimer (2016), who also found a modest influence of the M1 display on CDA amplitude. In line with this finding, the Experiment 2 behavioral data showed that items from M1 were less well remembered than items from M2, whereas no such difference was evident in Experiment 1.
DISCUSSION
Our findings provide strong support for the hypothesis that CDA activity reflects the active contents of WM (WM storage account) rather than the most recent focus of spatial attention (attentional activation account). We used a change detection task in which memory items were presented in two successive displays (M1, M2). The total memory load of M1 and M2 combined was four in all trials. In Experiment 1, the probe displays were spatially congruent with the memory displays, such that the position of each item was held constant across the memory and the probe display. In contrast, the displays in Experiment 2 were modeled after those in Berggren and Eimer (2016), such that the items from M1 and M2 were spatially translated and interleaved in the probe display. Our hypothesis was that the spatially congruent displays in Experiment 1 would be conducive to the integration—and thus concurrent storage—of the items in the M1 and M2 displays. In contrast, we reasoned that the displays from Experiment 2 would be harder to integrate and that this would encourage participants to represent M1 items with lower priority in WM when the M2 items were encoded.
In line with our hypothesis, in Experiment 1, we found that when M1 and M2 items were presented on the same side, the CDA amplitude observed in the second retention interval was identical, regardless of whether 1 + 3, 2 + 2, or 3 + 1 items were presented. Moreover, the CDA amplitude in the second encoding episode was identical to the sum of the CDA for one and three items, two and two items, or three and one items, in the first encoding episode, respectively. This is in line with the WM storage account of the CDA and supports previous studies that used sequential loading paradigms where relevant items appeared on the same side and were found to be additive (Ikkai et al., 2010; Vogel et al., 2005). The attentional activation interpretation of the CDA (Berggren & Eimer, 2016) that assumes that the CDA is a lingering trace of internal spatial attention would have predicted that the CDA elicited by M2 should vary as a function of the number of items from M2, that is, increasing amplitude with increasing number of items in M2.
When M1 and M2 were presented in opposite hemifields, the CDA amplitude was identical to the difference of the CDA for items from M1 minus the CDA for items from M2. This means that the CDA amplitude for the second encoding episode was identical to the CDA in the first encoding episode for two items (3 + 1 condition), to zero (2 + 2 condition), or to the CDA for two items with inversed polarity (1 + 3 condition). Again, this supports the WM storage account of the CDA and complements previous findings that used sequential loading approach with all items to be stored being presented on the same side (Ikkai et al., 2010; Vogel et al., 2005). These findings are inconsistent with the attentional activation account, which predicted that CDA activity would be determined primarily by the contents of M2 alone. Thus, the results from Experiment 1 strongly support the hypothesis that CDA activity is determined by active storage in WM.
By changing only the spatial congruence between the memory and probe displays, Experiment 2 provided evidence for our conjecture that this factor alone might determine whether participants engaged in concurrent storage of the items from M1 and M2. When the probes were not spatially congruent to the memory displays (Experiment 2), the CDA was primarily determined by the number of items in M2, in line with the attentional activation account and results from Berggren and Eimer (2016). When items in M1 and M2 were presented on the same side, CDA amplitude was more strongly (but not entirely) shaped by the contents of M2. For example in the 2 + 2 condition when M1 and M2 were in opposite hemifields, CDA amplitude was negative contralateral to M2 rather than near zero as in Experiment 1. Thus, our model determined that, in Experiment 1, the CDA indexed the storage of items from both M1 and M2. In contrast, with spatially incongruent displays in Experiment 2, our findings were much more similar to those of Berggren and Eimer (2016), such that the CDA reflected about half of the items in M1 and almost full activity from M2.
Note that Experiments 1 and 2 were completely identical, except for the probe display. Any difference we observed during the retention interval can thus not be explained by physical differences in the sample displays and must be due to different memory strategies. What could these different memory strategies be? Our proposal is simple: In Experiment 1, spatially congruent displays encouraged the concurrent storage of the items from M1 and M2, yielding CDA activity that reflected the combined load across the two displays. In contrast, the interleaved probe displays in Experiment 2 encouraged participants to encode items from M1 and M2 as separate entities. When the M2 display appeared, M1 items may have been transferred to LTM or an “activity-silent” representation so that the CDA amplitude, reflecting current WM representations, was primarily determined by the contents of M2. Although this empirical pattern was interpreted by Berggren and Eimer (2016) as evidence against a storage role for the CDA, our conclusion is that—taken together with the results of Experiment 1—the reduced influence of M1 on CDA activity in Experiment 2 may be better understood as strategic maintenance of the M1 items outside WM. The finding that the CDA amplitude after M1 and M2 have been presented is roughly determined by half the memory load of M1 could allude to the possibility that (i) half of the M1 items are dropped, (ii) M1 items are represented half as strongly, or (iii) all M1 items are dropped in half the trials. We do not wish to make specific claims as to which of these possibilities applies but would like to point out that all of these are in line with the traditional notion that the CDA reflects WM storage. Note that because the contribution of M1 to the CDA was fairly substantial, the underrepresentation of M1 items did not necessarily have to be compensated through LTM or activity-silent representations. Another possibility that can account for the small contribution of M1 items to the CDA amplitude in Experiment 2 may be that the representation of M1 items becomes more bilateral or is even recoded into a less sensory format in preparation for a novel display incongruent to the memory items. This is in line with bilateral VSTM activity that was previously found in magnetoencephalography (e.g., Robitaille, Grimault, & Jolicoeur, 2009).
Why would spatially incongruent displays motivate the dropping/attenuation of representations of M1 items? Our speculation is that the interleaved probe displays in Experiment 2 made it much harder to maintain a single integrated representation of the M1 and M2 items together, because such an integrated representation would have required a difficult spatial translation of the four items. Thus, perhaps participants found it easier to view M1 and M2 as parts of different encoding events. Indeed, current theories of event cognition have proposed that event boundaries may motivate the “flushing” of WM to make room for the flood of information that is typically encountered at significant event boundaries (Kurby & Zacks, 2008). Thus, although further work is needed to elucidate why participants appear to drop the initial items in procedures like that of Experiment 2, the notion that M1 and M2 were encoded as separate episodes in that study provides a plausible explanation of why participants did not store M1 and M2 concurrently.
The present results may not entirely rule out the attentional activation account. It may be that the CDA amplitudes partially reflect an attentional bias that is determined by the anticipated location of probe displays. According to that notion, in situations where observers know that memory display locations will subsequently be probed, attention is maintained at these locations even after a second memory display is presented elsewhere (to facilitate subsequent probe encoding). When probes are always presented at different locations, however, attention is disengaged for M1 once M2 arrives because it is no longer needed at this old location. This, however, would not explain why CDA activity is maintained for M2 locations when M2 locations are not needed anymore. Furthermore, the attentional activation account is at odds with findings from a previous study using subsequent memory displays (Ikkai et al., 2010), showing that the CDA amplitude increases after M2 regardless whether items in M2 are presented at the same or at different locations from M1. Showing items at locations that are already attended should not require the establishment of a novel focus of attention and thus should not increase the CDA amplitude if the CDA solely represents the most recent focus of attention.
Our findings suggest that the CDA indexes WM storage rather than the current focus of spatial attention. Moreover, these studies highlight the flexible and context-dependent nature of encoding into this online memory system. Experiments 1 and 2 employed identical stimuli (during the encoding and maintenance phases when CDA activity was recorded) and the same change detection task. Nevertheless, the geometry of the expected probe display had a powerful effect on whether the initial items were actively maintained throughout the second delay period. Our findings demonstrate that active maintenance in WM may be contingent on the specific way in which the relevant information will be used. Thus, although the literature has focused strongly on core factors such as the number of relevant items or the type of information presented, further work examining the influence of task context and processing demands may provide useful insights into when this online memory system is deployed.
References
- Atkinson RC, Shiffrin RM. The control of short-term memory. Scientific American. 1971;225:82–91. doi: 10.1038/scientificamerican0871-82. [DOI] [PubMed] [Google Scholar]
- Balaban H, Luria R. Integration of distinct objects in visual working memory depends on strong objecthood cues even for different-dimension conjunctions. Cerebral Cortex. 2015;26:2093–2104. doi: 10.1093/cercor/bhv038. [DOI] [PubMed] [Google Scholar]
- Berggren N, Eimer M. Does contralateral delay activity reflect working memory storage or the current focus of spatial attention within visual working memory? Journal of Cognitive Neuroscience. 2016;28:2003–2020. doi: 10.1162/jocn_a_01019. [DOI] [PubMed] [Google Scholar]
- Drew T, Horowitz TS, Wolfe JM, Vogel EK. Neural measures of dynamic changes in attentive tracking load. Journal of Cognitive Neuroscience. 2012;24:440–450. doi: 10.1162/jocn_a_00107. [DOI] [PubMed] [Google Scholar]
- Drew T, Vogel EK. Neural measures of individual differences in selecting and tracking multiple moving objects. Journal of Neuroscience. 2008;28:4183–4191. doi: 10.1523/JNEUROSCI.0556-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Vogel EK, Mayr U, Awh E. Quantity, not quality: The relationship between fluid intelligence and working memory capacity. Psychonomic Bulletin & Review. 2010;17:673–679. doi: 10.3758/17.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikkai A, McCollough AW, Vogel EK. Contralateral delay activity provides a neural measure of the number of representations in visual working memory. Journal of Neurophysiology. 2010;103:1963–1968. doi: 10.1152/jn.00978.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Olson IR, Chun MM. Organization of visual short-term memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:683. doi: 10.1037//0278-7393.26.3.683. [DOI] [PubMed] [Google Scholar]
- Kuo B-C, Stokes MG, Nobre AC. Attention modulates maintenance of representations in visual short-term memory. Journal of Cognitive Neuroscience. 2012;24:51–60. doi: 10.1162/jocn_a_00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurby CA, Zacks JM. Segmentation in the perception and memory of events. Trends in Cognitive Sciences. 2008;12:72–79. doi: 10.1016/j.tics.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria R, Balaban H, Awh E, Vogel EK. The contralateral delay activity as a neural measure of visual working memory. Neuroscience and Biobehavioral Reviews. 2016;62:100–108. doi: 10.1016/j.neubiorev.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Why does working memory capacity predict variation in reading comprehension? On the influence of mind wandering and executive attention. Journal of Experimental Psychology: General. 2012;141:302. doi: 10.1037/a0025250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille N, Grimault S, Jolicoeur P. Bilateral parietal and contralateral responses during maintenance of unilaterally encoded objects in visual short term memory: Evidence from magnetoencephalography. Psychophysiology. 2009;46:1090–1099. doi: 10.1111/j.1469-8986.2009.00837.x. [DOI] [PubMed] [Google Scholar]
- Rose NS, LaRocque JJ, Riggall AC, Gosseries O, Starrett MJ, Meyering EE, et al. Reactivation of latent working memories with transcranial magnetic stimulation. Science. 2016;354:1136–1139. doi: 10.1126/science.aah7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JT, Elliott EM, Matthews RA, Hill BD, Gouvier WM. The relationships of working memory, secondary memory, and general fluid intelligence: Working memory is special. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:813. doi: 10.1037/a0019046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MG. ‘Activity-silent’ working memory in prefrontal cortex: A dynamic coding framework. Trends in Cognitive Sciences. 2015;19:394–405. doi: 10.1016/j.tics.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychological Review. 2007;114:104–132. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Fukuda K, Awh E, Vogel EK. Working memory and fluid intelligence: Capacity, attention control, and secondary memory retrieval. Cognitive Psychology. 2014;71:1–26. doi: 10.1016/j.cogpsych.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. Storage of features, conjunctions, and objects in visual working memory. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]