Abstract
Context
Circulating thyroglobulin antibodies (TgAb) can confound measurement of serum thyroglobulin and impair thyroid cancer surveillance. Few data exist on the significance of TgAb in pediatric thyroid cancer.
Objective
To describe the prevalence, natural history, and clinical significance of TgAb in children with thyroid cancer.
Design
Retrospective cohort study.
Setting
Single academic pediatric center.
Patients
Seventy-three consecutive children (≤18 years) with nonmedullary thyroid cancer who had serum TgAb measured within 6 months after diagnosis.
Main Outcome Measures
Prevalence and natural history of TgAb; association of TgAb status and resolution with patient and disease characteristics.
Results
TgAb were detected in 41% of subjects (30 of 73) and were associated with lymph node metastasis (83% vs 53%, P = 0.01) but not distant metastasis. In patients with TgAb, resolution occurred in 44% (11 of 25) over a median follow-up of 3.8 years. Median time to clear TgAb was 10.7 months, and 10 of 11 patients who cleared (91%) did so within 2 years. Resolution of TgAb was associated with lower initial TgAb level (median 4.5 vs 76 normalized units, P = 0.003). TgAb positivity at diagnosis was not independently associated with persistent or recurrent disease (odds ratio 3.20, 95% confidence interval 0.95 to 10.80, P = 0.06).
Conclusions
TgAb are common at diagnosis in children with thyroid cancer but resolve in nearly half of patients within 1 to 2 years. TgAb are associated with the presence of lymph node metastasis at diagnosis, but the long-term prognostic significance remains to be determined.
In children with thyroid cancer, thyroglobulin antibodies are common but often transient, and are associated with more advanced disease at diagnosis.
Thyroid cancer is the most common endocrine malignancy in both children and adults. Although the risk of mortality from thyroid cancer is low, lifelong surveillance is mandatory due to the risk of disease recurrence, which may occur many years after initial treatment and apparent cure. This issue is especially salient in children with thyroid cancer because of the decades of monitoring required.
Because many differentiated thyroid cancers produce thyroglobulin (Tg), measurement of serum Tg can detect thyroid cancer recurrence or progression and is therefore an important element of long-term surveillance in patients with thyroid cancer (1). However, circulating Tg antibodies (TgAb) can confound conventional Tg assays and thereby prevent accurate measurement of Tg levels (2, 3). TgAb therefore represent a significant clinical issue in thyroid cancer monitoring (4). Persistent or rising TgAb titers have been associated with disease recurrence, whereas early clearance of TgAb may be associated with decreased risk of recurrence (2, 5–11). For this reason, longitudinal monitoring of TgAb levels as a surrogate tumor marker is recommended in patients in whom TgAb prohibit accurate measurement of Tg levels (1).
Although the prevalence of TgAb in pediatric thyroid cancer is unclear, it may be higher than the rate of 20 to 30% in adults with thyroid cancer (2, 4, 12, 13). Lymphocytic infiltration of the thyroid is common either within or remote from a thyroid tumor, and the presence and concentration of TgAb correlate with the degree of lymphocytic thyroiditis in adults with thyroid cancer (14). The fact that lymphocytic thyroiditis occurs in 42 to 88% of pediatric patients (10, 15–17) compared with 23 to 40% of adult patients (18–20) suggests that children may have a more robust autoimmune response to thyroid cancer, which could result in a higher prevalence of TgAb in children than in adults.
Given the clinical relevance of TgAb and the extended duration of monitoring required in pediatric thyroid cancer patients, we retrospectively analyzed the prevalence and natural history of TgAb in 73 consecutive children with thyroid cancer seen in our multidisciplinary pediatric thyroid clinic.
Subjects and Methods
Subjects
We reviewed the hospital records of all pediatric patients (≤18 years of age) with nonmedullary thyroid carcinoma seen between 1999 and 2014 in our multidisciplinary pediatric thyroid program located at a large academic medical center. Patients were included in the study if serum TgAb were measured between 1 month before and 6 months after initial surgery. Patients with very low-risk disease, defined as papillary thyroid carcinoma <1 cm without extrathyroidal extension or metastasis, were excluded from analysis.
Treatment and follow-up
Initial treatment consisted of near-total thyroidectomy (including lobectomy followed by completion thyroidectomy) or lobectomy alone. In all patients, central and/or lateral lymph node dissection was performed based on clinical findings. Postoperative radioactive iodine (RAI) ablation with 131I was performed in most patients. Thyrotropin suppression was used to a target of <0.1 mIU/L. Standard postoperative ultrasound surveillance was performed in all patients every 6 to 12 months.
In patients whose initial therapy included near-total thyroidectomy followed by RAI, we assessed the presence of persistent or recurrent disease within the first 3 years of follow-up, or until the end of the study period. A minimum of 6 months follow-up after RAI was required for inclusion in this analysis. Patients were classified as having no evidence of disease (NED) or persistent/recurrent disease. Patients without TgAb during follow-up (including those who were initially TgAb positive and later became TgAb negative) were considered to have NED if they had suppressed Tg <0.2 ng/mL or stimulated Tg <2 ng/mL, negative neck ultrasound, and (if performed) negative radioiodine whole-body scan. Patients with TgAb during follow-up were considered to have NED if they had both negative neck ultrasound and negative radioiodine whole-body scan. At the time of this study, our program did not routinely measure Tg levels by mass spectrometry in patients with circulating TgAb. Persistent/Recurrent disease was defined by either histopathological diagnosis of malignancy in surgically resected tissue, stimulated Tg >2 ng/mL, or abnormal uptake outside the thyroid bed on radioiodine whole-body scan. For patients with iodine-avid cervical lymph node metastases identified by initial RAI, we considered surgical resection of these metastases within 12 months after thyroidectomy to be part of initial treatment of the purposes of assessing the presence of persistent/recurrent disease.
Data collection and analysis
Patient features, including age, sex, and clinical and laboratory data, were obtained from hospital records and entered into the REDCap data capture tool (21). Based upon the standard of practice at our institution, surgical resection margin was considered to be negative for tumor unless specifically noted to be positive in the histopathology report. Presence of clinically significant TgAb was defined as a measured TgAb concentration above the upper limit of the assay reference range. For analysis of initial TgAb status, we included patients in whom TgAb were measured in a certified clinical laboratory by radioimmunoassay or immunochemiluminometric assay. To examine the natural history of TgAb, we analyzed only patients in whom all TgAb measurements were performed at our institution, because of the variability of TgAb measurement between assay methods (22). At our institution, TgAb were measured using a series of different chemiluminescent immunoassays over the course of the study period: Nichols Advantage (Nichols Institute Diagnostics, San Juan Capistrano, CA; reference range <2 IU/mL), 1999 to 2005; Beckman Access (Beckman Coulter, Fullerton, CA; reference range <5 IU/mL), 2005 to 2007; Beckman Access II (Beckman Coulter; reference range <4 IU/mL), 2007 to 2011; Roche Cobas E601 (Roche, Indianapolis, IN; reference range <19 IU/mL), 2011 to 2014. The manufacturer’s reference range was used for all TgAb assays except the Roche assay, for which a lower reference range was internally determined in our institution. To account for differences in TgAb assay methods over time, measured concentrations of TgAb were normalized to the upper limit of the reference range for each assay. In patients initially positive for TgAb, clearance of TgAb was defined as the date of the first measured TgAb concentration within the reference range.
Duration of follow-up and time to TgAb clearance were calculated from the date of initial surgery. Potential associations between subject features and TgAb presence or clearance were examined by Fisher exact test or by Wilcoxon rank sum test, and P < 0.05 was considered significant. Spearman correlation was used to assess the association between initial TgAb level and time to TgAb clearance due to the skewed distribution of initial TgAb levels. Associations between initial TgAb status and persistent/recurrent disease were assessed by Fisher exact test. Multiple logistic regression analysis was used to calculate the odds of persistent/recurrent disease based on TgAb status, controlling for patient characteristics found to be significantly associated with TgAb in univariate analysis. To account for potential referral bias (e.g., preferential referral of TgAb-positive patients to our tertiary care center), we performed a prespecified sensitivity analysis in which all analyses were repeated after excluding patients referred to our institution after their initial diagnosis of thyroid cancer. Because excluding these patients did not alter the results, only the results of the primary analysis are presented. SAS software (Cary, NC) was used for statistical computations. This research was approved by the Boston Children’s Hospital Institutional Review Board.
Results
Patient characteristics
A total of 96 patients ≤18 years of age with nonmedullary thyroid cancer was seen during the study period. Seventy-three patients met the study inclusion criteria, and their characteristics are shown in Table 1. The median (range) age at diagnosis was 14.8 (6.1 to 18.9) years, and 57 of 73 patients (78%) were female. Initial surgery consisted of near-total thyroidectomy in nearly all cases. Lobectomy alone was performed on only three patients (4%), one with a 2.5-cm follicular carcinoma containing capsular but no vascular invasion, and two others with 2.5- to 2.7-cm follicular variants of papillary thyroid carcinoma who initially declined completion thyroidectomy for personal reasons, but eventually underwent completion thyroidectomy 2 and 6 years later, respectively. Most patients had papillary thyroid carcinoma (92%), and the remainder had follicular thyroid carcinoma. The median (range) tumor size was 2.5 (0.3 to 6.5) cm. Based on our inclusion criteria, three patients with dominant tumors measuring <1 cm were included due to the presence of locoregional lymph node metastases.
Table 1.
Comparison of Patients With and Without TgAb at Initial Thyroid Cancer Diagnosis
| Characteristic | Total (73)a | TgAb Negative (43)a | TgAb Positive (30)a | P b |
|---|---|---|---|---|
| Age, y | 14.8 (6.1–18.9) | 15.0 (6.1–18.9) | 14.6 (7.5–17.6) | 0.46 |
| Sex | 0.41 | |||
| Female | 57 [78] | 32 [74] | 25 [83] | |
| Male | 16 [22] | 11 [26] | 5 [17] | |
| Surgical approach | 0.26 | |||
| Lobectomy | 3 [4] | 3 [7] | 0 [0] | |
| Near-total thyroidectomy | 70 [96] | 40 [93] | 30 [100] | |
| RAI ablation | 0.73 | |||
| No | 9 [12] | 6 [14] | 3 [10] | |
| Yes | 64 [88] | 37 [86] | 27 [90] | |
| Size of primary tumor, cm | 2.5 (0.3–6.5) | 2.5 (0.8–6.5) | 2.1 (0.3–6.5) | 0.71 |
| Type of cancer | 0.04 | |||
| Papillary thyroid cancer | 67 [92] | 37 [86] | 30 [100] | |
| Follicular thyroid cancer | 6 [8] | 6 [14] | 0 [0] | |
| Resection margin | 0.06 | |||
| Negative | 53 [73] | 35 [81] | 18 [60] | |
| Positive | 20 [27] | 8 [19] | 12 [40] | |
| Lymph node metastasis | 0.01 | |||
| Absent | 25 [34] | 20 [47] | 5 [17] | |
| Present | 48 [66] | 23 [53] | 25 [83] | |
| Distant metastasis | 1 | |||
| Absent | 68 [93] | 40 [93] | 28 [93] | |
| Present | 5 [7] | 3 [7] | 2 [7] |
N [% of column total] or median (minimum to maximum).
Testing for difference in distribution of characteristic between antibody-positive and antibody-negative patients, by Wilcoxon rank sum or Fisher’s exact test.
Prevalence of TgAb at diagnosis
TgAb were present in the initial serum sample in 30 of 73 (41%) patients. Table 1 shows the relationship of subject characteristics to the presence or absence of TgAb at diagnosis. The prevalence of TgAb at diagnosis did not vary by age at diagnosis or sex. The presence of TgAb was associated with a higher prevalence of lymph node metastasis (83% vs 53%, P = 0.01). The association between TgAb and positive surgical resection margin approached statistical significance (40% vs 19%, P = 0.06). Tumor size, distant metastasis, and initial treatment (including surgical approach and use of RAI) were not associated with the presence of TgAb.
TgAb were present in 30 of 67 (45%) patients with papillary thyroid cancer but absent in all six patients with follicular thyroid cancer (P = 0.04). In a post hoc analysis excluding patients with follicular thyroid cancer, the difference between TgAb-positive and TgAb-negative subjects in the prevalence of lymph node metastasis decreased from 30% (83% vs 53%, P = 0.01) to 21% (83% vs 62%, P = 0.06). Other associations reported in Table 1 were unaffected by exclusion of patients with follicular thyroid cancer.
Natural history of TgAb
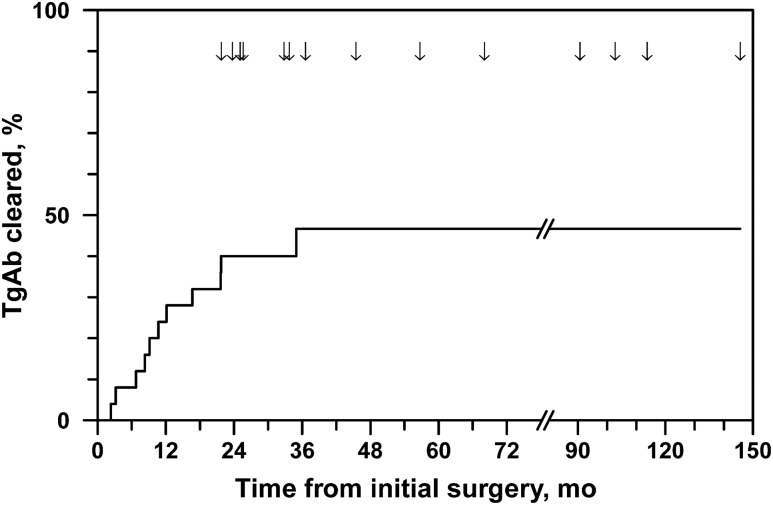
The median (range) duration of follow-up for the overall study cohort was 72.7 (2.9 to 238) months after initial surgery. Of the 30 patients initially positive for TgAb, five were excluded from analysis of natural history due to lack of follow-up TgAb measurements at our institution. The remaining 25 TgAb-positive patients were followed for a median (range) of 45.5 (22 to 146) months. During this time, 11 of 25 (44%) patients became TgAb negative, with a median (range) time to clearance of 10.7 (2.3 to 35) months (Fig. 1). All patients who cleared their TgAb did so in <22 months, except one patient who cleared after 35 months. In only one patient did apparent clearance of TgAb coincide with a change of TgAb assay method.
Figure 1.
Clearance of TgAb after initial treatment in pediatric patients with thyroid cancer. Arrows indicate when patients exited follow-up.
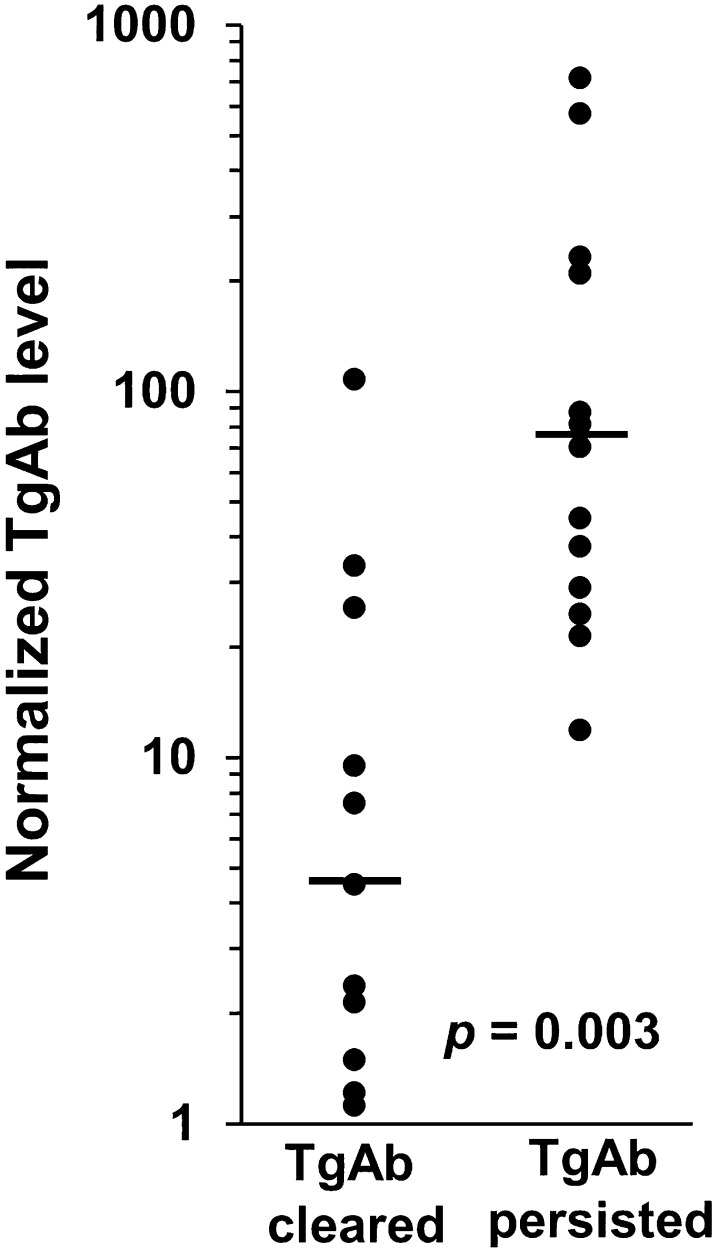
Patients who cleared their TgAb had lower median initial TgAb levels than those who did not clear (4.5 vs 76 normalized units, P = 0.003; Fig. 2), although there was substantial overlap in initial TgAb levels between the two groups. TgAb eventually cleared in all patients whose initial TgAb level was <10-fold the upper reference limit (n = 8). Among patients whose TgAb resolved, the time to TgAb clearance was directly correlated with initial TgAb level (Spearman r = 0.6, P = 0.05), and all patients with initial TgAb levels <threefold above the upper reference limit had resolution of TgAb within 1 year. There was no association between the likelihood of TgAb resolution and any other patient or disease characteristics we examined, including age, sex, surgical approach, treatment with RAI, tumor size or type, positivity of surgical resection margin, or lymph node or distant metastasis.
Figure 2.
Initial levels of TgAb are lower in patients who eventually clear their TgAb (median 4.5, range 1.1 to 108) than in patients whose TgAb persist (median 76, range 12 to 719), although there is substantial overlap between groups. Each TgAb level is normalized and expressed as a fold-change relative to the upper limit of the reference range for the specific TgAb assay by which it was measured.
Association of TgAb with persistent/recurrent disease
To assess the association between initial TgAb status and the risk of persistent/recurrent disease, we analyzed the subset of patients in our cohort who had received more extensive initial treatment consisting of near-total thyroidectomy and postoperative RAI ablation. Of the 73 patients in the full cohort, 12 were excluded from the analysis of persistent/recurrent disease because of treatment with lobectomy only (n = 3); no treatment with RAI due to loss to follow-up (n = 2), patient preference (n = 2), or low-risk disease (follicular variant of papillary thyroid carcinoma <1.5 cm, n = 2); or follow-up duration <6 months after RAI (n = 3).
The remaining 61 patients were assessed for the presence of persistent/recurrent disease during the first 3 years after RAI (Table 2). Of these, 36 patients (59%) were TgAb negative and 25 patients (41%) were TgAb positive at diagnosis. Overall, 25 of 61 patients (41%) had persistent/recurrent disease, which was confirmed by malignant histopathology on surgical resection of locoregional metastases (n = 15); presence of iodine-avid disease in the lungs (n = 5) or lateral neck/mediastinum (n = 4); or stimulated Tg 8 ng/mL (n = 1). Persistent/Recurrent disease was more prevalent in patients who were initially TgAb positive (60%) than in those who were initially TgAb negative (28%), a significant difference [odds ratio (OR) 3.90, 95% confidence interval 1.29–11.78, P = 0.02]. Clinical features that could account for this relation include those shown in Table 1 to be associated with TgAb status: type of cancer, resection margin, and lymph node metastasis. Of these, only lymph node metastasis showed the potential for confounding by virtue of a strong association with persistent/recurrent disease (P = 0.008). The relation between persistent/recurrent disease and TgAb status was no longer statistically significant after adjustment for lymph node metastasis (OR 3.20, 95% confidence interval 0.95 to 10.80, P = 0.06). Median duration of follow-up was equal in TgAb-negative and TgAb-positive patients, but a higher proportion of TgAb-positive was followed for <3 years due to having been diagnosed <3 years before the end of the study period (Table 2).
Table 2.
Assessment of Persistent/Recurrent Disease in First 3 Years in Patients With and Without TgAb at Initial Thyroid Cancer Diagnosis
| Characteristic | Totala | TgAb Negativea | TgAb Positivea | P b |
|---|---|---|---|---|
| Number (%) | 61 | 36 (59) | 25 (41) | |
| Age at diagnosis, y | 14.8 (6.1–18.9) | 14.8 (6.1–18.9) | 14.7 (7.5–17.6) | 0.77 |
| Duration of follow-up, mo | 36 (17–36) | 36 (17–36) | 36 (26–36) | 0.006 |
| Diagnosis <3 years prior to end of study | 13 (21) | 3 (8) | 10 (40) | 0.004 |
| Disease status | 0.02 | |||
| No evidence of disease | 36 [59] | 26 [72] | 10 [40] | |
| Persistent recurrent disease | 25 [41] | 10 [28] | 15 [60] |
N [% of column total] or median (minimum to maximum).
Testing for difference in distribution of characteristic between antibody-positive and antibody-negative patients, by Wilcoxon rank sum or Fisher’s exact test.
Whole-body radioiodine scintigraphy was performed in all TgAb-positive patients (as a condition of inclusion in this analysis) and in 23 of 36 TgAb-negative patients. Among the 13 of 36 TgAb-negative patients who did not undergo scintigraphy, persistent/recurrent disease was diagnosed in three patients based on abnormal ultrasound findings followed by histopathologic confirmation in resected tissue, whereas the remaining patients were confirmed to have NED based on stimulated Tg <2 ng/mL (n = 8) or suppressed Tg levels <0.1 ng/mL (n = 2).
Discussion
Measurement of serum Tg is a critical component of disease surveillance for adults and children with differentiated thyroid cancer (1, 23). However, circulating TgAb can confound serum Tg measurement, which may limit the ability to assess response to treatment or delay the detection of persistent or recurrent disease. Although recent consensus guidelines recommend an approach to TgAb in pediatric thyroid cancer similar to that recommended in adults (23), minimal data exist regarding the role of TgAb in children, and extrapolation from adult data may not be appropriate. This study describes the prevalence, natural history, and clinical significance of TgAb in pediatric thyroid cancer.
In our cohort, TgAb were present at diagnosis in 41% of children with thyroid cancer, a prevalence higher than that reported in adults (20% to 30%) (2, 4, 12, 13). Because the presence of TgAb at diagnosis was associated with the presence of lymph node metastases, it is possible that a higher prevalence of TgAb in children could reflect the tendency of pediatric thyroid cancer to present with more advanced disease than in adults, including more local and distant metastasis (24–26). Indeed, in our cohort, the prevalence of lymph node metastasis (66%) was significantly higher than in reports of adults with thyroid cancer (11, 27), but similar to that of other recent pediatric series (28, 29). The mechanism underlying the association between TgAb and lymph node metastasis is not clear, but potential reasons might include advanced tumors generating a more robust immune response in the thyroid or within lymph nodes, or differences in Tg expression or immunogenicity in more aggressive tumors.
After initial treatment of thyroid cancer, TgAb concentrations tended to decrease in our patients over time. TgAb eventually resolved in nearly half of patients over a median follow-up of 3.8 years, and all but one patient who cleared their TgAb did so within 2 years after initial surgery. Furthermore, the initial TgAb level was inversely related to the likelihood of TgAb resolution and directly related to the time to resolution. This indicates that, although TgAb may hinder early monitoring in a substantial proportion of children with thyroid cancer, this is a transient issue in about half of patients, and within a relatively short time the disappearance of TgAb will once again permit disease surveillance using Tg levels. Conversely, in the other half of pediatric patients, TgAb may persist over long periods, and this phenomenon appears to be more common than in adults. For example, although initial rates of TgAb clearance in children and adults appear similar, TgAb eventually resolve in 60 to 70% of adult patients over 3 to 5 years of follow-up (11–13). In contrast, of patients in our cohort in whom TgAb persisted for at least 2 years, only one of 13 (8%) had resolution of TgAb over a median (range) follow-up of 4.7 (2.1 to 12.1) years from diagnosis. This discrepancy might be due to age-related differences in immunologic response.
Although TgAb appear to be useful for longitudinal surveillance of thyroid cancer activity, the prognostic significance of TgAb status at diagnosis remains unclear. Some studies in adults suggest that TgAb may be associated with an increased risk of disease recurrence (10, 11), whereas others find no such association (27, 30). To address this issue in our pediatric cohort, we analyzed the prevalence of persistent/recurrent disease in the first 3 years after initial treatment in patients with or without TgAb at diagnosis. We found that persistent/recurrent disease was more than twice as common among TgAb-positive patients as among TgAb-negative patients. This result is consistent with a recent study of 1240 adults with thyroid cancer in whom TgAb were associated with a twofold increase in the rate of persistent disease (17% vs 8.4%) and a fourfold increase in recurrence rate (5.8% vs 1.4%) after 1 year (11).
Because our analysis does not account for all risk factors for persistent/recurrent disease proposed by current consensus guidelines (1, 23), whether TgAb represent an independent risk factor for disease persistence/recurrence remains unclear. TgAb are associated with lymph node metastasis, which is a known risk factor for persistent/recurrent disease in pediatric thyroid cancer (28, 29). After adjusting for the presence of lymph node metastasis, the association of persistence/recurrence with TgAb positivity was no longer statistically significant (P = 0.06); however, adjustment for lymph node metastases did not substantially alter the OR for this association (3.90 vs 3.20), suggesting that a significant relationship between TgAb positivity and persistence/recurrence has not been entirely excluded and might be detected in a larger sample with greater power. Importantly, this association was not due to differences in sex or distant metastasis—which are associated with persistence/recurrence (28) but not with TgAb (Table 1)—nor in treatment intensity (e.g., extent of initial surgery, use of RAI) or duration of surveillance between patients with and without TgAb. Although less-prevalent use of whole-body scintigraphy might theoretically have resulted in relative underdetection of persistent/recurrent disease in TgAb-negative patients, this is unlikely because disease status was determined with high confidence in all TgAb-negative patients who did not undergo scintigraphy. Therefore, although our finding of a possible association between TgAb and disease persistence/recurrence is provocative, its borderline statistical significance and possible confounders make it premature to conclude definitively that the presence of TgAb at diagnosis is an independent risk factor for disease persistence/recurrence or should be taken into account in initial treatment decisions.
More TgAb-positive patients in our cohort had follow-up for <3 full years, which may be related to the specific TgAb assay used during the last 3 years of the study. However, this potential difference is unlikely to have affected the analysis of disease persistence/recurrence for two reasons. First, shorter follow-up in TgAb-positive patients would, if anything, be expected to reduce detection of persistent/recurrent disease, leading to underestimation of the observed association. Second, of the nine TgAb-positive diagnosed within the last 3 years of the study, seven had TgAb concentrations in the range demonstrated to cause interference with serum Tg measurement (>115 mIU/L) (31), and therefore confidently can be considered TgAb positive. The remaining two patients had mildly positive TgAb that quickly cleared, and subsequently had NED based on negative ultrasound, scintigraphy, and stimulated Tg levels (<2 ng/mL). Thus, although these two patients might have been classified as initially TgAb negative on a different TgAb assay, their inclusion in the TgAb-positive group, if anything, underestimates the association between TgAb positivity and persistent/recurrent disease.
In our cohort, TgAb were detected only in patients with papillary thyroid cancer and not in those with follicular thyroid cancer. The attenuation we observed in the association between TgAb and lymph node metastasis after excluding the six patients with follicular carcinoma could be due to a number of factors. One potential factor is decreased sample size leading to insufficient power to detect a true association. Another possible factor is confounding by the presence of follicular carcinoma, which might have accounted for part of the association between TgAb and lymph node metastasis. Such confounding would necessarily be attributable to two associations: first, no patient with follicular carcinoma in this cohort had lymph node metastasis; second, follicular carcinoma may be less likely (for unknown reasons) to induce TgAb. Although the latter association is possible, perhaps due to differences between papillary and follicular carcinoma in antigen expression and/or tumor interaction with the immune system, at present it remains hypothetical, as we are aware of no prior evidence suggesting that follicular carcinoma is intrinsically less likely than papillary carcinoma to induce TgAb. Finally, we cannot exclude residual confounding between follicular carcinoma and unmeasured patient or disease characteristics that may be associated with TgAb.
This study has several limitations. The relatively small cohort size limits our power to detect differences between groups. Because our institution is a tertiary referral center, selection bias may be present due to the disproportionate inclusion of patients with more severe disease. However, our results were unchanged in a prespecified sensitivity analysis excluding patients referred from other institutions, making such selection bias unlikely. Finally, although this study was performed in a single center, over its 16-year time span different TgAb assays were used at different times. Because different TgAb assays may yield varying results even in the same patient sample (4, 32), assay changes might have contributed to variation in TgAb concentration within and between patients. We sought to minimize this issue by analyzing TgAb status dichotomously as positive or negative (not a continuous concentration) based on the upper limit of each assay’s reference range. However, the apparent presence or absence of TgAb may depend on the specific assay used, and TgAb reference ranges defined by assay manufacturers may not reflect the ability of circulating TgAb to interfere with measurement of serum Tg levels (31). This variability in the detection and functional significance of TgAb is a limitation of our study. Nevertheless, because this study was designed to inform clinical practice, we used the clinical laboratory reference range for TgAb, which is a strength in that it accurately reflects the reality of TgAb testing in clinical practice. Although a change between TgAb assays could theoretically have affected when TgAb appeared to resolve in individual patients, disappearance of TgAb coincided with a change in assay platform in only one patient, which is unlikely to have affected our results significantly.
In summary, this study directly assesses the behavior and clinical significance of TgAb in children with thyroid cancer. We show that the prevalence of TgAb is higher in pediatric compared with adult thyroid cancer, but that TgAb resolve over time in half of these children—particularly those with low TgAb levels—allowing Tg levels to be used for tumor surveillance within 1 to 2 years of initial diagnosis. TgAb were associated with more advanced disease at presentation. Although our data suggest that TgAb might represent a risk factor for short-term disease persistence/recurrence, this requires further investigation in larger studies that account for additional potential confounding variables. At this stage, our data do not support the use of more aggressive initial therapy (e.g., use of RAI or increased RAI dosage) solely on the basis of initial TgAb status; rather, management should be based on established risk stratification systems that have been validated in adult and pediatric disease (1, 23). Overall, these data support the concept that pediatric thyroid cancer has both similarities to and differences from adult thyroid cancer, and reinforce the importance of larger, prospective studies of pediatric thyroid cancer to inform the evidence-based care of these children.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- NED
no evidence of disease
- OR
odds ratio
- RAI
radioactive iodine
- Tg
thyroglobulin
- TgAb
Tg antibody.
References
- 1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spencer CA, Takeuchi M, Kazarosyan M, Wang CC, Guttler RB, Singer PA, Fatemi S, LoPresti JS, Nicoloff JT. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998;83(4):1121–1127. [DOI] [PubMed] [Google Scholar]
- 3. Locsei Z, Szabolcs I, Rácz K, Kovács GL, Horváth D, Toldy E. Serum thyroglobulin antibody levels within or near to the reference range may interfere with thyroglobulin measurement. Biochem Med (Zagreb). 2012;22(3):365–370. [PMC free article] [PubMed] [Google Scholar]
- 4. Spencer CA. Clinical review: clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J Clin Endocrinol Metab. 2011;96(12):3615–3627. [DOI] [PubMed] [Google Scholar]
- 5. Chung JK, Park YJ, Kim TY, So Y, Kim SK, Park DJ, Lee DS, Lee MC, Cho BY. Clinical significance of elevated level of serum antithyroglobulin antibody in patients with differentiated thyroid cancer after thyroid ablation. Clin Endocrinol (Oxf). 2002;57(2):215–221. [DOI] [PubMed] [Google Scholar]
- 6. Kim WG, Yoon JH, Kim WB, Kim TY, Kim EY, Kim JM, Ryu JS, Gong G, Hong SJ, Shong YK. Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulin-negative patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2008;93(12):4683–4689. [DOI] [PubMed] [Google Scholar]
- 7. Hsieh CJ, Wang PW. Sequential changes of serum antithyroglobulin antibody levels are a good predictor of disease activity in thyroglobulin-negative patients with papillary thyroid carcinoma. Thyroid. 2014;24(3):488–493. [DOI] [PubMed] [Google Scholar]
- 8. Tsushima Y, Miyauchi A, Ito Y, Kudo T, Masuoka H, Yabuta T, Fukushima M, Kihara M, Higashiyama T, Takamura Y, Kobayashi K, Miya A, Kikumori T, Imai T, Kiuchi T. Prognostic significance of changes in serum thyroglobulin antibody levels of pre- and post-total thyroidectomy in thyroglobulin antibody-positive papillary thyroid carcinoma patients. Endocr J. 2013;60(7):871–876. [DOI] [PubMed] [Google Scholar]
- 9. Verburg FA, Luster M, Cupini C, Chiovato L, Duntas L, Elisei R, Feldt-Rasmussen U, Rimmele H, Seregni E, Smit JW, Theimer C, Giovanella L. Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: a clinical position statement. Thyroid. 2013;23(10):1211–1225. [DOI] [PubMed] [Google Scholar]
- 10. Soyluk O, Boztepe H, Aral F, Alagol F, Özbey NC. Papillary thyroid carcinoma patients assessed to be at low or intermediary risk after primary treatment are at greater risk of long term recurrence if they are thyroglobulin antibody positive or do not have distinctly low thyroglobulin at initial assessment. Thyroid. 2011;21(12):1301–1308. [DOI] [PubMed] [Google Scholar]
- 11. Durante C, Tognini S, Montesano T, Orlandi F, Torlontano M, Puxeddu E, Attard M, Costante G, Tumino S, Meringolo D, Bruno R, Trulli F, Toteda M, Redler A, Ronga G, Filetti S, Monzani F; PTC Study Group . Clinical aggressiveness and long-term outcome in patients with papillary thyroid cancer and circulating anti-thyroglobulin autoantibodies. Thyroid. 2014;24(7):1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiovato L, Latrofa F, Braverman LE, Pacini F, Capezzone M, Masserini L, Grasso L, Pinchera A. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med. 2003;139(5):346–351. [DOI] [PubMed] [Google Scholar]
- 13. Görges R, Maniecki M, Jentzen W, Sheu SN, Mann K, Bockisch A, Janssen OE. Development and clinical impact of thyroglobulin antibodies in patients with differentiated thyroid carcinoma during the first 3 years after thyroidectomy. Eur J Endocrinol. 2005;153(1):49–55. [DOI] [PubMed] [Google Scholar]
- 14. Latrofa F, Ricci D, Montanelli L, Rocchi R, Piaggi P, Sisti E, Grasso L, Basolo F, Ugolini C, Pinchera A, Vitti P. Lymphocytic thyroiditis on histology correlates with serum thyroglobulin autoantibodies in patients with papillary thyroid carcinoma: impact on detection of serum thyroglobulin. J Clin Endocrinol Metab. 2012;97(7):2380–2387. [DOI] [PubMed] [Google Scholar]
- 15. O’Gorman CS, Hamilton J, Rachmiel M, Gupta A, Ngan BY, Daneman D. Thyroid cancer in childhood: a retrospective review of childhood course. Thyroid. 2010;20(4):375–380. [DOI] [PubMed] [Google Scholar]
- 16. Van Savell H Jr, Hughes SM, Bower C, Parham DM. Lymphocytic infiltration in pediatric thyroid carcinomas. Pediatr Dev Pathol. 2004;7(5):487–492. [DOI] [PubMed] [Google Scholar]
- 17. Fridman MV, Savva NN, Krasko OV, Zborovskaya AA, Mankovskaya SV, Schmid KW, Demidchik YE. Clinical and pathologic features of “sporadic” papillary thyroid carcinoma registered in the years 2005 to 2008 in children and adolescents of Belarus. Thyroid. 2012;22(10):1016–1024. [DOI] [PubMed] [Google Scholar]
- 18. Matsubayashi S, Kawai K, Matsumoto Y, Mukuta T, Morita T, Hirai K, Matsuzuka F, Kakudoh K, Kuma K, Tamai H. The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab. 1995;80(12):3421–3424. [DOI] [PubMed] [Google Scholar]
- 19. Jeong JS, Kim HK, Lee CR, Park S, Park JH, Kang SW, Jeong JJ, Nam KH, Chung WY, Park CS. Coexistence of chronic lymphocytic thyroiditis with papillary thyroid carcinoma: clinical manifestation and prognostic outcome. J Korean Med Sci. 2012;27(8):883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: a meta-analysis. Eur J Endocrinol. 2013;168(3):343–349. [DOI] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spencer CA, Bergoglio LM, Kazarosyan M, Fatemi S, LoPresti JS. Clinical impact of thyroglobulin (Tg) and Tg autoantibody method differences on the management of patients with differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2005;90(10):5566–5575. [DOI] [PubMed] [Google Scholar]
- 23. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M, Parisi MT, Rachmiel M, Thompson GB, Yamashita S; American Thyroid Association Guidelines Task Force . Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Welch Dinauer CA, Tuttle RM, Robie DK, McClellan DR, Svec RL, Adair C, Francis GL. Clinical features associated with metastasis and recurrence of differentiated thyroid cancer in children, adolescents and young adults. Clin Endocrinol (Oxf). 1998;49(5):619–628. [DOI] [PubMed] [Google Scholar]
- 25. Frankenthaler RA, Sellin RV, Cangir A, Goepfert H. Lymph node metastasis from papillary-follicular thyroid carcinoma in young patients. Am J Surg. 1990;160(4):341–343. [DOI] [PubMed] [Google Scholar]
- 26. Vassilopoulou-Sellin R, Klein MJ, Smith TH, Samaan NA, Frankenthaler RA, Goepfert H, Cangir A, Haynie TP. Pulmonary metastases in children and young adults with differentiated thyroid cancer. Cancer. 1993;71(4):1348–1352. [DOI] [PubMed] [Google Scholar]
- 27. McLeod DS, Cooper DS, Ladenson PW, Ain KB, Brierley JD, Fein HG, Haugen BR, Jonklaas J, Magner J, Ross DS, Skarulis MC, Steward DL, Maxon HR, Sherman SI; The National Thyroid Cancer Treatment Cooperative Study Group . Prognosis of differentiated thyroid cancer in relation to serum thyrotropin and thyroglobulin antibody status at time of diagnosis. Thyroid. 2014;24(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pires BP, Alves PA Jr, Bordallo MA, Bulzico DA, Lopes FP, Farias T, Dias F, Lima RA, Santos Gisler IC, Coeli CM, Carvalhaes de Oliveira RV, Corbo R, Vaisman M, Vaisman F. Prognostic factors for early and long-term remission in pediatric differentiated thyroid carcinoma: the role of sex, age, clinical presentation, and the newly proposed American Thyroid Association risk stratification system. Thyroid. 2016;26(10):1480–1487. [DOI] [PubMed] [Google Scholar]
- 29. Sung TY, Jeon MJ, Lee YH, Lee YM, Kwon H, Yoon JH, Chung KW, Kim WG, Song DE, Hong SJ. Initial and dynamic risk stratification of pediatric patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2017;102(3):793–800. [DOI] [PubMed] [Google Scholar]
- 30. Souza SL, Montalli Da Assumpção LV, Ward LS. Impact of previous thyroid autoimmune diseases on prognosis of patients with well-differentiated thyroid cancer. Thyroid. 2003;13(5):491–495. [DOI] [PubMed] [Google Scholar]
- 31. Spencer C, Petrovic I, Fatemi S. Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2011;96(5):1283–1291. [DOI] [PubMed] [Google Scholar]
- 32. Netzel BC, Grebe SK, Carranza Leon BG, Castro MR, Clark PM, Hoofnagle AN, Spencer CA, Turcu AF, Algeciras-Schimnich A. Thyroglobulin (Tg) testing revisited: Tg assays, TgAb assays, and correlation of results with clinical outcomes. J Clin Endocrinol Metab. 2015;100(8):E1074–E1083. [DOI] [PMC free article] [PubMed] [Google Scholar]