Abstract
Thyroid hormone (TH) is present in the systemic circulation and thus should affect all cells similarly in the body. However, tissues have a complex machinery that allows tissue-specific optimization of local TH action that calls for the assessment of TH action in a tissue-specific manner. Here, we report the creation of a TH action indicator (THAI) mouse model to study tissue-specific TH action. The model uses a firefly luciferase reporter readout in the context of an intact transcriptional apparatus and all elements of TH metabolism and transport and signaling. The THAI mouse allows the assessment of the changes of TH signaling in tissue samples or in live animals using bioluminescence, both in hypothyroidism and hyperthyroidism. Beyond pharmacologically manipulated TH levels, the THAI mouse is sufficiently sensitive to detect deiodinase-mediated changes of TH action in the interscapular brown adipose tissue (BAT) that preserves thermal homeostasis during cold stress. The model revealed that in contrast to the cold-induced changes of TH action in the BAT, the TH action in this tissue, at room temperature, is independent of noradrenergic signaling. Our data demonstrate that the THAI mouse can also be used to test TH receptor isoform-specific TH action. Thus, THAI mouse constitutes a unique model to study tissue-specific TH action within a physiological/pathophysiological context and test the performance of thyromimetics. In conclusion, THAI mouse provides an in vivo model to assess a high degree of tissue specificity of TH signaling, allowing alteration of tissue function in health and disease, independently of changes in circulating levels of TH.
The generated transgenic mouse model [thyroid hormone (TH) action indicator mouse] assesses TH action and allows the detection of local, tissue-specific changes of TH action.
Thyroid hormones (THs) orchestrate a wide variety of biological processes, including development, growth, and metabolism via transcriptional control of hundreds of TH-dependent genes. Specific regulatory elements within these genes [TH response element (TRE)] attract two isoforms of the TH receptor (TRα and TRβ) and their coregulators, thus allowing for transcriptional control (1–5). An unoccupied TR remains bound to the gene and is associated with corepressors–silencing genes that are positively regulated by TH. Upon TH binding, corepressors associated with the TRs are replaced by coactivators, relieving repression and promoting gene transactivation. Therefore, TH availability within the nuclear environment defines a switch between TH-dependent gene repression and activation.
Two THs exist: the prohormone T4 that can be converted to the TR-binding molecule T3 via deiodination. Relatively steady circulating levels of TH are maintained by the hypothalamus-pituitary-thyroid axis that is tightly regulated by the negative-feedback effects of TH. However, the existence of multiple mechanisms that customize TH availability at the cellular level provides tissue specificity to TH action. These mechanisms include TH transmembrane transporters, enzymes that can activate or inactivate TH within the target cells, i.e., the deiodinases, and differential expression of TR subtypes (6–8). The coordinated actions of TH-activating or -inactivating deiodinases (6) allow for quick modification of T3 levels within the target cells, rapidly affecting the expression of TH-responsive genes without antecedent changes in circulating T3 levels.
A corollary is that measurement of plasma TH levels under physiological and pathological conditions does not uniformly reflect TH actions in all tissues. This is suggested by conditions, such as the syndrome of resistance to TH (mutant TRα or TRβ) (2, 9), the X-linked mental retardation Allan-Herndon-Dudley syndrome caused by impaired transmembrane TH transport (mutant monocarboxylate transporter 8) (10, 11), and the commonly observed nonthyroidal illness syndrome, where circulating TH levels are uncoupled from local TH action in specific tissues (7, 12). In addition, there is evidence that TH signaling is specifically downregulated in the liver of obese subjects and in mice placed on a high-fat diet (13). Tissue specificity of TH action could also explain why some hypothyroid patients remain symptomatic despite exhibiting normal plasma thyroid-stimulating hormone levels (14, 15).
The discovery of cellular mechanisms that allow for tissue specificity of TH action inspired the development of TR isoform- and tissue-specific TH analogs (16, 17). For example, TRβ-selective TH analogs act predominantly in the liver and can be useful in treatment of hypercholesterolemia and obesity. However, progress in this field has been empirical, given current inability to assess tissue-specific TH action (15) within a physiological or pathophysiological context. Here, we developed and characterized a mammalian model: the TH action indicator (THAI) mouse, to assess tissue-specific TH signaling.
Materials and Methods
The transgenic THAI construct
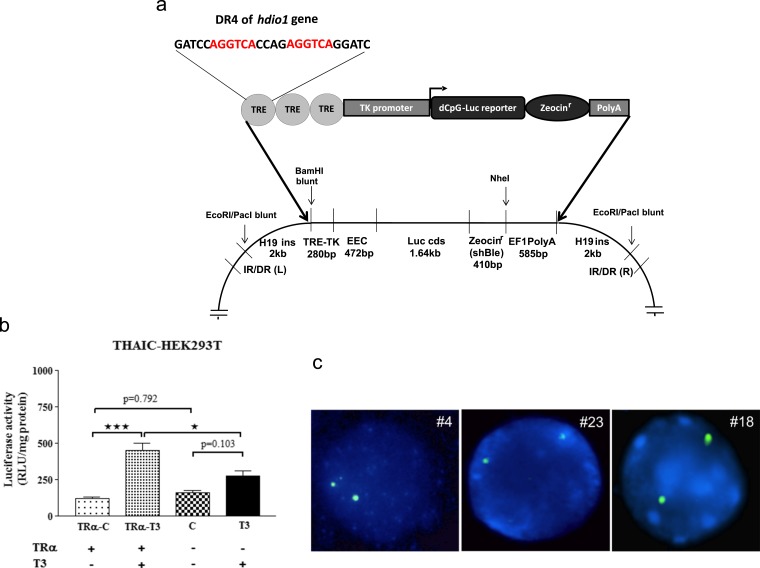
Three copies of a direct repeat 4 (DR-4) TRE (of the human DIO1 5′-flanking region) (18) were cloned upstream to the Herpes simplex virus thymidine kinase minimal promoter (19). This DNA segment was linked upstream of the luciferase coding region, containing a codon-optimized and methylation-resistant dCpG luciferase-ShBle fusion protein upstream of an EF1 pAn polyadenylation cassette. The use of the dCpG luciferase reporter that is resistant to T3-induced promoter-independent downregulation avoids a phenomenon that can interfere with TH-mediated changes of the classical firefly luciferase reporter protein (19). The cassette was then assembled in a pWHERE vector (InvivoGen, San Diego, CA) that flanked the insert with H19 insulators, followed by subcloning between the half-sites of the blunted EcoRI site of the pt2-BH transposon-cloning vector (Addgene, Cambridge, MA) containing multiple cloning sites flanked by indirect repeats. This resulted in the generation of the THAI expression vector (Fig. 1a). Next, human embryonic kidney (HEK)293T cells stably expressing the THAI construct (THAIC) and transiently expressing TRα were incubated with T3 for 24 hours. There was readily measurable baseline luciferase activity that was 3.7-fold stimulated after T3 exposure, whereas no stimulation was observed in the absence of TRα coexpression, indicating that induction was TR dependent (Fig. 1b).
Figure 1.
(a) The recombinant THAIC. (Lower) The structure of the targeting construct generated in the pt2-BH vector. The coding sequence encodes luciferase (62 kDa) fused to ShBle (14 kDa), allowing selection based on resistance to zeocin. (b) THAIC responds to T3 in a TR-dependent manner. The THAIC was stably expressed in human embryonic kidney (HEK)293T cells using Sleeping Beauty–mediated recombination followed by zeocin selection. TRα was transiently transfected, and THAIC-HEK293T cells were treated with 100 nM T3 for 24 hours. Means ± SEM; (n = 3). *P < 0.05, ***P < 0.001 by one-way analysis of variance (ANOVA), followed by Tukey post hoc test. (c) Fluorescence in situ hybridization (FISH) on homozygote THAI mouse, lines 4, 23, and 18. Hybridization with the green-colored dCpG luciferase-specific probe shows two equal-sized signals in interphase cells of homozygous animals, demonstrating the presence of two copies of the transgene in lymphocyte cultures of all three lines, indicating that a single copy was inserted into the genome of founders. C, control; EEC, expression enhancer cassette; luciferase reporter-zeocin sequences encoding a luciferase/zeocin resistance fusion protein, IR/DR (L) and (R), inverted repeat left and right; TK promoter, thymidine kinase minimal promoter.
Generation of the THAIC-HEK293T cell line
HEK293T cells were cultured in Dulbecco’s modified Eagle medium + 10% fetal bovine serum and transfected with the targeting THAIC (Fig. 1a) along with the pcGlobinSB100 transposase-encoding vector (20) (provided by Zsuzsanna Izsvák and Lajos Mátes) to allow Sleeping Beauty transposase-assisted genomic integration. Clone selection was performed in the presence of 150 μg/mL zeocin. Clones were cultured for 24 hours in charcoal-stripped fetal bovine serum (21), followed by the induction with 100 nM T3 for 24 hours in the presence of transiently coexpressed mouse TRα. Cells were lysed in passive lysis buffer of the dual luciferase assay system (Promega, Madison, WI) before luciferase assay.
Generation of transgenic mouse lines by transposon-mediated technology
Genomic insertion of the transgenic cassette was performed with the Sleeping Beauty transposase (22). The pronucleus of fertilized FVB/Ant (FVB.129P2-Pde6b+ Tyrc-ch/AntJ) (23) oocytes was injected with a mixture containing the plasmid harboring the targeting transposon cassette (Fig. 1a; 1 ng/µL) and the in vitro-transcribed messenger RNA (mRNA) encoding the Sleeping Beauty transposase (24) (5 ng/μL). Four females were identified as founders using TaqMan assay on tail DNA with a luciferase probe and crossed with wild-type FVB/Ant (FVB.129P2-Pde6b+ Tyrc-ch/AntJ) male mice, followed with inbred coupling of F1 generation. Indirect evidence obtained with luciferase TaqMan polymerase chain reaction (PCR) on different lines and generations suggests that a single copy of the transgenic cassette was inserted into the genome of all lines. All animal experiments were conducted in compliance with the European Communities Council Directive (2010/63/EU) and approved by the Animal Care and Use Committee of the Institute of Experimental Medicine (Hungarian Academy of Sciences, Budapest).
Characterization of transgenic lines
The F2 generation was screened for tissue luciferase activity. To characterize basal and TH-induced luciferase activity of peripheral tissues and brain regions, 60- to 70-day-old males were injected intraperitoneally (IP) with 5 µg/day/mouse of L-T4 or vehicle control for 3 days or 1 μg/g body weight (bw) of L-T3 for 1 day. Hypothyroid mice were generated by adding 0.1% sodium perchlorate and 0.5% methimazole in drinking water for 3 weeks combined with low-iodine diet (Research Diets, New Brunswick, NJ). After decapitation, tissues were removed, snap frozen on dry ice, and stored at −80°C for luciferase activity measurement. White blood cells were isolated as described (25). Bone and muscle samples originated from Tibia and musculusgastrocnemius, respectively. Three of the four lines (4, 18, and 23) were found to be TH responsive. Homozygous mice showed a twofold higher luciferase activity compared with heterozygous animals, demonstrating that activity of the reporter correlates directly with gene dosage (not shown), and were born following a Mendelian distribution. In accordance, the animals did not exhibit any identifiable phenotype.
Mouse lymphocyte cell culture and fluorescence in situ hybridization
Lymphocytes derived from the spleen of homozygote THAI mouse lines #4, 18, and 23 were cultured as described (26, 27). Individual spleens were homogenized to release the lymphocytes, and erythrocytes were lysed briefly (2 to 3 minutes) in sterile, distilled water, followed by the addition of physiological saline. Cell suspensions was centrifuged 5 minutes, 500 g at room temperature. After removing the supernatant, cells were transferred into ready-to-use LymphoChrome Karyotyping Media (Lonza Verviers, Belgium). The cells were incubated at 37°C for 24 hours, and 0.05 μg/mL Colcemid (Gibco/Thermo Fisher,) was added, 3 hours before harvest. Chromosomal preparations were performed according to a standard Rothfels-Siminovich procedure using 0.067 M KCl, followed by fixation in methanol/acetic acid. Chromosome analysis was performed on metaphase cells G-banded with trypsin and Wright Giemsa stain. A 1045-base pair fragment of the dCpG luciferase coding sequence (nucleotides 599 to 1644) was labeled with digoxigenin using Dig-Nick translation mix (Roche, Mannheim, Germany) and precipitated with mouse COT-1 DNA (Invitrogen), according to the manufacturer’s instructions. The precipitated probe was mixed with an equal volume of hybridization buffer. Fluorescence in situ hybridization (FISH) was performed on methanol/acetic acid-fixed cell suspensions. Slides/DNA preparation and hybridization were carried out according to standard techniques. Hybridized sections were incubated overnight at room temperature with antidigoxigenin-POD (1:100) antibody (Roche) in 1% bovine serum albumin solution. The immunoreaction signal was amplified using the tyramide signal amplification kit (Perkin Elmer Life and Analytical Sciences, Waltham, MA), according to the manufacturer’s instructions. The deposited reaction product was detected with fluorescein isothiocyanate/dichlorotriazinylamino fluorescein–streptavidin antibody (Jackson ImmunoResearch, West Grove, PA). The analysis was done in at least 100 interphase nuclei and in a few metaphases, if present on the hybridization area. The interphase or metaphase cells were evaluated for each sample with an Axioskop 2 Mot Plus Microscope (Carl Zeiss GmbH, Oberkochen, Germany) and Cytovision 3.6 computer analysis system (Applied Imaging International Ltd., Newcastle, United Kingdom).
Splinkerette PCR
Splinkerette PCR was performed, as described (28). In brief, genomic DNA was digested with BstYI. A universal Splinkerette oligonucleotide was used to amplify the region between the 5′ end of the cassette and the first BstYI site. The cassette-specific oligonucleotide was specific for the multiple cloning site of the PT/2BH vector, located 3′ to the 5′ inverted terminal repeat sequence. Nested PCR was performed to reamplify the PCR product, followed by sequencing. The reporter cassette was found inserted into a region of repeat family L1 into the genome of THAI mouse line 4, as shown by Splinkerette PCR, indicating that the cassette did not alter the structure of any endogenous gene.
GC24 treatment
Seventy-day-old male THAI #4 mice were treated, as described previously, for 2 weeks to inhibit endogenous TH production. Hypothyroid mice were treated with a single, IP injection of 1.53 nM/g bw GC24 (a kind gift of Dr. Tom Scanlan; equimolar to 1 μg/g bw T3) or dimethyl sulfoxide as vehicle, 24 hours before tissue sampling. After decapitation, liver (TRβ-dominant tissue) and heart (TRα-dominant tissue) (16) were removed and snap frozen on dry ice.
GW3965 treatment
GW3965 treatment was performed IP, as described with modifications, as follows (29). Sixty-five-day-old male THAI #4 mice were subject to two IP injections of 20 µg/g bw GW3965 HCl (homogenous suspension in 10% dimethyl sulfoxide–90% saline; Adooq Bioscience, Irvine, CA) or with solvent, 3.5 and 7 hours before tissue sampling. After decapitation, the liver was removed and snap frozen on dry ice. Samples were subjected to TaqMan real-time quantitative PCR.
Luciferase assay
Tissue samples were milled to powder in liquid nitrogen and sonicated in luciferase lysis buffer (100 mM KPO4, 4 mM EGTA, 4 mM EDTA, pH 7.8), freshly prepared with 0.7 mM phenylmethylsulfonyl fluoride and 0.1 mM dithiothreitol. Brain tissue was lysed in one step, with brief sonication in lysis buffer. After a 10-minute centrifugation at 14,000 g at 4°C, supernatant was removed for luciferase activity measurement. Assay on tissue samples or THAIC-HEK293T cells was performed with luciferase assay system reagent (Promega, Madison, WI) on a Luminoskan Ascent (Thermo Electron Corp. Labsystems, Vantaa, Finland) luminometer. Detected relative light unit (RLU) was normalized to protein content of the sample.
Sympathetic denervation of interscapular brown adipose tissue and cold stress
Interscapular brown adipose tissue (iBAT) was unilaterally denervated with surgical transection of its sympathetic nerves (30). Cold stress was applied 3 days after surgery. During cold stress, single housed animals were kept under standard light conditions at 4°C; water and food were available ad libitum. Control animals were kept at room temperature (22°C) under same conditions. After decapitation, samples from the iBAT were collected on dry ice and stored at −80°C for norepinephrine (NE) measurement, deiodination assay, and TaqMan analysis.
Analysis of NE content of iBAT samples
NE concentrations of mouse iBAT were measured using surfactant-assisted online solid-phase extraction sample preparation, followed by column-switching liquid chromatographic separation with electrochemical detection, as described (31). To prevent fat precipitation in the aqueous extraction solvent phase, the nonionic surfactant Triton X-100 was used. The native tissue was homogenized by sonication in 300 µl, 0.1 M, ice-cold perchloric acid, which contained 10 mM theophylline as an internal standard, 0.1% sodium metabisulfite as an antioxidant, and 0.01% (volume-to-volume ratio) Triton X-100 as an emulsifier. The NE content was expressed as picomoles per milligram protein.
Deiodination assay
iBAT tissue samples were homogenized with sonication in 0.1 M potassium phosphate, 1 mM EDTA, pH 6.9, buffer containing 0.25 M sucrose and 10 mM dithiothreitol. iBAT tissue lysates (100 μg protein) were assayed for 120 minutes at 37°C in duplicate to determine type 2 deiodination activity, as previously described (32).
TaqMan real-time quantitative PCR
Total RNA was isolated from iBAT tissue samples with the RNeasy Lipid Tissue Mini kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions, whereas for liver samples, the Qiazol Lysis Reagent was used, followed by isolation with the Macherey-Nagel (Düren, Germany) Nucleospin RNA kit. Total RNA (1 μg) was reverse transcribed with the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA). Complementary DNA (cDNA) concentration was determined with the Qubit single-stranded DNA assay kit. cDNA (10 ng) was used in each TaqMan reaction. mRNA expression of target genes was detected with TaqMan Gene expression probe sets using TaqMan Fast Universal PCR Mastermix (Thermo Fisher Scientific) and compared with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) housekeeping gene expression. Expression of GAPDH was also analyzed under all challenge conditions used. Cold stress had no effect on GAPDH expression, either in intact or in denervated iBAT lobes. Sympathetic denervation, however, consistently downregulated GAPDH expression twofold; thus, this was taken into account when calculating results of the denervation experiments by dividing fold changes of luciferase/GAPDH and type 2 deiodinase (D2)/GAPDH rations by two. Accession numbers of used TaqMan probes are listed in Supplemental Table 1. Reactions were assayed on a Viia 7 Real-Time PCR instrument (Applied Biosystems, Waltham, MA).
iBAT measurements
All measurements, including NE concentration, D2 activity, and mRNA expression of the luciferase reporter and D2, were performed from the same iBAT samples obtained from animals undergoing cold stress and unilateral sympathetic denervation, whereas the nondenervated, intact iBAT lobe, obtained from the same animal, was used for comparison.
In vivo imaging of luciferase activity
In vivo imaging of iBAT of T3-treated and cold-stressed animals was performed 24 hours after 1 μg/g bw IP T3 injection or after 24 hours exposure to 4°C. Live imaging was obtained in ketamine–xylazine (ketamine 50 μg/g, xylazine 10 μg/g bw, IP)-anesthetized mice whose fur over the iBAT and abdominal organs had been removed with depilatory cream. All animals were moved to room temperature, 30 minutes before imaging. d-Luciferin (Gold Biotechnology, St. Louis, MO) was dissolved in PBS and IP injected (150 and 750 μg/g bw for the T3-treated and cold-stressed animals, respectively), 15 minutes before in vivo imaging. The animals were placed on the heated plate of the imager, 5 minutes before imaging. Emitted light was detected with an IVIS Lumina II In vivo Imaging System (PerkinElmer, Waltham, MA) and quantitated in all animals using a region of interest of identical size and shape. Data are reported as emitted photon/s. T3-treated or cold-stressed animals were studied using identical imaging parameters (binning and exposure time) as their corresponding controls in both dorsal and ventral positions.
Indirect calorimetry measurements
Eleven-week-old old male THAI #4 and wild-type control mice were analyzed for whole-energy expenditure, oxygen consumption, and carbon dioxide production using calorimetric cages (PhenoMaster/LabMaster, TSE Systems GmbH, Bad Homburg, Germany). Body-composition analyses were performed using an EchoMRI Whole Body Composition Analyzer (EchoMRI, Houston, TX).
TH measurements
Serum-free T4 and T3 levels were measured with AccuLite CLIA Microwells kit (Monobind, Lake Forest, CA), according to the manufacturer’s instructions.
Statistics
Groups were compared with unpaired two-sample Student t test using a 95% level of confidence. For multiple comparison, one-way analysis of variance (ANOVA) or repeated-measures ANOVA was used, followed by Newman-Keuls or Tukey post hoc tests.
Results
The THAI mouse model integrates a highly TH-responsive reporting system that functions in the setting of endogenous levels of TH transporters, deiodinases, and TRs. We obtained three THAI mouse founders harboring a single copy of the transgenic cassette (lines 4, 23, and 18; Fig. 1c). THAI lines exhibit normal serum TH levels and bw, as well as other TH-dependent parameters, i.e., lean body mass, fat mass, respiratory exchange ratio, and oxygen consumption (Supplemental Tables 2 and 3). These animals were used to study TH action using two general approaches: (1) measuring luciferase activity and mRNA in tissue sonicates or (2) measuring bioluminescence in live animals.
The THAI mouse model reports differences in TH action caused by altered TH levels or the selective actions of TH analogs
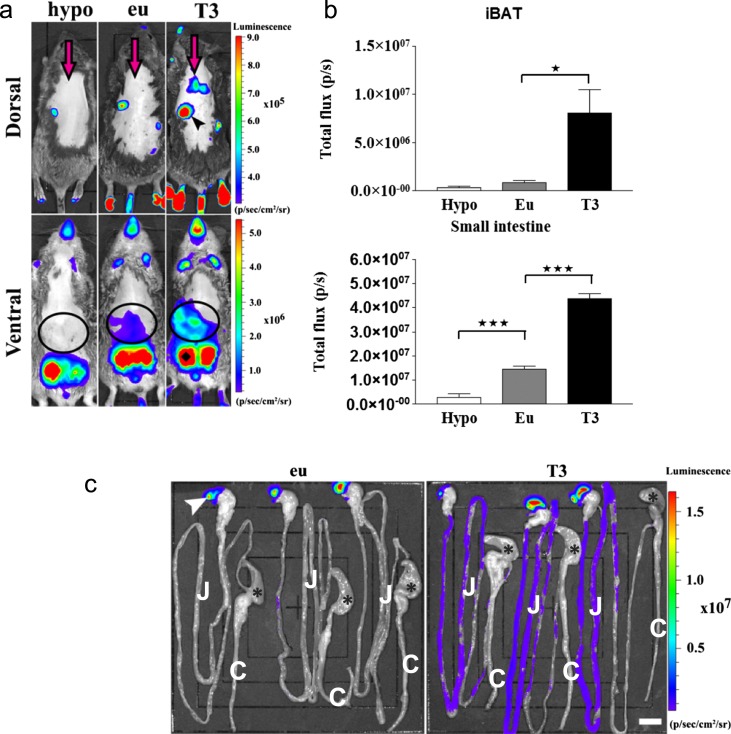
We first defined tissue-specific baseline values for the reporting system. Second, we measured the responsiveness of this system to a decrease or increase in TH levels. Within each mouse line, basal luciferase activity varied among different tissues. For example, there were up to approximately six orders-of-magnitude differences between liver and testicle, whereas in most other tissues, the variability ranged within two orders of magnitude (Tables 1 and 2 ). These differences are likely to reflect the overall level of transcriptional activity of the insertion site. Furthermore, when using in vivo bioluminescence, variable degrees of baseline activity were detected in the iBAT, anterior and posterior foot pads, tail, intestine, saccus cecum of the stomach, mandibular salivary gland, mouth, nose, skin, and testicles (Fig. 2a and 2b). To assess whether this reporting system detects a reduction in TH signaling, line 4 animals were rendered hypothyroid, and the reporter activity was measured. There was an ∼50% to 65% decrease of luciferase activity in different brain regions (e.g., the hypothalamus and cerebral cortex), the liver, and white blood cells; 75% in iBAT; 80% in the pituitary; 85% in the heart and bone; and 90% in the small intestine (Table 3). Line 4 was also studied viain vivo bioluminescence imaging, and an ∼80% reduction in signal was observed in the intestine in live hypothyroid animals (Fig. 2a and 2b).
Table 1.
Responsiveness of THAI #4, 23, and 18 Mouse Lines to T4
| Tissue | THAI #4 (T4) | THAI #23 (T4) | THAI #18 (T4) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eu (RLU/mg Protein) | Hyper (RLU/mg Protein) | Fold (Means ± SEM) | P Value | Eu (RLU/mg Protein) | Hyper (RLU/mg Protein) | Fold (Means ± SEM) | P Value | Eu (RLU/mg Protein) | Hyper (RLU/mg Protein) | Fold (Means ± SEM) | P Value | |
| Pit | 0.337 | 0.791 | 2.3 ± 0.16 | 0.0001a | 1.932 | 16.926 | 8.8 ± 0.62 | 0.0001a | 0.066 | 0.54 | 8.2 ± 0.36 | 0.0001a |
| MBH | 0.206 | 0.368 | 1.8 ± 0.20 | 0.013a | 0.142 | 0.24 | 1.7 ± 0.29 | 0.103 | 0.372 | 0.602 | 2.2 ± 0.25 | 0.005a |
| HC | 0.06 | 0.116 | 1.9 ± 0.03 | 0.0001a | 0.035 | 0.047 | 1.5 ± 0.35 | 0.201 | 2.308 | 3.756 | 2.1 ± 0.25 | 0.013a |
| HT | 0.377 | 0.488 | 1.3 ± 0.08 | 0.031a | 0.65 | 0.78 | 1.2 ± 0.11 | 0.176 | 0.755 | 1.589 | 2.8 ± 0.35 | 0.004a |
| CTX | 0.194 | 0.359 | 1.9 ± 0.30 | 0.035a | 0.179 | 0.253 | 1.4 ± 0.13 | 0.017a | 1.617 | 2.586 | 1.9 ± 1.18 | 0.005a |
| CER | 0.938 | 2.162 | 2.3 ± 0.41 | 0.019a | 0.012 | 0.023 | 1.9 ± 0.28 | 0.045a | 0.045 | 0.063 | 1.8 ± 0.31 | 0.073 |
| Liver | 0.002 | 0.005 | 1.9 ± 0.14 | 0.0007a | 0.001 | 0.003 | 2.9 ± 0.86 | 0.064 | No detectable expression | |||
| Heart | 0.004 | 0.03 | 8.5 ± 0.31 | 0.0001a | 0.001 | 0.001 | 1.0 ± 0.25 | – | No detectable expression | |||
| BAT | 0.028 | 1.82 | 64.6 ± 8.1 | 0.0002a | 0.005 | 0.038 | 7.2 ± 1.54 | 0.007a | No detectable expression | |||
| Bone | 0.031 | 0.066 | 2.2 ± 0.18 | 0.0015a | 0.01 | 0.011 | 0.7 ± 0.11 | 0.057 | No detectable expression | |||
| Muscle | 0.025 | 0.276 | 11.2 ± 5.7 | 0.0041a | 0.004 | 0.003 | 0.7 ± 0.18 | 0.115 | No detectable expression | |||
| MSG | 0.052 | 0.399 | 7.6 ± 2.60 | 0.044a | 0.004 | 0.011 | 2.7 ± 0.77 | 0.072 | Not determined | |||
| Thyroid | 0.115 | 0.176 | 1.5 ± 0.07 | 0.002a | 0.026 | 0.03 | 0.8 ± 0.18 | 0.734 | Not determined | |||
| Testicle | 4876 | 4086 | 0.8 ± 0.06 | 0.108 | 2909 | 2509 | 0.9 ± 0.11 | 0.253 | Not determined | |||
| Intestine | 4.571 | 9.48 | 2.1 ± 0.30 | 0.012a | 0.041 | 0.212 | 5.2 ± 1.6 | 0.029a | Not determined | |||
| Skin | 0.696 | 2.798 | 4.0 ± 0.76 | 0.001a | 2.358 | 1.878 | 0.8 ± 0.17 | 0.544 | Not determined | |||
Euthyroid animals were treated with L-T4 (IP 5 μg/d per animal for 3 days). Luciferase activity was assessed in tissue samples with luciferase assay and expressed as RLU per milligram protein. Luciferase activity of corresponding tissues of TH vs vehicle-treated animals was performed by Student t test (n ≥ 4).
Abbreviations: CER, cerebellum; CTX, cerebral cortex; Eu, euthyroid; HC, hippocampus; HT, hypothalamus; Hyper, hyperthyroid; MBH, mediobasal hypothalamus; MSG, mandibular salivary gland; Pit, pituitary.
P < 0.05.
Figure 2.
Detection of TH action in live animals. (a) In vivo bioluminescence imaging on euthyroid, L-T3-treated (1 μg/g bw injected IP, 24 hours before imaging), and hypothyroid THAI #4 mice, followed by IP luciferin administration. Representative images of hypothyroid (hypo), euthyroid (eu), and L-T3-treated (T3) mice and light intensity diagram of iBAT and small intestine. T3 injection increased light intensity in iBAT. In the small intestine, hypothyroidism decreased, whereas T3 treatment increased light intensity. Dorsal image arrow: iBAT (arrowhead), saccus cecum of the stomach; ventral image (circles), small intestine; ♦, testicles. (b) Light intensity diagram of luciferase activity [photons/s (p/s)] in iBAT and small intestine of hypothyroid, euthyroid, and T3-treated THAI #4 mice. Means of p/s ± SEM (n = 3). *P < 0.05; ***P < 0.001 by one-way ANOVA, followed by Newman-Keuls post hoc test. (c) Bioluminescence of the gastrointestinal tract is confined to the small intestine. Imaging on an isolated gastrointestinal tract of three euthyroid and three L-T3-treated (1 μg/g bw injected IP, 24 hours before imaging) THAI #4 animals. T3-mediated light-intensity upregulation is mainly restricted to the small intestine, whereas the colon shows an order of magnitude lower luciferase activity. *, cecum; arrowhead, saccus cecum of the stomach. Original scale bar, 1 cm. C, colon; J, jejunum.
Table 3.
Responsiveness of Different Tissues and Brain Regions of THAI #4 to Hypothyroidism
| Tissue | THAI #4 (Hypothyroid) | |||
|---|---|---|---|---|
| Eu (RLU/mg Protein) | Hypo (RLU/mg Protein) | Fold (Means ± SEM) | P value | |
| Pit | 0.347 | 0.072 | 0.21 ± 0.02 | 0.0005a |
| MBH | 0.153 | 0.076 | 0.49 ± 0.04 | 0.0099a |
| HT | 0.315 | 0.136 | 0.43 ± 0.05 | 0.0032a |
| CTX | 0.163 | 0.056 | 0.34 ± 0.06 | 0.0003a |
| Liver | 0.003 | 0.001 | 0.44 ± 0.07 | 0.0446a |
| Heart | 0.009 | 0.001 | 0.16 ± 0.06 | 0.0001a |
| BAT | 0.037 | 0.008 | 0.23 ± 0.1 | 0.0051a |
| Bone | 0.031 | 0.008 | 0.26 ± 0.04 | 0.0034a |
| Muscle | 0.008 | 0.007 | – | 0.776 |
| Thyroid | 0.071 | 0.078 | – | 0.439 |
| Intestine | 1.683 | 0.166 | 0.1 ± 0.01 | 0.0001a |
| WBC | 0.013 | 0.005 | 0.42 ± 0.08 | 0.0412a |
Animals were rendered hypothyroid by adding 0.1% sodium perchlorate and 0.5% methimazole in drinking water for 3 weeks combined with a low-iodine diet. Luciferase activity was assessed with luciferase assay and expressed as RLU per milligram protein in tissue samples. Luciferase activity of corresponding tissues of hypothyroid vs euthyroid animals was compared with Student t test (n = 6).
Abbreviations: CTX, cerebral cortex; Eu, euthyroid; HT, hypothalamus; Hypo, hypothyroid; MBH, mediobasal hypothalamus; Pit, pituitary; WBC, white blood cell.
P < 0.05.
Next, all three mouse lines were treated with L-T4 for 3 days. Responsiveness to TH varied among mouse lines and among tissues within the same mouse line. For example, responsiveness in line 4 varied from 0.8-fold in the testes, 1.5-twofold in the brain (hypothalamus, hippocampus, cerebellum, cortex), to 64-fold in iBAT. Other highly responsive tissues were skeletal muscle, heart, mandibular salivary gland, and skin (four- to 11-fold; Table 1). Lines 23 and 18 exhibited marked response to TH in the pituitary, and line 18 showed the highest brain baseline activity (Table 1). In this setting, the mouse lines showed greater responsiveness to L-T3 compared with L-T4; e.g., line 4 responded to L-T3 with a fivefold stimulation of reporter activity in the liver and 46-fold in the heart, whereas the mediobasal hypothalamus of line 23 responded to L-T3 with an ∼12-fold induction of reporter activity (Table 2). In line 4 animals, a single L-T3 injection, 24 hours before imaging, induced luciferase activity in iBAT by approximately ninefold, in the small intestine by approximately threefold, and to a lesser extent, in the mandibular salivary gland; the footpads and tail showed a tendency to increase 2.2- and 2.5-fold, respectively (euthyroid vs hyperthyroid), which became important when hypo- and hyperthyroid animals were compared, showing eight- and 46-fold changes for footpads and tails, respectively (Fig. 2a and 2b). The iBAT was confirmed to be the source of the bioluminescence of the interscapular region (Supplemental Fig. 1a), and signal detected in the intestine was predominantly confined to the small intestine (duodenum and jejunum; Fig. 2c). Notably, despite very high basal reporter activity, luciferase activity in the testicles was not inducible by L-T3 injection (Fig. 2a). Live THAI mouse line 23 also responded to L-T3 injection, with elevated reporter activity in the iBAT and small intestine (Supplemental Fig. 2). Thus, THAI mice allow the assessment of TH action both in live animals and in tissue samples. Line 4 permits the study of TH action in a wide variety of tissues, whereas the two other lines hold additional value for specific applications, represented by high reporter response in the pituitary (lines 18 and 23) and higher basal activity in the brain (18), as a result of their different insertion sites.
Table 2.
Responsiveness of THAI #4 and 23 Mouse Lines to T3
| THAI #4 (T3) | ||||
|---|---|---|---|---|
| Tissue | Eu (RLU/mg Protein) | Hyper (RLU/mg Protein) | Fold (Means ± SEM) | P Value |
| Liver | 0.003 | 0.017 | 5.0 ± 0.6 | 0.009a |
| Heart | 0.006 | 0.284 | 45.9 ± 5.65 | 0.002a |
| #23 (T3) | ||||
|---|---|---|---|---|
| Tissue | Eu (RLU/mg Protein) | Hyper (RLU/mg Protein) | Fold (Means ± SEM) | P Value |
| MBH | 0.18 | 2.222 | 12.31 ± 2.4 | 0.003a |
Euthyroid animals were treated with L-T3 (IP 1 μg/g bw for 1 day). Luciferase activity was assessed in tissue samples with luciferase assay and expressed as RLU per milligram protein. Luciferase activity of corresponding tissues of TH vs vehicle-treated animals was performed by Student t test (n ≥ 4).
Abbreviation: MBH, mediobasal hypothalamus.
P < 0.05.
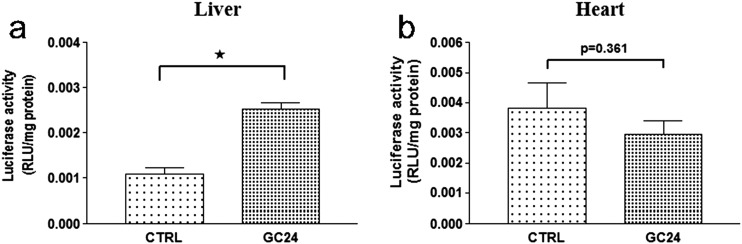
One of the aspects that provide specificity in TH signaling is differential expression of TRα and TRβ isoforms among tissues; e.g., TRβ predominates in the liver, whereas the TRα isoform predominates in the brain, skeletal muscle, and heart (1, 33). To test if TR subtype specificity could be assessed with the THAI mouse, line 4 animals were treated with GC24, a highly selective TRβ agonist (34). Treatment with GC24 increased luciferase activity by ∼2.5-fold in the liver (Fig. 3a) but did not affect TH signaling in the heart (Fig. 3b). As TRE is based on a DR-4 DNA motive, we also tested whether induction of liver X receptor (LXR) signaling, representing another DR-4-dependent pathway, could also be detected in THAI mouse. Administration of the LXR agonist GW3965 to the THAI #4 mouse induced hepatic luciferase expression by ∼2.3-fold [control: 1 ± 0.394; GW3965: 2.324 ± 0.362; means ± standard error of the mean (SEM); luciferase/GAPDH (n = 5); *P < 0.05 by Student t test].
Figure 3.
Effect of the TH analog GC24 on tissue-specific TH action. (a) Single injection of the TRβ agonist GC24 (1.53 nM/g bw IP) significantly increased luciferase activity after 24 hours in hypothyroid (0.1% sodium perchlorate and 0.5% methimazole in drinking water for 3 weeks combined with low-iodine diet) THAI #4 mice in the TRβ isoform-dominant liver, whereas (b) luciferase activity did not change in the TRα isoform-dominant heart. Luciferase activity of liver and heart samples was assayed 24 hours after GC24 injection. Means ± SEM (n = 10). *P < 0.0001 by Student t test. CTRL, control.
iBAT-specific activation of TH signaling in cold-stimulated THAI mice
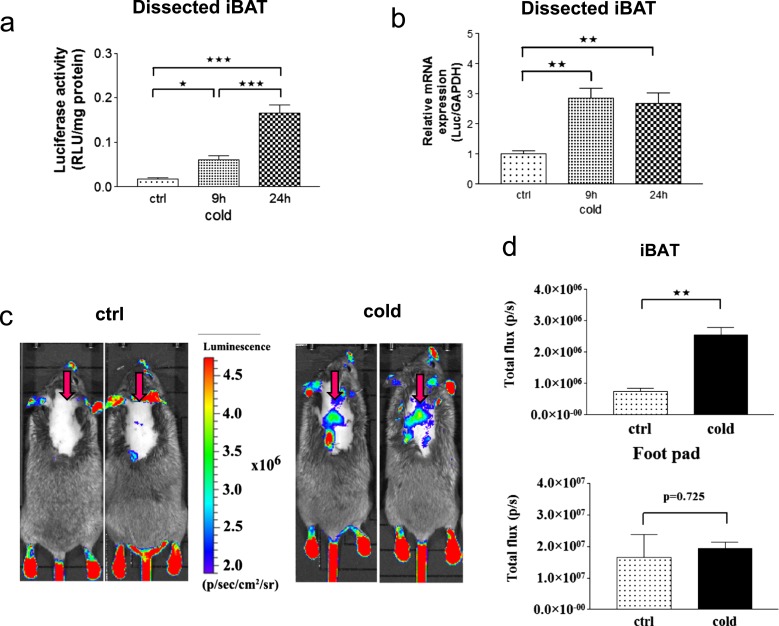
Cold exposure is associated with BAT sympathetic activation and several-fold stimulation of the type 2 deiodinase (D2) activity. This saturates local TR without affecting systemic levels of circulating T3 (35). However, it has been challenging to study TH signaling in iBAT under these conditions, given that virtually all downstream targets of T3 in this tissue are equally affected by cyclic adenosine monophosphate. For example, a mouse with disruption of D2-encoding dio2 only provided limited information, given the compensatory elevation in iBAT sympathetic activity (36). To approach this problem, line 4 mice were exposed to cold (4°C) for 9 and 24 hours. The animals exhibited an ∼3.4- and 9.3-fold increase in luciferase activity in iBAT at 9 and 24 hours vs control, respectively, whereas the luciferase mRNA was increased 2.8- and 2.7-fold vs control in the two time points (Fig. 4a and 4b). TH signaling in iBAT was also detected in live animals imaged for bioluminescence, with a 3.2-fold increase in signal after 24 hours of cold exposure. At all times, TH action in footpad skin remained unchanged. This indicates a highly selective increase in iBAT TH signaling during cold exposure (Fig. 4c and 4d).
Figure 4.
Cold-induced TH action in the iBAT of THAI #4 mice. (a) luciferaseiferase activity is significantly elevated in dissected iBAT samples of the THAI #4 line after 9 hours at 4°C and further increased after 24 hours of cold stress. *P < 0.05; ***P < 0.001 by one-way ANOVA, followed by Newman-Keuls post hoc test. Means ± SEM (n = 5). (b) Luciferase mRNA increased after 9 hours in dissected iBAT samples of the THAI #4 line; **P < 0.01 by one-way ANOVA, followed by Newman-Keuls post hoc test. Means ± SEM (n = 5). (c) In vivo bioluminescence imaging on iBAT of THAI #4 subjected to cold stress for 24 hours at 4°C, followed by IP luciferin administration. Representative images of two control and two cold-exposed mice. Arrows indicate iBAT. (d) Light intensity diagram of luciferase activity in iBAT and foot pads of control and cold-induced THAI #4 mice. Luciferase activity of skin above the non–BAT-containing region was deducted from iBAT reporter values. Means of photon/s ± SEM (n = 4). **P < 0.001 by Student t test.
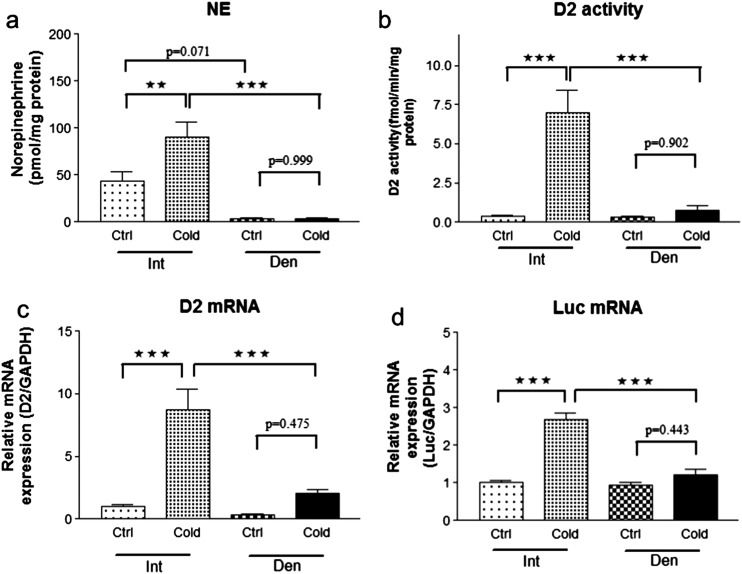
That the increase in TH signaling was caused by sympathetic activation was confirmed in THAI mice undergoing cold exposure after unilateral iBAT surgical denervation, which resulted in a strong tendency for a decrease in NE levels in iBAT (Fig. 5a) and prevented the 9-hour, cold exposure-induced, ∼twofold increase in NE content that was only observed in the intact iBAT lobe. Furthermore, only the intact iBAT lobe exhibited a ninefold increase in D2 expression and 18-fold acceleration in D2 activity (Fig. 5b and 5c), which then increased local TH signaling. Indeed, only in the intact lobe did luciferase mRNA levels increase by ∼2.7-fold (Fig. 5d). It is notable that baseline TH action was not affected by denervation in the animals kept at room temperature.
Figure 5.
Sympathetic regulation of TH action in iBAT of THAI #4 mice. (a) Nine-hour cold stress increased NE levels by twofold in intact (Int) BAT lobe, whereas the NE level was not influenced in the denervated (Den) lobe. (b) D2 enzymatic activity and (c) mRNA levels were increased by cold stress in dissected, intact iBAT, whereas the denervated side remained unresponsive. (d) Luciferase mRNA level increased 2.7-fold in the intact lobe in response to cold, but TH action was not influenced by cold on the denervated side. Despite sympathetic denervation, luciferase expression did not decrease at room temperature in the denervated lobe compared with the intact lobe. ***P < 0.001; **P < 0.01 by repeated-measures ANOVA, followed by Tukey post hoc test. Data are shown as means ± SEM (n = 6).
Discussion
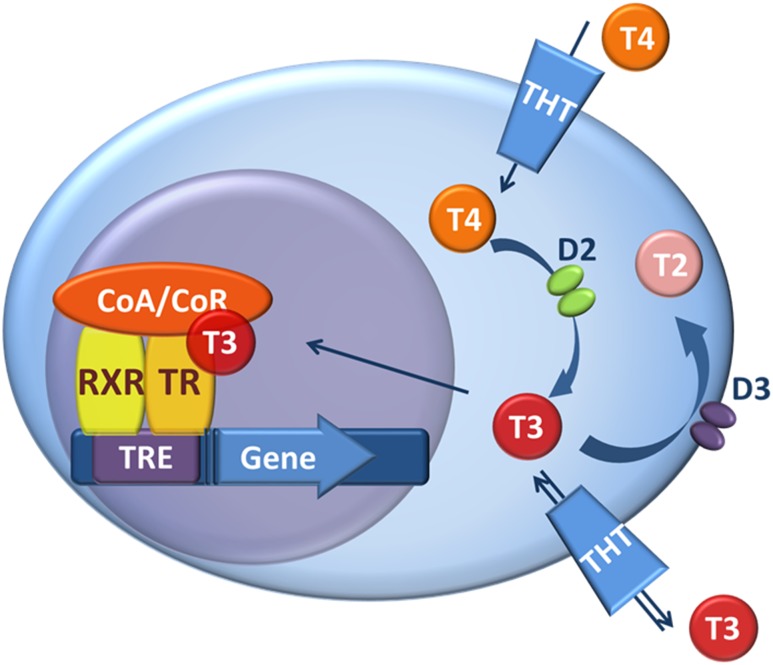
Under most circumstances, TH levels in the circulation are relatively stable, suggesting that TH signaling is homogeneous and steady throughout the body. Although indirect evidence exists that suggests that this is not the case, as a result of the lack of appropriate tools, direct measurement of tissue-specific TH action was not possible. Here, we developed and used a unique transgenic mouse model to show that TH signaling is tissue specific and fluctuates rapidly in a tissue-specific manner. These findings are physiologically relevant given the assessment of TH action using a reporter cassette that operates in the context of endogenously expressed levels of TH transporters, TRs, and transcriptional factors (Fig. 6). That this reporter system reflects physiological levels of TH signaling is illustrated by the fact that the THAI mouse model is sufficiently sensitive to detect a reduction of TH signaling in hypothyroid animals. In addition, the system can detect rapid, tissue-specific changes in TH signaling, such as in the cold-stimulated iBAT. Thus, the THAI model reveals how a studied condition/treatment can change TH action in a specific tissue. However, the model is not meant to establish and compare absolute levels of TH signaling in various tissues of the same or different THAI lines.
Figure 6.
TH action and its regulation by TH transporters and deiodinases. T3 binds the TR/RXR heterodimer attached to a TRE in the regulatory region of TH-responsive genes. The ability of TR/RXR to affect gene transcription is modulated by coactivators/corepressors (CoA/CoR) and factors regulating intracellular TH availability, TH transporters (THT) and TH deiodinases. As the first step in the regulation of TH action, D2 activates T4 by converting it to T3. Type 3 deiodinase (D3) catalyses the inactivating pathway by degrading T3 and converting T4 to the inactive reverse T3. The expression of the two main deiodinases, D2 and D3, varies according to cell type.
In vivo, the DR-4 element does not work exclusively as TRE but can also represent a binding site for other nuclear receptors; e.g., it can be also bind to LXR, a cholesterol-mediated nuclear receptor retinoid X receptor (RXR), farnesoid X receptor, pregnane X receptor, and constitute-androstane receptor (37–39). Our finding, that GW3965, an LXR agonist, could induce hepatic luciferase expression approximately twofold in THAI #4 mice, indicates that similar to the endogenous situation, the inserted DR-4 elements can respond to other DR-4-dependent pathways upon pharmacological induction. Thus, TH specificity of the THAI system should be viewed the same way as that of endogenous TREs that do not exclusively respond to TH but might also be affected by other DR-4-dependent, but not/or not directly TH-dependent, pathways. Therefore, under specific conditions, the biological output of a DR-4 response could be the net result of different DR-4-related signals.
Studies of deiodinase-mediated changes in TH signaling have been hindered by the lack of a sufficiently specific and sensitive reporter system, a problem that is now resolved with the development of this mouse model. Up until now, one could only assess tissue-specific TH signaling by measuring mRNA levels of TH-responsive genes (32). However, this approach has limited value, as multiple factors affect the expression of endogenously expressed, TH-responsive genes. Well-known TH-responsive genes, such as spot-14, malic enzyme, and ectonucleotide pyrophosphatase/phosphodiesterase 2, are also regulated by many other factors, including insulin and 17β-estradiol, for example (40, 41). Therefore, expression of these genes could authentically report on TH signaling only in atypical cases, when exclusively, TH levels are altered in the animal. However, typically, it cannot be determined to what extent these genes were regulated by changes of local TH levels and by other factors. This indicates the need for alternative systems that monitor TH signaling. To avoid problems associated with the responsiveness of endogenous TH-regulated promoters to TH-unrelated signals, we did not perform knockin-powered, targeted insertion of the reporter, 3′ to an endogenous promoter, but rather, applied Sleeping Beauty transposase-mediated delivery of a minimal viral promoter that was made TH responsive with the addition of TREs. As in the 5′−flanking region of many endogenous, TH-responsive genes, two or three TREs are present (18, 42, 43), we inserted three TREs in our transgenic construct. In contrast to our model, the available FINDT3 mouse model uses an artificial chimeric Gal4DNA-binding domain, a TRα ligand-binding domain fusion protein that works independently of endogenous TR and TRE (44). Thus, this system detects T3 availability but cannot assess the changes of physiologically relevant TH action that is a far more complex event and cannot be directly deducted from T3 availability alone. A 2× TRE β-galactosidase mouse model has been reported to assess TH action at embryonic stages, but it is not suitable for studies in the adult brain, and information in adult peripheral organs is missing (45). Furthermore, in contrast to the THAI mouse, neither model allows for quantification of the tissue-specific changes of TH signaling in live animals.
As the THAI model uses the endogenous TRs, it also allows the examination of TH signaling triggered by TH analogs, such as TR-selective agonists; i.e., the TRβ-selective GC24 markedly stimulated activity in the liver but not in the TRα-predominant heart. The observed reporter activity represents the net result of effects shaping the in vivo ability of a compound to act in a specific tissue, including tissue and cell penetration, local metabolism of the compound, and interaction with TRs and cofactors.
TRs are distinctive in that even when unoccupied by their ligands, they remain bound to the regulatory regions of TH-dependent genes, slowing down the transcriptional process through the recruitment of corepressors, a process known as gene repression. The binding of T3 increases gene expression through de-repression (uncoupling of TR and corepressors) and transactivation (coupling of TR with coactivators). It is remarkable that the THAI mouse exhibits a sufficient signal-to-noise ratio to assess corepression, i.e., a decrease in TH signaling observed during hypothyroidism. Indeed, in the hypothyroid THAI mouse, there is an approximate two- to 10-fold decrease in TH signaling in different brain regions, the pituitary, and other peripheral organs, including liver, heart, bone, iBAT, white blood cells, and small intestine. The latter is particularly interesting, as it illustrates the high degree of TH responsiveness exhibited by the intestinal epithelium, which is consistent with the observation that patients carrying TRα-inactivating mutations exhibit marked constipation (46). The measurement of TR-mediated gene repression with the THAI mouse in a tissue-specific manner provides an approach to physiological studies of TH signaling and the impact of the deiodinases and transporters.
Although the dosage of L-T4 and L-T3, as used in the present studies, is standard across studies of TH signaling, it is certainly well above the physiological range (32). However, the increase in luciferase activity in cold-stimulated iBAT provides strong evidence that the THAI mouse gauges TH signaling within a physiological context. Protein translation requires time; therefore, an increase of reporter activity can be slower than that of its encoding mRNA. This could explain that whereas an increase of luciferase mRNA reaches its plateau at 9 hours, luciferase activity continues to increase until 24 hours. The model also allowed direct tissue-specific assessment of TH action in iBAT and provided insights into the crosstalk between TH and noradrenergic signaling by revealing that in contrast to the cold-induced changes of TH action, the basal TH action at room temperature is independent of noradrenergic signaling in this tissue (35, 47). These data also demonstrate how robustly THAI mice reveal local tissue-specific up- or downregulation of TH action, independently from circulating TH levels.
The translatability of the present findings is straightforward. Patients with different forms of thyroid disease suffer from momentary or life-long dysregulation of TH signaling. Thus, if we are to correct TH signaling in such patients, we should be able to understand how major elements of TH metabolism and action affect T3-dependent biological effects. For example, >300 million patients with hypothyroidism worldwide are maintained on levothyroxine-replacement therapy (48). The rationale for using only levothyroxine is based on the idea that the deiodinases regulate the amount of T3 required by individual tissues. It has recently been recognized that there are limits to the extent that deiodinases normalize systemic and intracellular T3 during treatment with levothyroxine (15). As a result, patients on levothyroxine are ∼10 pounds heavier, 30% to 40% less active, and more likely to be on antidepressives, statins, and beta-blockers, despite normal thyroid-stimulating hormone serum levels (49). We are now in a position to understand why and in which tissues TH signaling is dysregulated during levothyroxine supplementation.
In conclusion, the generated THAI model can assess TH action in live animals and tissue samples. The model is able to capture both increased and decreased levels of endogenous TH signaling. It can be used to study the tissue-specific impact of TH analogs and has the potential to serve as a useful model for testing the impact of endocrine disruptors on the TH economy. Whereas the THAI model overcomes the interfering effect of numerous signals modulating the expression of endogenous, TH-responsive marker genes via various promoter-binding sites, its reporter activity can certainly be affected under specific conditions by DR-4-dependent, but not/or not directly TH-related, signals, the same way as endogenous DR-4 works in vivo. The THAI mouse reveals that during the preservation of thermal homeostasis, the activation of the sympathetic nervous system induced elevation of TH action in the iBAT of mice in a cold environment, but the sympathetic nervous system has no effect on TH action in the iBAT of mice at room temperature, indicating highly different regulation of TH action under these two conditions.
Supplementary Material
Acknowledgments
The technical help of A. Juhász is gratefully acknowledged. We thank Dr. Zs. Izsvák (Berlin, Germany) and L. Mátes (Szeged, Hungary) for the SB100X construct, Perry Hackett of Addgene for the pt2-BH transposon cloning vector (plasmid no. 26554), and Dr. T. Scanlan (Portland, OR) for GC24.
Financial Support: This work was supported by the Hungarian National Brain Research Program [Grants KTIA_13_NAP_A_I/3 (to C.F.) and KTIA_13_NAP_A_I/4 (to B.G.)] and Hungarian Scientific Research Fund [Grants OTKA K109415 (to B.G.) and K109710 (to C.F.)].
Author Contributions: P.M. conducted experiments, analyzed results, and wrote the manuscript. M.B., B.B., M.T., I.H., Z.H., B.S., Z.T., and R.S. conducted experiments. F.E. and G.S. performed founder generation. R.M.L. and A.C.B. analyzed results and wrote the manuscript. C.F. and B.G. conceived of the project, designed and performed experiments, analyzed results, wrote the manuscript, and acquired the funding.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- ANOVA
analysis of variance
- BAT
brown adipose tissue
- bw
body weight
- cDNA
complementary DNA
- D2
type 2 deiodinase
- DR-4
direct repeat 4
- FISH
fluorescence in situ hybridization
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HEK
human embryonic kidney
- iBAT
interscapular brown adipose tissue
- IP
intraperitoneal(ly)
- LXR
liver X receptor
- mRNA
messenger RNA
- NE
norepinephrine
- PCR
polymerase chain reaction
- RLU
relative light unit
- RXR
retinoid X receptor
- SEM
standard error of the mean
- TH
thyroid hormone
- THAI
thyroid hormone action indicator
- THAIC
thyroid hormone action indicator construct
- TR
thyroid hormone receptor
- TRE
thyroid hormone response element.
References
- 1. Zhang J, Lazar MA. The mechanism of action of thyroid hormones. Annu Rev Physiol. 2000;62(1):439–466. [DOI] [PubMed] [Google Scholar]
- 2. Ortiga-Carvalho TM, Sidhaye AR, Wondisford FE. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol. 2014;10(10):582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brent GA, Larsen PR, Harney JW, Koenig RJ, Moore DD. Functional characterization of the rat growth hormone promoter elements required for induction by thyroid hormone with and without a co-transfected beta type thyroid hormone receptor. J Biol Chem. 1989;264(1):178–182. [PubMed] [Google Scholar]
- 4. Glass CK, Franco R, Weinberger C, Albert VR, Evans RM, Rosenfeld MG. A c-erb-A binding site in rat growth hormone gene mediates trans-activation by thyroid hormone. Nature. 1987;329(6141):738–741. [DOI] [PubMed] [Google Scholar]
- 5. Chatonnet F, Guyot R, Benoît G, Flamant F. Genome-wide analysis of thyroid hormone receptors shared and specific functions in neural cells. Proc Natl Acad Sci USA. 2013;110(8):E766–E775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29(7):898–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014;35(2):159–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Visser WE, Friesema EC, Jansen J, Visser TJ. Thyroid hormone transport in and out of cells. Trends Endocrinol Metab. 2008;19(2):50–56. [DOI] [PubMed] [Google Scholar]
- 9. Refetoff S, DeWind LT, DeGroot LJ. Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab. 1967;27(2):279–294. [DOI] [PubMed] [Google Scholar]
- 10. Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364(9443):1435–1437. [DOI] [PubMed] [Google Scholar]
- 11. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74(1):168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boelen A, Kwakkel J, Fliers E. Beyond low plasma T3: local thyroid hormone metabolism during inflammation and infection. Endocr Rev. 2011;32(5):670–693. [DOI] [PubMed] [Google Scholar]
- 13. Pihlajamäki J, Boes T, Kim EY, Dearie F, Kim BW, Schroeder J, Mun E, Nasser I, Park PJ, Bianco AC, Goldfine AB, Patti ME. Thyroid hormone-related regulation of gene expression in human fatty liver. J Clin Endocrinol Metab. 2009;94(9):3521–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Werneck de Castro JP, Fonseca TL, Ueta CB, McAninch EA, Abdalla S, Wittmann G, Lechan RM, Gereben B, Bianco AC. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Invest. 2015;125(2):769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gereben B, McAninch EA, Ribeiro MO, Bianco AC. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat Rev Endocrinol. 2015;11(11):642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tancevski I, Rudling M, Eller P. Thyromimetics: a journey from bench to bed-side. Pharmacol Ther. 2011;131(1):33–39. [DOI] [PubMed] [Google Scholar]
- 17. Verge CF, Konrad D, Cohen M, Di Cosmo C, Dumitrescu AM, Marcinkowski T, Hameed S, Hamilton J, Weiss RE, Refetoff S. Diiodothyropropionic acid (DITPA) in the treatment of MCT8 deficiency. J Clin Endocrinol Metab. 2012;97(12):4515–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toyoda N, Zavacki AM, Maia AL, Harney JW, Larsen PR. A novel retinoid X receptor-independent thyroid hormone response element is present in the human type 1 deiodinase gene. Mol Cell Biol. 1995;15(9):5100–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kollár A, Kvárta-Papp Z, Egri P, Gereben B.. Different types of luciferase reporters show distinct susceptibility to T3-evoked downregulation. Thyroid 2016;26(1):179–182. [DOI] [PubMed] [Google Scholar]
- 20. Mátés L, Chuah MK, Belay E, Jerchow B, Manoj N, Acosta-Sanchez A, Grzela DP, Schmitt A, Becker K, Matrai J, Ma L, Samara-Kuko E, Gysemans C, Pryputniewicz D, Miskey C, Fletcher B, VandenDriessche T, Ivics Z, Izsvák Z. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat Genet. 2009;41(6):753–761. [DOI] [PubMed] [Google Scholar]
- 21. Egri P, Gereben B. Minimal requirements for ubiquitination-mediated regulation of thyroid hormone activation. J Mol Endocrinol. 2014;53(2):217–226. [DOI] [PubMed] [Google Scholar]
- 22. Ivics Z, Mátés L, Yau TY, Landa V, Zidek V, Bashir S, Hoffmann OI, Hiripi L, Garrels W, Kues WA, Bösze Z, Geurts A, Pravenec M, Rülicke T, Izsvák Z. Germline transgenesis in rodents by pronuclear microinjection of Sleeping Beauty transposons. Nat Protoc. 2014;9(4):773–793. [DOI] [PubMed] [Google Scholar]
- 23. Errijgers V, Van Dam D, Gantois I, Van Ginneken CJ, Grossman AW, D’Hooge R, De Deyn PP, Kooy RF. FVB.129P2-Pde6b(+) Tyr(c-ch)/Ant, a sighted variant of the FVB/N mouse strain suitable for behavioral analysis. Genes Brain Behav. 2007;6(6):552–557. [DOI] [PubMed] [Google Scholar]
- 24. Mátés L. Rodent transgenesis mediated by a novel hyperactive Sleeping Beauty transposon system. Methods Mol Biol. 2011;738:87–99. [DOI] [PubMed] [Google Scholar]
- 25. Coligan JE, Bierer B, Margulies DH, Shevach EM, Strober W, eds. Current Protocols in Immunology. New York, NY: Wiley Online; 2017. [Google Scholar]
- 26. Koprowski H, Mocarelli P, Wiktor TJ. Antibody response in vitro to an animal virus: production of rabies virus neutralizing antibodies by mouse cells in culture. Proc Natl Acad Sci USA. 1972;69(9):2433–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kanda R, Shang Y, Tsuji S, Eguchi-Kasai K, Hayata I. An improved culture system of mouse peripheral blood lymphocytes for analysis of radiation-induced chromosome aberrations. Biosci Rep. 2004;24(6):641–650. [DOI] [PubMed] [Google Scholar]
- 28. Potter CJ, Luo L. Splinkerette PCR for mapping transposable elements in Drosophila. PLoS One. 2010;5(4):e10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morales JR, Ballesteros I, Deniz JM, Hurtado O, Vivancos J, Nombela F, Lizasoain I, Castrillo A, Moro MA. Activation of liver X receptors promotes neuroprotection and reduces brain inflammation in experimental stroke. Circulation. 2008;118(14):1450–1459. [DOI] [PubMed] [Google Scholar]
- 30. Pulinilkunnil T, He H, Kong D, Asakura K, Peroni OD, Lee A, Kahn BB. Adrenergic regulation of AMP-activated protein kinase in brown adipose tissue in vivo. J Biol Chem. 2011;286(11):8798–8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horváth G, Gölöncsér F, Csölle C, Király K, Andó RD, Baranyi M, Koványi B, Máté Z, Hoffmann K, Algaier I, Baqi Y, Müller CE, Von Kügelgen I, Sperlágh B. Central P2Y12 receptor blockade alleviates inflammatory and neuropathic pain and cytokine production in rodents. Neurobiol Dis. 2014;70:162–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bianco AC, Anderson G, Forrest D, Galton VA, Gereben B, Kim BW, Kopp PA, Liao XH, Obregon MJ, Peeters RP, Refetoff S, Sharlin DS, Simonides WS, Weiss RE, Williams GR. American thyroid association guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid. 2014;24(1):88–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moran C, Chatterjee K. Resistance to thyroid hormone due to defective thyroid receptor alpha. Best Pract Res Clin Endocrinol Metab. 2015;29(4):647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borngraeber S, Budny MJ, Chiellini G, Cunha-Lima ST, Togashi M, Webb P, Baxter JD, Scanlan TS, Fletterick RJ. Ligand selectivity by seeking hydrophobicity in thyroid hormone receptor. Proc Natl Acad Sci USA. 2003;100(26):15358–15363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bianco AC, Silva JE. Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest. 1987;79(1):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Christoffolete MA, Linardi CC, de Jesus L, Ebina KN, Carvalho SD, Ribeiro MO, Rabelo R, Curcio C, Martins L, Kimura ET, Bianco AC. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes. 2004;53(3):577–584. [DOI] [PubMed] [Google Scholar]
- 37. Echchgadda I, Song CS, Oh T, Ahmed M, De La Cruz IJ, Chatterjee B. The xenobiotic-sensing nuclear receptors pregnane X receptor, constitutive androstane receptor, and orphan nuclear receptor hepatocyte nuclear factor 4alpha in the regulation of human steroid-/bile acid-sulfotransferase. Mol Endocrinol. 2007;21(9):2099–2111. [DOI] [PubMed] [Google Scholar]
- 38. Quack M, Frank C, Carlberg C. Differential nuclear receptor signalling from DR4-type response elements. J Cell Biochem. 2002;86(3):601–612. [DOI] [PubMed] [Google Scholar]
- 39. Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol. 2006;68(1):159–191. [DOI] [PubMed] [Google Scholar]
- 40. Ishihara A, Matsumoto E, Horikawa K, Kudo T, Sakao E, Nemoto A, Iwase K, Sugiyama H, Tamura Y, Shibata S, Takiguchi M. Multifactorial regulation of daily rhythms in expression of the metabolically responsive gene spot14 in the mouse liver. J Biol Rhythms. 2007;22(4):324–334. [DOI] [PubMed] [Google Scholar]
- 41. Sárvári M, Kalló I, Hrabovszky E, Solymosi N, Rodolosse A, Vastagh C, Auer H, Liposits Z. Hippocampal gene expression is highly responsive to estradiol replacement in middle-aged female rats. Endocrinology. 2015;156(7):2632–2645. [DOI] [PubMed] [Google Scholar]
- 42. Xiong S, Chirala SS, Hsu MH, Wakil SJ. Identification of thyroid hormone response elements in the human fatty acid synthase promoter. Proc Natl Acad Sci USA. 1998;95(21):12260–12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hartong R, Wang N, Kurokawa R, Lazar MA, Glass CK, Apriletti JW, Dillmann WH. Delineation of three different thyroid hormone-response elements in promoter of rat sarcoplasmic reticulum Ca2+ATPase gene. Demonstration that retinoid X receptor binds 5′ to thyroid hormone receptor in response element 1. J Biol Chem. 1994;269(17):13021–13029. [PubMed] [Google Scholar]
- 44. Quignodon L, Legrand C, Allioli N, Guadaño-Ferraz A, Bernal J, Samarut J, Flamant F. Thyroid hormone signaling is highly heterogeneous during pre- and postnatal brain development. J Mol Endocrinol. 2004;33(2):467–476. [DOI] [PubMed] [Google Scholar]
- 45. Nucera C, Muzzi P, Tiveron C, Farsetti A, La Regina F, Foglio B, Shih SC, Moretti F, Della Pietra L, Mancini F, Sacchi A, Trimarchi F, Vercelli A, Pontecorvi A. Maternal thyroid hormones are transcriptionally active during embryo-foetal development: results from a novel transgenic mouse model. J Cell Mol Med. 2010;14(10):2417–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bochukova E, Schoenmakers N, Agostini M, Schoenmakers E, Rajanayagam O, Keogh JM, Henning E, Reinemund J, Gevers E, Sarri M, Downes K, Offiah A, Albanese A, Halsall D, Schwabe JW, Bain M, Lindley K, Muntoni F, Vargha-Khadem F, Dattani M, Farooqi IS, Gurnell M, Chatterjee K. A mutation in the thyroid hormone receptor alpha gene [published correction appears in N Engl J Med. 2012;367(15):1474] N Engl J Med. 2012;366(3):243–249. [DOI] [PubMed] [Google Scholar]
- 47. Silva JE, Larsen PR. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature. 1983;305(5936):712–713. [DOI] [PubMed] [Google Scholar]
- 48. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM, American Thyroid Association Task Force on Thyroid Hormone Replacement . Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peterson SJ, McAninch EA, Bianco AC. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy? J Clin Endocrinol Metab. 2016;101(12):4964–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.