The objective is to formulate clinical practice guidelines for the assessment, treatment, and prevention of pediatric obesity.
Abstract
Cosponsoring Associations:
The European Society of Endocrinology and the Pediatric Endocrine Society. This guideline was funded by the Endocrine Society.
Objective:
To formulate clinical practice guidelines for the assessment, treatment, and prevention of pediatric obesity.
Participants:
The participants include an Endocrine Society–appointed Task Force of 6 experts, a methodologist, and a medical writer.
Evidence:
This evidence-based guideline was developed using the Grading of Recommendations, Assessment, Development, and Evaluation approach to describe the strength of recommendations and the quality of evidence. The Task Force commissioned 2 systematic reviews and used the best available evidence from other published systematic reviews and individual studies.
Consensus Process:
One group meeting, several conference calls, and e-mail communications enabled consensus. Endocrine Society committees and members and co-sponsoring organizations reviewed and commented on preliminary drafts of this guideline.
Conclusion:
Pediatric obesity remains an ongoing serious international health concern affecting ∼17% of US children and adolescents, threatening their adult health and longevity. Pediatric obesity has its basis in genetic susceptibilities influenced by a permissive environment starting in utero and extending through childhood and adolescence. Endocrine etiologies for obesity are rare and usually are accompanied by attenuated growth patterns. Pediatric comorbidities are common and long-term health complications often result; screening for comorbidities of obesity should be applied in a hierarchal, logical manner for early identification before more serious complications result. Genetic screening for rare syndromes is indicated only in the presence of specific historical or physical features. The psychological toll of pediatric obesity on the individual and family necessitates screening for mental health issues and counseling as indicated. The prevention of pediatric obesity by promoting healthful diet, activity, and environment should be a primary goal, as achieving effective, long-lasting results with lifestyle modification once obesity occurs is difficult. Although some behavioral and pharmacotherapy studies report modest success, additional research into accessible and effective methods for preventing and treating pediatric obesity is needed. The use of weight loss medications during childhood and adolescence should be restricted to clinical trials. Increasing evidence demonstrates the effectiveness of bariatric surgery in the most seriously affected mature teenagers who have failed lifestyle modification, but the use of surgery requires experienced teams with resources for long-term follow-up. Adolescents undergoing lifestyle therapy, medication regimens, or bariatric surgery for obesity will need cohesive planning to help them effectively transition to adult care, with continued necessary monitoring, support, and intervention. Transition programs for obesity are an uncharted area requiring further research for efficacy. Despite a significant increase in research on pediatric obesity since the initial publication of these guidelines 8 years ago, further study is needed of the genetic and biological factors that increase the risk of weight gain and influence the response to therapeutic interventions. Also needed are more studies to better understand the genetic and biological factors that cause an obese individual to manifest one comorbidity vs another or to be free of comorbidities. Furthermore, continued investigation into the most effective methods of preventing and treating obesity and into methods for changing environmental and economic factors that will lead to worldwide cultural changes in diet and activity should be priorities. Particular attention to determining ways to effect systemic changes in food environments and total daily mobility, as well as methods for sustaining healthy body mass index changes, is of importance.
Summary of Recommendations
1.0 Diagnosing overweight and obesity
-
1.1
We recommend using body mass index (BMI) and the Centers for Disease Control and Prevention (CDC) normative BMI percentiles to diagnose overweight or obesity in children and adolescents ≥2 years of age. (1|⊕⊕⊕○)
-
1.2
We recommend diagnosing a child or adolescent >2 years of age as overweight if the BMI is ≥85th percentile but <95th percentile for age and sex, as obese if the BMI is ≥95th percentile, and as extremely obese if the BMI is ≥120% of the 95th percentile or ≥35 kg/m2 (1|⊕⊕○○). We suggest that clinicians take into account that variations in BMI correlate differently to comorbidities according to race/ethnicity and that increased muscle mass increases BMI. (2|⊕○○○)
-
1.3
We suggest calculating, plotting, and reviewing a child’s or adolescent’s BMI percentile at least annually during well-child and/or sick-child visits. (Ungraded Good Practice Statement)
-
1.4
We suggest that a child <2 years of age be diagnosed as obese if the sex-specific weight for recumbent length is ≥97.7th percentile on the World Health Organization (WHO) charts, as US and international pediatric groups accept this method as valid. (2|⊕○○○)
-
1.5
We recommend against routine laboratory evaluations for endocrine etiologies of pediatric obesity unless the patient’s stature and/or height velocity are attenuated (assessed in relationship to genetic/familial potential and pubertal stage). (1|⊕⊕⊕○)
-
1.6
We recommend that children or adolescents with a BMI of ≥85th percentile be evaluated for potential comorbidities (see Table 2 and Fig. 1). (1|⊕⊕⊕○)
-
1.7
We recommend against measuring insulin concentrations when evaluating children or adolescents for obesity. (1|⊕⊕⊕○)
Table 2.
Screening for Comorbidities of Pediatric Overweight or Obesity
| Comorbidity | Tests and Interpretation | Source |
|---|---|---|
| Prediabetes HbA1c | 5.7% to <6.5% (39 to <48 mmol/mol) (note the unpredictability of this test in pediatrics in the text)a | American Diabetes Association (59) |
| IFG (verify fasting status) | Fasting plasma glucose of ≥100 but <126 mg/dL (≥5.6 but <7.0 mmol/L) | |
| IGT (if OGTT is used) | Two-hour glucose of ≥140 but <200 mg/dL (≥7.8 but <11.1 mmol/L) | |
| Diabetes mellitus | HbA1c ≥ 6.5% (≥48 mmol/mol)a,b | American Diabetes Association (59) |
| Fasting plasma glucose of ≥126 mg/dL (≥7.0 mmol/L) (fasting is defined as no caloric intake for 8 h)b | ||
| Two-hour plasma glucose of ≥200 mg/dL (≥11.1 mmol/L) during an OGTTb | ||
| In a patient with classic symptoms of hyperglycemia, a random plasma glucose of ≥200 mg/dL | ||
| Dyslipidemia | Fasting lipids | Expert Panel Summary Report (58) |
| Triglycerides (mg/dL) (multiply by 0.0113 to convert to mmol/L): 0–9 y < 75 (acceptable), 75–99 (borderline high), ≥100 (high); 10–19 y < 90 (acceptable), 90–129 (borderline high), ≥130 (high) | ||
| LDL cholesterol (mg/dL) (multiply by 0.0259 to convert to mmol/L): <110 (acceptable), 110–129 (borderline high), ≥130 (high) | ||
| Total cholesterol (mg/dL) (multiply by 0.0259 to convert to mmol/L): <170 (acceptable), 170–199 (borderline high), ≥200 (high) | ||
| HDL cholesterol (mg/dL) (multiply by 0.0259 to convert to mmol/L): <40 (low), 40–45 (borderline low), >45 (acceptable) | ||
| Non–HDL cholesterol (mg/dL) (multiply by 0.0259 to convert to mmol/L) (can be nonfasting) | ||
| <120 (acceptable), 120–144 (borderline high), ≥145 (high) | ||
| Prehypertension and hypertension | 3–11 y: (standardized according to sex, age, and height percentile) | Expert Panel Summary Report (58); Mancia et al., 2013 (61) |
| BP > 90th percentile to <95th percentile = prehypertension | ||
| BP ≥ 95th percentile to <99th percentile + 5 mm Hg = stage 1 HTN | ||
| BP ≥ 99th percentile + 5 mm Hg = stage 2 HTN | ||
| 12–17 y: (standardized according to sex, age, and height percentile) | ||
| BP of >90th percentile to <95th percentile or >120/80 = prehypertension | ||
| BP ≥ 95th percentile to <99th percentile + 5 mm Hg = stage 1 HTN | ||
| BP ≥ 99th percentile + 5 mm Hg = stage 2 HTN | ||
| 18 to 21 y: | ||
| BP ≥ 120/80 to 139/89 mm Hg = prehypertension | ||
| BP ≥ 140/90 to 159/99 mm Hg = stage 1 HTN | ||
| BP ≥ 160/100 to 179/109 mm Hg = stage 2 HTN | ||
| BP > 180/110 mm Hg = stage 3 HTN | ||
| NAFLD | ALT > 25 U/L (boys) and >22 U/L (girls) | Schwimmer et al., 2010 (62) |
| PCOS | Free and total testosterone and SHBG, per Endocrine Society PCOS guidelinesc | Legro et al., 2013 (63) |
| Obstructive sleep apnea | If positive history, refer to pulmonary for nocturnal polysomnography and if not available overnight oximetry | Wise et al., 2011 (48) |
| Psychiatric | If positive history, refer to mental health specialist | Zamethkin et al., 2004 (51) |
To convert mg/dL to mmol/L, multiply by 0.0555 for glucose, 0.0259 for cholesterol, and 0.0113 for triglycerides.
Abbreviations: BP, blood pressure; HDL, high-density lipoprotein; HTN, hypertension; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; LDL, low-density lipoprotein; NAFLD, non-alcoholic fatty liver disease; OGTT, oral glucose tolerance test (1.75 g/kg, maximum 75 g); PCOS, polycystic ovary syndrome.
The test should be performed in a laboratory using a method that is NGSP certified and standardized to the DCCT assay.
In the absence of unequivocal hyperglycemia, should be confirmed by repeat testing.
Given variability in testosterone levels and the poor standardization of assays, it is difficult to define an absolute level that is diagnostic of PCOS or other causes of hyperandrogenism (familiarity with local assays recommended) (63). The preferred assay is HPLC tandem mass spectroscopy (64). [Derived from (a) ADA, 2014 (60); (b) Expert Panel 2011 (58); (c) Schwimmer et al., 2010 (62); (d) Legro et al., 2013 (63); (e) Wise et al., 2011 (48); (f) Zametkin et al., 2004 (51)].
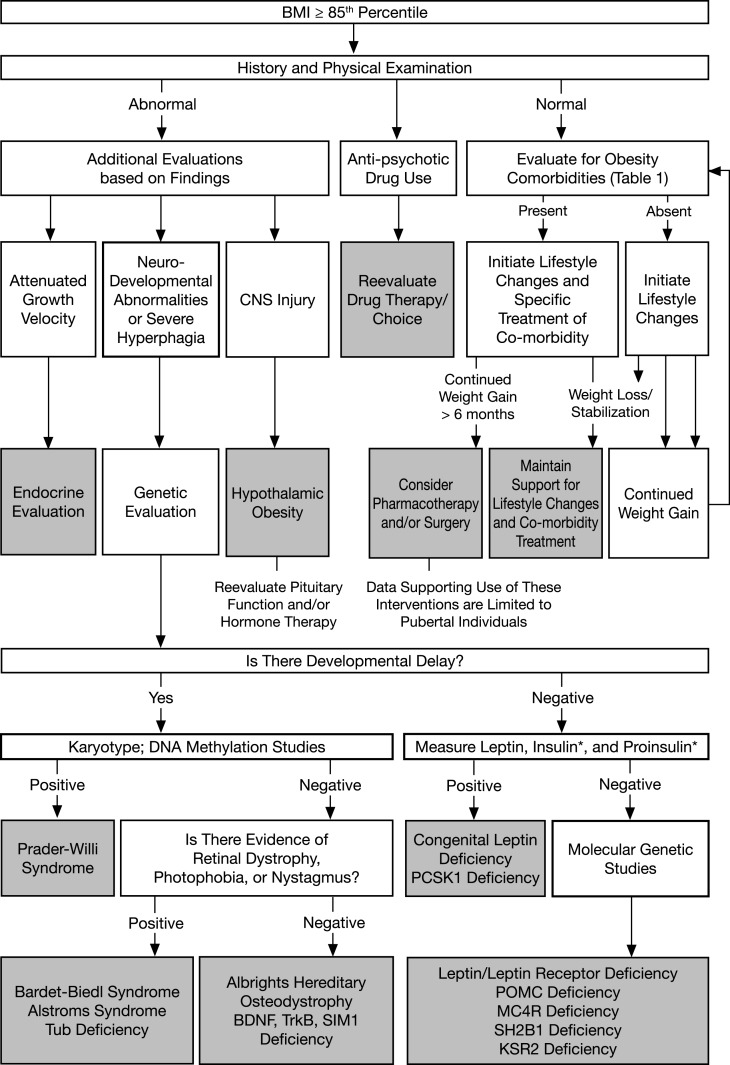
Figure 1.
Diagnosis and management flowchart. *Measure insulin and proinsulin in patients with clinical features of PCSK1 deficiency. [Adapted from August GP et al. (86) with permission, © Endocrine Society.] [Republished with permission of Springer Science and Bus Media BV from Farooqi S and O'Rahilly S (87); permission conveyed through Copyright Clearance Center, Inc.]
2.0 Genetic obesity syndromes
-
2.1
We suggest genetic testing in patients with extreme early onset obesity (before 5 years of age) and that have clinical features of genetic obesity syndromes (in particular extreme hyperphagia) and/or a family history of extreme obesity. (2|⊕⊕○○)
3.0 Prevention of obesity
-
3.1
We suggest that clinicians promote and participate in the ongoing healthy dietary and activity education of children and adolescents, parents, and communities, and encourage schools to provide adequate education about healthy eating (1). (2|⊕○○○)
-
3.2
We recommend that clinicians prescribe and support healthy eating habits such as:
-
•
avoiding the consumption of calorie-dense, nutrient-poor foods (e.g., sugar-sweetened beverages, sports drinks, fruit drinks, most “fast foods” or those with added table sugar, high-fructose corn syrup, high-fat or high-sodium processed foods, and calorie-dense snacks)
-
•
encouraging the consumption of whole fruits rather than fruit juices. (1|⊕⊕○○)
-
•
-
3.3
We recommend that children and adolescents engage in at least 20 minutes, optimally 60 minutes, of vigorous physical activity at least 5 days per week to improve metabolic health and reduce the likelihood of developing obesity. (1|⊕⊕○○)
-
3.4
We suggest fostering healthy sleep patterns in children and adolescents to decrease the likelihood of developing obesity due to changes in caloric intake and metabolism related to disordered sleep. (2|⊕⊕○○)
-
3.5
We recommend balancing unavoidable technology-related screen time in children and adolescents with increased opportunities for physical activity. (1|⊕⊕○○)
-
3.6
We suggest that a clinician’s obesity prevention efforts enlist the entire family rather than only the individual patient. (2|⊕○○○)
-
3.7
We suggest that clinicians assess family function and make appropriate referrals to address family stressors to decrease the development of obesity. (2|⊕⊕○○)
-
3.8
We suggest using school-based programs and community engagement in pediatric obesity prevention. (2|⊕⊕○○)
-
3.9
We recommend using comprehensive behavior-changing interventions to prevent obesity. Such programs would be integrated with school- or community-based programs to reach the widest audience. (1|⊕⊕○○)
-
3.10
We recommend breast-feeding in infants based on numerous health benefits. However, we can only suggest breast-feeding for the prevention of obesity, as evidence supporting the association between breast-feeding and subsequent obesity is inconsistent. (2|⊕○○○)
4.0 Treating obesity
Lifestyle: general considerations
-
4.1
We recommend that clinicians prescribe and support intensive, age-appropriate, culturally sensitive, family-centered lifestyle modifications (dietary, physical activity, behavioral) to promote a decrease in BMI. (1|⊕⊕⊕○)
-
4.2
We recommend that clinicians prescribe and support healthy eating habits in accordance with the following guidelines of the American Academy of Pediatrics and the US Department of Agriculture:
-
•
decreased consumption of fast foods
-
•
decreased consumption of added table sugar and elimination of sugar-sweetened beverages
-
•
decreased consumption of high-fructose corn syrup and improved labeling of foods containing high-fructose corn syrup
-
•
decreased consumption of high-fat, high-sodium, or processed foods
-
•
consumption of whole fruit rather than fruit juices
-
•
portion control education
-
•
reduced saturated dietary fat intake for children and adolescents >2 years of age
-
•
US Department of Agriculture recommended intake of dietary fiber, fruits, and vegetables
-
•
timely, regular meals, and avoiding constant “grazing” during the day, especially after school and after supper
-
•
recognizing eating cues in the child’s or adolescent’s environment, such as boredom, stress, loneliness, or screen time
-
•
encouraging single portion packaging and improved food labeling for easier use by consumers. (Ungraded Good Practice Statement)
-
•
-
4.3
We recommend that clinicians prescribe and support the reduction of inactivity and also a minimum of 20 minutes of moderate to vigorous physical activity daily, with a goal of 60 minutes, all in the context of a calorie-controlled diet. (1|⊕⊕○○)
-
4.4
We suggest that clinicians encourage and support patients to limit nonacademic screen time to 1 to 2 hours per day and decrease other sedentary behaviors, such as digital activities. (2|⊕○○○)
-
4.5
We suggest that the health care team identify maladaptive rearing patterns related to diet and activity and educate families about healthy food and exercise habits. (2|⊕○○○)
-
4.6
We suggest that the health care team probe for and diagnose unhealthy intrafamily communication patterns and support rearing patterns that seek to enhance the child’s or adolescent’s self-esteem. (2|⊕○○○)
-
4.7
We suggest that the health care team evaluate for psychosocial comorbidities and prescribe assessment and counseling when psychosocial problems are suspected. (2|⊕○○○)
-
4.8
We suggest pharmacotherapy for children or adolescents with obesity only after a formal program of intensive lifestyle modification has failed to limit weight gain or to ameliorate comorbidities (2|⊕○○○). We recommend against using obesity medications in children and adolescents <16 years of age who are overweight but not obese, except in the context of clinical trials. (1|⊕○○○)
-
4.9
We suggest that Food and Drug Administration (FDA)–approved pharmacotherapy for obesity be administered only with a concomitant lifestyle modification program of the highest intensity available and only by clinicians who are experienced in the use of anti-obesity agents and are aware of the potential for adverse reactions. (2|⊕○○○)
-
4.10
We suggest that clinicians should discontinue medication and reevaluate the patient if the patient does not have a >4% BMI/BMI z score reduction after taking antiobesity medication for 12 weeks at the medication’s full dosage. (2|⊕○○○)
-
4.11
We suggest bariatric surgery only under the following conditions:
-
•
the patient has attained Tanner 4 or 5 pubertal development and final or near-final adult height, the patient has a BMI of >40 kg/m2 or has a BMI of >35 kg/m2 and significant, extreme comorbidities
-
•
extreme obesity and comorbidities persist despite compliance with a formal program of lifestyle modification, with or without pharmacotherapy
-
•
psychological evaluation confirms the stability and competence of the family unit [psychological distress due to impaired quality of live (QOL) from obesity may be present, but the patient does not have an underlying untreated psychiatric illness]
-
•
the patient demonstrates the ability to adhere to the principles of healthy dietary and activity habits
-
•
there is access to an experienced surgeon in a pediatric bariatric surgery center of excellence that provides the necessary infrastructure for patient care, including a team capable of long-term follow-up of the metabolic and psychosocial needs of the patient and family. (2|⊕⊕○○)
-
•
-
4.12
We suggest against bariatric surgery in preadolescent children, pregnant or breast-feeding adolescents (and those planning to become pregnant within 2 years of surgery), and in any patient who has not mastered the principles of healthy dietary and activity habits and/or has an unresolved substance abuse, eating disorder, or untreated psychiatric disorder. (2|⊕○○○)
Method of Development of Evidence-Based Clinical Practice Guidelines
The Clinical Guidelines Subcommittee of the Endocrine Society deemed prevention and treatment of pediatric obesity a priority area in need of practice guidelines and appointed a Task Force to formulate evidence-based recommendations. The Task Force followed the approach recommended by the Grading of Recommendations, Assessment, Development, and Evaluation group, an international group with expertise in the development and implementation of evidence-based guidelines (2). A detailed description of the grading scheme has been published elsewhere (3). The Task Force used the best available research evidence to develop the recommendations. The Task Force also used consistent language and graphical descriptions of both the strength of a recommendation and the quality of evidence. In terms of the strength of a recommendation, strong recommendations use the phrase “we recommend” and the number 1, and weak recommendations ue the phrase “we suggest” and the number 2. Cross-filled circles indicate the quality of the evidence, such that ⊕○○○ denotes very low quality evidence; ⊕⊕○○, low quality; ⊕⊕⊕○, moderate quality; and ⊕⊕⊕⊕, high quality. The Task Force has confidence that persons who receive care according to the strong recommendations will derive, on average, more good than harm. Weak recommendations require more careful consideration of the person’s circumstances, values, and preferences to determine the best course of action. Linked to each recommendation is a description of the evidence and the values that the Task Force considered in making the recommendation; in some instances, there are remarks, a section in which the Task Force offers technical suggestions for testing conditions, dosing, and monitoring. These technical comments reflect the best available evidence applied to a typical person being treated. Often this evidence comes from the unsystematic observations of the Task Force and their values and preferences; therefore, one should consider these remarks as suggestions.
In this guideline, the Task Force made several statements to emphasize the importance of shared decision making, general preventive care measures, and basic principles of pediatric obesity prevention and treatment. They labeled these as “Ungraded Good Practice Statement.” Direct evidence for these statements was either unavailable or not systematically appraised, and thus considered out of the scope of this guideline. The intention of these statements is to draw attention and remind providers of these principles; one should not consider these statements as graded recommendations (4).
The Endocrine Society maintains a rigorous conflict-of-interest review process for developing clinical practice guidelines. All Task Force members must declare any potential conflicts of interest by completing a conflict-of-interest form. The Clinical Guidelines Subcommittee reviews all conflicts of interest before the Society’s Council approves the members to participate on the Task Force and periodically during the development of the guideline. All others participating in the guideline’s development must also disclose any conflicts of interest in the matter under study, and most of these participants must be without any conflicts of interest. The Clinical Guidelines Subcommittee and the Task Force have reviewed all disclosures for this guideline and resolved or managed all identified conflicts of interest.
Conflicts of interest are defined as remuneration in any amount from commercial interests; grants; research support; consulting fees; salary; ownership interests [e.g., stocks and stock options (excluding diversified mutual funds)]; honoraria and other payments for participation in speakers’ bureaus, advisory boards, or boards of directors; and all other financial benefits. Completed forms are available through the Endocrine Society office.
The Endocrine Society provided the funding for this guideline; the Task Force received no funding or remuneration from commercial or other entities.
Commissioned Systematic Review
The Task Force commissioned 2 systematic reviews to support this guideline [Treatments of Pediatric Obesity: An Umbrella Systematic Review (5); The Association of Weight Loss and Cardiometabolic Outcomes in Obese Children: Systematic Review and Meta-Regression (6)]. The first was an umbrella review of randomized controlled trials (RCTs) that had a duration >6 months and evaluated medication, surgery, lifestyle, or community-based interventions in overweight or obese children or adolescents. The purpose of this review was to estimate the effectiveness of these interventions and to rate the quality of supporting evidence. This review summarized data from 133 RCTs enrolling 30,445 patients and provided an evidence profile for each intervention. The second was a study-level meta-regression that identified changes in BMI associated with cardiometabolic changes (lipid panel, liver function tests, systolic blood pressure, diastolic blood pressure, hemoglobin A1c (HbA1c), and fasting blood glucose) in pediatric overweight and obese subjects.
The Problem With Obesity
Pediatric obesity is a persistent, epidemic, international problem, and preventing pediatric obesity and its comorbidities is of paramount importance. Treating children or adolescents is difficult and requires changes in diet, activity, and environment. Intensive lifestyle interventions, contacting both patient and family at least monthly (and weekly if possible) for the first 3 months, and providing dietary and nutritional education, a physical activity prescription, and behavioral therapy are poorly reimbursed, which often impedes these services. Additionally, there is inadequate national and international recognition of the value of addressing global obesity prevention and treatment, and we must work with key policymakers to improve this. Elevated BMI among US children and adolescents 6 to 19 years of age is associated with 1.4 billion dollars of additional health care dollars for outpatient visits and other health care expenditures compared with children and adolescents with normal BMIs (7). The Brookings Institution predicted that if all 12.7 million US children and adolescents with obesity became obese adults, the individual average cost would be >$92,000, and the societal costs during their lifetimes might be >$1.1 trillion (8).
1.0 Diagnosing overweight and obesity
-
1.1
We recommend using BMI and the CDC normative BMI percentiles to diagnose overweight or obesity in children and adolescents ≥2 years of age. (1|⊕⊕⊕○)
-
1.2
We recommend diagnosing a child or adolescent >2 years of age as overweight if the BMI is ≥85th percentile but <95th percentile for age and sex, as obese if the BMI is ≥95th percentile, and as extremely obese if the BMI is ≥120% of the 95th percentile or ≥35 kg/m2 (1|⊕⊕○○). We suggest that clinicians take into account that variations in BMI correlate differently to comorbidities according to race/ethnicity and that increased muscle mass increases BMI. (2|⊕○○○)
-
1.3
We suggest calculating, plotting, and reviewing a child’s or adolescent’s BMI percentile at least annually during well-child and/or sick-child visits. (Ungraded Good Practice Statement)
-
1.4
We suggest that a child <2 years of age be diagnosed as obese if the sex-specific weight for recumbent length is ≥97.7th percentile on the WHO charts, as US and international pediatric groups accept this method as valid. (2|⊕○○○)
Definitions
Children and adolescents ≥2 years of age are diagnosed as overweight if the BMI is ≥85th percentile but <95th percentile and obese if the BMI is ≥95th percentile for age and gender on the revised 2000 CDC charts. A child <2 years of age is obese if the weight for recumbent length is ≥97.7th percentile of WHO growth standards (9). Extreme obesity is defined as a BMI ≥120% of the 95th percentile or ≥35 kg/m2 (10). A recent proposal suggests redefining this state as class 2 obesity, as it relates to the definition of class 2 obesity in adults; class 3 pediatric obesity is proposed (but not yet fully accepted) to be BMI ≥140% of the 95th percentile or ≥40 kg/m2, as this is considered to represent an even higher risk group. Class 2 and class 3 obesity are increasing significantly in girls of all ages, most clearly between 6 and 11 years of age, and in boys between 12 and 19 years of age with a nonsignificant trend in boys <12 years of age (11).
Evidence
The CDC BMI charts (12) are the accepted standards for US children and adolescents ≥2 years of age and provide a means for determining changes in pediatric obesity prevalence. The US Preventive Services Task Force found that the BMI of children and adolescents correlates reasonably well to percentile rankings of percent body fat measured by more direct methods (13). However, BMI cannot differentiate muscle from adipose tissue, and thus cannot differentiate between excess adipose tissue and increased lean muscle mass when classifying a child or adolescent as overweight or obese. Pediatric racial/ethnic differences in the percentage of fat at a specific BMI further complicate BMI measures; for example, non-Hispanic black children and adolescents have a lower percentage body fat than do comparable non-Hispanic whites or Mexican Americans at the same BMI, and they are less likely to have high adiposity (14). Additionally, Singapore Chinese adolescents have a higher percentage fat at the same BMI than do white comparison groups (15). Furthermore, in the 1999–2002 National Health and Nutrition Examination Survey obese male Hispanic adolescents had a higher risk of hepatic steatosis than did girls and other ethnic groups, indicating the limits of BMI alone as a risk factor (16). A systematic review found differences in regional mass and body composition in adults between race/ethnic groups when BMI and height are held constant and further differences within the same gender and race/ethnic group by age (17). Therefore, although we do recommend using BMI in clinical practice, it is not an infallible indicator of overweight or obesity. Clinicians should consult endocrinologists when questions arise.
The prevalence of pediatric overweight and obesity in all racial and ethnic groups increased between the 1960s and 1970s until about 2000 when it leveled off in most groups (Table 1). As of 2014, the prevalence of obesity in subjects 2 to 19 years old is 17%. The reason 17% of the population is above the 95th percentile for age is that the CDC only uses weight data prior to 1980 (using NHANES II data) for ages >6 years (before the obesity epidemic developed) and uses height data up to the end of 1994 (the end of NHANES III data collection) for the stature charts. Some recent data suggest a decrease in the prevalence of overweight and obesity in children <5 years of age, but the durability of this potential decline remains unknown. This trend may be explained by the oversampling of Asian preschoolers in that particular dataset; these children had a lower overall BMI.
Table 1.
Prevalence of Pediatric Overweight and Obesity in the United States
| Age | Obesity |
Combined Overweight and Obesity |
|||||
|---|---|---|---|---|---|---|---|
| 1963–1970 | 1999–2000 | 2004 | 2011–2014 | 1999–2000 | 2003–2004 | 2011–2012 | |
| 0–23 mo | 7.20% | 11.60% | 8.10% | ||||
| 2–5 y | 5 | 10.50% | 13.90% | 8.90% | 22.0% | 26.50% | 22.80% |
| 6–11 y | 4.20% | 15.30% | 18.80% | 17.50% | 29.8% | 37.20% | 34.20% |
| 12–19 y | 4.60% | 15.50% | 17.40% | 20.50% | 30.0% | 34.30% | 34.50% |
| 2–19 y | 13.9% | 17.1% | 17.0% | 28.2% | 33.60% | 31.80% | |
| 12–19 y by race | Hispanic | 21.90% | 43.3 | 34.3% | 38.1 | ||
| Boys | 22.4 | 43.6 | 37.3% | 39.6% | |||
| Girls | 221.4 | 42.9 | 31.1% | 36.5% | |||
| African American | 19.50% | 39.5 | 36.5% | 39.8% | |||
| Boys | 18.40% | 35.6 | 31.4% | 37.3% | |||
| Girls | 20.70% | 43.7 | 42.1% | 42.5% | |||
| White | 14.70% | 26.2 | 34.7% | 31.2% | |||
| Boys | 14.30% | 27.4 | 38.70% | 31.5% | |||
| Girls | 15.10% | 24.8 | 30.4% | 31.0% | |||
| Asian | 8.60% | — | — | 24.6% | |||
| Boys | 11.80% | — | — | 33.9% | |||
| Girls | 5.30% | — | — | 15.0% | |||
| All | 16.90% | 30.0 | 34.30% | 34.5% | |||
| Boys | 16.90% | 30.0 | 36.8% | 35.1% | |||
| Girls | 17.10% | 30.0 | 31.70% | 33.8% | |||
Different racial and ethnic populations demonstrate differences in the prevalence of obesity and overweight and in the trajectory of change during the last decades (Table 1). Thus, using these BMI definitions may underestimate risk to the health of pediatric Asian patients. Furthermore, a recent meta-analysis including 53,521 patients between a mean age of 4 to 18 years demonstrated that using these BMI cutoffs led to a specificity of 0.93 but a sensitivity of only 0.73 when compared with reference standard methods for measuring body adiposity, such as dual energy X-ray absorptiometry, hydrostatic weighing, air-displacement plethysmography, isotope dilution, bioelectrical impedance analysis, and skin-fold thickness measurements. This suggests that most children and adolescents diagnosed as obese by BMI do indeed have excess fat, but that a normal BMI is compatible with excess body fat in ∼25% of subjects (22). Clinical judgment must augment the definitions of obesity based on BMI alone to determine which children or adolescents are actually overfat.
The odds ratio of adult obesity increases for obese adolescents as they approach 18 years of age. The odds ratio of adult obesity rises progressively with the number of parents who are obese, but the greatest predictive effect of parent obesity is found in infancy regardless of the infant’s weight (23). Determining overweight or obesity in young children may also help identify which individuals are most likely to become overweight or obese in adulthood. There is an increase in BMI in the first year followed by a fall and then a second rise in BMI at about 6 years of age (termed the adiposity rebound); an early BMI rebound before 5 years of age carries a higher risk for adult obesity. Recent analysis suggests that BMI (or possibly just height) at 7 years of age may provide equally robust predictive ability (24). Longitudinal data from 7738 participants from the National Center for Educational Statistics, Early Childhood Longitudinal Study, Kindergarten Class of 1998–1999 demonstrated the greatest incidence (new onset) of obesity and overweight between the first to third grades; furthermore, there was a fourfold higher risk for obesity at age 14 years in the subjects overweight in kindergarten. These data support a focus on prevention before 9 years of age (25). However, a longitudinal study of 4884 subjects from the National Longitudinal Survey of Youth, the Population Study of Income Dynamics, and the National Health and Nutrition Evaluation Surveys demonstrated that screening for obesity at 5 years of age would miss 50% of those who became obese by 18 years of age, whereas screening at 15 years of age would miss only 9%; the authors recommend using universal prevention methods instituted at a young age and continuing through childhood and adolescence, rather than focusing only on overweight young children (26). These contrasting study conclusions demonstrate a continued need for research into childhood prediction of obesity.
Values and preferences
The Task Force placed a high value on the ease of calculating BMI and familiarity with this measure among providers and patients over other limitations of using BMI. BMI currently is the most reasonable measure for evaluating overweight and obesity, guiding proper management, and determining the need for specialist referral (when values rise toward the extreme). The utility of predicting adult obesity and comorbidities from childhood and adolescent BMI calculations may be somewhat limited, supporting a universal prevention approach to obesity that begins in early childhood.
-
1.5
We recommend against routine laboratory evaluations for endocrine etiologies of pediatric obesity unless the patient’s stature and/or height velocity are attenuated (assessed in relationship to genetic/familial potential and pubertal stage). (1|⊕⊕⊕○)
Evidence
Endocrine and syndromic disorders as a cause of overweight/obesity are rare in children and adolescents and are associated with additional symptoms (26). The distinguishing feature of endocrine causes of obesity, such as growth hormone (GH) deficiency, hypothyroidism, or Cushing syndrome, is that stature and height velocity are decreased, whereas a normal or increased growth rate generally excludes endocrine causes. However, Albright hereditary osteodystrophy/pseudohypoparathyroidism, although associated with short stature in adolescence, may be associated with increased growth velocity in the first 2 to 3 years of life. Pediatric overweight/obesity is also associated with earlier breast development, pubarche, and menarche in girls, and advanced skeletal development in boys that will lead to increased growth rate (27–30). The evidence is stronger in girls than boys because a subgroup of boys with obesity exhibit delayed testicular development (31). Thus, clinicians should not test for endocrine causes of obesity unless the patient is short relative to genetic potential and has decreased growth velocity against the backdrop of continued weight gain (26, 32).
This rule is not inviolable, however, as acquired hypothalamic obesity is a syndrome of intractable weight gain caused by hypothalamic damage from a tumor or its treatment with surgery or radiotherapy (33). Such patients may have adequate growth velocity even when GH deficient but have tumor-related signs and symptoms or have already undergone tumor treatment.
Values and preferences
The Task Force placed a high value on limiting endocrine assessments for the etiology of pediatric overweight or obesity to those rare patients who are obese and short or with decreased height velocity and placed a low value on the unnecessary diagnostic endocrine laboratory screening of children and adolescents who are obese without other signs or symptoms or contributory neurosurgical history.
Remarks
Clinicians can determine a deceleration in height velocity (as needed to account for the stage of puberty) either by using a height velocity (34) curve normalized for age and/or stage of puberty or by observing that the patient is crossing height percentile curves downward on the standardized height attainment charts (12) for average-maturing, early-maturing, and late-maturing children (35). Clinicians should refer maturing children who are obese with short stature and decreased growth velocity despite continued weight gain to a pediatric endocrinologist, as these patients may have an endocrinopathy.
Evidence
Pediatric overweight and obesity is associated with substantial comorbidities, including prediabetes/type 2 diabetes mellitus (T2DM); dyslipidemia; prehypertension/hypertension; sleep apnea; nonalcoholic fatty liver disease (NAFLD); proteinuria and focal segmental glomerulosclerosis; early subclinical atherosclerosis; hyperandrogenemia/polycystic ovary syndrome (PCOS); slipped capital femoral epiphysis and pseudotumor cerebri (36–42); and cardiovascular disease (CVD) morbidity, and premature mortality in adulthood (43–47). The greater the severity of obesity, the higher the risks of cardiometabolic risk factors, particularly among boys (11). Importantly, the risks of CVD outcomes among children and adolescents who were obese and became nonobese by adulthood appear similar to those who were never obese (46). Thus, clinicians should carefully examine medical and family histories and laboratory assessments of children and adolescents who are overweight or obese to identify comorbidities early and initiate appropriate management.
Values and preferences
The Task Force placed a high value on identifying adiposity-related complications and screening for comorbidities because of their high prevalence and their association with morbidity and mortality. The Task Force also placed a high value on reducing unnecessary testing and evaluation, such as the routine measurements of fasting insulin, because of lack of scientific evidence for its usefulness in clinical practice by general providers.
Remarks
A thorough medical and family history is crucial for assessing obese youths, because obesity and associated comorbidities may be asymptomatic/subclinical but have familial tendencies. The family history should encompass obesity; bariatric surgery (typically not revealed by families unless specifically asked); T2DM; gestational diabetes; dyslipidemia; hypertension; NAFLD; cirrhosis; sleep apnea and use of continuous positive airway pressure; premature CVD events/deaths (such as heart attacks or strokes); and (in women) infertility, PCOS, or hyperandrogenism-associated signs and symptoms. Clinicians should assess the presence of polyuria/polydipsia, blurry vision, fungal vaginitis/discharge in girls, and unexplained weight loss, all of which could be indicative of hyperglycemia. Clinicians should also look for the presence of frequent unexplained headaches, which raise the possibility of hypertension or sleep apnea; habitual snoring, restless sleep, morning headaches, generalized tiredness, and/or excessive daytime sleepiness, as well as hyperactive inattentive behavior in young children as manifestations of sleep apnea (48); gastrointestinal discomfort as a manifestation of NAFLD (39); musculoskeletal symptoms (49); and (in pubertal girls) acne, hirsutism (including the recent use of hair removal techniques that would mask the degree of hirsutism at the time of the examination), and onset and pattern of menses to screen for the possibility of PCOS. Clinicians should obtain a careful history for psychiatric disorders, because children and adolescents who are overweight or obese are more likely to suffer from mental health disorders than their normal weight counterparts (50, 51). Furthermore, clinicians should obtain a history of second-generation antipsychotics use, such as clozapine, risperidone, olanzapine, and quetiapine, because of their association with weight gain (52, 53). Although the various techniques assessing dietary intake are unreliable and subject to error (9, 54), it is still important to estimate the type and quantity of beverage intake, the frequency of dining out and where, and the frequency and type of snacks (among other dietary issues). Clinicians should also obtain a history of sedentary behaviors, such as hours spent on screen activities, and physical activity (e.g., duration, frequency, in school and at home, sports participation, walking to school and stores).
Clinicians should evaluate the following:
-
•
weight, height, and BMI calculation [Even though the International Diabetes Federation includes waist circumference (an indicator of insulin resistance measured at the level of the iliac crest ≥ 90th percentile) as a defining factor for metabolic syndrome in children and adolescents 10 to 16 years of age and as a finding of concern in children 6 to 10 years old (55, 56), given the intermeasurement variability of waist circumference measurements in a clinical setting performed by different support staff, this research tool does not add significantly to what we learn from BMI (57).]
-
•
blood pressure [using height/age/sex percentile normalized blood pressure tables to interpret the findings (58)]
-
•
acanthosis nigricans and skin tags
-
•
extreme acne and hirsutism in pubertal girls
-
•
fundoscopic examination for pseudotumor cerebri
-
•
tenderness and range of motion of the knee, leg, or foot
-
•
peripheral edema, thyroid examination for goiter
-
•
physical findings associated with syndromic obesity, particularly if there is a neurodevelopmental abnormality (see section 3).
We list suggested screening tests in Table 2.
In 2009 an International Expert Committee recommended using HbA1c to diagnose diabetes and prediabetes (65). It recommended classifying asymptomatic individuals as having diabetes if they had HbA1c ≥ 6.5% (≥48 mmol/mol) on 2 separate occasions and classifying asymptomatic individuals with prediabetes if they had HbA1c ≥ 6.0% (≥42 mmol/mol) (65), or HbA1c of 5.7% to <6.5% (39 to <48 mmol/mol) (66). Although they based these recommendations on studies in adults with no validation in pediatrics (65), the committee recommended that the same criteria be applied in adolescents. However, several studies have demonstrated poor performance of HbA1c in diagnosing prediabetes or diabetes in pediatrics, underestimating the prevalence of both (67–69). Another pitfall in using the HbA1c is the unresolved issue of racial/ethnic disparities in the correlation between HbA1c and ambient blood glucose (70). Given such drawbacks, HbA1c screening (alone) in overweight or obese children and adolescents is a poor diagnostic tool for prediabetes and T2DM. Additional definitive testing (fasting or random glucose or oral glucose tolerance test) may be necessary in high-risk youths based on medical history, familial risk, race/ethnicity, and/or the presence of additional risk factors for diabetes (71). In a cost effectiveness analysis of various screening strategies for identifying pediatric diabetes and dysglycemia, the preferred strategy for dysglycemia was the 2-hour oral glucose tolerance test with 100% effectiveness (proportion of cases identified) and efficiency (cost per case identified) at $390 per case, and the least effective and efficient was HbA1c (ranges, 7% to 32% and $938 to $3370 per case) (72).
NAFLD is usually asymptomatic and thus requires screening for detection. Presently, no screening guidelines exist outside of recognizing those at risk by weight categorization (BMI ≥ 85% for age and sex) (39). Recently new normative standards were proposed for alanine aminotransferase (ALT) concentrations (≤25 U/L for boys and ≤ 22U/L for girls) (62), because pediatric liver biopsy specimens from patients with normal or mildly elevated ALT (≥26 to 50 U/L for boys and ≥23 to 44 U/L for girls) had significant histologic abnormalities, including advanced fibrosis (73). Using highly sensitive research methods of magnetic resonance spectroscopy or magnetic resonance imaging, fatty liver is likely present in most pediatric obesity whether the liver enzymes are high or not. High ALT levels would suggest a more advanced stage of NAFLD, hepatitis, or fibrotic changes. Thus, even though ALT elevation underestimates liver injury in NAFLD, it is still an easily available screen for clinicians to use when assessing children and adolescents who are overweight or obese.
Many clinicians measure insulin values thinking it adds to the diagnosis of comorbidities. In fact it does not, and such measurements are not recommended. Although obesity is associated with insulin resistance/hyperinsulinemia, attempts to diagnose insulin resistance by measuring plasma insulin concentration or any other surrogate (74) in the clinical setting has no merit because it has no diagnostic value. Fasting insulin concentrations show considerable overlap between insulin-resistant and insulin-sensitive youths (74). Therefore, there is no well-defined cut point differentiating normal from abnormal and no universally accepted, clinically useful, numeric expression that defines insulin resistance (75), unlike the case for glucose or lipids. A major requirement for any screening program is the availability of an accurate, reliable, reproducible, standardized, and easily applicable method of measurement. Adult studies have shown that measures of fasting insulin explain no more than 5% to 50% of the variability in insulin sensitivity in nondiabetic subjects (76). Different studies have proposed different cutoffs for so called “insulin resistance values” varying by 2.5-fold (76). In pediatrics, the transient puberty-related insulin resistance that occurs with the completion of puberty further complicates this (77, 78). Moreover, measuring insulin is hampered by the lack of standardized insulin assays, and poor reproducibility of even the same assay (79). Further limitations include race/ethnicity-related differences in insulin concentrations due to differences in the metabolic clearance rate of insulin (80) and the cross-reactivity between insulin and proinsulin. In youths with T2DM, despite severe deficiency in insulin secretion, fasting insulin concentrations are higher than in youths without diabetes (81). Importantly, fasting insulin concentrations are similar in youths who are obese with normal glucose tolerance vs impaired glucose tolerance (82), allowing for the possible danger of missing a diagnosis of impaired glucose tolerance if one uses fasting insulin concentrations as a screening tool. Because of these limitations, measuring plasma insulin concentrations remains a research tool with no clinical value for evaluation of obesity. Measuring fasting insulin concentrations to try to diagnose insulin resistance within general practice should be abandoned.
2.0 Genetic obesity syndromes
-
2.1
We suggest genetic testing in patients with extreme early onset obesity (before 5 years of age) and that have clinical features of genetic obesity syndromes (in particular extreme hyperphagia) and/or a family history of extreme obesity. (2|⊕⊕○○)
Evidence
In addition to the obvious environmental drivers, multiple common and rare genetic variants contribute to substantial heritability for BMI and waist circumference (83, 84). Approximately 7% of patients with extreme pediatric obesity may have rare chromosomal abnormalities and/or highly penetrant genetic mutations that drive their obesity (85). This percentage is likely to increase with newer methods for genetic testing.
Values and preferences
When assessing children and adolescents with extreme obesity, clinicians should consider potentially treatable causes and genetic conditions (Fig. 1). The diagnosis of a genetic obesity syndrome can provide information that helps the family and health care providers appropriately manage the child’s or adolescent’s health and possibly lessen the social stigma. Additionally, clinicians can provide genetic counseling. A genetic diagnosis can inform management, including the possibility of bariatric surgery (many such patients are relatively resistant to weight loss through changes in diet and exercise).
Remarks
It is currently useful to categorize genetic obesity syndromes as those with developmental delay and/or dysmorphism and those without these features, although the clinical spectrum can be quite variable (Table 3). Clinicians should obtain a careful family history to identify potential consanguineous relationships, a family history of severe obesity/bariatric surgery, the ethnic and geographical origin of the child or adolescent, and family members to guide the appropriate use of diagnostic tests (Fig. 1).
Table 3.
Genetic Obesity Syndromes With and Without Developmental Delay
| Genetic Obesity Syndrome | Clinical Features |
|---|---|
| Obesity with developmental delay | |
| Dominant | |
| Prader-Willi syndrome | Hypotonia, failure to thrive in infancy followed by weight gain, short stature (due to GH deficiency), hyperphagia, hypogonadotropic hypogonadism, sleep disturbance, obsessive behaviors |
| Albright hereditary osteodystrophy | Short stature in some but not all patients, skeletal defects, impaired olfaction, and hormone resistance (e.g., parathyroid hormone) if a mutation is maternally inherited |
| SIM1 deficiency | Hyperphagia with autonomic dysfunction (characterized by low systolic blood pressure), speech and language delay, neurobehavioral abnormalities, including autistic type behaviors |
| BDNF/TrkB deficiency | Hyperactivity, impaired concentration, limited attention span, impaired short-term memory and pain sensation |
| Recessive | |
| Bardet-Biedl syndrome | Dysmorphic extremities (syndactyly/brachydactyly/polydactyly), retinal dystrophy or pigmentary retinopathy, hypogonadism, renal abnormalities/impairment |
| TUB deficiency | Retinal dystrophy, deafness |
| Obesity without developmental delay | |
| Dominant | |
| Alström syndrome | Retinal dystrophy; extreme insulin resistance; deafness; dilated cardiomyopathy; progressive pulmonary, hepatic, and renal dysfunction |
| MC4R deficiency | Hyperphagia, accelerated linear growth, disproportionate hyperinsulinemia, low/normal blood pressure |
| SH2B1 deficiency | Hyperphagia, disproportionate hyperinsulinemia, early speech and language delay that often resolves, behavioral problems including aggression |
| KSR2 deficiency | Mild hyperphagia and reduced basal metabolic rate, insulin resistance often with acanthosis nigricans, irregular menses, early development of T2DM |
| Recessive | |
| Leptin deficiency | Extreme hyperphagia, frequent infections, hypogonadotropic hypogonadism, mild hypothyroidism |
| Leptin receptor deficiency | Extreme hyperphagia, frequent infections, hypogonadotropic hypogonadism, mild hypothyroidism |
| POMC deficiency | Hyperphagia, cholestatic jaundice or adrenal crisis due to ACTH deficiency, pale skin, and red hair in whites |
| PCSK1 deficiency | Small bowel enteropathy, hypoglycemia, hypothyroidism, ACTH deficiency, diabetes insipidus |
[Republished from Farooqi and O’Rahilly (87) with permission of Springer Science and Bus Media BV, permission conveyed through the Copyright Clearance Center.]
Abbreviations: ACTH, adrenocorticotropic hormone; BDNF, brain-derived neurotrophic factor; GH, growth hormone; POMC, proopiomelanocortin; T2DM, type 2 diabetes mellitus.
Obesity syndromes with developmental delay
Dominant disorders
Prader-Willi syndrome is a methylation disorder caused by the deletion of a critical segment on the paternally inherited chromosome 15q11.2-q12, loss of the entire paternal chromosome 15 with the presence of 2 maternal copies (uniparental maternal disomy), or an imprinting defect that can be sporadic or due to a mutation of the paternally derived imprinting control site of the 15q13 region (88). Plasma ghrelin levels are markedly elevated in children, adolescents, and adults with Prader-Willi syndrome, although the physiological relevance of this finding is unknown (89). GH treatments decrease body fat and increase linear growth, muscle mass, and energy expenditure (90).
Maternal transmission of heterozygous mutations in GNAS1 leads to classical Albright hereditary osteodystrophy and resistance to several hormones that activate heterotrimeric G proteins in their target tissues, whereas paternal transmission leads only to Albright hereditary osteodystrophy (91).
Chromosomal rearrangements and heterozygous mutations involving single-minded 1 brain-derived neurotrophic factor (92, 93), or its receptor, TrkB, lead to hyperphagia and developmental and behavioral abnormalities (94, 95). Clinicians should consider de novo mutations if both parents are of normal weight and intelligence quotient.
Recessive disorders
Homozygous mutations that disrupt 1/some of the 16 Bardet-Biedl syndrome genes lead to Bardet-Biedl syndrome (96). Other recessive disorders affecting proteins localized to the basal body of the monocilium, such as Alström syndrome and TUB gene mutations (97), are also associated with obesity.
Obesity syndromes without developmental delay
Rare copy number variants (deletions/duplications) that disrupt multiple genes can cause extreme pediatric obesity without learning difficulties (98). Mutations in specific genes, mostly involving the leptin–melanocortin pathway, cause extreme obesity characterized by hyperphagia (increased drive to eat) and impaired satiety (reduced sensation of fullness after a meal) (Table 3). Clinicians should take a careful history to identify food-seeking behavior, searching for/stealing food, waking at night to find food, and eating food others leave behind, which should prompt genetic investigation (neurologic causes should be excluded in patients with a new history of these behaviors). These behaviors typically occur as a result of the disruption of hypothalamic pathways involved in the regulation of energy balance. Pica syndrome is evident in only a small subset of children and adolescents with hyperphagia.
Dominant disorders
Heterozygous mutations in the melanocortin 4 receptor are found in 2% to 5% of subjects with extreme pediatric obesity, making this the most common genetic form of obesity (99, 100) (Table 3). Homozygous mutations in melanocortin 4 receptor have also been identified in offspring from consanguineous families (101). Heterozygous missense mutations affecting proopiomelanocortin-derived peptides and rare variants in melanocortin 2 receptor accessory protein 2 may also contribute to extreme obesity by modulating melanocortin signaling (102, 103). In the near future, selective melanocortin receptor agonists may be feasible therapies for patients with mutations in the melanocortin pathway. Several studies have shown that adolescents and adults with heterozygous melanocortin 4 receptor mutations lose weight following Roux-en-Y gastric bypass (RYGB) surgery (104).
Recessive disorders
Homozygous mutations that reduce the production, secretion, or biological activity of leptin are associated with extreme hyperphagia, frequent infections, hypogonadotropic hypogonadism, and mild hypothyroidism; these features can be fully treated with subcutaneous injections of recombinant human leptin (105–107). Recombinant human leptin is currently available on a named patient basis through selected centers.
Serum leptin is a useful test in patients with severe obesity, as undetectable serum leptin is highly suggestive of congenital leptin deficiency. Mutations that result in detectable but bioinactive leptin are rare (107). Serum leptin concentrations are usually appropriate for the degree of obesity in most patients with homozygous mutations in the leptin receptor gene that have comparable clinical features (108) (Table 3).
Children and adolescents who are homozygous or compound heterozygous for mutations in the proopiomelanocortin gene require long-term corticosteroid replacement, as proopiomelanocortin is a precursor of adrenocorticotropic hormone in the pituitary gland (102). Compound heterozygous or homozygous mutations in the PCSK1 gene, which encodes the processing enzyme (prohormone convertase 1/3), may present in infancy with persistent diarrhea requiring parenteral feeding. An abnormally high level of plasma proinsulin (compared with mature insulin) indicates this possible diagnosis (109).
3.0 Prevention of obesity
The prime objective in addressing the obesity epidemic should be prevention to avoid the comorbidities of obesity. Although beyond the scope of this statement, which addresses postnatal prevention, preconception and prenatal interventions are also of major importance, and the Task Force supports the recommendations of the WHO to address this area of prevention (110).
-
3.1
We suggest that clinicians promote and participate in the ongoing healthy dietary and activity education of children and adolescents, parents, and communities, and encourage schools to provide adequate education about healthy eating (1). (2|⊕○○○)
Evidence
The authors of the Endocrine Society’s previous guideline on pediatric obesity commissioned a meta-analysis (111) which summarized evidence from RCTs that measured the impact of lifestyle interventions to prevent pediatric obesity. The study found modest effects of these interventions; there was decreased sedentary behavior in long-term trials (P = 0.05) with a significantly greater effect when directed toward children in contrast to adolescents (P = 0.02), reduced unhealthy dietary habits (P = 0.02), but only a trend towards increased physical activity (P = 0.06–0.07). These beneficial effects did not translate into important changes in BMI (111), but the Task Force recognized that weight maintenance in a growing child or adolescent is as effective as weight loss in an adult. The present committee updated and expanded upon these findings as listed below and in Table 4.
Table 4.
Factors Associated With Prevention of Pediatric Obesity
| Study Format | Relationship | Source | Relationship to the Development of Obesity or Metabolic Improvement |
|---|---|---|---|
| 4.2 Increased sugar sweetened beverages intake | |||
| 2- to 5-y-old children from various periods of the National Health and Nutrition Examination Surveys | There was a decrease of 57 calories/d intake of sugar-sweetened beverages between 2003–2004 and 2009–2010 with no appreciable change in sugar intake thereafter up to 2011–2012 | Ford et al., 2015 (112) | Probable + |
| Cross-sectional analysis of 4880 children between 3 and 11 y from the National Health and Nutrition Examination Survey between 1999 and 2004 | Sugar-sweetened beverage intake was independently associated with decreased HDL, increased C-reactive protein, and increased waist circumference | Kosova et al., 2013 (113) | + |
| Longitudinal study of 9600 children in the Early Childhood Longitudinal Survey–Birth Cohort | There was a 1.4 odds ratio for being obese if a 5-y-old child drinks 4 or 5 sugar-sweetened beverages per day but no such risk for 2-y-old; however there was a significant influence on drinking sugar-sweetened beverages at 2 y of age and an increase in BMI z score during the next 2 y | DeBoer et al., 2013 (114) | + |
| Randomized controlled study of 224 teenagers that reduced sugar sweetened beverage intake | There was a decrease in the change in BMI and weight at 1 y but no difference at 2 y | Ebbeling et al., 2012 (115) | + |
| Eighteen-month study of 642 primarily normal-weight Dutch children aged 4 y 10 mo to 11 y 11 mo who were divided into groups receiving 8 ounces of sugar-free drink or 105 kcal containing sugar-sweetened drinks | There was an increased weight gain and increase in BMI in the sugar-sweetened group | de Ruyter et al., 2102 (116) | + |
| One hundred forty-six 7- to 11-y-olds drinking sugar-free or sugar-sweetened beverages | There was no difference in the level of satiety experienced; the conclusion is that the child will not compensate for all calories missing from nonsweetened drinks, which may partly explain a lower degree of weight gain with nonsweetened drink ingestion | de Ruyter et al., 2012 (116) | + |
| 4.3 Higher level of activitya | |||
| Meta-analysis of 11 RCTs of activity ranging in length from 20 min to >1 h/d and ranging in frequency from twice a week to every day of the school week | There was little effect on BMI, but there were decreases in triglycerides and systolic and diastolic blood pressure when the intervention lasted at least 6 mo; total cholesterol, however, did increase during some studies | Cesa et al., 2014 (117); Vasconcellos et al., 2014 (118) | − |
| Nine randomized controlled pediatric studies (n = 367) included in a meta-analysis | At least 3 mo of exercise in 3 sessions per week of 60 min each led to decreased fasting glucose and insulin and body fat | Garcia-Hermoso et al., 2014 (119) | − |
| Meta-analysis of 24 studies of fasting insulin levels and 12 studies on insulin resistance in pediatric normal weight overweight and obese | There was a small but positive effect in improving fasting insulin resistance in children, with the greatest effect occurring in those with the highest BMI standard deviation values | Fedewa et al., 2014 (120) | − |
| Systematic review of 16 studies of school-based jumping exercises | There was small positive effect of bone-targeted exercise on fat mass (SMD, −0.248; 95% CI, −0.406 to −0.089) and lean mass (SMD, 0.159; 95% CI, −0.076 to 0.394), but there are few studies | Nogueira and Hrovat, 2014 (121) | − |
| Meta-analysis of 40 studies on the effect of resistance training in pediatric overweight or obese | Resistance training in children and adolescents who are overweight and obese appears to generally have very small to small effects on body composition and moderate to large effects on strength | Schranz et al., 2013 (122) | − |
| Systematic review of 2 aggregate data meta-analyses representing 14 and 17 studies in 481 and 701 boys and girls, respectively | Exercise decreased the percentage of body fat but does not necessarily have an effect on BMI; therefore, replacing fat tissue with muscle may not necessarily be reflected by characteristic clinic-based anthropomorphic data | Kelley and Kelley, 2013 (123) | − |
| Randomized controlled pediatric study of >200 subjects who experienced 20 or 40 min of fun but nonetheless aerobic activity 5 d/wk during 13 wk | There was a dose response decrease in insulin resistance measured by the area under the curve of an oral glucose tolerance test, decreased total body fat and visceral fat, and a similar improvement in fitness measured by peak VO2; the conclusion is that there is benefit for a child who is obese if the child will actually engage in at least 20 min of aerobic exercise 5 d/wk, (and we expect this may extend to the prevention of obesity) | Davis et al., 2011 (124) | − |
| 4.4 Decreased sleep duration or variation | |||
| A systematic review and unbiased meta-analysis of 11 longitudinal studies of 24,821 children and adolescents | There was a twofold increase in risk for obesity with “short” sleep duration according to sleep standards | Fatima et al., 2015 (125) | + |
| Sleep duration in a cross-sectional pediatric study (n = 676) | Energy density of the diet, added sugar, and SSBs decreased with increased sleep | Kjeldsen et al., 2014 (126) | + |
| Variability in sleep duration of 10 min per night | This was positively associated with energy density (P = 0.04), sugar-sweetened beverages intake (P = 0.03), and Children's Sleep Habits Questionnaire score independent of sleep duration | Kjeldsen et al., 2014 (126) | + |
| One hour decrease in pediatric sleep duration (n = 441) during 200 d | There was a higher intake of added sugar (P = 0.001) and sugar-sweetened beverages (P = 0.002) with no change in energy density of the diet (P = 0.78) | Hjorth et al., 2014 (127); Kjeldsen et al., 2014 (126) | + |
| Sleeping <10 h at 16 mo of age in 1303 twins | There was a 50 kcal increased intake | Fisher et al., 2014 (128) | + |
| Increasing pediatric sleep duration an average of 2 h 20 min (n = 37) | There was decreased caloric intake by 134 kcal/d and lowered plasma leptin | Hart et al., 2013 (129) | + |
| Three hundred eleven term infants; sleep duration at 9 mo, 18 mo, and 3 y of age | There was no relationship between sleep duration and adiposity indicators in 9- to 36-mo-old children: the SKOT cohort | Klingenberg et al., 2013 (130) | None |
| Eight hundred two 4- to 14-y-old children and adolescents; sleep and intake followed for 7 d | There was no relationship between sleep duration and energy intake, but there was a trend toward a positive association with intake of dietary fiber and vegetables and a negative association with intake of poultry, and a trend toward a negative association with intake of liquid “discretionary calories” | Hoppe et al., 2013 (131) | None |
| Longitudinal cohort study of 550 children of average age 9.6 y | There was an odds ratio of 2.08 for obesity with <10 h sleep | Chaput et al., 2011 (132) | + |
| A meta-analysis of 12 studies including 20,003 children | There was a 1.86 odds ratio for obesity with “short” duration of sleep | Cappuccio et al., 2008 (133) | + |
| 4.5 Increased screen time | |||
| Measurements at ∼12 y of age of 234 parents from a previously established cohort were compared with 382 of their offspring for screen time and measures of adiposity | Both generations demonstrated a relationship between screen time and obesity at about 12.5 y of age, demonstrating a need to target high-risk families across generations | Steffen et al., 2013 (134) | + |
| A systematic review of 7 prospective studies on television time and 1 study on computer use | Six studies of varying quality demonstrated a positive relationship between screen time and the development of obesity | te Velde et al., 2012 (135) | + |
| Seventy children studied every 6 mo during 2 y in a randomized controlled study to decrease television viewing 50% and decrease sedentary activity in the intervention group of 35 | The intervention decreased sedentary activity especially in lower socioeconomic group children; there was relationship between decreased television viewing, decreased BMI, and decreased energy, but not increased activity | Epstein et al., 2008 (136) | + |
| 4.6 Increased family involvement in prevention | |||
| Fifteen RCTs of family-based lifestyle interventions for children and adolescents | Family-based interventions based in behavior theory had more effect than did those theoretically connected to family systems theory | Sung-Chan et al., 2013 (137) | − |
| A systematic review including 24 studies including parental involvement in long-term weight-control interventions with a nutritional focus | Although there were inadequate data to determine whether parental involvement in prevention programs is important, medium and high levels of parental involvement in obesity treatment programs improved outcomes, suggesting that parental involvement should be studied in prevention | van der Kruk et al., 2013 (138) | Probable + |
| Fifteen studies (7 were longitudinal) included measures of frequency of family meals although in an inconsistent manner | There was inconsistent and weak evidence of an inverse association between the frequency of family meals and risk of pediatric overweight; there is need for robust longitudinal studies on this topic | Valdes et al., 2013 (139) | None |
| A systematic review of 9 studies including portion manipulation interventions or portion education/training interventions | Most studies demonstrated increased intake with increased portion size, and that parents can be educated to estimate portion size more accurately, but there were other studies that contradicted both concepts | Small et al., 2013 (140) | − |
| 4.7 Disordered family function or abuse | |||
| Systematic review of 16 cross-sectional and 1 longitudinal study of family function | Lower levels of family functioning, including poor communication, poor behavior control, poor family cohesion, high levels of family conflict, and low family hierarchy values representing low authority, dominance, and decision power, showed low to moderate relationship to the subject’s classification of pediatric obese or overweight; however, out of 4 interventional studies only 2 showed that improved family functioning decreased the risk of obesity, but these studies were suboptimal | Halliday et al., 2014 (141) | + |
| A meta-analysis of 41 studies including 190,285 participants | Pediatric maltreatment was associated with a 1.36 increased risk ratio for pediatric obesity | Danese and Tam, 2014 (142) | + |
| Systematic review of 36 studies | Interpersonal violence increased the risk of obesity later in life | Midei et al., 2011 (143) | + |
| Systematic review of 6 prospective and 2 retrospective studies | Stressful environments during childhood and adolescence, including lack of good care, pediatric anxiety disorders, learning difficulties, low school achievement, and childhood/adolescence abuse, increased adult obesity risk, depression in adolescence, and increased the risk for obesity in girls only | Vamosi et al., 2010 (144) | + |
| 4.8 Increased school involvement | |||
| Nine community-based studies (5 RCTs and 4 non-RCTs) of which 1 was conducted only in the community setting, 3 were conducted in the community and school setting, and 5 were conducted in the community setting in combination with at least 1 other setting, such as the home | There was moderate strength of evidence that a combined diet and physical activity intervention in the community with a school component is effective at preventing obesity or overweight | Bleich et al., 2013 (145) | − |
| A systematic review of 16 studies involving school prevention programs with community involvement | School programs with more community involvement were more successful than those with less community involvement | Krishnaswami et al., 2012 (146) | − |
| Meta-analysis of 37 studies of 27,946 children generally between 6 and 12 y of age | There were beneficial effects of pediatric obesity prevention programs on BMI with school curriculum that includes healthy eating; physical activity and body image; increased sessions for physical activity and the development of fundamental movement skills throughout the school week; improvements in nutritional quality of the food supply in schools; environments and cultural practices that support children eating healthier foods and being active throughout each day; support for teachers and other staff to implement health promotion strategies and activities, as well as parent support and home activities that encourage children to be more active, eat more nutritious foods, and spend less time in screen-based activities; however, weaknesses in studies and potential bias point to the necessity for improved studies in the future | Waters et al., 2011 (147) | − |
| 4.10 Increased breast feeding | |||
| Meta-analysis of 25 studies with a total of 226,508 participants | Breast-feeding was protective of the development of obesity with a dose response effect in 17 studies | Yan et al., 2014 (148); Kramer et al., 2009 (149) | + |
| A cluster-randomized trial of a breast-feeding promotion intervention of 13,889 subjects (81.5%) followed up at 6.5 y from 31 Belarusian maternity hospitals and affiliated clinics | Although there were substantial increases in the duration and exclusivity of breast-feeding, there was no reduction in obesity at age 6.5 y | Kramer et al., 2009 (149) | None |
| Meta-analysis of 10 studies of breast-feeding | Five studies showed protective effects and 5 did not; likewise, there were mixed findings on length of breast-feeding and time of introduction of complementary food | Weng et al., 2012 (150) | Mixed |
| Cohort analyses of 11,998 teenagers from the National Longitudinal Study of Adolescent Health | There was a decreased risk of obesity in girls breast-fed at least 9 mo with similar, but less significant, effects in boys; however, analysis of sibling pairs eliminated any significance from the relationship, demonstrating the effect of confounding effects on cohort analyses | Nelson et al., 2005 (151) | + |
Note: Numbers 4.2–4.8 and 4.10 refer to numbered recommendations in the manuscript.
Abbreviations: AOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; RCT, randomized controlled trial; SKOT, Scottish Childhood Obesity Treatment Trial; SMD, standardized mean difference; TV, television; VO2, oxygen consumption.
Some studies included obese children and adolescents, but results may relate to prevention.
Decreasing caloric intake by consuming more fruits and vegetables and reducing dietary fat and refined carbohydrate intake can decrease the risk of developing obesity and T2DM (152). Many children and adolescents eat fewer than 3 servings of fruits and vegetables a day rather than meeting the US Department of Agriculture dietary recommendation of 5 to 7 fruit and vegetable servings per day. Inadequate consumption of dietary fiber may contribute to excessive weight gain, highlighting the need to continue to address vegetable and whole fruit intake (153). Whole fruit intake increased and fruit juice intake decreased from 2003–2004 to 2009–2010 (154).
Children and adolescents in the public school system in the United States consume up to 40% of their calories at school, so attention to the composition of foods and drinks available to them during the school day is critical (155). New US federal guidelines are encouraging, in that they eliminate trans fat, limit saturated fat, and decrease total sugar content of foods served in schools (156).
Values
The committee places a high value on increasing vegetable and fruit intake to decrease the risk of developing obesity. Calorie-dense, nutrient-poor foods should not be available in the school and school sports environments, where their presence increases their consumption and implies adult assent.
-
3.2
We recommend that clinicians prescribe and support healthy eating habits such as:
-
•
avoiding the consumption of calorie-dense, nutrient-poor foods (e.g., sugar-sweetened beverages, sports drinks, fruit drinks, most “fast foods” or those with added table sugar, high-fructose corn syrup, high-fat or high-sodium processed foods, and calorie-dense snacks)
-
•
encouraging the consumption of whole fruits rather than fruit juices. (1|⊕⊕○○)
-
•
Evidence
Drinking sugar and sugar-sweetened beverages is associated with developing obesity (157, 158). Table sugar consists of 50% glucose and 50% fructose; sugar-sweetened beverages often have a higher percentage of fructose, sometimes up to 65%; and high-fructose corn syrup is found in many foods besides liquid beverages. Metabolic responses differ significantly between fructose and glucose.
Consuming nutrient-poor, calorie-dense, high-fat foods and sugar-sweetened beverages is a risk factor for obesity (156). Reducing sugared-beverage consumption (e.g., soda, fruit drinks, sports drinks, and excessive consumption of fruit juices) is an effective way to reduce ingested calories (159). However, children and adolescents currently consume, on average, 30% to 40% of calories from nutrient-poor, energy-dense foods and drinks (160). Although sugar-sweetened beverage intake is decreasing in younger children, it has actually increased since 2007 in adolescents (161). Fruit juice provides a more concentrated dose of carbohydrates than does whole fruit and may not lead to the feeling of satiety experienced after ingesting whole fruits. Thus, healthy children should limit fruit juice ingestion and children with dental caries or excessive weight should ingest less than the maximal recommended volumes. Therefore, fruit juice has no role in the diet of infants under 6 months of age. After 6 months of age, fruit juice must be limited to 4 to 6 ounces per day until children reach 6 years of age, after which 8 to 12 ounces is an acceptable serving, according to the American Academy of Pediatrics policy. In view of the fact that it is easy for children to exceed such limits, the Early Childhood Longitudinal Study—Birth Cohort of >4000 children demonstrated that daily ingestion of fruit juice at 2 years of age resulted in an increase in BMI at 4 years compared with children who had no or infrequent fruit juice. The study also demonstrated that whole fruit provides increased nutritional benefit over juice. This committee encourages the consumption of whole fruits rather than fruit juices (162, 163).
Since 1965, teens have doubled their consumption of sugar-sweetened and fruit-flavored beverages (156, 164). School-based interventions can reduce soda consumption and reduce weight in students at the highest BMI percentiles (152, 165). Although there has reportedly been a 95% decrease in the amount of regular sodas shipped to schools, other sweetened beverages (such as sports drinks) have become more available in schools (166).
However, as of 2014–2015, federal guidelines now restrict the use of such “competitive foods” in the school environment. Obese or normal weight children and adolescents who substituted noncaloric beverages in lieu of sugar-sweetened beverages had less of an increase in BMI at 1 year (115, 116). Because there was no difference in satiety between those who drank sugar-sweetened beverages and those who did not, it appears that a child or adolescent will not compensate for the decreased caloric intake of nonsweetened drinks by increasing his or her caloric intake via other foods or drinks. This lack of compensation may partly explain the reduced weight gain associated with nonsweetened drinks (115, 167).
Although there are reports that reducing glycemic load may have a beneficial effect in the prevention or treatment of obesity, a systematic review of epidemiologic, prospective, and intervention studies did not demonstrate consistent results (168).
Water is frequently recommended as a beneficial replacement for sugar-sweetened beverages. Whereas a systematic review found only a weak association between water consumption and weight control in longitudinal studies, the introduction of water jets to New York City elementary school students led to a 0.022 to 0.025 decrease in BMI and a 0.6% to 0.9% decrease in risk for overweight; this is possibly related to a 12.3% decrease in milk purchases (169). Water remains the most reasonable “drinking” choice for quenching thirst and changing behavior from high-sugar drinking habits (170).
Values
The Task Force placed a high value on decreasing access to sugar-sweetened beverages by children and adolescents as a means of obesity prevention and treatment and a high value on strengthening the message to families that these beverages contribute to pediatric obesity.
Remarks
The costs of comorbidities related to pediatric and adult obesity are spiraling, and we must explore measures to limit nonnutritive excess calories as one means of preventing obesity. No nation can afford the social and financial ramifications of increased obesity incidence left unchecked. The individual practitioner cannot prevent obesity alone; a multidisciplinary health care team including dieticians, mental health practitioners, and nurses provides the optimal setting.
However, the committee agrees with the WHO that such changes must reach beyond the clinical setting and require policy changes at the highest level, as well as the cooperation of commercial entities. The committee supports the suggestion by the WHO for worldwide tax leverage on calorie-dense, nutrient-poor foods (110, 171).
-
3.3
We recommend that children and adolescents engage in at least 20 minutes, optimally 60 minutes, of vigorous physical activity at least 5 days per week to improve metabolic health and reduce the likelihood of developing obesity. (1|⊕⊕○○)
Evidence
A common goal for preventing obesity is to increase physical activity and decrease sedentary time in addition to reducing energy intake. A meta-analysis showed a positive association between sedentary time and the risk for obesity, although the effects were small (172). The 2008 Physical Activity Guidelines for Americans (173) and other sources suggest 1 hour of activity per day for children and adolescents at a minimum; although this is a reasonable aspirational goal, the minimal achievable activity level that produces beneficial effects may be less. Shorter bursts of activity, such as 20 minutes a day 3 to 5 days per week, can improve metabolic measurements in obese children and adolescents in a 3 to 6 month period, and these lower activity levels may also prevent obesity (124). The beneficial effects of exercise are most consistent in the heaviest children and adolescents who previously had not engaged in activity. See Table 4 and section 5 on treatment for more information on how activity and sedentary time affect obesity.
Lack of activity may lead to obesity and overweight, but obesity also decreases the coordination and exercise capacity of affected and adolescents, as well as the likelihood of being chosen for team sports, resulting in an overall decreased desire for physical activity (174–177).
Values
The Task Force placed a high value on interventions with a low potential for adverse effects and burdens such as increasing physical activity and decreasing sedentary time. The benefits on metabolic fitness are regularly demonstrated, although changes in weight or BMI are less consistent.
-
3.4
We suggest fostering healthy sleep patterns in children and adolescents to decrease the likelihood of developing obesity due to changes in caloric intake and metabolism related to disordered sleep. (2|⊕⊕○○)
Evidence
Disordered sleep length and quality in adults, children, and adolescents affects appetite and decreases insulin sensitivity (150). Table 4 lists 8 studies that show how different sleep durations or changes in sleep duration affect dietary intake in children and adolescents. These results suggest that sleep duration affects obesity development, although 2 other studies challenge these findings, weakening the strength of the evidence (130, 131).
Values
The committee puts a high value on ensuring adequate sleep time for all children and adolescents, although the effect on dietary intake and weight gain is not definitive. The National Sleep Foundation recommends 8 to 11 hours of sleep for school age children and adolescents (178).
-
3.5
We recommend balancing unavoidable technology-related screen time in children and adolescents with increased opportunities for physical activity. (1|⊕⊕○○)
Evidence
A systemic analysis of 24 papers reviewing 15 studies demonstrated strong evidence for decreasing screen time and increasing physical activity to prevent obesity (135); another study reported that decreasing screen time decreases sedentary time (136). A 2-generation study associated increased BMI with >2 hours of screen time per day for both parents and offspring (134). Data from >11,000 preschool children 4 to 6 years of age linked increased caloric intake from snacks and sugar-sweetened beverages to increased screen time (135, 179, 180).
Values
There are frequent requirements for video screen use for schoolwork; as technology becomes more prevalent, such requirements will not decrease. However, the committee put a high value on adhering to the American Academy of Pediatrics guidelines limiting discretionary screen time for children (85, 181).
-
3.6
We suggest that a clinician’s obesity prevention efforts enlist the entire family rather than only the individual patient. (2|⊕○○○)
Evidence
A meta-analysis commissioned by the original Task Force demonstrated a nonsignificant trend associating family involvement with the prevention of obesity, especially if the child is <8 years of age (140, 181).
One recent meta-analysis suggested that family-based therapy is effective for treating obesity (137), and another highlighted the importance of the intensity of parental involvement in the success of family interventions to prevent and treat obesity (138). Furthermore, studies of weight loss in obese children and adolescents demonstrated the importance of including family members in the process; without parental inclusion, the effect on weight loss was not significant (182). However, there is a need for more research into the influence of family participation for the prevention or treatment of pediatric obesity (139, 140, 183). In spite of a general consensus that an authoritative parenting style is optimal and restrictive parenting in terms of food choice is not, there are insufficient data to determine what type of parenting approach is most effective in preventing pediatric obesity (184).
Values
The Task Force placed a high value on involving the entire family in obesity prevention efforts as a practical low-risk approach, while understanding that much of the evidence comes from treatment studies and even those studies are not unanimous on the effects of family intervention.
-
3.7
We suggest clinicians assess family function and make appropriate referrals to address family stressors to decrease the development of obesity. (2|⊕⊕○○)
Evidence
There is evidence for an association between the development of pediatric obesity and family dysfunction as well as exposure to stress (Table 4).
Values
The committee placed a high value on fostering healthy family functioning and minimizing pediatric stress, as adverse life events are linked to the development of obesity as well as numerous other complications throughout life.
-
3.8
We suggest using school-based programs and community engagement in pediatric obesity prevention. (2|⊕⊕○○)
Evidence
A school-based program offers the promise of standardization across multiple sites and also can reach large populations of children and adolescents during the early and teenage years.
Numerous school-based interventions focused on reducing obesity rates. The Cardiovascular Health in Children study improved outcomes by decreasing body fat and cholesterol (185). The Cardiovascular Health in Children II study was effective in reducing body fat and blood pressure in middle school children and adolescents (186).
A school-based intervention can reduce body fat and blood pressure in young adolescents (186). One reason the short-term Cardiovascular Health in Children interventions were successful in affecting physiological variables may be the increased time spent in moderate to vigorous physical activity in school (20 minutes per day in elementary schools, 30 minutes in middle schools). Both school design and adult supervision for physical activity affect the amount of physical activity that sixth to eighth graders engage in during free time (187). Additionally, school-based intervention for >4000 middle school children and adolescents at risk for T2DM in the HEALTHY Study Group demonstrated efficacy in decreasing overweight and obesity in both the intervention and control groups, and decreased BMI z score, fasting insulin, prevalence of obesity, and percentage of students with waist circumference > 90th percentile in the intervention group (188). School systems have begun to initiate before- and after-school lifetime fitness programs that appear to be helpful in controlling weight gain (189). As noted, evidence supports prevention efforts in the third grade, which could be carried out in an entire school and preschool environment.
There is moderate evidence that community-based pediatric obesity prevention programs, when combined with a school-based component, can have positive 1-year effects on preventing obesity (145). Community-based participatory research may help enhance school-community involvement, resulting in effective obesity prevention programs (146). A review of multiple settings (early care and education, school, community, health providers, and the home) demonstrated strength for each of these approaches and suggested that a combined approach holds more promise (190).
Values
In making these suggestions, the committee set a high value on the ability of school-based programs to reach a wide population that would benefit from obesity prevention and emphasized the need for additional community-based interventions that used techniques coordinated with a school setting.
-
3.9
We recommend using comprehensive behavior-changing interventions to prevent obesity. Such programs would be integrated with school- or community-based programs to reach the widest audience. (1|⊕⊕○○)
Evidence
A systematic review of RCTs using behavior change techniques to prevent or treat obesity demonstrated that 6 techniques held promise for preventing obesity during a period of at least 6 months. These techniques were:
-
•
providing individualized information on the consequences of behaviors conducive to the development of obesity
-
•
restructuring the environment to make individualized behavior change more successful
-
•
guiding practices expected to decrease the development of obesity
-
•
guiding the identification of role models or advocates to change behavior
-
•
implementing stress management/emotional control training
-
•
providing general communication skills training.
Values
The committee realized the difficulty in providing widespread exposure to behavior change programs but placed a high value on the pursuit of effective techniques of behavior change.
-
3.10
We recommend breast-feeding in infants based on numerous health benefits. However, we can only suggest breast-feeding for the prevention of obesity, as evidence supporting the association between breast-feeding and subsequent obesity is inconsistent. (1|⊕○○○)
The previous guidelines supported breast-feeding as an effective method of preventing obesity. However, reports on the effect of breast-feeding on preventing obesity are mixed during the last 10 to 15 years. In particular, sibling analyses point to confounding effects in interpreting the results of cohort studies (151). Furthermore, a 6.5-year-long longitudinal cluster-randomized study of 13,889 subjects demonstrated no effect of breast-feeding on the development of obesity, even among those with more sustained breast-feeding duration (149).
Likewise, 2 meta-analyses showed no strong evidence for the associating between the time of introducing complementary feeding and the development of pediatric overweight or obesity (191, 192).
Values
The committee places high value on promoting breast-feeding to improve infant health but can only suggest breast-feeding as a method for preventing obesity.
Remarks
For most children and adolescents and their families, lifestyle patterns related to eating and exercise are established early, affecting children and adolescents not only when they are young but also throughout life. Health care providers should follow universal prevention methods to avoid the harmful health consequences of less-than-optimal lifestyle choices, conveying to all patients and families in a culturally sensitive and language-appropriate manner the energy needs and essential nutrient requirements of young children, and the importance of physical activity. This is of particular importance when we consider the increased efficacy of prevention trials when directed toward younger children.
The intestinal microflora may influence the development of obesity. Although it is premature to discuss methods of altering the intestinal flora, evidence suggests that Bacteroides fragilis is more frequent in the stool of overweight vs normal weight children, adolescents, and adults (193). The intestinal microflora varies between vaginal and cesarean section birth and also due to the composition of early diets, including breast milk. Upcoming results of clinical trials, which modify the microbiome, may suggest new methods of obesity prevention and treatment.
A recent systematic review that looked at the way urban environments affect health behaviors or outcomes for children and adolescents reported some evidence of potential health benefits from urban environment interventions relating to road safety and active travel. However, evidence for the effectiveness of such interventions was weak due to study designs that were opportunistic and nonrandomized, used subjective outcome measures, and did not incorporate follow-up of study participants (194). Nonetheless, health care providers are encouraged to advocate for common sense changes, including providing safe walking/biking areas in parks, school routes, and neighborhoods and providing programs for active play in free time. Environmental change recommendations require additional research with more robust study designs incorporating objective outcome measures to inform meaningful policy change.
4.0 Treating obesity
Lifestyle: general considerations
-
4.1
We recommend clinicians prescribe and support intensive, age-appropriate, culturally sensitive, family-centered lifestyle modifications (dietary, physical activity, behavioral) to promote a decrease in BMI. (1|⊕⊕⊕○)
Evidence
The 2015 Endocrine Society Task Force commissioned a systematic review to evaluate the impact of weight change on metabolic outcomes in children and adolescents who are obese (5). The results showed that change in BMI was associated with improvements in triglycerides, high-density lipoprotein, and systolic blood pressure. This analysis is limited by the fact that it used aggregate data. Other studies also showed associations between weight change and other metabolic outcomes (Fig. 2) (195, 196).
Figure 2.
Change in metabolic outcome per unit change in BMI or weight. Abbreviations: HDL, high-density lipoprotein; SBP, systolic blood pressure; TG, triglyceride.
Successful weight management, through lifestyle interventions, delays the onset of T2DM in adults (197) and improves cardiovascular fitness (198, 199). Many pediatric weight management programs have found improved body composition and metabolic parameters (13, 200).
A commonly held belief is that lifestyle modification is not sufficiently efficacious. Children and adolescents may not lose weight, or despite initial success, children and adolescents might regain weight after the active phase of the program has ended (201). A factor in weight regain may be lack of continued exercise. The odds for weight regain are twofold greater in those who are sedentary (201). In a 10-year study of adults who participated in the National Weight Control Registry, >87% of participants maintained at least 10% weight loss for 5 to 10 years. A worse outcome was associated with decreased physical activity, decreased dietary restraint, decreased frequency self-weighing, increased energy intake as fat, and increased disinhibition (202).
There is sufficient evidence that intensive lifestyle modification programs can be effective tools for pediatric weight control in the short term (203, 204). Furthermore, implementing a formal maintenance program after the completed treatment phase can be important for maintaining achieved weight loss (205). This finding is consistent with the concept of obesity as a chronic disease (206).
A Task Force–commissioned meta-analysis of randomized pediatric trials of combined lifestyle interventions for treating obesity (diet and exercise) showed a modest but significant effect on obesity (equivalent to a decrease in BMI of 1.5 kg/m2; P < 0.00001) when interventions targeted family involvement. When parents were not specifically included, the effect on weight loss was not significant (182). These results suggest involving the family when delivering combined lifestyle interventions.
An additional meta-analysis of RCTs of lifestyle interventions (without an analysis of family involvement) found moderate positive effects from the interventions when compared with no treatment or information-only controls. These effects persisted for an average follow-up period of 15 months (207). Although there was overlap with the Task Force meta-analysis, each study contained reports not covered by the other.
An evidence-based position statement of the American Dietetic Association supports the utility of family-based lifestyle interventions for children and similar multicomponent programs for adolescents (208). These recommendations are consistent with conclusions of a combined CDC and American Medical Association expert committee (209) and an evidence-based review of pharmacological interventions for pediatric obesity that highlighted the importance of concomitant intensive lifestyle interventions, including dietary, exercise, and family counseling (210).
Values and preferences
In making this recommendation, the Task Force placed a high value on promoting healthy, safe pediatric lifestyle modification that included family involvement, with potential wide-reaching benefits.
Remarks
Clinicians should encourage BMI reduction for patients with obesity. A Task Force commissioned meta-analysis demonstrated favorable effects on systolic blood pressure, serum triglycerides, and serum high-density lipoprotein with decreasing BMI or weight (5). When interpreting these data, one must consider that the beneficial effects seen in the 133 RCTs and 16 systematic reviews are for averaged data, not individualized patients; other factors such as age, ethnicity, or genetics may modify individual responses. Very large changes may not be necessary. Although a BMI decrease of 1.5 kg/m2 (reported in the meta-analysis commissioned by the first Task Force) may seem small, if maintained for the long term, overweight or obese children and adolescents may benefit by maintaining weight as they grow; BMI will decline as linear growth proceeds, and lifestyle modification may reduce fat mass, increase lean body mass, and improve cardiovascular fitness (211). Seven percent weight loss may be a more realistic goal for children and adolescents with extreme obesity. Well-designed RCTs, with large numbers of patients, employing intensive lifestyle intervention and follow-up maintenance programs, will help develop refined techniques. A review of 25 years of behavioral therapy intervention in children and adolescents has demonstrated that long-term weight loss maintenance is possible (212). Other RCTs of diet, physical activity, and/or behavior modification have also demonstrated persistent changes in BMI (212, 213).
Dietary
-
4.2
We recommend that clinicians prescribe and support healthy eating habits in accordance with the following guidelines of the American Academy of Pediatrics and the US Department of Agriculture:
-
•
decreased consumption of fast foods
-
•
decreased consumption of added table sugar and elimination of sugar-sweetened beverages
-
•
decreased consumption of high-fructose corn syrup and improved labeling of foods containing high-fructose corn syrup
-
•
decreased consumption of high-fat, high-sodium, or processed foods
-
•
consumption of whole fruit rather than fruit juices
-
•
portion control education
-
•
reduced saturated dietary fat intake for children and adolescents >2 years of age
-
•
US Department of Agriculture recommended intake of dietary fiber, fruits, and vegetables
-
•
timely, regular meals, and avoiding constant “grazing” during the day, especially after school and after supper
-
•
recognizing eating cues in the child’s or adolescent’s environment, such as boredom, stress, loneliness, or screen time
-
•
encouraging single portion packaging and improved food labeling for easier use by consumers. (Ungraded Good Practice Statement)
-
•
Evidence
(Refer to section 3.2 for some of the evidence for recommendation 4.2.) Children and adolescents who are overweight are more likely to skip breakfast and consume few large meals per day (214) than do their leaner counterparts who are more likely to consume smaller, more frequent meals (215). Because snacks tend to be higher in calorie density than meals, frequent snacking (among children and adolescents) is associated with a high intake of fat, sugar, and calories and with overweight (216).
Educating families, children, and adolescents about the need to measure out single snack portions from multiserving packages and place them in single-serving containers can significantly change the amount of food children and adolescents consume (217).
Values and preferences
The committee placed a high value on decreasing snacking and decreasing overall caloric intake to reduce weight gain among children and adolescents.
Remarks
A meta-analysis in children and adolescents suggests that improved weight can be achieved regardless of the macronutrient composition of the diet, and this mirrors similar results found in adults (218). The WHO has recently recommended that adults, children, and adolescents limit sugar to <5% to 10% of total daily energy intake, unless the sugars are contained in fresh fruits and vegetables, which are lower in calories and higher in fiber than processed carbohydrates. The other carbohydrates, which they term “free sugars,” include honey; other sweeteners; glucose/fructose; and sugar added by a cook, consumer, or producer. This recommendation was termed “strongly” because of moderate quality evidence that increasing free sugars in one’s diet increases body weight, and decreasing free sugars decreases body weight (110).
A dietician familiar with the energy needs of growing children and adolescents should supervise calorie reduction for weight loss or maintenance in patients of this age group. Unbalanced hypocaloric diets (e.g., “fad diets”) may be deficient in essential vitamins and minerals.
Physical activity
-
4.3
We recommend that clinicians prescribe and support the reduction of inactivity and also a minimum of 20 minutes of moderate to vigorous physical activity daily, with a goal of 60 minutes, all in the context of a calorie-controlled diet. (1|⊕⊕○○)
Evidence
In the absence of caloric restriction, moderate exercise does not cause weight loss. However, in combination with decreased caloric intake, exercise can achieve and maintain significant weight loss. Studies performed in the school setting have shown beneficial effects of exercise in children and adolescents (204). The beneficial effects of both aerobic exercise and resistance training can be short-lived, and exercise must be sustained over months. Even 20 minutes of aerobic activity 5 days per week over 13 weeks can decrease body and visceral fat (124). Recent studies in Denmark and elsewhere have demonstrated benefits in mild intensity jogging and in small 10- to 15-minute intervals of exercise, which may be more readily achievable (219, 220).
Physical fitness, even without weight loss, may confer health benefits. Improvements in cardiovascular fitness were associated with improvements in body composition and diabetes risk factors in adolescents (220). In addition to improving metabolic fitness, exercise has been linked to improvements in cognitive function and concentration (124). (Refer to section 4.8 regarding school-based interventions to increase activity.)
Values
The Committee placed a high value on losing weight (in the form of body fat) by decreasing caloric intake and increasing energy expenditure.
Remarks
Although current recommendations state that school children and adolescents (who spend about half their waking hours in school) should receive a minimum of 30 to 60 minutes of moderately vigorous physical activity and at least 60 minutes of aerobic (moderate and vigorous) physical activity each school day, only 5% of school districts in the United States have a requirement for a specific amount of physical education (221–225). Clinicians should place emphasis on increasing a child’s or adolescent’s activity by helping facilitate:
-
•
the ability to safely walk to and from school
-
•
increased use of stairs (and improved signage to indicate their location)
-
•
increased breaks for movement in the classroom
-
•
increased movement during recess and gym.
Moderate to vigorous exercise is defined as causing some increase in breathing and heart rate; in a healthy person this is usually associated with brisk walking, dancing, swimming, or cycling on flat terrain. In exercise physiology terms, the energy expended should be at least 3 metabolic equivalents (85, 226). Moderate exercise allows talking but not singing, and vigorous exercise makes it impossible to sing and difficult to talk. This generalization should help families understand and identify the difference between moderate and vigorous exercise.
The use of motivational interviews to help an older child or adolescent and/or his or her parent set physical fitness or dietary goals may lead to greater success in decreasing BMI (218, 219). In spite of limitations inherent in the method, clinicians should assess a patient’s readiness for change when determining how to approach the family.
-
4.4
We suggest that clinicians encourage and support patients to limit nonacademic screen time to 1 to 2 hours per day and decrease other sedentary behaviors, such as digital activities. (2|⊕○○○)
Evidence
The 2009 Cochrane analysis reported that a combined behavioral approach incorporating both dietary and physical activity changes can produce a significant and clinically meaningful reduction in overweight in children and adolescents (204). A meta-analysis commissioned by the original Task Force of 3 randomized trials of interventions for reducing sedentary activity reported imprecise results (i.e., that these interventions had both a favorable and unfavorable impact on obesity outcomes) (182). Both girls and boys demonstrated small decreases in the amount of screen time in a German study, and these decreases did not correlate with increases in physical activity (227).
Values and preferences
The committee placed a high value on limiting digital access time and other efforts to decrease sedentary time. As our ever-increasing digital environment necessitates increased screen time, a plan for the world’s children and adolescents should complement necessary screen time with:
-
•
environments that demand and facilitate movement
-
•
monetary incentives for decreased caloric intake (such as taxes on sugar-sweetened beverages).
The committee agrees with research that finds an association with the presence of a television set in a child’s bedroom to increased screen time and increase caloric intake while weakening the positive influence of parents on promotion of healthy habits (228, 229).
Psychological complications of overweight and obesity
Psychosocial
-
4.5
We suggest that the health care team identify maladaptive rearing patterns related to diet and activity and educate families about healthy food and exercise habits. (2|⊕○○○)
-
4.6
We suggest that the health care team probe for and diagnose unhealthy intrafamily communication patterns and support rearing patterns that seek to enhance the child’s or adolescent’s self-esteem. (2|⊕○○○)
-
4.7
We suggest that the health care team evaluate for psychosocial comorbidities and prescribe assessment and counseling when psychosocial problems are suspected. (2|⊕○○○)
Evidence
In section 4 we discuss the importance of involving the whole family, and not just the child or adolescent, in prevention and treatment interventions.
How interactions between parents and children and adolescents and parenting styles contribute to unhealthy lifestyle habits is a subject of investigation (230, 231). An additional factor to overcome before initiating any intervention may be the parents’ inability to recognize that their child or adolescent is overweight, particularly for the preschool child (232–234).
Obesity is associated with QOL, with levels measured in obese children and adolescents equivalent to those seen in pediatric cancer or diabetes (235, 236). In addition to low QOL, children and adolescents with obesity have significant psychosocial comorbidities, including poor self-esteem (237–239), increased risk of depression and anxiety (240–242), and higher-than-average risk of eating disorders and substance abuse. Low self-esteem (243) and perceived or actual higher BMIs are associated with increased likelihood of smoking and alcohol consumption (244).
To remove the bias that might be seen in a clinic sample, the Childhood Growth and Development Study in Australia enrolled healthy weight (n = 158), overweight (n = 77), and obese (n = 27) children from the schools and from families asking to be referred (n = 19). Heights, weights, and psychological testing were done in the schools for the school-based cohort (245). Increasing BMI z scores were associated with decreasing self-worth and global self-esteem as well as with decreased athletic competency, social acceptance, and dissatisfaction with their physical appearance. These associations were reported as young as age 8 years, but the association with physical appearance was more pronounced in the older group (246). The presence of psychosocial distress in a population of school children and adolescents not seeking clinic referral, as well as those seeking referral, indicates that psychosocial issues are present in both clinical and nonclinical populations of youths who are obese.
A review of the literature found lower QOL scores for social acceptance, family life, physical appearance, school functioning, and physical functioning in all but 2 of the 34 publications included in the study. Factors influencing lower QOL included degree of obesity, symptoms of depression, lack of social support from classmates/family, and low socioeconomic status (247).
In general, low self-esteem does not seem to be a significant problem until adolescence, as self-esteem is similar between preteen children who are obese and normal weight. During adolescence, however, self-esteem becomes more closely tied to body image, and rapidly plummets, with those adolescent females who have higher BMIs and body image dissatisfaction having the lowest self-esteem (248).
Individuals with eating disorders tend to define self-worth by their body image (249), possibly explaining the association between eating disorders and youths who are overweight and obese. Surveys from 135 Hispanic and African American girls who are obese or overweight revealed that 52% had been teased about their weight by girls and 60% had been teased about their weight by boys. Of those who were teased, 70% skipped meals, dieted, or starved themselves; 12% reported binge eating; and 33% stated they had “emotional” eating. All of the girls surveyed stated they were unhappy about their weight and wanted to be thinner (250). Eating disorders, including binge eating and anorexia nervosa/bulimia, are more commonly seen in those who have depression, anxiety, and disruptive behavior (251, 252).
Parental reaction to their child’s weight affects how the child responds. Bullying by peers and families contributes to poor body image and impaired psychosocial functioning (253).
Some may harass their child, letting them know how unattractive he/she is, resulting in worsening body image and poor self-esteem. A retrospective Internet-based study of college students with great concern about their weight, body shape, and eating behaviors revealed that >80% had a history of parents or siblings making negative comments about their weight, shape, or eating behaviors. Most scored above average in psychometric emotional-abuse tests, with positive associations with negative parental comments and higher weight and negative associations with social support and self-esteem (254). Some parents are overly restrictive, potentially causing their children and adolescents to binge when they have access to unrestricted food (255, 256). Alternatively, adolescents with extreme obesity may develop anorexia bulimia, anorexia nervosa, or purging behaviors in an effort to lose weight. A cross-sectional cohort study of adolescents with extreme obesity and their parents found bulimic symptoms did not correlate with the degree of obesity but were associated with maternal psychopathology, including somatization and anxiety (257).
Youths who are obese are more likely to be teased and bullied and are less likely to have a “best friend” or be considered popular by classmates than their thinner peers (258). Parents, teachers, and peers indicate that youths who are obese are more isolated and have poorer social skills than do their thinner counterparts (259). Those with low self-esteem (243) and perceived or actual higher BMIs are more likely to smoke and drink alcohol than those with higher self-esteem (244). Additionally, they are less athletic and less likely to have romantic relationships, contributing to increased teasing, worsening of self-esteem, loneliness, depression, anxiety, and introverted behavior (260).
In general, those who are most obese report more psychological distress (246, 261). Girls become more depressed with increasing BMI than do boys, and some studies indicate that depression in African American boys is not linked to BMI but rather to peer teasing (246, 262). Race and socioeconomic status (in addition to sex) affect how children and adolescents react to obesity; however, there are conflicting reports on the effect of obesity on psychological status in different groups. One study found that African American children have more body image dissatisfaction and anxiety than do their same-weight white counterparts (262), whereas a study of adolescents found that African Americans and Hispanics are less stigmatized than whites (263). High socioeconomic status adolescents who are obese with psychopathology are less likely to seek help at a weight-loss program than are low socioeconomic status adolescents who are obese (252), possibly due to a more negative perception regarding obesity in high socioeconomic status families.
As adolescents who are obese consistently report high rates of depression, anxiety, and binge eating disorders, all overweight patients should be assessed for psychopathology. Assessment and counseling by a psychologist are often indicated. Clinicians should prescribe antidepressant medications with caution, as atypical antipsychotics cause rapid (often extreme) weight gain (264).
Diuretics, diet pills, and self-induced emesis are not uncommonly used to achieve rapid weight loss by adolescents. One study demonstrated that laxative use, self-induced vomiting, and diet pill ingestion were more common in adolescents who are obese compared with those who are normal weight and overweight (265). Six percent of 6957 middle school children and adolescents in North Carolina used diet pills and 7.1% used laxatives or self-induced vomiting. The case prevalence of diet pills was 3.4 in normal weight, 4.1 in overweight, and 9.5 in adolescents who are obese; the case prevalence of laxatives was 1.3, 0.7, and 3.2, respectively; and the case prevalence of self-induced vomiting was 3.4 in normal weight vs 7.6 in adolescents who are obese. Females more commonly abused substances for weight loss, such as tobacco, alcohol, and marijuana; they were also more likely to participate in risky sexual behaviors (265). Clinicians should discuss these maladaptive behaviors at clinic visits, as they are potentially harmful. It is important to emphasize moderation rather than restriction and to counsel against risky weight loss strategies
Remarks
As psychosocial issues are so prevalent, providers should psychologically screen all youths who are obese for the presence of mental health issues, asking questions regarding:
-
•
school absences/refusal
-
•
teasing by peers regarding weight/appearance
-
•
persistent anxiety
-
•
depression/self-harm
-
•
anger outbursts
-
•
sexual activity, alcohol, drug use
-
•
eating disorders—purging, anorexia, binge eating
-
•
family functioning/family attitudes about weight and specifically obesity/parent psychopathology.
Parents and/or children and adolescents should complete a mental health screening measure, such as the Pediatric Symptom Checklist (266). Clinicians can review this during patient visits and refer patients to a mental health professional when indicated. Obesity-related mental health issues are a pervasive problem, and a team-based approach is essential, involving school counselors, nurses, and teachers, as well as health care providers. It might also be helpful to consult with school personnel to initiate school-based counseling. A list of local programs (e.g., YMCA, Boys and Girls Clubs) that offer physical activity programs and healthy snacks is also helpful. Behavioral modification is helpful in determining the child’s readiness to change and potential barriers to achieving change (264).
Pharmacotherapy
-
4.8
We suggest pharmacotherapy for children or adolescents with obesity only after a formal program of intensive lifestyle modification has failed to limit weight gain or to ameliorate comorbidities (2|⊕○○○). We recommend against using obesity medications in children and adolescents <16 years of age who are overweight but not obese, except in the context of clinical trials. (1|⊕○○○)
-
4.9
We suggest that FDA-approved pharmacotherapy for obesity be administered only with a concomitant lifestyle modification program of the highest intensity available and only by clinicians who are experienced in the use of antiobesity agents and are aware of the potential for adverse reactions. (2|⊕○○○)
-
4.10
We suggest that clinicians should discontinue medication and re-evaluate the patient if the patient does not have a >4% BMI/BMI z score reduction after taking antiobesity medication for 12 weeks at the medication’s full dosage. (2|⊕○○○)
Evidence
The FDA recently approved a number of weight-loss medications for adults (216, 267, 268) and considers these medications to be appropriate for those ≥16 years of age who have BMI ≥ 30 kg/m2 or who have BMI ≥ 27 kg/m2 and at least 1 weight-related comorbid condition (e.g., hypertension or T2DM). However, although the utility of pharmacotherapy in pediatric obesity has been recently reviewed (269–271), there are no published data directly comparing adult and adolescent outcomes for obesity pharmacotherapy.
Physicians should be discouraged from prescribing weight loss medications off-label to those <16 years old because of: 1) the lack of FDA approval for use; 2) the limited number of well-controlled safety and efficacy studies in obese children and adolescents, 3) the limited efficacy demonstrated in adults for most agents, and 4) the need to weigh the relative risk of drug-induced adverse events in children and adolescents against a medication’s long-term theoretical potential for reducing obesity-related morbidity and mortality.
Despite these concerns, the negative health impact of pediatric obesity may justify long-term medication. However, pharmacotherapy should only be prescribed in combination with comprehensive lifestyle modification programs (210, 271–274) that have substantial efficacy (270). The limited available evidence suggests the best pediatric pharmacotherapy outcomes are among patients adherent to lifestyle program recommendations (275).
Among pharmacotherapeutic agents approved for adult obesity (Table 5), only orlistat is FDA approved for obesity treatment of ages 12 to 16 years. Orlistat (299–305) inhibits gastrointestinal lipases, reducing adolescent’s fat absorption by ∼30% (299). Orlistat reduces BMI significantly in adolescents by ∼0.7 to 1.7 kg/m2 (150, 318), but treatment is associated with significant gastrointestinal side effects (Table 5). Orlistat must be taken with each meal, thus reducing its utility in school-attending adolescents. Orlistat appears to affect the absorption of fat-soluble vitamins E and D (299). Available data suggest that ∼50% of pediatric patients that are prescribed orlistat discontinued it within 1 month, 75% stop using it by 3 months, and only 10% remain on orlistat after 6 months (319, 320). Given its limited efficacy and low long-term use, orlistat appears of limited benefit in practice.
Table 5.
Medications Studied for the Long-Term Treatment of Obesity
| Drug | Status | Common Side Effects | Monitoring and Contraindications | Source |
|---|---|---|---|---|
| Centrally acting anorexigenic agents | ||||
| Phentermine, diethylpropion, and mazindola | Approved only for short-term use in adults | Insomnia, elevation in heart rate, dry mouth, taste alterations, dizziness, tremors, headache, diarrhea, constipation, vomiting, gastrointestinal distress, anxiety, restlessness | Monitor HR, BP. These medications are contraindicated in uncontrolled hypertension, hyperthyroidism, glaucoma, agitated states, history of drug abuse, and MAOIs; use caution when prescribing to patients with even mild hypertension | Rauh and Lipp, 1968 (276); Lorber, 1966 (277); von Spranger, 1965 (278); Andelman et al., 1967 (279); Golebiowska et al., 1981 (280); Komorowski, 1982 (281) |
| Lisdexamfetamine dimesylatea | Not FDA approved for obesity. Approved for binge eating disorder in adults and for attention deficit hyperactivity disorder in patients 6 y of age and older | Dry mouth, sleeplessness (insomnia), increased heart rate, jittery feelings, constipation, anxiety | This medication is contraindicated with MAOIs. There is a risk for sudden death in people who have heart problems or heart defects, and stroke and heart attack in adults. Monitor blood pressure and heart rate. May produce psychotic or manic symptoms, such as hallucinations, delusional thinking, or mania. May worsen peripheral vasculopathy, including Raynaud phenomenon | McElroy et al., 2015 (282); McElroy et al., 2015 (283) |
| Sibutramine | Withdrawn in the US (increased risk of serious cardiovascular events). Still available in some countries such as Brazil | Tachycardia, hypertension, palpitations, insomnia, anxiety, nervousness, depression, diaphoresis | Monitor HR, BP. Do not use with other drugs, MAOIs | Berkowitz et al., 2003 (275); Godoy-Matos et al., 2005 (284); Berkowitz et al., 2006 (285) |
| Lorcaserina | Approved for long-term use in adults | Headache, dizziness, fatigue, nausea, dry mouth, cough, and constipation; back pain, cough, hypoglycemia in patients with T2DM | There is a risk for serotonin syndrome or neuroleptic malignant syndrome-like reactions. Evaluate patients for signs or symptoms of valvular heart disease. Euphoria, hallucination, and dissociation have been seen with supratherapeutic doses. Interactions with triptans, MAOIs, including linezolid, SSRIs, SNRIs, dextromethorphan, tricyclic antidepressants, bupropion, lithium, tramadol, tryptophan, and St. John’s wort | Smith et al., 2010 (286); Fidler et al., 2011 (287) |
| Liraglutidea | Approved for long-term use in adults | Nausea, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, increased lipase | Monitor heart rate at regular intervals. This medication is contraindicated in patients with a history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2. Discontinue promptly if pancreatitis is suspected | Zinman et al., 2009 (288); Wadden et al., 2013 (289); Astrup et al., 2009 (290) |
| Phentermine plus topiramatea | Approved for long-term use in adults | Paresthesias, dizziness, taste alterations, insomnia, constipation, dry mouth, elevation in heart rate, memory or cognitive changes | This medication is contraindicated in glaucoma, hyperthyroidism, MAOIs. Concerns about teratogenicity (increased risk of oral clefts) mandate effective contraceptive use and pregnancy test monitoring in females. Metabolic acidosis, hypokalemia, and elevated creatinine have been reported, and periodic monitoring is advised. Abrupt withdrawal of topiramate may cause seizures | Garvey et al., 2012 (291); Allison et al., 2011 (292) |
| Bupropion plus naltrexonea | Approved for long-term use in adults | Nausea, constipation, headache, vomiting, dizziness, insomnia, dry mouth, diarrhea | Monitor HR, BP. Do not administer to patients with a history of seizure disorders or with anorexia or bulimia nervosa or to patients who are using opioids or abruptly discontinuing use of alcohol, benzodiazepines, barbiturates, or antiseizure medications. There is potential increased risk of suicidality | Greenway et al., 2010 (293); Padwal, 2009 (294) |
| Drugs in development or used off-label that may act centrally as anorexigenic medications | ||||
| Recombinant human leptin, metreleptina | This drug is under investigation. In monotherapy it was successful for treating leptin deficiency | Headache, abdominal pain | This drug is useful only in leptin deficiency. Antibodies with neutralizing activity have been identified in patients treated with metreleptin. T cell lymphoma has been reported in patients with acquired generalized lipodystrophy. A risk evaluation and mitigation strategy should be in place to prevent inappropriate prescription | Farooqi et al., 2002 (105); Farooqi et al., 1999 (295) |
| Exenatidea | Not FDA approved for obesity | Nausea, vomiting, diarrhea, feeling jittery, dizziness, headache, dyspepsia | Acute pancreatitis, including fatal and nonfatal hemorrhagic or necrotizing pancreatitis, has been reported. Observe patients carefully for signs and symptoms of pancreatitis. Discontinue promptly if pancreatitis is suspected. Contraindicated in patients with severe renal impairment | Rosenstock et al., 2010 (296); Kelly et al., 2013 (297); Kelly et al., 2012 (298) |
| Drugs affecting nutrient trafficking | ||||
| Orlistat | This drug is FDA approved for treatment of obesity in adolescents ≥12 y old | Oily spotting, flatus with discharge, fecal urgency, fatty/oily stool, increased defecation, fecal incontinence | This drug is contraindicated in chronic malabsorption syndromes and cholestasis. Cholelithiasis and, rarely, severe liver injury, including hepatocellular necrosis and acute hepatic failure leading to death, have been reported. It decreases drug concentrations of cyclosporine and levothyroxine. Doses should be temporally separated from orlistat. Fat-soluble vitamin absorption is decreased by orlistat. Use with caution in those at risk for renal insufficiency. MVI supplementation is strongly recommended. A low- dose preparation is approved for over-the-counter sale | McDuffie et al., 2002 (299); Zhi et al., 2003 (300); Norgren et al., 2003 (301); Ozkan et al., 2004 (302); McDuffie et al., 2004 (303); Chanoine et al., 2005 (304); Maahs et al., 2006 (305) |
| Drugs affecting internal milieu/metabolic control | ||||
| Metformina | This drug is not FDA approved for obesity. It is approved for ≥10 y of age for T2DM | Nausea, flatulence, bloating, diarrhea; usually resolves | Do not use in renal failure or with i.v. contrast. MVI supplementation is strongly recommended. Potential risk for vitamin B12 deficiency when used long-term. Avoid alcohol intake | Freemark and Bursey, 2001 (306); Atabeck and Pirgon, 2008 (307); Love-Osborne et al., 2008 (308); Wilson et al., 2010 (309); Yanovski et al., 2011 (310); Kendall et al., 2013 (311) |
| Octreotide (for hypothalamic obesity)a | This drug is not FDA approved for obesity | Cholelithiasis (can be prevented by concurrent ursodiol), diarrhea, edema, abdominal cramps, nausea, bloating, reduction in T4 concentrations, decreased GH but normal IGF-I | Monitor fasting glucose, FT4, HbA1c. Useful only for hypothalamic obesity. Ursodiol coadministration is strongly recommended | Gambineri et al., 2005 (312); Haqq et al., 2003 (313); Lustig et al., 2001 (314); Lustig et al., 1999 (315); Lustig et al., 2006 (316) |
| Recombinant human GHa | This drug is not FDA approved for obesity. It is FDA approved in Prader-Willi syndrome to increase height velocity | Edema, carpal tunnel syndrome, death in patients with preexisting obstructive sleep apnea | GH should be used only after screening to rule out obstructive sleep apnea in patients with Prader-Willi syndrome. Clinicians must closely monitor pulmonary function, adrenal function, glucose, HbA1c | Shadid and Jensen, 2003 (317) |
Note: All agents are contraindicated in pregnancy. See full prescribing information for all adverse effects, cautions, and contraindications. Pharmacotherapy is not usually considered if the BMI is below the 95th percentile, but there are additional factors to consider. If we initiate pharmacotherapy early in the course of obesity, we may prevent extreme weight gain and metabolic complications, but we may treat an excess of children and adolescents, raise the rate of unwarranted side effects, and increase the costs to individuals and to society. Alternatively, if we begin medication late in the course of obesity, we run the risk of runaway weight gain and long-term morbidity. One approach that reconciles these difficulties is to act aggressively with lifestyle intervention in overweight and mildly obese patients to prevent extreme obesity and to consider pharmacotherapy when the risk of complications is high or soon after complications emerge. The tipping point for pharmacotherapy could be if the family history is strongly positive for a major comorbidity. Lifestyle intervention should precede pharmacotherapy and should be maintained during pharmacotherapy. Derived from August et al. (86).
Abbreviations: BP, blood pressure; CNS, central nervous system; FT4, (plasma) free thyroxine; HR, heart rate; IV, intravenous; MAOI, monoamine oxidase inhibitor; MVI, multivitamins; SNRI, selective serotonin-norepinephrine reuptake inhibitors; SSRI, selective serotonin-reuptake inhibitors; T4, thyroxine.
The use for obesity treatment in children and adolescents <16 y of age of these non–FDA-approved agents should be restricted to large, well-controlled studies.
Additional medications not FDA approved for the treatment of pediatric obesity
Metformin (306–311, 321–334) is not FDA approved for obesity treatment. However, metformin reduces hepatic glucose production, increases peripheral insulin sensitivity, and may reduce appetite (335). Metformin decreases BMI, but with a mean decrease of only 1.16 kg/m2 over 6 to 12 months (336). Metformin may also possibly be useful in combating the weight gain observed in children and adolescents who are taking atypical psychotropic medications (337, 338) or who have PCOS (324, 331, 339). However, given its limited weight-loss efficacy, metformin is not a considered a weight-loss treatment.
Sibutramine (275, 284, 285, 340–346) was removed from the US market in 2010 because of concerns for cardiovascular safety but remains available in several other countries.
Other medications approved for obesity treatment of ≥16 years of age or under investigation generally have few relevant pediatric data (297, 298) (Table 5).
Some centrally active, amphetamine-like catecholaminergic and dopaminergic stimulants, such as phentermine and diethylpropion, are FDA approved as short-term monotherapy (a few weeks) for obesity in adults. Recently, lisdexamfetamine dimesylate was FDA approved to treat binge eating in adults (282, 283). Lisdexamfetamine treatment was associated with short-term weight loss, but this medication is not FDA approved for weight management. Because of adverse effect profiles (Table 5), abuse potential (347), and the absence of trials showing long-term weight loss efficacy, none of the amphetamine-like agents is recommended for obesity management in children and adolescents.
Although not FDA approved for the treatment of obesity, GH treatment of children and adolescents with Prader-Willi syndrome, particularly when started early (90), decreases body fat percentage and increases lean body mass (348), with effects that may be sustained for the long term (90). A summary of the benefits and risks of GH treatment (349) and consensus guidelines for GH therapy in Prader-Willi Syndrome are available (350).
Octreotide limits the opening of voltage-gated calcium channels in beta cells (351, 352), decreasing the magnitude of insulin response to glucose (353). In obese adults with insulin hypersecretion, treating with long-acting repeatable octreotide for 6 months resulted in ∼2% greater weight loss than in controls (316). Studies have reported weight stabilization, instead of significant weight gain, in children and adolescents with hypothalamic obesity treated with somatostatin analogs (315, 354). Given its side-effect profile, octreotide appears to be potentially beneficial only for those with hypothalamic obesity.
Liraglutide, a glucagon-like peptide 1 analog, is approved for long-term adult obesity treatment; the effective 3 mg dose produced an additional weight loss of 4.5% vs placebo at 1 year, with sustained effects for up to 2 years (355). Small trials suggest that another glucagon-like peptide 1 analog, exenatide, may potentially have efficacy in adolescent obesity; used for >3 months, exenatide reduced BMI by >1 kg/m2 (compared with control), with continued BMI reduction during a 3-month open-label phase (297, 298).
Leptin therapy in leptin-deficient patients produces significant loss of fat mass (295, 356, 357). Unfortunately, leptin therapy in adults who are not leptin deficient has little effect on body weight (358–360).
Agents that have been recently approved for long-term obesity treatment in adults (Table 5) currently lack pediatric-specific data. The additional weight loss (beyond that achieved with placebo) at 1 year among adults ranges from ∼3% (lorcaserin) to ∼10% (phentermine plus topiramate) (267), but none is without potential risks. If adult patients taking full-dose lorcaserin, bupropion plus naltrexone, liraglutide, or phentermine plus topiramate do not see clinically meaningful weight loss (>3% to 5% of body weight) after 12 weeks, clinicians should discontinue treatment, because significant weight loss after 1 year is unlikely. Similar results were found for adults given orlistat (361). In the largest adolescent orlistat trial (362), 21% of orlistat-treated adolescents decreased their body weight by ≥5% at 12 weeks and went on to decrease body weight by 7.8% after 1 year of treatment; however, those who lost <5% at 12 weeks had a 2.3% weight gain after 1 year (362). Thus, clinicians should discontinue pharmacotherapy agents when sufficient weight loss is not observed after 12 weeks.
Values and preferences
We placed a higher value on avoiding drug side effects and on achieving healthy weight through the incorporation of healthy behaviors. The suggestion to minimize the use of pharmacotherapy in children and adolescents reflects the limited efficacy and small number of long-term pediatric trials for existing agents, along with the imperative to manage pediatric obesity as a serious chronic condition in which long-term success overrides short-term gains.
Remarks
Drug efficacy is based only on reductions of BMI or BMI z scores. Antiobesity drugs may have differential effects on obesity-associated comorbidities based on their mechanisms of action. For example, certain medications (e.g., metformin) have more potent effects on glucose tolerance. Clinicians should tailor drug selection to the individual patient and pay strong attention to the patient’s concomitant medications, medical conditions, and family history, as well as each medication’s efficacy and adverse event profile. The benefits of any drug used to treat pediatric obesity should clearly outweigh its long-term risks. Clinicians should be aware that no obesity medication has been shown to reduce the incidence of cardiovascular morbidity or mortality (267).
The recommendation to discontinue medication when it appears relatively ineffective after 12 weeks of use is consistent with adult obesity pharmacotherapy labeling. The FDA label for liraglutide recommends discontinuation when adults have <4% weight reduction. Most drugs should be discontinued if a 5% decrease in BMI/BMI z score does not occur.
Although pediatricians prescribe many medications “off-label”, we think pharmacotherapeutic agents not yet approved for the treatment of pediatric obesity should be restricted to large, well-controlled clinical studies.
Bariatric surgery
-
4.11
We suggest bariatric surgery only under the following conditions:
-
•
the patient has attained Tanner 4 or 5 pubertal development and final or near-final adult height, the patient has a BMI of >40 kg/m2 or has a BMI of >35 kg/m2 and significant, extreme comorbidities
-
•
extreme obesity and comorbidities persist despite compliance with a formal program of lifestyle modification, with or without pharmacotherapy
-
•
psychological evaluation confirms the stability and competence of the family unit, psychological distress due to impaired QOL from obesity may be present, but the patient does not have an underlying untreated psychiatric illness
-
•
the patient demonstrates the ability to adhere to the principles of healthy dietary and activity habits
-
•
patient has access to an experienced surgeon in a pediatric bariatric surgery center of excellence providing the necessary infrastructure for patient care, including a team capable of long-term follow-up of the metabolic and psychosocial needs of the patient and family. (2|⊕○○○)
-
•
-
4.12
We suggest against bariatric surgery in preadolescent children; pregnant or breast-feeding adolescents (and those planning to become pregnant within 2 years of surgery); and in any patient who has not mastered the principles of healthy dietary and activity habits and/or has an unresolved substance abuse, eating disorder, or untreated psychiatric disorder. (2|⊕○○○)
Evidence
Clinicians prescribe bariatric procedures for weight loss in adolescents because of the poor success of nonsurgical treatment in achieving and maintaining weight loss in adolescents with extreme obesity.
Indications for weight loss surgery include BMI of >35 kg/m2 with major comorbidities of obesity (T2DM, moderate to extreme sleep apnea, pseudotumor cerebri, debilitating orthopedic problems, and nonalcoholic steatohepatitis with advanced fibrosis). Patients are also candidates for bariatric surgery if they have a BMI of >40 kg/m2 with mild comorbidities (hypertension, dyslipidemia, moderate orthopedic problems, mild sleep apnea, nonalcoholic steatohepatitis, and extreme psychological distress that is secondary to their obesity) (363).
Because of the beneficial effects on QOL, social relationships, and depression in studies of adolescents (364–367), some as long as 2 to 3 years in duration (368, 369), proponents of bariatric surgery suggest that extreme psychological distress is an indication for bariatric surgery (368, 370). Most guidelines now include obesity-related psychological distress an indication for bariatric surgery if the adolescent’s BMI is >40 kg/m2 (363, 370).
A psychologist must assess the bariatric surgery candidate to determine the severity of psychological impairment as well as ability to comply with the requirements for successful outcome. It is essential that all potential candidates have a stable home environment with good family support and the ability to carry out the necessary postoperative behaviors—adherence to dietary guidelines (including macronutrient administration) and physical activity recommendations. Adolescents who are unable to give assent; who have untreated or unstable psychiatric issues other than depression; who are substance abusers; or who are pregnant, planning pregnancy, or breastfeeding are not good candidates for bariatric surgery (370). All candidates for bariatric surgery should agree to psychological evaluation before surgery and in the perioperative period (371).
Surgery can be malabsorptive, restrictive, or combination procedures. Laparoscopic adjustable gastric banding (LAGB) (83) is a purely restrictive procedure that isolates the upper stomach by placing an adjustable silicone ring around the entrance to the stomach [Fig. 3(A)] (223). The LAGB procedure has high reoperation and long-term complication rates, which increase with time and thus it is rarely used anymore (373–375).
Figure 3.
Bariatric surgical procedures. (A) LAGB, (B) vertical banded gastroplasty, (C) RYGB, (D) biliopancreatic diversion, (E) biliopancreatic diversion with duodenal switch, (F) VSG, (G) ileal interposition with sleeve gastrectomy, and (H) Santoro III. (A), (C), and (F) are applicable to section 4 (Bariatric Surgery). [Reproduced from Nandagopal R et al. (372), with permission.]
Malabsorptive procedures decrease intestinal mucosal function by rearranging the anatomy of the intestine, resulting in malabsorption of nutrients. RYGB is a combination procedure in which the surgeon creates a small stomach pouch and the remainder of the stomach is bypassed. The surgeon inserts a segment of the jejunum in the small gastric pouch, which connects to the proximal portion of the jejunum that drains the bypassed portion of the stomach and the duodenum [Fig. 3(C)]. The RYGB has the restrictive properties of a partial gastrectomy while causing malabsorption and “dumping syndrome” by bypassing much of the stomach.
In vertical sleeve gastrectomy (VSG), a surgeon resects ∼85% of the stomach, removing the fundus and greater curvature, leaving a narrow gastric remnant [Fig. 3(F)]. There is no rearrangement of the anatomy, making it less likely that patients having VSG will have malabsorption of micronutrients or postoperative bowel obstruction, as compared with RYGB (370). Because VSG has less surgical complications than the RYGB, patients use it with increasing frequency (373, 376). The Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study (a prospective, multisite observational study at 5 academic centers) performed 52 RYGB and 1 VSG in 2008 vs 24 RYGB and 29 VSG in 2011 (377).
In addition to the anatomic effects of the procedures, both RYGB and VSG decrease the orexigenic hormone ghrelin (87, 378, 379) and increase the anorexogenic incretins glucagon-like peptide 1 and peptide YY (380, 381), thus decreasing appetite and improving insulin sensitivity (382).
Adolescents having VSG performed between 2008 and 2011 had 61.3% excess weight loss at 1 year (n = 41) and 62.3% excess weight loss at 2 years (n = 8) (383). The largest pediatric study to date found a similar BMI reduction with VSG (37%) (384) as with RYGB (35% to 37%) (385) at 1 year following surgery. This is consistent with the results of the Adolescent Morbid Obesity Study (376), a Swedish study of 81 adolescents with a mean BMI decrease from 45.5 to 29.7 kg/m2 2 years following RYGB (367). Three-year data from the Teen-LABS study found that the mean BMI decreased overall from 53 to 38 kg/m2 (a decrease of 27%) with a 28% BMI decrease in those receiving RYGB (n = 161) and a 26% decrease in the teens who had VSG (n = 67) (369).
Regardless of procedure, the percentage of weight loss is independent of initial BMI, so those who are extremely obese will still be obese following surgery (385). Even when obesity persists, most comorbidities associated with obesity improve markedly following the surgery. A study of 22 adolescents who were extremely obese showed positive effects of RYGB on glucose homeostasis parameters with 38% decline in BMI (61 to 39 kg/m2) 1 year after surgery (386). Positive effects of bariatric surgery have included the reversal of T2DM (387), improvements in glucose homeostasis in nondiabetics (379), improved insulin sensitivity and secretion (388), resolution of sleep apnea (389), improvements in nonalcoholic steatohepatitis (381), improvements in severe arthropathy (371), and improvements in cardiovascular risk factors [dyslipidemia, hypertension, and inflammation (390) and increased adiponeptin and decreased IL-1, IL-8, CRP, and TNF-α (391)], as well as decreased left ventricular mass index, improvements in left ventricular hypertrophy, improvements in diastolic function, and improved rate-pressure product, all of which suggest decreased cardiac workload (392).
The Teen-LABS study indicated that 39% of enrolled patients had more than 4 major comorbid conditions at baseline (376). Three-year follow-up of the patients enrolled in Teen-LABS found a 95% remission of T2DM (19 of 20 teens who had diabetes at the time of surgery), 76% remission of prediabetes (13 of 17 patients), 74% remission of hypertension (56 of 76 with initial high blood pressure), and a 66% normalization of dyslipidemia (84 of 128 patients) (369).
The Teen-LABS study assessed comorbid conditions and surgical complications in the perioperative period in 242 adolescents during the first month following surgery (377). There were no procedure-related deaths. Sixty-six percent had laparoscopic RYGB, 28% had VSG, and 6% had LAGB. Major complications occurring within the 30 days following surgery (gastrointestinal leaks, suicidal ideation, anticoagulation for pulmonary embolus) occurred in 7 patients (0.4%), and minor complications occurred in 27 patients (11.2%; 2.5% of patients undergoing RYGB, 3.0% VSG, and 7.1% AGB) (377). The most common complications with both RYGB and VSG were abdominal pain/diarrhea/nausea/dehydration followed by stricture with RYGB and wound infection with VSG (373). Nineteen of 242 patients had major complications within 30 days of surgery (9.3% with RYGB, 4.5% following VSG, and 7.1% with AGB). Late complications occurred in 10% to 15% of patients and included hernias at incision sites, cholelithiasis, small bowel obstruction, stomal stenosis, protein calorie malnutrition, vitamin and mineral deficiencies, and weight regain (376, 377, 393, 394). The Adolescent Morbid Obesity Surgery Study in Sweden had a 33% adverse event rate, with 15% (n = 12) requiring reoperation, 5 for internal hernias, 5 for cholecystectomy, 1 for adhesions, and 1 for pain without surgical findings (364). Seven percent had psychological sequelae, 2 with suicide attempts from medication overdose, 1 with self-destructive behavior and suicidal ideation, and 3 with depression and anxiety. All had psychological problems before surgery. Five patients had excessive use of addictive drugs (none of these patients disclosed the fact that they had preexisting addictions at the time of presurgery assessment) (367). Although most patients have improvement in QOL, self-esteem, anxiety, and depressive symptoms (365), these improvements were not universally maintained at 2 years following surgery (368). Suicidal ideation has been reported, possibly related to unrealistic expectations that their life would be completely different following surgery or to continued poor self-image with weight regain (367). The most recent Teen-LABS study, evaluating 242 adolescents at 3 years after RYGB or VSG, found the mean QOL improved from 63 to 83 based on the total score from the Impact of Weight on Quality of Life–Kids survey (369).
As these procedures all have potential adverse events, it is important to have life-long monitoring for complications. Adherence to prescribed nutritional guidelines is essential for all weight-loss surgery patients postoperatively because low levels of minerals and vitamins can occur due to restricted nutrient intake, decreased gastric acid production, decreased production of intrinsic factor and digestive enzymes, or food intolerance (especially following the dumping syndrome with RYGB) (121, 395). Iron deficiency is the most common mineral deficiency, as RYGB not only causes malabsorption but also has low gastric acid production, further impairing iron absorption (121, 370). Decreased bone mineralization is common, as RYGB decreases cholecalciferol absorption by 25% and calcium and phosphorous concentrations may be low, resulting in significant bone density loss (396). Vitamin deficiencies are common, including deficiencies of vitamins B12, B1, and folate, as RYGB and VSG both reduce the surface of the distal portion of the stomach, resulting in inadequate secretion of intrinsic factor. Annual screening is recommended for patients at risk for developing vitamin deficiencies. As RYGB can result in copper, selenium, and zinc deficiencies, it is recommended that all patients having bariatric surgery receive supplementation with a multivitamin with minerals (370). Patients need to be monitored long term for changes in bone density, hair loss secondary to zinc deficiency, and neurologic complications (363). It is recommended that they avoid alcohol and decrease the intake of sugar and fructose-containing drinks. Despite the importance of nutritional supplementation following bariatric surgery, the Adolescent Morbid Obesity Surgery study found a 67% noncompliance rate with prescribed vitamin and mineral intake at 2 years following surgery. Low ferritin levels were found in 12% of patients before surgery and in 39% of patients 2 years after surgery. Similarly, Vitamin B12 deficiency increased from 1.3% before surgery to 13% after surgery (367). The 3-year follow-up data from the Teen-LABS study found similar results. Low folate levels were found in 3% of youths at baseline and in 8% at 3 years, low vitamin B12 concentrations increased from <1% to 8%, low 25 hydroxyvitamin D levels increased from 37% to 43%, and the percentage of adolescents with low ferritin levels increased from 5% at baseline to 57% at 3 years (369).
These data emphasize the need for a multidisciplinary team that should include a bariatric surgeon, a pediatric obesity specialist to screen and manage the comorbidities, a dietitian to plan the diet and assure adequate nutritional intake, a mental health professional to perform the initial psychological assessment and provide counseling during the postoperative adjustment, a program coordinator to facilitate compliance and follow-up, and a social worker to provide resources to help overcome barriers to care and run support groups (373). Long-term follow-up is essential to maintain compliance with nutritional recommendations.
We agree with the expert panels (226, 227) that suggest bariatric surgery for adolescents with obesity-related comorbid conditions that threaten the adolescent’s health—a BMI of >35 kg/m2 and an extreme comorbidity or a BMI of >40 kg/m2 and less extreme comorbidity.
Remarks
As adolescents appear to have a greater rate of diabetes resolution and improvement in other obesity-related comorbidities than do adults, it may be beneficial to consider earlier surgery in obese teens, as they likely have less vascular damage than do older individuals.
Values and preferences
The Task Force suggestion of bariatric surgery in adolescents who are extremely obese with serious comorbidities places a high value on amelioration of life-threatening complications and lower value on surgical cost and perioperative complications.
Conclusion
Pediatric obesity remains an ongoing serious international health concern affecting ∼17% of US children and adolescents, threatening their adult health and longevity. Pediatric obesity has its basis in genetic susceptibilities influenced by a permissive environment starting in utero and extending through childhood and adolescence. Endocrine etiologies for obesity are rare and usually are accompanied by attenuated growth patterns. Pediatric comorbidities are common and long-term health complications often result; screening for comorbidities of obesity should be applied in a hierarchal, logical manner for early identification before more serious complications result. Genetic screening for rare syndromes is indicated only in the presence of specific historical or physical features. The psychological toll of pediatric obesity on the individual and family necessitates screening for mental health issues and counseling as indicated. The prevention of pediatric obesity by promoting healthful diet, activity, and environment should be a primary goal, as achieving effective, long-lasting results with lifestyle modification once obesity occurs is difficult. Although some behavioral and pharmacotherapy studies report modest success, additional research into accessible and effective methods for preventing and treating pediatric obesity is needed. The use of weight loss medications during childhood and adolescence should be restricted to clinical trials. Increasing evidence demonstrates the effectiveness of bariatric surgery in the most seriously affected mature teenagers who have failed lifestyle modification, but it requires experienced teams with resources for long-term follow-up. Adolescents undergoing lifestyle therapy, medication regimens, or bariatric surgery for obesity will need cohesive planning to help them effectively transition to adult care, such as continued necessary monitoring, support, and intervention. Transition programs for obesity are an uncharted area requiring further research for efficacy.
Despite a significant increase in research on pediatric obesity since the initial publication of these guidelines 8 years ago, there remains an unmet need for further study of the genetic and biological factors that increase the risk of weight gain and influence the response to therapeutic interventions. Also needed are more studies to better understand the genetic and biological factors that cause an obese individual to manifest 1 comorbidity vs another or to be free of comorbidities. Continued investigation into the most effective methods of preventing and treating obesity and into methods for changing environmental and economic factors that will lead to worldwide cultural changes in diet and activity should be priorities. Particular attention to determining ways to effect systemic changes in food environs and total daily mobility, as well as methods for sustaining healthy BMI changes, is of importance.
Summary of Changes
Since the publication of the original guidelines 8 years ago there have been an additional 1778 references added to PubMed concerning pediatric obesity. We have incorporated the most relevant data from these to update and enhance the original text.
The epidemiology and definition section contains the latest statistics on trends in childhood obesity, including an apparent recent stabilization of the prevalence. New definitions for extreme obesity are added with a notation that this is the group that continues to rise. The prevalence in ethnic minorities as well a discussion of the limitations of applying the BMI equation to all ethnic groups are addressed.
The evaluation section provides the latest guidelines for utilizing laboratory evaluation for diagnosis and management of comorbidities of obesity. Special emphasis on avoiding endocrine evaluation in most children as well as avoiding measurement of insulin values is provided to prevent unnecessary laboratory testing.
The genetics section has been extensively revised with the latest genomic findings presented in table form and provides guidelines on when to invoke genetic testing in obese children, particularly those with early onset obesity, family history of extreme obesity, and hyperphagia. A combined flowchart demonstrating pathways of diagnosis from history and physical examination to genetic testing is included.
Prevention of obesity is discussed with numerous new studies that support most previous conclusions on lifestyle modification. However, although breast-feeding is beneficial for an infant in numerous ways and was supported as a recommendation to prevent obesity in the previous guidelines, recent data weaken support for breast-feeding as a means of preventing obesity and breast-feeding is now a suggestion.
The treatment section focuses on lifestyle changes as the basis of all efforts to treat childhood obesity and supports most previous recommendations and suggestions. A chart demonstrating how much change in systolic blood pressure and lipid values might be expected with a decrease in 1 unit of BMI (kg/m2) or a decrease of 1 kg of body weight is added.
A discussion of the significant toll childhood obesity takes on the psychological function of a child follows. Guidelines for evaluation of children and access to tools to evaluate child and family function are provided. Referral to appropriate counseling programs is emphasized when psychological problems or aberrant family dynamics are found.
Although noting that all but one of the pharmacological agents targeting obesity are not approved until 16 years of age, agents and their method of action are presented in detail in a table. Lifestyle modification is emphasized as a basis for any additional pharmacological therapy. Should pharmacological therapy be invoked, even off label, guidelines for use and for discontinuation in the case of lack of efficacy are provided. When pharmacotherapy is considered, only clinicians experienced in the use of the agents should use them.
The increasing information on the benefits and risks of bariatric surgery is presented along with a discussion of the types of procedures that might be used. There is emphasis upon contraindications in the use of bariatric surgery in growing children and immature teenagers. Emphasis that procedures should only be carried out in those mature pubertal individuals with severe comorbidities of obesity in the presence of a motivated and compliant patient and family and only in the hands of an experienced surgeon with a dedicated and experienced support team is provided.
The last section sets new goals for future research into the thorny questions of the best method to determine the etiology of childhood obesity and methods to prevent and treat childhood obesity and its comorbidities.
Financial Disclosures of the Task Force*
Dennis M. Styne (chair)—Financial and Business/Organizational Interests: None declared, Significant Financial Interest or Leadership Position: Teva, Bristol Myers, Organovo (Ownership Intrests). Silva A. Arslanian—Financial and Business/Organizational Interests: Aegerion (Consultant), Boehringer-Ingelheim (Advisory Board & Data Safety Monitoring), Bristol-Myers Squibb (Advisory Board), INTARCIA Therapeutics, Inc. (Consultant), Lilly USA, LLC (Advisory Board), Novo Nordisk (Advisory Board), Rambaxy (Consultant), Sanofi-aventis (Advisory Board), Janssen Pharmaceutical (Principal Investigator), NIH (Grantee and Reviewer), Significant Financial Interest or Leadership Position: Aegerion (Consultant), Boehringer-Ingelheim (Advisory Board & Data Safety Monitoring), Bristol-Myers Squibb (Advisory Board), INTARCIA Therapeutics, Inc. (Consultant), Lilly USA, LLC (Advisory Board), Novo Nordisk (Advisory Board), Rambaxy (Consultant), Sanofi-aventis (Advisory Board), Janssen Pharmaceutical (Principal Investigator), NIH (Grantee and Reviewer). Ellen L. Connor—Financial and Business/Organizational Interests: Pediatric Endocrine Society (Obesity Committee Chair), Significant Financial Interest or Leadership Position: none declared. Ismaa Sadaf Farooqi—Financial and Business/Organizational Interests: none declared, Significant Financial Interest or Leadership Position: none declared. M. Hassan Murad,**—Financial and Business/Organizational Interests: Mayo Clinic, Evidence-based Practice Center, Significant Financial Interest or Leadership Position: none declared. Janet H. Silverstein—Financial and Business/Organizational Interests: Pediatric Endocrine Society (Chair, MOC Committee, Member SCAMPS Committee), Daichi Sankyo (Clinical Trial [funds to UF]), Sanofi (Clinical Trial [funds to UF]), Significant Financial Interest or Leadership Position: Daichi Sankyo (Clinical Trial [funds to UF]), Sanofi (Clinical Trial to UF). Jack A. Yanovski—Financial and Business/Organizational Interests: Zafgen Inc. (Principal Investigator [funds to NIH]), Significant Financial Interest or Leadership Position: None declared.
* Financial, business, and organizational disclosures of the Task Force cover the year prior to publication. Disclosures prior to this time period are archived.
**Evidence-based reviews for this guideline were prepared by the Mayo Clinic, Evidence-based Practice Center under contract with the Endocrine Society.
Acknowledgments
We offer special thanks to the committee that developed the original version of these guidelines (76). We also offer thanks to Drs. David B. Allen and George A. Bray for careful review and thoughtful suggestions and Eric Vohr, medical writer, for excellent editorial assistance.
Disclosure Summary: See Financial Disclosures.
Abbreviations:
- ALT
alanine aminotransferase
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- CVD
cardiovascular disease
- FDA
Food and Drug Administration
- GH
growth hormone
- HbA1c
hemoglobin A1c
- LAGB
laparoscopic adjustable gastric banding
- NAFLD
nonalcoholic fatty liver disease
- PCOS
polycystic ovary syndrome
- QOL
quality of life
- RCT
randomized controlled trial
- RYGB
Roux-en-Y gastric bypass
- T2DM
type 2 diabetes mellitus
- VSG
vertical sleeve gastrectomy
- WHO
World Health Organization
References
- 1. Daniels SR, Hassink SG; Committee on Nutrition . The role of the pediatrician in primary prevention of obesity. Pediatrics. 2015;136:e275–e292. [DOI] [PubMed] [Google Scholar]
- 2. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swiglo BA, Murad MH, Schunemann HJ, Kunz R, Vigersky RA, Guyatt GH, Montori VM. A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab. 2008;93:666–673. [DOI] [PubMed] [Google Scholar]
- 4. Guyatt GH, Schunemann HJ, Djulbegovic B, Akl EA. Guideline panels should not GRADE good practice statements. J Clin Epidemiol. 2015;68:597–600. [DOI] [PubMed] [Google Scholar]
- 5. Rajjo T, Mohammed K, Alsawas M, Ahmed AT, Farah W, Asi N, Almasri J, Prokop LJ, Murad MH. Treatment of pediatric obesity: an umbrella systematic review. J Clin Endocrinol Metab. 2017;102:763–775. [DOI] [PubMed]
- 6. Rajjo T, Almasri J, Al Notal A, Farah W, Alsawas M, Ahmed AT, Mohammed K, Kanwar A, Asi N, Wang Z, Prokopi J, Murad MH. The association of weight loss and cardiometabolic outcomes in obese children: systematic review and meta-regression. J Clin Endocrinol Metab. 2017;102(3):761–765. [DOI] [PubMed] [Google Scholar]
- 7. Trasande L, Chatterjee S. The impact of obesity on health service utilization and costs in childhood. Obesity (Silver Spring). 2009;17:1749–1754. [DOI] [PubMed] [Google Scholar]
- 8.Kasman MHR, Werman A, Mack-Crane A, McKinnon R. An in-depth look at the lifetime economic cost of obesity. Available at: http://www.brookings.edu/~/media/Events/2015/05/12-economic-costs-of-obesity/0512-Obesity-Presentation-v6-RM.pdf?la=en. Accessed 17 March 2016.
- 9. Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl 4):S193–S228. [DOI] [PubMed] [Google Scholar]
- 10. Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, Urbina EM, Ewing LJ, Daniels SR; American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young, Council on Nutrition, Physical Activity, and Metabolism, and Council on Clinical Cardiology . Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128:1689–1712. [DOI] [PubMed] [Google Scholar]
- 11. Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med. 2015;373:1307–1317. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. National Center for Health Statistics. Available at: http://www.cdc.gov/growthcharts/. Accessed 17 March 2016.
- 13. Whitlock EP, O’Conner EA, Williams SB, Beil TL, Lutz KW. Effectiveness of Primary Care Interventions for Weight Management in Children and Adolescents: An Updated, Targeted Systematic Review for the USPSTF. Rockville, MD: Agency for Healthcare Research and Quality; 2010. [PubMed] [Google Scholar]
- 14. Flegal KM, Ogden CL, Yanovski JA, Freedman DS, Shepherd JA, Graubard BI, Borrud LG. High adiposity and high body mass index-for-age in US children and adolescents overall and by race-ethnic group. Am J Clin Nutr. 2010;91:1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deurenberg P, Bhaskaran K, Lian PL. Singaporean Chinese adolescents have more subcutaneous adipose tissue than Dutch Caucasians of the same age and body mass index. Asia Pac J Clin Nutr. 2003;12:261–265. [PubMed] [Google Scholar]
- 16. Graham RC, Burke A, Stettler N. Ethnic and sex differences in the association between metabolic syndrome and suspected nonalcoholic fatty liver disease in a nationally representative sample of US adolescents. J Pediatr Gastroenterol Nutr. 2009;49:442–449. [DOI] [PubMed] [Google Scholar]
- 17. Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM Jr. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes Rev. 2016;17:262–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015;(219):1–8. [PubMed] [Google Scholar]
- 19. Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. [DOI] [PubMed] [Google Scholar]
- 20. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. [DOI] [PubMed] [Google Scholar]
- 22. Javed A, Jumean M, Murad MH, Okorodudu D, Kumar S, Somers VK, Sochor O, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: a systematic review and meta-analysis. Pediatr Obes. 2015;10:234–244. [DOI] [PubMed] [Google Scholar]
- 23. Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. [DOI] [PubMed] [Google Scholar]
- 24. Freedman DS, Kettel Khan L, Serdula MK, Srinivasan SR, Berenson GS. BMI rebound, childhood height and obesity among adults: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2001;25:543–549. [DOI] [PubMed] [Google Scholar]
- 25. Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reinehr T, Hinney A, de Sousa G, Austrup F, Hebebrand J, Andler W. Definable somatic disorders in overweight children and adolescents. J Pediatr. 2007; 150:618–622, 622.e1–5. [DOI] [PubMed]
- 27. Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123:84–88. [DOI] [PubMed] [Google Scholar]
- 28. Mamun AA, Hayatbakhsh MR, O’Callaghan M, Williams G, Najman J. Early overweight and pubertal maturation—pathways of association with young adults’ overweight: a longitudinal study. Int J Obes. 2009;33:14–20. [DOI] [PubMed] [Google Scholar]
- 29. Johnson W, Stovitz SD, Choh AC, Czerwinski SA, Towne B, Demerath EW. Patterns of linear growth and skeletal maturation from birth to 18 years of age in overweight young adults. Int J Obes. 2012;36:535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crocker MK, Stern EA, Sedaka NM, Shomaker LB, Brady SM, Ali AH, Shawker TH, Hubbard VS, Yanovski JA. Sexual dimorphisms in the associations of BMI and body fat with indices of pubertal development in girls and boys. J Clin Endocrinol Metab. 2014;99:E1519–E1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wagner IV, Sabin MA, Pfaffle RW, Hiemisch A, Sergeyev E, Korner A, Kiess W. Effects of obesity on human sexual development. Nat Rev Endocrinol. 2012;8:246–254. [DOI] [PubMed] [Google Scholar]
- 32. Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375:1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steele CA, Cuthbertson DJ, MacFarlane IA, Javadpour M, Das KS, Gilkes C, Wilding JP, Daousi C. Hypothalamic obesity: prevalence, associations and longitudinal trends in weight in a specialist adult neuroendocrine clinic. Eur J Endocrinol. 2013;168:501–507. [DOI] [PubMed] [Google Scholar]
- 34. Kelly A, Winer KK, Kalkwarf H, Oberfield SE, Lappe J, Gilsanz V, Zemel BS. Age-based reference ranges for annual height velocity in US children. J Clin Endocrinol Metab. 2014;99:2104–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107:317–329. [DOI] [PubMed] [Google Scholar]
- 36. Reinehr T, Wiegand S, Siegfried W, Keller KM, Widhalm K, l’Allemand D, Zwiauer K, Holl RW. Comorbidities in overweight children and adolescents: do we treat them effectively? Int J Obes. 2013;37:493–499. [DOI] [PubMed] [Google Scholar]
- 37. Flechtner-Mors M, Thamm M, Wiegand S, Reinehr T, Schwab KO, Kiess W, Widhalm K, Holl RW. Comorbidities related to BMI category in children and adolescents: German/Austrian/Swiss Obesity Register APV compared to the German KiGGS Study. Horm Res Paediatr. 2012;77:19–26. [DOI] [PubMed] [Google Scholar]
- 38. Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr. 2013;162:496–500.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang JS, Barlow SE, Quiros-Tejeira RE, Scheimann A, Skelton J, Suskind D, Tsai P, Uko V, Warolin JP, Xanthakos SA. Childhood obesity for pediatric gastroenterologists. J Pediatr Gastroenterol Nutr. 2013;56:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alonso-Alvarez ML, Cordero-Guevara JA, Teran-Santos J, Gonzalez-Martinez M, Jurado-Luque MJ, Corral-Penafiel J, Duran-Cantolla J, Kheirandish-Gozal L, Gozal D. Obstructive sleep apnea in obese community-dwelling children: the NANOS study. Sleep. 2014;37:943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McGill HC Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, Strong JP. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105:2712–2718. [DOI] [PubMed] [Google Scholar]
- 42. Christensen SB, Black MH, Smith N, Martinez MM, Jacobsen SJ, Porter AH, Koebnick C. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril. 2013;100:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eriksson JG, Kajantie E, Lampl M, Osmond C. Trajectories of body mass index amongst children who develop type 2 diabetes as adults. J Intern Med. 2015;278:219–226. [DOI] [PubMed] [Google Scholar]
- 44. Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357:2371–2379. [DOI] [PubMed] [Google Scholar]
- 45. Tirosh A, Shai I, Afek A, Dubnov-Raz G, Ayalon N, Gordon B, Derazne E, Tzur D, Shamis A, Vinker S, Rudich A. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364:1315–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JS, Dwyer T, Raitakari OT. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–1885. [DOI] [PubMed] [Google Scholar]
- 47. Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wise MS, Nichols CD, Grigg-Damberger MM, Marcus CL, Witmans MB, Kirk VG, D’Andrea LA, Hoban TF. Executive summary of respiratory indications for polysomnography in children: an evidence-based review. Sleep. 2011;34:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taylor ED, Theim KR, Mirch MC, Ghorbani S, Tanofsky-Kraff M, Adler-Wailes DC, Brady S, Reynolds JC, Calis KA, Yanovski JA. Orthopedic complications of overweight in children and adolescents. Pediatrics. 2006;117:2167–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. BeLue R, Francis LA, Colaco B. Mental health problems and overweight in a nationally representative sample of adolescents: effects of race and ethnicity. Pediatrics. 2009;123:697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zametkin AJ, Zoon CK, Klein HW, Munson S. Psychiatric aspects of child and adolescent obesity: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2004;43:134–150. [DOI] [PubMed] [Google Scholar]
- 52. Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014;9:e94112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Musil R, Obermeier M, Russ P, Hamerle M. Weight gain and antipsychotics: a drug safety review. Expert Opin Drug Saf. 2015;14:73–96. [DOI] [PubMed] [Google Scholar]
- 54. Caulfield LE. Methodological challenges in performing targeting: assessing dietary risk for WIC participation and education. J Nutr. 2005;135:879–881. [DOI] [PubMed] [Google Scholar]
- 55. Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S. The metabolic syndrome in children and adolescents—an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. [DOI] [PubMed] [Google Scholar]
- 56. Lee S, Bacha F, Arslanian SA. Waist circumference, blood pressure, and lipid components of the metabolic syndrome. J Pediatr. 2006;149:809–816. [DOI] [PubMed] [Google Scholar]
- 57. Daniels SR. Should pediatricians be measuring waist circumference? J Pediatr. 2006;149:A1. [Google Scholar]
- 58. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 60. American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 61. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 62. doi: 10.1053/j.gastro.2009.12.052. Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, Sirlin CB. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010;138:1357–1364.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413. [DOI] [PubMed] [Google Scholar]
- 65. International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.American Diabetes Association. Standards of medical care in diabetes—2010. Available at: http://care.diabetesjournals.org/content/33/Supplement_1/S4.full. Accessed 17 March 2016. [DOI] [PMC free article] [PubMed]
- 67. Lee JM, Gebremariam A, Wu EL, LaRose J, Gurney JG. Evaluation of nonfasting tests to screen for childhood and adolescent dysglycemia. Diabetes Care. 2011;34:2597–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nowicka P, Santoro N, Liu H, Lartaud D, Shaw MM, Goldberg R, Guandalini C, Savoye M, Rose P, Caprio S. Utility of hemoglobin A1c for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care. 2011;34:1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. doi: 10.1016/j.jpeds.2010.11.026. Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr. 2011; 158:947–952.e1–3. doi: 10.1016/j.jpeds.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dagogo-Jack S. Pitfalls in the use of HbA1c as a diagnostic test: the ethnic conundrum. Nat Rev Endocrinol. 2010;6:589–593. [DOI] [PubMed] [Google Scholar]
- 71.American Diabetes Association. Standards of medical care in diabetes—2014. Available at: http://care.diabetesjournals.org/content/37/Supplement_1/S5.full. Accessed 17 March 2016. [DOI] [PMC free article] [PubMed]
- 72. Wu EL, Kazzi NG, Lee JM. Cost-effectiveness of screening strategies for identifying pediatric diabetes mellitus and dysglycemia. JAMA Pediatr. 2013;167:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Molleston JP, Schwimmer JB, Yates KP, Murray KF, Cummings OW, Lavine JE, Brunt EM, Scheimann AO, Unalp-Arida A. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. J Pediatr. 2014;164:707–713.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab. 2011;96:2136–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Levy-Marchal C, Arslanian S, Cutfield W, Sinaiko A, Druet C, Marcovecchio ML, Chiarelli F. Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab. 2010;95:5189–5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Monzillo LU, Hamdy O. Evaluation of insulin sensitivity in clinical practice and in research settings. Nutr Rev. 2003;61:397–412. [DOI] [PubMed] [Google Scholar]
- 77. Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res. 2006;60:759–763. [DOI] [PubMed] [Google Scholar]
- 78. Ball GD, Huang TT, Gower BA, Cruz ML, Shaibi GQ, Weigensberg MJ, Goran MI. Longitudinal changes in insulin sensitivity, insulin secretion, and β-cell function during puberty. J Pediatr. 2006;148:16–22. [DOI] [PubMed] [Google Scholar]
- 79. Robbins DC, Andersen L, Bowsher R, Chance R, Dinesen B, Frank B, Gingerich R, Goldstein D, Widemeyer HM, Haffner S, Hales CN, Jarett L, Polonsky K, Porte D, Skyler J, Webb G, Gallagher K. Report of the American Diabetes Association’s Task Force on standardization of the insulin assay. Diabetes. 1996;45:242–256. [DOI] [PubMed] [Google Scholar]
- 80. Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51:3014–3019. [DOI] [PubMed] [Google Scholar]
- 81. Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, β-cell failure, or both? Diabetes Care. 2005;28:638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bacha F, Lee S, Gungor N, Arslanian SA. From pre-diabetes to type 2 diabetes in obese youth: pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes Care. 2010;33:2225–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Magi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Hua Zhao J, Zhao W, Chen J, Fehrmann R, Hedman AK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Mateo Leach I, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stancakova A, Strawbridge RJ, Ju Sung Y, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Arnlov J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Bluher M, Bohringer S, Bonnycastle LL, Bottcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Ida Chen YD, Clarke R, Daw EW, de Craen AJ, Delgado G, Dimitriou M, Doney AS, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Grassler J, Gronberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga JJ, James AL, Jeff JM, Johansson A, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindstrom J, Sin Lo K, Lobbens S, Lorbeer R, Lu Y, Mach F, Magnusson PK, Mahajan A, McArdle WL, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Muller G, Muller-Nurasyid M, Musk AW, Nagaraja R, Nothen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Vernon Smith A, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundstrom J, Swertz MA, Swift AJ, Syvanen AC, Tan ST, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh HW, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q; LifeLines Cohort Study, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gadin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee JY, Liang L, Lifton RP, Ma B, McCarroll SA, McKnight AJ, min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, Perry JR, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Tanaka T, Van’t Hooft FM, Vinkhuyzen AA, Westra HJ, Zheng W, Zondervan KT; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; Magic Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium, Heath AC, Arveiler D, Bakker SJ, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Cupples LA, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrieres J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Hovingh GK, Humphries SE, Hunt SC, Hypponen E, Illig T, Jacobs KB, Jarvelin MR, Jockel KH, Johansen B, Jousilahti P, Jukema JW, Jula AM, Kaprio J, Kastelein JJ, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Le Marchand L, Lehtimaki T, Lyssenko V, Mannisto S, Marette A, Matise TC, McKenzie CA, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Madden PA, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, Schwarz PE, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tonjes A, Tregouet DA, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Volker U, Waeber G, Willemsen G, Witteman JC, Zillikens MC, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PI, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, Marz W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njolstad I, Oostra BA, Palmer CN, Pedersen NL, Perola M, Perusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann HE, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O’Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, McCarthy MI, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, Loos RJ, Speliotes EK. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr. 2008;87:398–404. [DOI] [PubMed] [Google Scholar]
- 85. Farooqi IS, O’Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab. 2008;4:569–577. [DOI] [PubMed] [Google Scholar]
- 86. August GP, Caprio S, Fennoy I, Freemark M, Kaufman FR, Lustig RH, Silverstein JH, Speiser PW, Styne DM, Montori VM. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab. 2008;93:4576–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Farooqi SOR, O’Rahilly S. Genetic obesity syndromes. In: Grant S, ed. The Genetics of Obesity. New York, NY: Springer; 2104:23–32. [Google Scholar]
- 88. Sahoo T, del Gaudio D, German JR, Shinawi M, Peters SU, Person RE, Garnica A, Cheung SW, Beaudet AL. Prader-Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nat Genet. 2008;40:719–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basdevant A, Weigle DS. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8:643–644. [DOI] [PubMed] [Google Scholar]
- 90. Bakker NE, Kuppens RJ, Siemensma EP, Tummers-de Lind van Wijngaarden RF, Festen DA, Bindels-de Heus GC, Bocca G, Haring DA, Hoorweg-Nijman JJ, Houdijk EC, Jira PE, Lunshof L, Odink RJ, Oostdijk W, Rotteveel J, Schroor EJ, Van Alfen AA, Van Leeuwen M, Van Pinxteren-Nagler E, Van Wieringen H, Vreuls RC, Zwaveling-Soonawala N, de Ridder MA, Hokken-Koelega AC. Eight years of growth hormone treatment in children with Prader-Willi syndrome: maintaining the positive effects. J Clin Endocrinol Metab. 2013;98:4013–4022. [DOI] [PubMed] [Google Scholar]
- 91. Weinstein LS, Chen M, Liu J. Gsα mutations and imprinting defects in human disease. Ann N Y Acad Sci. 2002;968:173–197. [DOI] [PubMed] [Google Scholar]
- 92. Ramachandrappa S, Raimondo A, Cali AM, Keogh JM, Henning E, Saeed S, Thompson A, Garg S, Bochukova EG, Brage S, Trowse V, Wheeler E, Sullivan AE, Dattani M, Clayton PE, Datta V, Bruning JB, Wareham NJ, O’Rahilly S, Peet DJ, Barroso I, Whitelaw ML, Farooqi IS. Rare variants in single-minded 1 (SIM1) are associated with severe obesity. J Clin Invest. 2013;123:3042–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bonnefond A, Raimondo A, Stutzmann F, Ghoussaini M, Ramachandrappa S, Bersten DC, Durand E, Vatin V, Balkau B, Lantieri O, Raverdy V, Pattou F, Van Hul W, Van Gaal L, Peet DJ, Weill J, Miller JL, Horber F, Goldstone AP, Driscoll DJ, Bruning JB, Meyre D, Whitelaw ML, Froguel P. Loss-of-function mutations in SIM1 contribute to obesity and Prader-Willi-like features. J Clin Invest. 2013;123:3037–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yeo GS, Connie Hung CC, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, O’Rahilly S, Farooqi IS. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7:1187–1189. [DOI] [PubMed] [Google Scholar]
- 95. Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, Adler-Wailes DC, Sanford EL, Lacbawan FL, Uhl GR, Rennert OM, Yanovski JA. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. 2008;359:918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Beales PL, Warner AM, Hitman GA, Thakker R, Flinter FA. Bardet-Biedl syndrome: a molecular and phenotypic study of 18 families. J Med Genet. 1997;34:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Borman AD, Pearce LR, Mackay DS, Nagel-Wolfrum K, Davidson AE, Henderson R, Garg S, Waseem NH, Webster AR, Plagnol V, Wolfrum U, Farooqi IS, Moore AT. A homozygous mutation in the TUB gene associated with retinal dystrophy and obesity. Hum Mutat. 2014;35:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, Saeed S, Hamilton-Shield J, Clayton-Smith J, O’Rahilly S, Hurles ME, Farooqi IS. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463:666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O’Rahilly S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Farooqi IS, Keogh JM, Yeo GS, Lank EJ, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003; 348: 1085–1095. [DOI] [PubMed]
- 102. Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME, Wraight V, Sims M, Vatin V, Meyre D, Shield J, Burren C, Ibrahim Z, Cheetham T, Swift P, Blackwood A, Hung CC, Wareham NJ, Froguel P, Millhauser GL, O’Rahilly S, Farooqi IS. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab. 2006;3:135–140. [DOI] [PubMed] [Google Scholar]
- 103. Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, Ramanathan V, Strochlic DE, Ferket P, Linhart K, Ho C, Novoselova TV, Garg S, Ridderstrale M, Marcus C, Hirschhorn JN, Keogh JM, O’Rahilly S, Chan LF, Clark AJ, Farooqi IS, Majzoub JA. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341:275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hatoum IJ, Stylopoulos N, Vanhoose AM, Boyd KL, Yin DP, Ellacott KL, Ma LL, Blaszczyk K, Keogh JM, Cone RD, Farooqi IS, Kaplan LM. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J Clin Endocrinol Metab. 2012;97:E1023–E1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O’Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O’Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, Krauss RM, Veldhuis JD, Wagner AJ, DePaoli AM, McCann SM, Wong ML. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA. 2004;101:4531–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wabitsch M, Funcke JB, Lennerz B, Kuhnle-Krahl U, Lahr G, Debatin KM, Vatter P, Gierschik P, Moepps B, Fischer-Posovszky P. Biologically inactive leptin and early-onset extreme obesity. N Engl J Med. 2015;372:48–54. [DOI] [PubMed] [Google Scholar]
- 108. Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O’Rahilly S. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, Hutton JC, O’Rahilly S. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16:303–306. [DOI] [PubMed] [Google Scholar]
- 110.World Health Organization. 2015 Guideline: sugars intake for adults and children. Available at: http://www.who.int/nutrition/publications/guidelines. Accessed 10 January 2016. [PubMed]
- 111. Kamath CC, Vickers KS, Ehrlich A, McGovern L, Johnson J, Singhal V, Paulo R, Hettinger A, Erwin PJ, Montori VM. Clinical review: behavioral interventions to prevent childhood obesity: a systematic review and metaanalyses of randomized trials. J Clin Endocrinol Metab. 2008;93:4606–4615. [DOI] [PubMed] [Google Scholar]
- 112. Ford CN, Ng SW, Popkin BM. Ten-year beverage intake trends among US preschool children: rapid declines between 2003 and 2010 but stagnancy in recent years. Pediatr Obes. 2016;11:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kosova EC, Auinger P, Bremer AA. The relationships between sugar-sweetened beverage intake and cardiometabolic markers in young children. J Acad Nutr Diet. 2013;113:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. DeBoer MD, Scharf RJ, Demmer RT. Sugar-sweetened beverages and weight gain in 2- to 5-year-old children. Pediatrics. 2013;132:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, Ludwig DS. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367:1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. de Ruyter JC, Olthof MR, Kuijper LD, Katan MB. Effect of sugar-sweetened beverages on body weight in children: design and baseline characteristics of the Double-blind, Randomized INtervention study in Kids. Contemp Clin Trials. 2012;33:247–257. [DOI] [PubMed] [Google Scholar]
- 117. Cesa CC, Sbruzzi G, Ribeiro RA, Barbiero SM, de Oliveira Petkowicz R, Eibel B, Machado NB, Marques R, Tortato G, dos Santos TJ, Leiria C, Schaan BD, Pellanda LC. Physical activity and cardiovascular risk factors in children: meta-analysis of randomized clinical trials. Prev Med. 2014;69:54–62. [DOI] [PubMed] [Google Scholar]
- 118. Vasconcellos F, Seabra A, Katzmarzyk PT, Kraemer-Aguiar LG, Bouskela E, Farinatti P. Physical activity in overweight and obese adolescents: systematic review of the effects on physical fitness components and cardiovascular risk factors. Sports Med. 2014;44:1139–1152. [DOI] [PubMed] [Google Scholar]
- 119. Garcia-Hermoso A, Saavedra JM, Escalante Y, Sanchez-Lopez M, Martinez-Vizcaino V. Endocrinology and adolescence: aerobic exercise reduces insulin resistance markers in obese youth: a meta-analysis of randomized controlled trials. Eur J Endocrinol. 2014;171:R163–R171. [DOI] [PubMed] [Google Scholar]
- 120. Fedewa MV, Gist NH, Evans EM, Dishman RK. Exercise and insulin resistance in youth: a meta-analysis. Pediatrics. 2014;133:e163–e174. [DOI] [PubMed] [Google Scholar]
- 121. Nogueira I, Hrovat K. Adolescent bariatric surgery: review on nutrition considerations. Nutr Clin Pract. 2014;29:740–746. [DOI] [PubMed] [Google Scholar]
- 122. Schranz N, Tomkinson G, Olds T. What is the effect of resistance training on the strength, body composition and psychosocial status of overweight and obese children and adolescents? A systematic review and meta-analysis. Sports Med. 2013;43:893–907. [DOI] [PubMed] [Google Scholar]
- 123. Kelley GA, Kelley KS. Effects of exercise in the treatment of overweight and obese children and adolescents: a systematic review of meta-analyses. J Obes. 2013;2013:783103. [DOI] [PMC free article] [PubMed]
- 124. Davis CL, Tomporowski PD, McDowell JE, Austin BP, Miller PH, Yanasak NE, Allison JD, Naglieri JA. Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized, controlled trial. Health Psychol. 2011;30:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fatima Y, Doi SAR, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2015;16:137–149. [DOI] [PubMed] [Google Scholar]
- 126. Kjeldsen JS, Hjorth MF, Andersen R, Michaelsen KF, Tetens I, Astrup A, Chaput JP, Sjodin A. Short sleep duration and large variability in sleep duration are independently associated with dietary risk factors for obesity in Danish school children. Int J Obes. 2014;38:32–39. [DOI] [PubMed] [Google Scholar]
- 127. Hjorth MF, Quist JS, Andersen R, Michaelsen KF, Tetens I, Astrup A, Chaput JP, Sjodin A. Change in sleep duration and proposed dietary risk factors for obesity in Danish school children. Pediatr Obes. 2014;9:e156–e159. [DOI] [PubMed] [Google Scholar]
- 128. Fisher A, McDonald L, van Jaarsveld CH, Llewellyn C, Fildes A, Schrempft S, Wardle J. Sleep and energy intake in early childhood. Int J Obes. 2014;38:926–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hart CN, Carskadon MA, Considine RV, Fava JL, Lawton J, Raynor HA, Jelalian E, Owens J, Wing R. Changes in children’s sleep duration on food intake, weight, and leptin. Pediatrics. 2013;132:e1473–e1480. [DOI] [PubMed] [Google Scholar]
- 130. Klingenberg L, Christensen LB, Hjorth MF, Zangenberg S, Chaput JP, Sjodin A, Molgaard C, Michaelsen KF. No relation between sleep duration and adiposity indicators in 9-36 months old children: the SKOT cohort. Pediatr Obes. 2013;8:e14–e18. [DOI] [PubMed] [Google Scholar]
- 131. Hoppe C, Rothausen BW, Biltoft-Jensen A, Matthiessen J, Groth MV, Chaput JP, Tetens I. Relationship between sleep duration and dietary intake in 4- to 14-year-old Danish children. J Nutr Sci. 2013;2:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chaput JP, Lambert M, Gray-Donald K, McGrath JJ, Tremblay MS, O’Loughlin J, Tremblay A. Short sleep duration is independently associated with overweight and obesity in Quebec children. Can J Public Health. 2011;102:369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Steffen LM, Sinaiko AR, Zhou X, Moran A, Jacobs DR Jr, Korenfeld Y, Dengel DR, Chow LS, Steinberger J. Relation of adiposity, television and screen time in offspring to their parents. BMC Pediatr. 2013;13:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. te Velde SJ, van Nassau F, Uijtdewilligen L, van Stralen MM, Cardon G, De Craemer M, Manios Y, Brug J, Chinapaw MJ. Energy balance-related behaviours associated with overweight and obesity in preschool children: a systematic review of prospective studies. Obes Rev. 2012;13(Suppl 1):56–74. [DOI] [PubMed] [Google Scholar]
- 136. Epstein LH, Roemmich JN, Robinson JL, Paluch RA, Winiewicz DD, Fuerch JH, Robinson TN. A randomized trial of the effects of reducing television viewing and computer use on body mass index in young children. Arch Pediatr Adolesc Med. 2008;162:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sung-Chan P, Sung YW, Zhao X, Brownson RC. Family-based models for childhood-obesity intervention: a systematic review of randomized controlled trials. Obes Rev. 2013;14:265–278. [DOI] [PubMed] [Google Scholar]
- 138. van der Kruk JJ, Kortekaas F, Lucas C, Jager-Wittenaar H. Obesity: a systematic review on parental involvement in long-term European childhood weight control interventions with a nutritional focus. Obes Rev. 2013;14:745–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Valdés J, Rodriguez-Artalejo F, Aguilar L, Jaen-Casquero MB, Royo-Bordonada MA. Frequency of family meals and childhood overweight: a systematic review. Pediatr Obes. 2013;8:e1–e13. [DOI] [PubMed] [Google Scholar]
- 140. Small L, Lane H, Vaughan L, Melnyk B, McBurnett D. A systematic review of the evidence: the effects of portion size manipulation with children and portion education/training interventions on dietary intake with adults. Worldviews Evid Based Nurs. 2013;10:69–81. [DOI] [PubMed] [Google Scholar]
- 141. Halliday JA, Palma CL, Mellor D, Green J, Renzaho AM. The relationship between family functioning and child and adolescent overweight and obesity: a systematic review. Int J Obes (Lond). 2014;38:480–493. [DOI] [PubMed]
- 142. Danese A, Tan M. Childhood maltreatment and obesity: systematic review and meta-analysis. Mol Psychiatry. 2014;19:544–554. [DOI] [PubMed] [Google Scholar]
- 143. Midei AJ, Matthews KA. Interpersonal violence in childhood as a risk factor for obesity: a systematic review of the literature and proposed pathways. Obes Rev. 2011;12:e159–e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Vámosi M, Heitmann BL, Kyvik KO. The relation between an adverse psychological and social environment in childhood and the development of adult obesity: a systematic literature review. Obes Rev. 2010;11:177–184. [DOI] [PubMed] [Google Scholar]
- 145. Bleich SN, Segal J, Wu Y, Wilson R, Wang Y. Systematic review of community-based childhood obesity prevention studies. Pediatrics. 2013;132:e201–e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Krishnaswami J, Martinson M, Wakimoto P, Anglemeyer A. Community-engaged interventions on diet, activity, and weight outcomes in U.S. schools: a systematic review. Am J Prev Med. 2012;43:81–91. [DOI] [PubMed] [Google Scholar]
- 147. Waters E, de Silva-Sanigorski A, Hall BJ, Brown T, Campbell KJ, Gao Y, Armstrong R, Prosser L, Summerbell CD. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2011;Cd001871. [DOI] [PubMed] [Google Scholar]
- 148. Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kramer MS, Matush L, Vanilovich I, Platt RW, Bogdanovich N, Sevkovskaya Z, Dzikovich I, Shishko G, Collet JP, Martin RM, Smith GD, Gillman MW, Chalmers B, Hodnett E, Shapiro S. A randomized breast-feeding promotion intervention did not reduce child obesity in Belarus. J Nutr. 2009;139:417S–421S. [DOI] [PubMed] [Google Scholar]
- 150. Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Nelson MC, Gordon-Larsen P, Adair LS. Are adolescents who were breast-fed less likely to be overweight? Analyses of sibling pairs to reduce confounding. Epidemiology. 2005;16:247–253. [DOI] [PubMed] [Google Scholar]
- 152. James J, Thomas P, Cavan D, Kerr D. Preventing childhood obesity by reducing consumption of carbonated drinks: cluster randomised controlled trial. BMJ. 2004;328:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Pereira MA, Ludwig DS. Dietary fiber and body-weight regulation. Observations and mechanisms. Pediatr Clin North Am. 2001;48:969–980. [DOI] [PubMed] [Google Scholar]
- 154. Kim SA, Moore LV, Galuska D, Wright AP, Harris D, Grummer-Strawn LM, Merlo CL, Nihiser AJ, Rhodes DG. Vital signs: fruit and vegetable intake among children—United States, 2003–2010. MMWR Morb Mortal Wkly Rep. 2014;63:671–676. [PMC free article] [PubMed] [Google Scholar]
- 155. Glickman D, Parker L, Sim L, Del Valle Cook H, Miller EA, eds. Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation. Washington, DC: National Academies Press; 2012. [PubMed] [Google Scholar]
- 156.Williams C. Children’s dietary intakes. Available at: http://www.cnpp.usda.gov/sites/default/files/dietary_guidelines_for_americans/Resource1-Children.pdf. Accessed 10 January 2016.
- 157. Bray GA, Popkin BM. Calorie-sweetened beverages and fructose: what have we learned 10 years later. Pediatr Obes. 2013;8:242–248. [DOI] [PubMed] [Google Scholar]
- 158. Caprio S. Calories from soft drinks—do they matter? N Engl J Med. 2012;367:1462–1463. [DOI] [PubMed] [Google Scholar]
- 159. Dietz WH. Sugar-sweetened beverages, milk intake, and obesity in children and adolescents. J Pediatr. 2006;148:152–154. [DOI] [PubMed] [Google Scholar]
- 160. Council on School Health, Committee on Nutrition Snacks, sweetened beverages, added sugars, and schools. Pediatrics. 2015;135(3):575–583. [DOI] [PubMed] [Google Scholar]
- 161. Babey SH, Jones M, Yu H, Goldstein H. Bubbling over: soda consumption and its link to obesity in California. Policy Brief UCLA Cent Health Policy Res. 2009;(PB2009-5):1–8. [PubMed] [Google Scholar]
- 162. Shefferly A, Scharf RJ, DeBoer MD. Longitudinal evaluation of 100% fruit juice consumption on BMI status in 2–5-year-old children. Pediatr Obes. 2016;11:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. American Academy of Pediatrics The use and misuse of fruit juice in pediatrics. Pediatrics. 2001;107:1210–1213. [DOI] [PubMed] [Google Scholar]
- 164. Reedy J, Krebs-Smith SM. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J Am Diet Assoc. 2010;110:1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006;117:673–680. [DOI] [PubMed] [Google Scholar]
- 166. Centers for Disease Control and Prevention Availability of less nutritious snack foods and beverages in secondary schools—selected States, 2002–2008. MMWR Morb Mortal Wkly Rep. 2009;58:1102–1104. [PubMed] [Google Scholar]
- 167. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367:1397–1406. [DOI] [PubMed] [Google Scholar]
- 168. Rouhani MH, Kelishadi R, Hashemipour M, Esmaillzadeh A, Azadbakht L. Glycemic index, glycemic load and childhood obesity: A systematic review. Adv Biomed Res. 2014;3:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Schwartz AE, Leardo M, Aneja S, Elbel B. Effect of a school-based water intervention on child body mass index and obesity. JAMA Pediatr. 2016;170:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Muckelbauer R, Barbosa CL, Mittag T, Burkhardt K, Mikelaishvili N, Muller-Nordhorn J. Association between water consumption and body weight outcomes in children and adolescents: a systematic review. Obesity (Silver Spring). 2014;22:2462–2475. [DOI] [PubMed] [Google Scholar]
- 171.World Health Organization. Using price policies to promote healthier diets. Available at: http://www.euro.who.int/en/publications/abstracts/using-price-policies-to-promote-healthier-diets. Accessed 8 March 2016.
- 172. Tremblay MS, LeBlanc AG, Kho ME, Saunders TJ, Larouche R, Colley RC, Goldfield G, Connor Gorber S. Systematic review of sedentary behaviour and health indicators in school-aged children and youth. Int J Behav Nutr Phys Act. 2011;8:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Office of Disease Prevention and Health Promotion. 2008 Physical activity guidelines for Americans summary. Available at: http://health.gov/paguidelines/guidelines/summary.aspx. Accessed 17 March 2016.
- 174. D’Hondt E, Deforche B, Gentier I, De Bourdeaudhuij I, Vaeyens R, Philippaerts R, Lenoir M. A longitudinal analysis of gross motor coordination in overweight and obese children versus normal-weight peers. Int J Obes. 2013;37:61–67. [DOI] [PubMed] [Google Scholar]
- 175. Norman AC, Drinkard B, McDuffie JR, Ghorbani S, Yanoff LB, Yanovski JA. Influence of excess adiposity on exercise fitness and performance in overweight children and adolescents. Pediatrics. 2005;115:e690–e696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Olds TS, Ferrar KE, Schranz NK, Maher CA. Obese adolescents are less active than their normal-weight peers, but wherein lies the difference? J Adolesc Health. 2011;48:189–195. [DOI] [PubMed] [Google Scholar]
- 177. Zabinski MF, Saelens BE, Stein RI, Hayden-Wade HA, Wilfley DE. Overweight children’s barriers to and support for physical activity. Obes Res. 2003;11:238–246. [DOI] [PubMed] [Google Scholar]
- 178.National Sleep Foundation. National Sleep Foundation recommends new sleep durations. Available at: https://sleepfoundation.org/media-center/press-release/national-sleep-foundation-recommends-new-sleep-times. Accessed 8 March 2016.
- 179. Olafsdottir S, Berg C, Eiben G, Lanfer A, Reisch L, Ahrens W, Kourides Y, Molnar D, Moreno LA, Siani A, Veidebaum T, Lissner L. Young children’s screen activities, sweet drink consumption and anthropometry: results from a prospective European study. Eur J Clin Nutr. 2014;68:223–228. [DOI] [PubMed] [Google Scholar]
- 180. Boyland EJ, Whalen R. Food advertising to children and its effects on diet: a review of recent prevalence and impact data. Pediatr Diabetes. 2015;16:331–337. [DOI] [PubMed] [Google Scholar]
- 181. American Academy of Pediatrics Children, adolescents, and television. Pediatrics. 2001;107:423–426. [DOI] [PubMed] [Google Scholar]
- 182. McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C, Erwin PJ, Montori VM. Clinical review: treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J Clin Endocrinol Metab. 2008;93:4600–4605. [DOI] [PubMed] [Google Scholar]
- 183. Upton P, Taylor C, Erol R, Upton D. Family-based childhood obesity interventions in the UK: a systematic review of published studies. Community Pract. 2014;87:25–29. [PubMed] [Google Scholar]
- 184. Faith MS, Van Horn L, Appel LJ, Burke LE, Carson JA, Franch HA, Jakicic JM, Kral TV, Odoms-Young A, Wansink B, Wylie-Rosett J. Evaluating parents and adult caregivers as “agents of change” for treating obese children: evidence for parent behavior change strategies and research gaps: a scientific statement from the American Heart Association. Circulation. 2012;125:1186–1207. [DOI] [PubMed] [Google Scholar]
- 185. Harrell JS, McMurray RG, Gansky SA, Bangdiwala SI, Bradley CB. A public health vs a risk-based intervention to improve cardiovascular health in elementary school children: the Cardiovascular Health in Children Study. Am J Public Health. 1999;89:1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. McMurray RG, Harrell JS, Bangdiwala SI, Bradley CB, Deng S, Levine A. A school-based intervention can reduce body fat and blood pressure in young adolescents. J Adolesc Health. 2002;31:125–132. [DOI] [PubMed] [Google Scholar]
- 187. Sallis JF, Conway TL, Prochaska JJ, McKenzie TL, Marshall SJ, Brown M. The association of school environments with youth physical activity. Am J Public Health. 2001;91:618–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Foster GD, Linder B, Baranowski T, Cooper DM, Goldberg L, Harrell JS, Kaufman F, Marcus MD, Trevino RP, Hirst K. A school-based intervention for diabetes risk reduction. N Engl J Med. 2010;363:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Yin Z, Moore JB, Johnson MH, Barbeau P, Cavnar M, Thornburg J, Gutin B. The Medical College of Georgia Fitkid project: the relations between program attendance and changes in outcomes in year 1. Int J Obes. 2005;29(Suppl 2):S40–S45. [DOI] [PubMed] [Google Scholar]
- 190. Foltz JL, May AL, Belay B, Nihiser AJ, Dooyema CA, Blanck HM. Population-level intervention strategies and examples for obesity prevention in children. Annu Rev Nutr. 2012;32:391–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Pearce J, Taylor MA, Langley-Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes. 2013;37:1295–1306. [DOI] [PubMed] [Google Scholar]
- 192. Pearce J, Langley-Evans SC. The types of food introduced during complementary feeding and risk of childhood obesity: a systematic review. Int J Obes. 2013;37:477–485. [DOI] [PubMed] [Google Scholar]
- 193. Kozyrskyj AL, Kalu R, Koleva PT, Bridgman SL. Fetal programming of overweight through the microbiome: boys are disproportionately affected. J Dev Orig Health Dis. 2016;7:25–34. [DOI] [PubMed] [Google Scholar]
- 194. Audrey S, Batista-Ferrer H. Healthy urban environments for children and young people: a systematic review of intervention studies. Health Place. 2015;36:97–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Tucker CM, Butler A, Kaye LB, Nolan SE, Flenar DJ, Marsiske M, Bragg M, Hoover E, Daly K. Impact of a culturally sensitive health self-empowerment workshop series on health behaviors/lifestyles, BMI, and blood pressure of culturally diverse overweight/obese adults. Am J Lifestyle Med. 2014;8:122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Li JS, Barnett TA, Goodman E, Wasserman RC, Kemper AR. Approaches to the prevention and management of childhood obesity: the role of social networks and the use of social media and related electronic technologies: a scientific statement from the American Heart Association. Circulation. 2013;127:260–267. [DOI] [PubMed] [Google Scholar]
- 197. Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, Clark NG. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care. 2004;27:2067–2073. [DOI] [PubMed] [Google Scholar]
- 198. Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the U.S. Am J Prev Med. 2013;44:S346–S351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes. 2005;29:1153–1167. [DOI] [PubMed] [Google Scholar]
- 200. Harder-Lauridsen NM, Birk NM, Ried-Larsen M, Juul A, Andersen LB, Pedersen BK, Krogh-Madsen R. A randomized controlled trial on a multicomponent intervention for overweight school-aged children—Copenhagen, Denmark. BMC Pediatr. 2014;14:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33:34–40. [DOI] [PubMed] [Google Scholar]
- 202. Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med. 2014;46:17–23. [DOI] [PubMed] [Google Scholar]
- 203. Lloyd-Richardson EE, Jelalian E, Sato AF, Hart CN, Mehlenbeck R, Wing RR. Two-year follow-up of an adolescent behavioral weight control intervention. Pediatrics. 2012;130:e281–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O’Malley C, Stolk RP, Summerbell CD. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;Cd001872. [DOI] [PubMed] [Google Scholar]
- 205. Wilfley DE, Stein RI, Saelens BE, Mockus DS, Matt GE, Hayden-Wade HA, Welch RR, Schechtman KB, Thompson PA, Epstein LH. Efficacy of maintenance treatment approaches for childhood overweight: a randomized controlled trial. JAMA. 2007;298:1661–1673. [DOI] [PubMed] [Google Scholar]
- 206. Rhodes ET, Ludwig DS. Childhood obesity as a chronic disease: keeping the weight off. JAMA. 2007;298:1695–1696. [DOI] [PubMed] [Google Scholar]
- 207. Wilfley DE, Tibbs TL, Van Buren DJ, Reach KP, Walker MS, Epstein LH. Lifestyle interventions in the treatment of childhood overweight: a meta-analytic review of randomized controlled trials. Health Psychol. 2007;26:521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Hoelscher DM, Kirk S, Ritchie L, Cunningham-Sabo L. Position of the Academy of Nutrition and Dietetics: interventions for the prevention and treatment of pediatric overweight and obesity. J Acad Nutr Diet. 2013;113:1375–1394. [DOI] [PubMed] [Google Scholar]
- 209. Spear BA, Barlow SE, Ervin C, Ludwig DS, Saelens BE, Schetzina KE, Taveras EM. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl 4):S254–S288. [DOI] [PubMed] [Google Scholar]
- 210. Freemark M. Pharmacotherapy of childhood obesity: an evidence-based, conceptual approach. Diabetes Care. 2007;30:395–402. [DOI] [PubMed] [Google Scholar]
- 211. Goldschmidt AB, Wilfley DE, Paluch RA, Roemmich JN, Epstein LH. Indicated prevention of adult obesity: how much weight change is necessary for normalization of weight status in children? JAMA Pediatr. 2013;167:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Goldschmidt AB, Stein RI, Saelens BE, Theim KR, Epstein LH, Wilfley DE. Importance of early weight change in a pediatric weight management trial. Pediatrics. 2011;128:e33–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Arora M, Nazar GP, Gupta VK, Perry CL, Reddy KS, Stigler MH. Association of breakfast intake with obesity, dietary and physical activity behavior among urban school-aged adolescents in Delhi, India: results of a cross-sectional study. BMC Public Health. 2012;12:881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Grydeland M, Bergh IH, Bjelland M, Lien N, Andersen LF, Ommundsen Y, Klepp KI, Anderssen SA. Correlates of weight status among Norwegian 11-year-olds: the HEIA study. BMC Public Health. 2012;12:1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Yanovski SZ, Yanovski JA. Naltrexone extended-release plus bupropion extended-release for treatment of obesity. JAMA. 2015;313:1213–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Wansink B, Painter JE, North J. Bottomless bowls: why visual cues of portion size may influence intake. Obes Res. 2005;13:93–100. [DOI] [PubMed] [Google Scholar]
- 218. Gow ML, Ho M, Burrows TL, Baur LA, Stewart L, Hutchesson MJ, Cowell CT, Collins CE, Garnett SP. Impact of dietary macronutrient distribution on BMI and cardiometabolic outcomes in overweight and obese children and adolescents: a systematic review. Nutr Rev. 2014;72:453–470. [DOI] [PubMed] [Google Scholar]
- 219. Schnohr P, O’Keefe JH, Marott JL, Lange P, Jensen GB. Dose of jogging and long-term mortality: the Copenhagen City Heart Study. J Am Coll Cardiol. 2015;65:411–419. [DOI] [PubMed] [Google Scholar]
- 220. Lee DC, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol. 2014;64:472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.National Physical Activity Plan Alliance. The National Physical Activity Plan. Available at: http://www.physicalactivityplan.org/theplan/about.html. Accessed 17 March 2016.
- 222. Dentro KN, Beals K, Crouter SE, Eisenmann JC, McKenzie TL, Pate RR, Saelens BE, Sisson SB, Spruijt-Metz D, Sothern MS, Katzmarzyk PT. Results from the United States’ 2014 report card on physical activity for children and youth. J Phys Act Health. 2014;11(Suppl 1):S105–S112. [DOI] [PubMed] [Google Scholar]
- 223.Chriqui J, Resnick E, Chaloupka F. Bridging the Gap. School district wellness policies: evaluating progress and potential for improving children’s health five years after the federal mandate. Volume 3. February 2013. Available at: http://www.bridgingthegapresearch.org/_asset/13s2jm/WP_2013_report.pdf. Accessed 17 March 2016.
- 224.Johnston LD, O’Malley PM, Terry-McElrath YM, Colabianchi N. Bridging the Gap. School policies and practices to improve health and prevent obesity: National secondary school survey results. Volume 3. March 2013. Available at: http://www.bridgingthegapresearch.org/_asset/gqq408/SS_2013_report.pdf. Accessed 17 March 2016.
- 225.Centers for Disease Control and Prevention. Youth risk behavior surveillance—United States, 2011. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/ss6104a1.htm. Accessed 17 March 2016.
- 226. Pate RR, Davis MG, Robinson TN, Stone EJ, McKenzie TL, Young JC. Promoting physical activity in children and youth: a leadership role for schools: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism (Physical Activity Committee) in collaboration with the Councils on Cardiovascular Disease in the Young and Cardiovascular Nursing. Circulation. 2006;114:1214–1224. [DOI] [PubMed] [Google Scholar]
- 227. Bucksch J, Inchley J, Hamrik Z, Finne E, Kolip P. Trends in television time, non-gaming PC use and moderate-to-vigorous physical activity among German adolescents 2002–2010. BMC Public Health. 2014;14:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Veldhuis L, van Grieken A, Renders CM, HiraSing RA, Raat H. Parenting style, the home environment, and screen time of 5-year-old children; the “be active, eat right” study. PLoS One. 2014;9:e88486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Schwartz MB, Gilstad-Hayden K, Henderson KE, Luedicke J, Carroll-Scott A, Peters SM, McCaslin C, Ickovics JR. The relationship between parental behaviors and children’s sugary drink consumption is moderated by a television in the child’s bedroom. Child Obes. 2015;11:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Rhee KE, Lumeng JC, Appugliese DP, Kaciroti N, Bradley RH. Parenting styles and overweight status in first grade. Pediatrics. 2006;117:2047–2054. [DOI] [PubMed] [Google Scholar]
- 231. Jordan AB, Hersey JC, McDivitt JA, Heitzler CD. Reducing children’s television-viewing time: a qualitative study of parents and their children. Pediatrics. 2006;118:e1303–e1310. [DOI] [PubMed] [Google Scholar]
- 232. Hearst MO, Sherwood NE, Klein EG, Pasch KE, Lytle LA. Parental perceptions of their adolescent’s weight status: the ECHO study. Am J Health Behav. 2011;35:248–255. [DOI] [PMC free article] [PubMed]
- 233. Huang JS, Becerra K, Oda T, Walker E, Xu R, Donohue M, Chen I, Curbelo V, Breslow A. Parental ability to discriminate the weight status of children: results of a survey. Pediatrics. 2007;120:e112–e119. [DOI] [PubMed] [Google Scholar]
- 234. Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106:52–58. [DOI] [PubMed] [Google Scholar]
- 235. Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. 2003;289:1813–1819. [DOI] [PubMed] [Google Scholar]
- 236. Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 generic core scales. Health Qual Life Outcomes. 2007;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282–304. [DOI] [PubMed] [Google Scholar]
- 238. Franklin J, Denyer G, Steinbeck KS, Caterson ID, Hill AJ. Obesity and risk of low self-esteem: a statewide survey of Australian children. Pediatrics. 2006;118:2481–2487. [DOI] [PubMed] [Google Scholar]
- 239. Nowicka P, Hoglund P, Birgerstam P, Lissau I, Pietrobelli A, Flodmark CE. Self-esteem in a clinical sample of morbidly obese children and adolescents. Acta Paediatr. 2009;98:153–158. [DOI] [PubMed] [Google Scholar]
- 240. Britz B, Siegfried W, Ziegler A, Lamertz C, Herpertz-Dahlmann BM, Remschmidt H, Wittchen HU, Hebebrand J. Rates of psychiatric disorders in a clinical study group of adolescents with extreme obesity and in obese adolescents ascertained via a population based study. Int J Obes Relat Metab Disord. 2000;24:1707–1714. [DOI] [PubMed] [Google Scholar]
- 241. Vila G, Zipper E, Dabbas M, Bertrand C, Robert JJ, Ricour C, Mouren-Simeoni MC. Mental disorders in obese children and adolescents. Psychosom Med. 2004;66:387–394. [DOI] [PubMed] [Google Scholar]
- 242. Erermis S, Cetin N, Tamar M, Bukusoglu N, Akdeniz F, Goksen D. Is obesity a risk factor for psychopathology among adolescents? Pediatr Int. 2004;46:296–301. [DOI] [PubMed] [Google Scholar]
- 243. Braet C, Mervielde I, Vandereycken W. Psychological aspects of childhood obesity: a controlled study in a clinical and nonclinical sample. J Pediatr Psychol. 1997;22:59–71. [DOI] [PubMed] [Google Scholar]
- 244. Koval JJ, Pederson LL, Zhang X, Mowery P, McKenna M. Can young adult smoking status be predicted from concern about body weight and self-reported BMI among adolescents? Results from a ten-year cohort study. Nicotine Tob Res. 2008;10:1449–1455. [DOI] [PubMed] [Google Scholar]
- 245. Gibson LY, Byrne SM, Davis EA, Blair E, Jacoby P, Zubrick SR. The role of family and maternal factors in childhood obesity. Med J Aust. 2007;186:591–595. [DOI] [PubMed] [Google Scholar]
- 246. Gibson LY, Byrne SM, Blair E, Davis EA, Jacoby P, Zubrick SR. Clustering of psychosocial symptoms in overweight children. Aust N Z J Psychiatry. 2008;42:118–125. [DOI] [PubMed] [Google Scholar]
- 247. Zeller MH, Modi AC. Predictors of health-related quality of life in obese youth. Obesity (Silver Spring). 2006;14:122–130. [DOI] [PubMed] [Google Scholar]
- 248. Tiggemann M. Body dissatisfaction and adolescent self-esteem: prospective findings. Body Image. 2005;2:129–135. [DOI] [PubMed] [Google Scholar]
- 249. Fairburn CG, Harrison PJ. Eating disorders. Lancet. 2003;361:407–416. [DOI] [PubMed] [Google Scholar]
- 250. Olvera N, McCarley K, Matthews-Ewald MR, Fisher F, Jones M, Flynn EG. Pathways for disordered eating behaviors in minority girls: the role of adiposity, peer weight-related teasing, and desire to be thinner. J Early Adolesc. 2015;October 2015:1–20. [Google Scholar]
- 251. Eddy KT, Tanofsky-Kraff M, Thompson-Brenner H, Herzog DB, Brown TA, Ludwig DS. Eating disorder pathology among overweight treatment-seeking youth: clinical correlates and cross-sectional risk modeling. Behav Res Ther. 2007;45:2360–2371. [DOI] [PubMed] [Google Scholar]
- 252. Van Vlierberghe L, Braet C, Goossens L, Mels S. Psychiatric disorders and symptom severity in referred versus non-referred overweight children and adolescents. Eur Child Adolesc Psychiatry. 2009;18:164–173. [DOI] [PubMed] [Google Scholar]
- 253. Gray WN, Janicke DM, Ingerski LM, Silverstein JH. The impact of peer victimization, parent distress and child depression on barrier formation and physical activity in overweight youth. J Dev Behav Pediatr. 2008;29:26–33. [DOI] [PubMed]
- 254. Taylor CB, Bryson S, Celio Doyle AA, Luce KH, Cunning D, Abascal LB, Rockwell R, Field AE, Striegel-Moore R, Winzelberg AJ, Wilfley DE. The adverse effect of negative comments about weight and shape from family and siblings on women at high risk for eating disorders. Pediatrics. 2006;118:731–738. [DOI] [PubMed] [Google Scholar]
- 255. Birch LL, Fisher JO, Davison KK. Learning to overeat: maternal use of restrictive feeding practices promotes girls’ eating in the absence of hunger. Am J Clin Nutr. 2003;78:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Rollins BY, Loken E, Savage JS, Birch LL. Effects of restriction on children’s intake differ by child temperament, food reinforcement, and parent’s chronic use of restriction. Appetite. 2014;73:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Isnard P, Quantin L, Cortese S, Falissard B, Musher-Eizenman D, Guedeney A, Frelut ML, Mouren MC. Bulimic behaviours and psychopathology in obese adolescents and in their parents. Int J Pediatr Obes. 2010;5:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Zeller MH, Reiter-Purtill J, Ramey C. Negative peer perceptions of obese children in the classroom environment. Obesity (Silver Spring). 2008;16:755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Fox CL, Farrow CV. Global and physical self-esteem and body dissatisfaction as mediators of the relationship between weight status and being a victim of bullying. J Adolesc. 2009;32:1287–1301. [DOI] [PubMed] [Google Scholar]
- 260. Pearce MJ, Boergers J, Prinstein MJ. Adolescent obesity, overt and relational peer victimization, and romantic relationships. Obes Res. 2002;10:386–393. [DOI] [PubMed] [Google Scholar]
- 261. Pinhas-Hamiel O, Singer S, Pilpel N, Fradkin A, Modan D, Reichman B. Health-related quality of life among children and adolescents: associations with obesity. Int J Obes. 2006;30:267–272. [DOI] [PubMed] [Google Scholar]
- 262. Young-Hyman D, Tanofsky-Kraff M, Yanovski SZ, Keil M, Cohen ML, Peyrot M, Yanovski JA. Psychological status and weight-related distress in overweight or at-risk-for-overweight children. Obesity (Silver Spring). 2006;14:2249–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. McClure AC, Tanski SE, Kingsbury J, Gerrard M, Sargent JD. Characteristics associated with low self-esteem among US adolescents. Acad Pediatr. 2010;10:238–244.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Rojas A, Storch EA. Psychological complications of obesity. Pediatr Ann. 2010;39:174–180. [DOI] [PubMed] [Google Scholar]
- 265. Garry JP, Morrissey SL, Whetstone LM. Substance use and weight loss tactics among middle school youth. Int J Eat Disord. 2003;33:55–63. [DOI] [PubMed] [Google Scholar]
- 266. Jellinek MM, J. Pediatric Symptom Checklist. 2015. Available at: http://www.massgeneral.org/psychiatry/services/psc_home.aspx. Accessed 13 January 2017.
- 267. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: A systematic and clinical review. JAMA. 2014;311:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Clements JN, Shealy KM. Liraglutide: an injectable option for the management of obesity. Ann Pharmacother. 2015;49:938–944. [DOI] [PubMed] [Google Scholar]
- 269. Viner RM, Hsia Y, Tomsic T, Wong IC. Efficacy and safety of anti-obesity drugs in children and adolescents: systematic review and meta-analysis. Obes Rev. 2010;11:593–602. [DOI] [PubMed] [Google Scholar]
- 270. Peirson L, Fitzpatrick-Lewis D, Morrison K, Warren R, Usman Ali M, Raina P. Treatment of overweight and obesity in children and youth: a systematic review and meta-analysis. CMAJ Open. 2015;3:E35–E46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Sherafat-Kazemzadeh R, Yanovski SZ, Yanovski JA. Pharmacotherapy for childhood obesity: present and future prospects. Int J Obes. 2013;37:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Speiser PW, Rudolf MC, Anhalt H, Camacho-Hubner C, Chiarelli F, Eliakim A, Freemark M, Gruters A, Hershkovitz E, Iughetti L, Krude H, Latzer Y, Lustig RH, Pescovitz OH, Pinhas-Hamiel O, Rogol AD, Shalitin S, Sultan C, Stein D, Vardi P, Werther GA, Zadik Z, Zuckerman-Levin N, Hochberg Z. Obesity consensus working G. Childhood obesity. J Clin Endocrinol Metab. 2005;90:1871–1887. [DOI] [PubMed] [Google Scholar]
- 273. Centre for Public Health Excellence at NICE (UK); National Collaborating Centre for Primary Care (UK) . Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London, England: National Institute for Health and Clinical Excellence (UK); 2006. [PubMed] [Google Scholar]
- 274. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213–S256. [DOI] [PMC free article] [PubMed]
- 275. Berkowitz RI, Wadden TA, Tershakovec AM, Cronquist JL. Behavior therapy and sibutramine for the treatment of adolescent obesity: a randomized controlled trial. JAMA. 2003;289:1805–1812. [DOI] [PubMed] [Google Scholar]
- 276. Rauh JL, Lipp R. Chlorphentermine as an anorexigenic agent in adolescent obesity. Report of its efficacy in a double-blind study of 30 teen-agers. Clin Pediatr (Phila). 1968;7:138–140. [DOI] [PubMed] [Google Scholar]
- 277. Lorber J. Obesity in childhood. A controlled trial of anorectic drugs. Arch Dis Child. 1966;41:309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. von Spranger J. Phentermine resinate in obesity. Clinical trial of Mirapront in adipose children. Munch Med Wochenschr. 1965;107:1833–1834. [PubMed] [Google Scholar]
- 279. Andelman MB, Jones C, Nathan S. Treatment of obesity in underprivileged adolescents. Comparison of diethylpropion hydrochloride with placebo in a double-blind study. Clin Pediatr (Phila). 1967;6:327–330. [DOI] [PubMed] [Google Scholar]
- 280. Golebiowska M, Chlebna-Sokol D, Kobierska I, Konopinska A, Malek M, Mastalska A, Zwaigzne-Raczynska J. Clinical evaluation of Teronac (mazindol) in the treatment of obesity in children. Part II. Anorectic properties and side effects (author’s transl). Przegl Lek. 1981;38:355–358. [PubMed] [Google Scholar]
- 281. Komorowski JM, Zwaigzne-Raczynska J, Owczarczyk I, Golebiowska M, Zarzycki J. Effect of mazindol (teronac) on various hormonal indicators in children with simple obesity. Pediatr Pol. 1982;57:241–246. [PubMed] [Google Scholar]
- 282. McElroy SL, Hudson JI, Mitchell JE, Wilfley D, Ferreira-Cornwell MC, Gao J, Wang J, Whitaker T, Jonas J, Gasior M. Efficacy and safety of lisdexamfetamine for treatment of adults with moderate to severe binge-eating disorder: a randomized clinical trial. JAMA Psychiatry. 2015;73:235–246. [DOI] [PubMed] [Google Scholar]
- 283. McElroy SL, Guerdjikova AI, Mori N, Keck PE Jr. Psychopharmacologic treatment of eating disorders: emerging findings. Curr Psychiatry Rep. 2015;17:35. [DOI] [PubMed] [Google Scholar]
- 284. Godoy-Matos A, Carraro L, Vieira A, Oliveira J, Guedes EP, Mattos L, Rangel C, Moreira RO, Coutinho W, Appolinario JC. Treatment of obese adolescents with sibutramine: a randomized, double-blind, controlled study. J Clin Endocrinol Metab. 2005;90:1460–1465. [DOI] [PubMed] [Google Scholar]
- 285. Berkowitz RI, Fujioka K, Daniels SR, Hoppin AG, Owen S, Perry AC, Sothern MS, Renz CL, Pirner MA, Walch JK, Jasinsky O, Hewkin AC, Blakesley VA. Effects of sibutramine treatment in obese adolescents: a randomized trial. Ann Intern Med. 2006;145:81–90. [DOI] [PubMed] [Google Scholar]
- 286. Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. [DOI] [PubMed] [Google Scholar]
- 287. Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, Anderson CM. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067–3077. [DOI] [PubMed] [Google Scholar]
- 288. Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32:1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, Aronne L, Investigators NN. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes. 2013;37:1443–1451. [DOI] [PubMed] [Google Scholar]
- 290. Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME, Group NNS. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. [DOI] [PubMed] [Google Scholar]
- 291. Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, Schwiers M, Day WW, Bowden CH. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. doi: 10.1038/oby.2011.330. Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, Tam PY, Troupin B, Day WW. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2011;20:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. [DOI] [PubMed] [Google Scholar]
- 294. Padwal R. Contrave, a bupropion and naltrexone combination therapy for the potential treatment of obesity. Curr Opin Investig Drugs. 2009;10:1117–1125. [PubMed] [Google Scholar]
- 295. Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. [DOI] [PubMed] [Google Scholar]
- 296. Rosenstock J, Klaff LJ, Schwartz S, Northrup J, Holcombe JH, Wilhelm K, Trautmann M. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care. 2010;33:1173–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Kelly AS, Rudser KD, Nathan BM, Fox CK, Metzig AM, Coombes BJ, Fitch AK, Bomberg EM, Abuzzahab MJ. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013;167:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Kelly AS, Metzig AM, Rudser KD, Fitch AK, Fox CK, Nathan BM, Deering MM, Schwartz BL, Abuzzahab MJ, Gandrud LM, Moran A, Billington CJ, Schwarzenberg SJ. Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Obesity (Silver Spring). 2012;20:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. McDuffie JR, Calis KA, Uwaifo GI, Sebring NG, Fallon EM, Hubbard VS, Yanovski JA. Three-month tolerability of orlistat in adolescents with obesity-related comorbid conditions. Obes Res. 2002;10:642–650. [DOI] [PubMed] [Google Scholar]
- 300. Zhi J, Moore R, Kanitra L. The effect of short-term (21-day) orlistat treatment on the physiologic balance of six selected macrominerals and microminerals in obese adolescents. J Am Coll Nutr. 2003;22:357–362. [DOI] [PubMed] [Google Scholar]
- 301. Norgren S, Danielsson P, Jurold R, Lotborn M, Marcus C. Orlistat treatment in obese prepubertal children: a pilot study. Acta Paediatr. 2003;92:666–670. [DOI] [PubMed] [Google Scholar]
- 302. Ozkan B, Bereket A, Turan S, Keskin S. Addition of orlistat to conventional treatment in adolescents with severe obesity. Eur J Pediatr. 2004;163:738–741. [DOI] [PubMed] [Google Scholar]
- 303. McDuffie JR, Calis KA, Uwaifo GI, Sebring NG, Fallon EM, Frazer TE, Van Hubbard S, Yanovski JA. Efficacy of orlistat as an adjunct to behavioral treatment in overweight African American and Caucasian adolescents with obesity-related co-morbid conditions. J Pediatr Endocrinol Metab. 2004;17:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293:2873–2883. [DOI] [PubMed] [Google Scholar]
- 305. Maahs D, de Serna DG, Kolotkin RL, Ralston S, Sandate J, Qualls C, Schade DS. Randomized, double-blind, placebo-controlled trial of orlistat for weight loss in adolescents. Endocr Pract. 2006;12:18–28. [DOI] [PubMed] [Google Scholar]
- 306. Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107:e55. [DOI] [PubMed] [Google Scholar]
- 307. Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: a 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab. 2008;21:339–348. [DOI] [PubMed] [Google Scholar]
- 308. Love-Osborne K, Sheeder J, Zeitler P. Addition of metformin to a lifestyle modification program in adolescents with insulin resistance. J Pediatr. 2008;152:817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Wilson DM, Abrams SH, Aye T, Lee PD, Lenders C, Lustig RH, Osganian SV, Feldman HA. Metformin extended release treatment of adolescent obesity: a 48-week randomized, double-blind, placebo-controlled trial with 48-week follow-up. Arch Pediatr Adolesc Med. 2010;164:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Yanovski JA, Krakoff J, Salaita CG, McDuffie JR, Kozlosky M, Sebring NG, Reynolds JC, Brady SM, Calis KA. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes. 2011;60:477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Kendall D, Vail A, Amin R, Barrett T, Dimitri P, Ivison F, Kibirige M, Mathew V, Matyka K, McGovern A, Stirling H, Tetlow L, Wales J, Wright N, Clayton P, Hall C. Metformin in obese children and adolescents: the MOCA trial. J Clin Endocrinol Metab. 2013;98:322–329. [DOI] [PubMed] [Google Scholar]
- 312. Gambineri A, Patton L, De Iasio R, Cantelli B, Cognini GE, Filicori M, Barreca A, Diamanti-Kandarakis E, Pagotto U, Pasquali R. Efficacy of octreotide-LAR in dieting women with abdominal obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:3854–3862. [DOI] [PubMed] [Google Scholar]
- 313. Haqq AM, Stadler DD, Rosenfeld RG, Pratt KL, Weigle DS, Frayo RS, LaFranchi SH, Cummings DE, Purnell JQ. Circulating ghrelin levels are suppressed by meals and octreotide therapy in children with Prader-Willi syndrome. J Clin Endocrinol Metab. 2003;88:3573–3576. [DOI] [PubMed] [Google Scholar]
- 314.Lustig RH, Hinds PS, Ringwald-Smith K, Christensen RK, Kaste SC, Schreiber RE, Rai SN, Lensing SY, Wu S, Xiong X. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab 2003;88(6):2586–2592. [DOI] [PubMed] [Google Scholar]
- 315. Lustig RH, Rose SR, Burghen GA, Velasquez-Mieyer P, Broome DC, Smith K, Li H, Hudson MM, Heideman RL, Kun LE. Hypothalamic obesity caused by cranial insult in children: altered glucose and insulin dynamics and reversal by a somatostatin agonist. J Pediatr. 1999;135:162–168. [DOI] [PubMed] [Google Scholar]
- 316. Lustig RH, Greenway F, Velasquez-Mieyer P, Heimburger D, Schumacher D, Smith D, Smith W, Soler N, Warsi G, Berg W, Maloney J, Benedetto J, Zhu W, Hohneker J. A multicenter, randomized, double-blind, placebo-controlled, dose-finding trial of a long-acting formulation of octreotide in promoting weight loss in obese adults with insulin hypersecretion. Int J Obes. 2006;30:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Shadid S, Jensen MD. Effects of growth hormone administration in human obesity. Obes Res. 2003;11:170–175. [DOI] [PubMed] [Google Scholar]
- 318. Czernichow S, Lee CM, Barzi F, Greenfield JR, Baur LA, Chalmers J, Woodward M, Huxley RR. Efficacy of weight loss drugs on obesity and cardiovascular risk factors in obese adolescents: a meta-analysis of randomized controlled trials. Obes Rev. 2010;11:150–158. [DOI] [PubMed] [Google Scholar]
- 319. Viner RM, Hsia Y, Neubert A, Wong IC. Rise in antiobesity drug prescribing for children and adolescents in the UK: a population-based study. Br J Clin Pharmacol. 2009;68:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Sun AP, Kirby B, Black C, Helms PJ, Bennie M, McLay JS. Unplanned medication discontinuation as a potential pharmacovigilance signal: a nested young person cohort study. BMC Pharmacol Toxicol. 2014;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Lutjens A, Smit JL. Effect of biguanide treatment in obese children. Helv Paediatr Acta. 1977;31:473–480. [PubMed] [Google Scholar]
- 322. Kay JP, Alemzadeh R, Langley G, D’Angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism. 2001;50:1457–1461. [DOI] [PubMed] [Google Scholar]
- 323. Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87:1555–1559. [DOI] [PubMed] [Google Scholar]
- 324. Allen HF, Mazzoni C, Heptulla RA, Murray MA, Miller N, Koenigs L, Reiter EO. Randomized controlled trial evaluating response to metformin versus standard therapy in the treatment of adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2005;18:761–768. [DOI] [PubMed] [Google Scholar]
- 325. Schwimmer JB, Middleton MS, Deutsch R, Lavine JE. A phase 2 clinical trial of metformin as a treatment for non-diabetic paediatric non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;21:871–879. [DOI] [PubMed] [Google Scholar]
- 326. Bridger T, MacDonald S, Baltzer F, Rodd C. Randomized placebo-controlled trial of metformin for adolescents with polycystic ovary syndrome. Arch Pediatr Adolesc Med. 2006;160:241–246. [DOI] [PubMed] [Google Scholar]
- 327. De Leo V, Musacchio MC, Morgante G, Piomboni P, Petraglia F. Metformin treatment is effective in obese teenage girls with PCOS. Hum Reprod. 2006;21:2252–2256. [DOI] [PubMed] [Google Scholar]
- 328. Srinivasan S, Ambler GR, Baur LA, Garnett SP, Tepsa M, Yap F, Ward GM, Cowell CT. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab. 2006;91:2074–2080. [DOI] [PubMed] [Google Scholar]
- 329. Fu JF, Liang L, Zou CC, Hong F, Wang CL, Wang XM, Zhao ZY. Prevalence of the metabolic syndrome in Zhejiang Chinese obese children and adolescents and the effect of metformin combined with lifestyle intervention. Int J Obes. 2007;31:15–22. [DOI] [PubMed] [Google Scholar]
- 330. Burgert TS, Duran EJ, Goldberg-Gell R, Dziura J, Yeckel CW, Katz S, Tamborlane WV, Caprio S. Short-term metabolic and cardiovascular effects of metformin in markedly obese adolescents with normal glucose tolerance. Pediatr Diabetes. 2008;9:567–576. [DOI] [PubMed] [Google Scholar]
- 331. Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93:4299–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Clarson CL, Mahmud FH, Baker JE, Clark HE, McKay WM, Schauteet VD, Hill DJ. Metformin in combination with structured lifestyle intervention improved body mass index in obese adolescents, but did not improve insulin resistance. Endocrine. 2009;36:141–146. [DOI] [PubMed] [Google Scholar]
- 333. Rezvanian H, Hashemipour M, Kelishadi R, Tavakoli N, Poursafa P. A randomized, triple masked, placebo-controlled clinical trial for controlling childhood obesity. World J Pediatr. 2010;6:317–322. [DOI] [PubMed] [Google Scholar]
- 334. Wiegand S, l’Allemand D, Hubel H, Krude H, Burmann M, Martus P, Gruters A, Holl RW. Metformin and placebo therapy both improve weight management and fasting insulin in obese insulin-resistant adolescents: a prospective, placebo-controlled, randomized study. Eur J Endocrinol. 2010;163:585–592. [DOI] [PubMed] [Google Scholar]
- 335. Adeyemo MA, McDuffie JR, Kozlosky M, Krakoff J, Calis KA, Brady SM, Yanovski JA. Effects of metformin on energy intake and satiety in obese children. Diabetes Obes Metab. 2015;17:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. McDonagh MS, Selph S, Ozpinar A, Foley C. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014;168:178–184. [DOI] [PubMed] [Google Scholar]
- 337. Morrison JA, Cottingham EM, Barton BA. Metformin for weight loss in pediatric patients taking psychotropic drugs. Am J Psychiatry. 2002;159:655–657. [DOI] [PubMed] [Google Scholar]
- 338. Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163:2072–2079. [DOI] [PubMed] [Google Scholar]
- 339. Önalan G, Goktolga U, Ceyhan T, Bagis T, Onalan R, Pabuccu R. Predictive value of glucose-insulin ratio in PCOS and profile of women who will benefit from metformin therapy: obese, lean, hyper or normoinsulinemic? Eur J Obstet Gynecol Reprod Biol. 2005;123:204–211. [DOI] [PubMed] [Google Scholar]
- 340. Violante-Ortíz R, Del-Rio-Navarro BE, Lara-Esqueda A, Perez P, Fanghanel G, Madero A, Berber A. Use of sibutramine in obese Hispanic adolescents. Adv Ther. 2005;22:642–649. [DOI] [PubMed] [Google Scholar]
- 341. García-Morales LM, Berber A, Macias-Lara CC, Lucio-Ortiz C, Del-Rio-Navarro BE, Dorantes-Alvarez LM. Use of sibutramine in obese mexican adolescents: a 6-month, randomized, double-blind, placebo-controlled, parallel-group trial. Clin Ther. 2006;28:770–782. [DOI] [PubMed] [Google Scholar]
- 342. Reisler G, Tauber T, Afriat R, Bortnik O, Goldman M. Sibutramine as an adjuvant therapy in adolescents suffering from morbid obesity. Isr Med Assoc J. 2006;8:30–32. [PubMed] [Google Scholar]
- 343. Budd GM, Hayman LL, Crump E, Pollydore C, Hawley KD, Cronquist JL, Berkowitz RI. Weight loss in obese African American and Caucasian adolescents: secondary analysis of a randomized clinical trial of behavioral therapy plus sibutramine. J Cardiovasc Nurs. 2007;22:288–296. [DOI] [PubMed] [Google Scholar]
- 344. Daniels SR, Long B, Crow S, Styne D, Sothern M, Vargas-Rodriguez I, Harris L, Walch J, Jasinsky O, Cwik K, Hewkin A, Blakesley V. Cardiovascular effects of sibutramine in the treatment of obese adolescents: results of a randomized, double-blind, placebo-controlled study. Pediatrics. 2007;120:e147–e157. [DOI] [PubMed] [Google Scholar]
- 345. Danielsson P, Janson A, Norgren S, Marcus C. Impact sibutramine therapy in children with hypothalamic obesity or obesity with aggravating syndromes. J Clin Endocrinol Metab. 2007;92:4101–4106. [DOI] [PubMed] [Google Scholar]
- 346. Van Mil EG, Westerterp KR, Kester AD, Delemarre-van de Waal HA, Gerver WJ, Saris WH. The effect of sibutramine on energy expenditure and body composition in obese adolescents. J Clin Endocrinol Metab. 2007;92:1409–1414. [DOI] [PubMed] [Google Scholar]
- 347. Klein-Schwartz W. Abuse and toxicity of methylphenidate. Curr Opin Pediatr. 2002;14:219–223. [DOI] [PubMed] [Google Scholar]
- 348. Carrel AL, Myers SE, Whitman BY, Allen DB. Benefits of long-term GH therapy in Prader-Willi syndrome: a 4-year study. J Clin Endocrinol Metab. 2002;87:1581–1585. [DOI] [PubMed] [Google Scholar]
- 349. Wolfgram PM, Carrel AL, Allen DB. Long-term effects of recombinant human growth hormone therapy in children with Prader-Willi syndrome. Curr Opin Pediatr. 2013;25:509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Deal CL, Tony M, Hoybye C, Allen DB, Tauber M, Christiansen JS; the 2011 Growth Hormone in Prader-Willi Syndrome Clinical Care Guidelines Workshop. Growth Hormone Research Society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab. 2013;98:E1072–E1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Hsu WH, Xiang HD, Rajan AS, Kunze DL, Boyd AE III. Somatostatin inhibits insulin secretion by a G-protein-mediated decrease in Ca2+ entry through voltage-dependent Ca2+ channels in the beta cell. J Biol Chem. 1991;266:837–843. [PubMed] [Google Scholar]
- 352. Mitra SW, Mezey E, Hunyady B, Chamberlain L, Hayes E, Foor F, Wang Y, Schonbrunn A, Schaeffer JM. Colocalization of somatostatin receptor sst5 and insulin in rat pancreatic β-cells. Endocrinology. 1999;140:3790–3796. [DOI] [PubMed] [Google Scholar]
- 353. Bertoli A, Magnaterra R, Borboni P, Marini MA, Barini A, Fusco A, Bollea MR. Dose-dependent effect of octreotide on insulin secretion after OGTT in obesity. Horm Res. 1998;49:17–21. [DOI] [PubMed] [Google Scholar]
- 354. Lustig RH, Hinds PS, Ringwald-Smith K, Christensen RK, Kaste SC, Schreiber RE, Rai SN, Lensing SY, Wu S, Xiong X. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2003;88:2586–2592. [DOI] [PubMed] [Google Scholar]
- 355. Scott LJ. Liraglutide: a review of its use in the management of obesity. Drugs. 2015;75:899–910. [DOI] [PubMed] [Google Scholar]
- 356. Gibson WT, Farooqi IS, Moreau M, DePaoli AM, Lawrence E, O’Rahilly S, Trussell RA. Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy. J Clin Endocrinol Metab. 2004;89:4821–4826. [DOI] [PubMed] [Google Scholar]
- 357. Paz-Filho G, Wong ML, Licinio J. Ten years of leptin replacement therapy. Obes Rev. 2011;12:e315–e323. [DOI] [PubMed] [Google Scholar]
- 358. Shetty GK, Matarese G, Magkos F, Moon HS, Liu X, Brennan AM, Mylvaganam G, Sykoutri D, Depaoli AM, Mantzoros CS. Leptin administration to overweight and obese subjects for 6 months increases free leptin concentrations but does not alter circulating hormones of the thyroid and IGF axes during weight loss induced by a mild hypocaloric diet. Eur J Endocrinol. 2011;165:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Moon HS, Matarese G, Brennan AM, Chamberland JP, Liu X, Fiorenza CG, Mylvaganam GH, Abanni L, Carbone F, Williams CJ, De Paoli AM, Schneider BE, Mantzoros CS. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60:1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Korner J, Conroy R, Febres G, McMahon DJ, Conwell I, Karmally W, Aronne LJ. Randomized double-blind placebo-controlled study of leptin administration after gastric bypass. Obesity (Silver Spring). 2013;21:951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Rissanen A, Lean M, Rossner S, Segal KR, Sjostrom L. Predictive value of early weight loss in obesity management with orlistat: an evidence-based assessment of prescribing guidelines. Int J Obes Relat Metab Disord. 2003;27:103–109. [DOI] [PubMed] [Google Scholar]
- 362. Chanoine JP, Richard M. Early weight loss and outcome at one year in obese adolescents treated with orlistat or placebo. Int J Pediatr Obes. 2011;6:95–101. [DOI] [PubMed] [Google Scholar]
- 363. Nobili V, Vajro P, Dezsofi A, Fischler B, Hadzic N, Jahnel J, Lamireau T, McKiernan P, McLin V, Socha P, Tizzard S, Baumann U. Indications and limitations of bariatric intervention in severely obese children and adolescents with and without nonalcoholic steatohepatitis: ESPGHAN Hepatology Committee position statement. J Pediatr Gastroenterol Nutr. 2015;60:550–561. [DOI] [PubMed] [Google Scholar]
- 364. Sarr MG. Medical indications for weight-loss surgery in adolescents: but are there other equally important indications? JAMA Pediatr. 2014;168:11–12. [DOI] [PubMed] [Google Scholar]
- 365. Zeller MH, Modi AC, Noll JG, Long JD, Inge TH. Psychosocial functioning improves following adolescent bariatric surgery. Obesity (Silver Spring). 2009;17:985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Loux TJ, Haricharan RN, Clements RH, Kolotkin RL, Bledsoe SE, Haynes B, Leath T, Harmon CM. Health-related quality of life before and after bariatric surgery in adolescents. J Pediatr Surg. 2008;43:1275–1279. [DOI] [PubMed] [Google Scholar]
- 367. Olbers T, Gronowitz E, Werling M, Marlid S, Flodmark CE, Peltonen M, Gothberg G, Karlsson J, Ekbom K, Sjostrom LV, Dahlgren J, Lonroth H, Friberg P, Marcus C. Two-year outcome of laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity: results from a Swedish nationwide study (AMOS). Int J Obes. 2012;36:1388–1395. [DOI] [PubMed] [Google Scholar]
- 368. Zeller MH, Reiter-Purtill J, Ratcliff MB, Inge TH, Noll JG. Two-year trends in psychosocial functioning after adolescent Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2011;7:727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, Harmon CM, Zeller MH, Chen MK, Xanthakos SA, Horlick M, Buncher CR. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Wasserman H, Inge TH. Bariatric surgery in obese adolescents: opportunities and challenges. Pediatr Ann. 2014;43:e230–e236. [DOI] [PubMed] [Google Scholar]
- 371. Hsia DS, Fallon SC, Brandt ML. Adolescent bariatric surgery. Arch Pediatr Adolesc Med. 2012;166:757–766. [DOI] [PubMed] [Google Scholar]
- 372. Nandagopal R, Brown RJ, Rother KI. Resolution of type 2 diabetes following bariatric surgery: implications for adults and adolescents. Diabetes Technol Ther. 2010;12:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Barnett SJ. Surgical management of adolescent obesity. Adv Pediatr. 2013;60:311–325. [DOI] [PubMed] [Google Scholar]
- 374. Widhalm K, Fritsch M, Widhalm H, Silberhumer G, Dietrich S, Helk O, Prager G. Bariatric surgery in morbidly obese adolescents: long-term follow-up. Int J Pediatr Obes. 2011;6(Suppl 1):65–69. [DOI] [PubMed] [Google Scholar]
- 375. Himpens J, Cadiere GB, Bazi M, Vouche M, Cadiere B, Dapri G. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg. 2011;146:802–807. [DOI] [PubMed] [Google Scholar]
- 376. Rosenthal RJ, Diaz AA, Arvidsson D, Baker RS, Basso N, Bellanger D, Boza C, El Mourad H, France M, Gagner M, Galvao-Neto M, Higa KD, Himpens J, Hutchinson CM, Jacobs M, Jorgensen JO, Jossart G, Lakdawala M, Nguyen NT, Nocca D, Prager G, Pomp A, Ramos AC, Rosenthal RJ, Shah S, Vix M, Wittgrove A, Zundel N. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8:8–19. [DOI] [PubMed] [Google Scholar]
- 377. Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP, Harmon CM, Courcoulas A, Horlick M, Xanthakos SA, Dolan L, Mitsnefes M, Barnett SJ, Buncher R. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr. 2014;168:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Tritos NA, Mun E, Bertkau A, Grayson R, Maratos-Flier E, Goldfine A. Serum ghrelin levels in response to glucose load in obese subjects post-gastric bypass surgery. Obes Res. 2003;11:919–924. [DOI] [PubMed] [Google Scholar]
- 379. Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, Wardlaw SL. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–365. [DOI] [PubMed] [Google Scholar]
- 380. Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–407. [DOI] [PubMed] [Google Scholar]
- 381. Ramón JM, Salvans S, Crous X, Puig S, Goday A, Benaiges D, Trillo L, Pera M, Grande L. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg. 2012;16:1116–1122. [DOI] [PubMed] [Google Scholar]
- 382. Hafeez S, Ahmed MH. Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: a future treatment by choice or by chance? J Obes 2013;2013:839275. [DOI] [PMC free article] [PubMed]
- 383. Alqahtani AR, Antonisamy B, Alamri H, Elahmedi M, Zimmerman VA. Laparoscopic sleeve gastrectomy in 108 obese children and adolescents aged 5 to 21 years. Ann Surg. 2012;256:266–273. [DOI] [PubMed] [Google Scholar]
- 384. Al-Qahtani AR. Laparoscopic adjustable gastric banding in adolescent: safety and efficacy. J Pediatr Surg. 2007;42:894–897. [DOI] [PubMed] [Google Scholar]
- 385. Inge TH, Jenkins TM, Zeller M, Dolan L, Daniels SR, Garcia VF, Brandt ML, Bean J, Gamm K, Xanthakos SA. Baseline BMI is a strong predictor of nadir BMI after adolescent gastric bypass. J Pediatr. 2010;156:103–108.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Lawson ML, Kirk S, Mitchell T, Chen MK, Loux TJ, Daniels SR, Harmon CM, Clements RH, Garcia VF, Inge TH. One-year outcomes of Roux-en-Y gastric bypass for morbidly obese adolescents: a multicenter study from the Pediatric Bariatric Study Group. J Pediatr Surg. 2006;41:137–143, discussion 137–143. [DOI] [PubMed] [Google Scholar]
- 387. Inge TH, Miyano G, Bean J, Helmrath M, Courcoulas A, Harmon CM, Chen MK, Wilson K, Daniels SR, Garcia VF, Brandt ML, Dolan LM. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics. 2009;123:214–222. [DOI] [PubMed] [Google Scholar]
- 388. Inge TH, Prigeon RL, Elder DA, Jenkins TM, Cohen RM, Xanthakos SA, Benoit SC, Dolan LM, Daniels SR, D’Alessio DA. Insulin sensitivity and β-cell function improve after gastric bypass in severely obese adolescents. J Pediatr. 2015;167:1042–1048.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Kalra M, Inge T. Effect of bariatric surgery on obstructive sleep apnoea in adolescents. Paediatr Respir Rev. 2006;7:260–267. [DOI] [PubMed] [Google Scholar]
- 390. Michalsky MP, Inge TH, Simmons M, Jenkins TM, Buncher R, Helmrath M, Brandt ML, Harmon CM, Courcoulas A, Chen M, Horlick M, Daniels SR, Urbina EM. Cardiovascular risk factors in severely obese adolescents. JAMA Pediatr. 2015;169:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Holterman AX, Holterman M, Browne A, Henriques S, Guzman G, Fantuzzi G. Patterns of surgical weight loss and resolution of metabolic abnormalities in superobese bariatric adolescents. J Pediatr Surg. 2012;47:1633–1639. [DOI] [PubMed] [Google Scholar]
- 392. Ippisch HM, Inge TH, Daniels SR, Wang B, Khoury PR, Witt SA, Glascock BJ, Garcia VF, Kimball TR. Reversibility of cardiac abnormalities in morbidly obese adolescents. J Am Coll Cardiol. 2008;51:1342–1348. [DOI] [PubMed] [Google Scholar]
- 393. Michalsky M, Kramer RE, Fullmer MA, Polfuss M, Porter R, Ward-Begnoche W, Getzoff EA, Dreyer M, Stolzman S, Reichard KW. Developing criteria for pediatric/adolescent bariatric surgery programs. Pediatrics. 2011;128(Suppl 2):S65–S70. [DOI] [PubMed] [Google Scholar]
- 394. Strauss RS, Bradley LJ, Brolin RE. Gastric bypass surgery in adolescents with morbid obesity. J Pediatr. 2001;138:499–504. [DOI] [PubMed] [Google Scholar]
- 395. Sugerman HJ, Sugerman EL, DeMaria EJ, Kellum JM, Kennedy C, Mowery Y, Wolfe LG. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003;7:102–108. [DOI] [PubMed] [Google Scholar]
- 396. Kaulfers AM, Bean JA, Inge TH, Dolan LM, Kalkwarf HJ. Bone loss in adolescents after bariatric surgery. Pediatrics. 2011;127:e956–e961. [DOI] [PMC free article] [PubMed] [Google Scholar]