Abstract
With increased life expectancy, women will spend over three decades of life postmenopause. The menopausal transition increases susceptibility to metabolic diseases such as obesity, diabetes, cardiovascular disease, and cancer. Thus, it is more important than ever to develop effective hormonal treatment strategies to protect aging women. Understanding the role of estrogens, and their biological actions mediated by estrogen receptors (ERs), in the regulation of cardiometabolic health is of paramount importance to discover novel targeted therapeutics. In this brief review, we provide a detailed overview of the literature, from basic science findings to human clinical trial evidence, supporting a protective role of estrogens and their receptors, specifically ERα, in maintenance of cardiometabolic health. In so doing, we provide a concise mechanistic discussion of some of the major tissue-specific roles of estrogens signaling through ERα. Taken together, evidence suggests that targeted, perhaps receptor-specific, hormonal therapies can and should be used to optimize the health of women as they transition through menopause, while reducing the undesired complications that have limited the efficacy and use of traditional hormone replacement interventions.
A brief detailed overview of the literature from basic science to human clinical trials supporting a protective role of estrogens, specifically signaling through ERα, in cardiometabolic health.
With increased life expectancy, women are spending more time in the postmenopausal phase of life, characterized in part by ovarian insufficiency and reductions in circulating estrogens, than ever before. Whereas cardiovascular disease (CVD) remains the leading cause of mortality in both men and women in the United States, the risk among women escalates following menopause for reasons that remain incompletely understood (1). Impaired estrogen signaling has been viewed as a major causal factor (2, 3). Meanwhile, the mechanistic role of estrogens and estrogen receptors (ERs) in regulating women’s cardiometabolic health remains largely unknown; and, despite decades of research, there is still confusion about the best therapeutic approaches to preserve cardiometabolic health during and following menopause. Indeed, hormone therapies have been shown to exert both positive and negative effects on a variety of endpoints in settings of ovarian hormone insufficiency. Most of our current understanding of the biological actions of estrogens is based on studies of classic estrogen signaling through its main receptors, ERα and ERβ. Although classical estrogen signaling results in estrogen-mediated gene expression changes, it has become apparent that estrogen receptors activate both genomic and nongenomic (i.e., rapid signaling) pathways (1, 4). In this review, emerging findings pointing to a central role for ERα signaling in cardiometabolic health will be discussed. Improved understanding regarding how ERα signaling impacts cellular metabolism and integrative physiology will advance our understanding of disease pathobiology and lay the foundation for improved preventive care and treatment strategies for the maintenance of cardiometabolic health of women throughout the life span. Given the brevity of this review and the ever-expanding body of data on the role of ERs in cardiometabolic health, we apologize for any important studies that we may have missed.
Estrogen Receptors: Structure, Function, and Location
The ERs were among the first of the nuclear receptor superfamilies to be cloned, second only to the glucocorticoid receptor superfamily cloned in 1985 (5–7). Tissue-specific gene targets and mechanisms of action, including activation or repression of genes involved in the integrative regulation, are an area of intense investigation. There are 30 nuclear receptor genes, and they can be grouped into three subfamilies, as follows: (1) thyroid hormone and retinoic acid receptors, (2) orphan receptors, and (3) steroid hormone receptors. The cellular effects of estrogens are mediated predominately by two ERs: ESR1 (the gene that encodes ERα) and ESR2 (the gene that encodes ERβ). ESR1 was identified in 1958 (8), and ESR2 was first identified in the rat prostate and ovary in 1996 (9). Different splice variants of each receptor have been identified, and each exhibits distinct tissue-specific expression patterns and functions (10, 11). All ERs, with the exception of the ERαD3 isoform, are composed of six functional domains, from A to F, which contain the NH2-terminal domain, the DNA-binding domain, and the COOH-terminal ligand-binding domain. Two regions, named activation functions (AFs), have been identified as crucial for the transcriptional response of the ERs; the first one is localized at the NH2-terminal domain, whereas the second one is in the ligand-binding domain (12). ESR1 and ESR2 are structurally different in the ligand-binding pockets, which has facilitated the development of receptor-specific selective ligands (11). An important function of all ERs is to act as ligand-mediated transcriptional factors (11, 13–15).
ESR1 is broadly expressed in the central nervous system (CNS) (16–18) and in peripheral tissues, including adipose tissue (19), skeletal muscle, the cardiovascular system, and immune cells (20); ESR2 is the predominant receptor in the ovary, lung, bladder, hematopoietic cells, and gastrointestinal tract (20–22). In tissues in which both ERs are present, such as adipose tissue and the cardiovascular system, ERs are found in varying distribution in the different adipose tissue depots and blood vessel types (23–25). Recently, it was discovered that both ERs are also present in human fetal brown adipose tissue (26) with ERS1 being the more prominent receptor. ESR1 in the CNS influences adipose tissue distribution and locomotor activity (27). In the liver, ESR1 is significantly more expressed than ESR2, in which receptor activation is thought to be protective against insulin resistance in mice (28–30). Although both ERs, including splice variants (e.g., ERα 46 and 66), are expressed in the heart and the vascular wall (31), there are conflicting data regarding the localization of ESR2 (32). Both ERs are thought to be involved in the development of cardiac and vascular dysfunction.
There are some data suggesting that the cellular localization of receptors mediates their function and action (15, 33, 34). Importantly, estrogens mediate most of their biological effects through ERs at the level of gene regulation by interacting through its site-specific DNA and with other coregulatory proteins. In the nucleus, ERs up- or downregulate the expression of target genes by interacting through their site-specific DNA and with other coregulatory proteins that include coactivators and corepressors (35–37). The classical hormone/receptor paradigm includes ER monomers in the cytosol that form protein complexes with chaperone heat-shock proteins. Ligand-mediated activation of ER promotes dissociation from these complexes, and dimerization with other free monomers follows. Homodimers are most often formed; however, ERα/ERβ heterodimers have also been observed (38). ER dimers enter the nucleus, where they bind DNA either directly via estrogen response elements (ERE) in the promoter of target genes, or indirectly, through protein–protein tethering (39). A host of genes can be induced or repressed depending on the cell type, the presence of transcriptional cofactors, the type and concentration of the ligand, as well as the type of ER dimer formed (40). Splice variants, including the truncation of AF domains, are shown to manifest unique metabolic phenotypes and target gene signatures.
In 2000, pioneering work done by O’Malley and colleagues (37) demonstrated that ERs function as ligand-activated transcription factors. The trans-activation activity of ERs initiates through the ligand-bound receptor to its cognate, cis-acting enhancers, ERE (38). The consensus palindromic element ERE was this perfect ERE sequence that was shown to function in an orientation- and distance-independent manner, both of which are properties of an enhancer (7, 41). When ER directly interacts with the promoter/enhancer, binding to a full ERE is apparently the dominant mode of interaction. The human full EREs have a 3-bp spacer between the two half sites, the exceptions being response elements in the human transforming growth factor-α promoter, with a 4-bp spacer, and in the promoter of the rat luteinizing hormone β gene, with a 5-bp spacer (41). Controversy still exists concerning ER DNA binding via ERE half sites, although a number of examples exist (42–45).
Since the identification of a canonical ERE, several computational approaches have been undertaken to identify target genes based on the presence of EREs within promoter-proximal regions (46). For instance, for the 38 estrogen-responsive genes reviewed by Klinge (7), most of the functional EREs located within the promoters or 3′-untranslated regions are not the traditional consensus sequence. Thus, many target genes contain response elements that bear little similarity to consensus EREs. In one of the most comprehensive studies, Bourdeau and coworkers (47) screened for all EREs in the human and mouse genomes and identified in excess of >70,000 EREs within the human genome, >17,000 of which were within 15 kb of messenger RNA start sites. Elimination of EREs that were not conserved between the human and mouse genomes reduced the number of gene-proximal EREs to 660. A number of these sites were validated as genuine ER interaction sites, supporting the use of computational models to predict putative ER target genes to some degree (48). A variety of transcription factors, whose activity is controlled by protein phosphorylation events, are influenced by nongenomic ER action in a cell-specific context (29, 49). Membrane (i.e., nongenomic) ERs are found in caveolae and lipid rafts, where they interact with other proteins to mediate rapid intracellular signaling pathways through proteins, including growth factor receptors (such as insulin-like growth factor-1), G proteins, and tyrosine kinases (such as SRC and RAS) (29, 49). ERα interaction with caveolin-1 (in caveolae) and nongenomic receptor activities can be controlled by receptor palmitoylation (41). Rapid, nongenomic, estrogenic signaling has also been shown to occur via a membrane-bound G protein–coupled estrogen receptor 30 (GPR30) (42). GPR30 is also involved in a wide range of physiological and pathological conditions, including some evidence for it playing a protective role in CVD (43).
ERs and cardiometabolic risk: mechanistic evidence from animal studies
Much of the functional significance observed for ERs in mediating cardiometabolic risk has been obtained in rodents, where female mice carrying ESR1 mutations develop age-dependent vascular dysfunction and features of the metabolic syndrome, including obesity, glucose intolerance, and insulin resistance (44–46, 48), reminiscent of the human condition (described below). Total body ERα knockout (KO) mice (αERKO) recapitulate a remarkable metabolic dysfunction similar to that observed in humans with rare inactivating receptor mutations and genetic polymorphisms in the receptor. Not only do these mice have increased adiposity caused by reductions in energy expenditure and increased food intake, but they also exhibit glucose intolerance, insulin resistance, and reduced endothelial-derived nitric oxide production (vasculoprotective molecule), thus demonstrating the critical role for ESR1 in regulating energy, metabolic, and vascular homeostasis (44, 50, 51). Additionally, ESR1 is required to mediate the beneficial metabolic actions of estradiol. Indeed, estradiol exerts a protective effect against high-fat diet (HFD)–induced glucose intolerance in wild-type but not αERKO mice (52).
Another interesting mouse model is the total body ESR1 AF deletion mutant. Male and female mice lacking the AF2 domain of ERS1 show a phenotype similar to ESR1 KO mice, suggesting that this region of the ER is involved in estrogen-mediated transcriptional activation. Additionally, the AF2 region, but not the AF1, appears essential in preventing insulin resistance and glucose intolerance in mice fed a regular chow diet or following exposure to HFD (53), and in preventing atherosclerosis in low-density lipoprotein receptor-deficient mice fed a hypercholesterolemic diet (54). Specifically, it was recently observed that mice deficient of AF1, when exposed to estradiol, still maintain estradiol-related vasculoprotective functions, including estradiol’s ability to induce re-endothelialization capacity, the ability to produce nitric oxide, and the possibility of preventing atheroma generation (55); however, the specific actions of ER-AF regions still remain partially unknown because their function appears uniquely cell-type dependent (56).
ESR1 deletion from selected brain regions is shown to influence a variety of metabolic functions associated with cardiometabolic risk, including the following: body fat distribution, energy expenditure, reproduction, blood pressure, and food intake (57–60). In addition to expression and action of ERα in the CNS, ESR1 expression in the periphery is also shown to regulate metabolic homeostasis and insulin action. For example, ERS1 KO female mice have a lower insulin-stimulated glucose uptake in skeletal muscle tissue when compared with control/wild-type mice (48). Additionally, it was recently shown that muscle-specific ESR1 KO in female mice recapitulates a similar degree of metabolic dysfunction, as seen in the global null mouse model, including impaired muscle fatty acid oxidation, lipid accumulation, insulin resistance, and obesity (61). Muscle lipid accumulation and reduced muscle oxidative metabolism characteristic of muscle-specific ESR1 KO mice were in part a consequence of mitochondrial dysfunction (61). Indeed, ESR1 promotes mitochondrial DNA replication, mitochondrial biogenesis, and the turnover of dysfunctional organelles (by a process called mitophagy) in skeletal muscle, and, when these processes are impaired due to ESR1 deficiency, the accumulation of dysfunctional mitochondria drives oxidative stress and tissue inflammation, molecular underpinnings involved in the development of insulin resistance and atherosclerosis (61, 62).
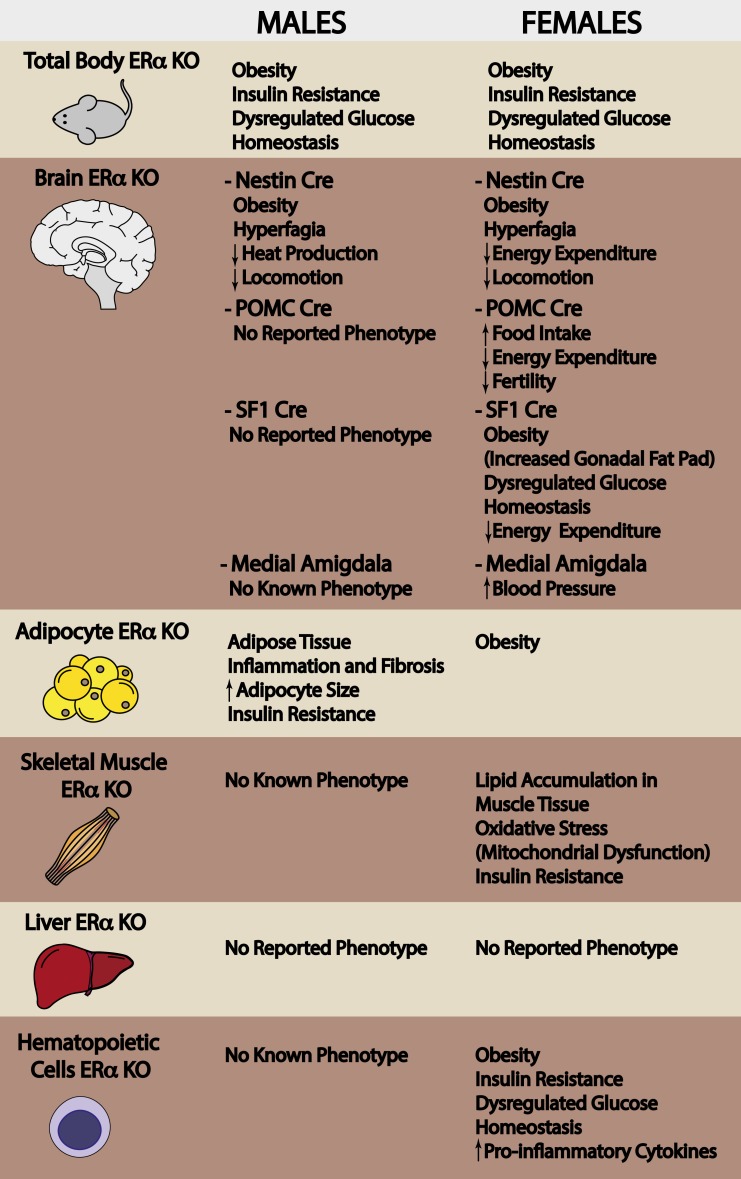
Given that immune cells infiltrate and reside in almost every tissue, it is not surprising that immune cells were found to play an important role in regulating adiposity, metabolic, and vascular homeostasis. In recent years, extensive research has been performed to clarify the communication between immune cells (specifically macrophages) and adipocytes, to better understand the influence of immune cell infiltration on cardiometabolic risk. The Hevener laboratory observed increased chemoattractant signals in adipose tissue of myeloid-specific ESR1 KO mice, and this was paralleled by increased immune cell infiltration (63). Deletion of ESR1 from myeloid cells promoted increased body weight, glucose intolerance, insulin resistance, and altered plasma adipokine and cytokine levels in female mice (63). In macrophages, ESR1 controls inflammation and oxidative metabolism and regulates the expression of a variety of genes and signaling networks involved in regulating metabolic homeostasis and providing atherosclerosis protection (63). Secreted factors from ESR1 KO macrophages were shown to promote increased lipid storage, inflammation, and impaired insulin action in a variety of cell types in culture. In vivo, female mice with a hematopoietic deletion of ESR1 showed increased susceptibility for atherosclerosis with a twofold increase in atherosclerotic lesion area when fed a Western diet (63). In summary, these tissue-selective and cell-specific deletion studies have allowed us to more elegantly interrogate the functions of ERα to better understand the tissue-specific actions of this hormone receptor in controlling cardiometabolic risk. Figure 1 provides a summary of the phenotype of ESR1 KO and tissue-specific ESR1 KO male mice.
Figure 1.
Tissue-specific effects of estrogen receptor deletion. Total body ERα deletion in mice promotes obesity and tissue-specific metabolic dysfunction. To better understand the involvement of ERα in the development of metabolic disease, in the last two decades, tissue-specific ERα knockout mice have been generated.
Fewer studies have investigated the effects of ESR2 mutations compared with those that have investigated ESR1 mutations. In contrast to the findings from animals with mutations in ESR1, whole-body ESR2/ERβ KO mice (ERKO) have not been shown to exhibit impaired insulin sensitivity, glucose intolerance, or increased body weight or adiposity compared with wild-type mice (50), further supporting the notion that ESR1 is more important in driving the positive cardiometabolic actions of estrogens. However, βERKO do develop sustained systolic and diastolic hypertension with aging (64). Evidence suggests that ESR2 may play a compensatory role in driving adipose tissue accumulation in the context of a HFD via inhibition of peroxisome proliferator-activated receptor (PPAR)γ, a transcription factor controlling broad adipocyte-specific gene expression (65). Interestingly, in that study, the effect of ESR2 to augment PPAR signaling in adipose tissue was accompanied by reduced accumulation of triglycerides and preserved insulin sensitivity in liver and skeletal muscle (65). Conversely, in an additional study, authors demonstrated that protein levels of ESR2 are increased, whereas PPAR-γ decreased, in visceral adipose tissues of rats following ovariectomy (66), thus suggesting that declines in circulating estrogen levels, mimicked in rats following ovariectomy, might increase adiposity in postmenopausal women, as well as increase metabolic and cardiovascular disease risk when compared with ovarian-intact rodents or cycling women.
Although the animal data supporting a role for ESR1 as a critical regulator in energy homeostasis are overwhelming, it is important to remember that most studies to date have only altered the expression levels of one of the receptors and not controlled for the actions of the other. Indeed, significantly less attention has focused on the role of ESR2, which has also been shown to mediate some metabolically protective effects of estradiol (28). Furthermore, it will be important to determine whether there are coactivating or coinhibitory roles influenced by heterodimerization of receptors or alternative mechanisms for activating/suppressing gene targets.
Total body GPR30 KO mice manifest a phenotype characterized by moderate obesity associated with reduced energy expenditure (67), impaired glucose tolerance (68). Specifically, deletion of G protein–coupled ER increased atherosclerosis progression, total and low-density lipoprotein cholesterol levels, and inflammation, while reducing vascular nitric oxide bioactivity in rodents following exposure to a high-fat atherogenic diet (69). GPR30 KO female mice have increased adipose tissue mass with aging (67). Interestingly, GPR30 KO females are less responsive to estradiol replacement following ovariectomy when compared with wild-type mice (67), suggesting a potential role for GPR30 in modulating estrogen sensitivity. In addition, female GPR30 KO mice are more susceptible to perturbations in glucose homeostasis as a consequence of estradiol insufficiency, and this phenotype is due to defects in pancreatic function (70). Although estradiol preserved pancreatic β-cell mass in female mice following streptozotocin-induced type-1 diabetes, this beneficial effect of estrogen was lost in GPR30 KO animals (70). Recently, it was shown that GPR30 agonist treatment induces vasodilation in female rats, although the mechanism mediating this effect is unclear (71). Together, these findings demonstrate extensive roles for GPR30 in mediating estrogenic effects in the maintenance of glucose homeostasis and vascular function.
ERs and cardiometabolic risk: mechanistic evidence from human studies
It is clear that estrogen loss in women leads to a cluster of cardiometabolic abnormalities that greatly increase risk of diseases, including CVD, type 2 diabetes, and certain forms of cancer. Consistently, in rodents fed a HFD, ovariectomy promotes CVD and weight gain and reduces insulin sensitivity, effects that are prevented by selective activation of ESR1 with 4,4′,4′′-(4-propyl-[1H]-pyrazole-1,3,5-triyl) (72). Surprisingly little is known, however, about the role that ERs play in age-related cardiometabolic disease pathology in humans. What makes research on the role of ER signaling in cardiometabolic disease in humans difficult is the fact that ER expression is not static, and the balance and levels of ERs (ERα isoforms and ERβ) can change with aging, disease, and prolonged estrogen deficiency, all of which can alter the response to estrogen (73, 74). For example, ERs have been reported to be lower in postmortem coronary arteries from postmenopausal women compared with premenopausal women and lower in atherosclerotic coronary arteries compared with normal coronary arteries regardless of menopausal state (75).
Although hormone replacement therapy (HRT) is sometimes highly effective at mitigating the negative cardiometabolic consequences of estrogen loss, it has also been associated with adverse cardiometabolic effects, such as an increase in insulin resistance and cardiac events (76, 77). Time lapse between ovarian failure and onset of therapy (i.e., duration of estrogen deficiency) is thought to play a role in determining who benefits from hormone therapy (78). Indeed, experiments in ovariectomized rats showed that early-onset, but not late-onset estrogen replacement therapy prevents oxidative stress and alteration in brain glucose uptake caused by estrogen withdrawal and by the increase in the ERα/β ratio (79). In humans, clinical data now suggest that HRT might be protective in preventing menopausal symptoms when used in women close to menopause transition, whereas null or adverse effect occurs when HRT is initiated in older women after the onset of menopause (12, 80–83).
Another current limitation associated with HRT is it lacks tissue specificity, and therefore acts on different tissues in the body ranging from the brain and adipose tissue (where it exerts positive effects) to the breast, endometrium, and endothelium (where it might exert toxic effects) (84). Furthermore, the precise mechanism(s) by which HRT imparts its effects on tissues has not been fully elucidated, but it most likely involves differences in receptor biochemistry (78).
ESR1 expression is reduced in endothelial cells harvested from the antecubital veins of healthy estrogen-deficient postmenopausal compared with premenopausal women (85), as well as in skeletal muscle from women with the metabolic syndrome. Reductions in endothelial cell ERα have been associated with impaired endothelial function (85), the key antecedent for atherosclerosis development. Moreover, skeletal muscle ESR1 expression was inversely correlated with adiposity and fasting insulin, surrogate markers of cardiometabolic health (i.e., low muscle ESR1 expression levels are associated with metabolic dysfunction). Thus, maintenance of vascular or skeletal ERα expression, or activation of muscle ESR1, could serve as an effective means to improve cardiometabolic health and combat diseases associated with metabolic dysfunction (61). Observational studies by Nilsson et al. (86) support this idea, showing reduced ESR1 expression in adipose tissue from obese women suffering from metabolic dysfunction.
In humans, genetic variations in the genes coding for ERα and ERβ have been associated with a myriad of diseases, including cardiovascular disease and premature ischemic heart disease (87–89). The Insulin Resistance Atherosclerosis Family Study identified a positive association between single-nucleotide polymorphisms (SNPs) in ESR1 and the metabolic syndrome, type 2 diabetes (T2DM), and adiposity (obesity) (90). The correlation between SNPs in ERS1 and the metabolic syndrome was confirmed in Japanese and Chinese women who were part of the Study of Women's Health Across the Nation (91). Specifically, in this study they were able to identify an association between SNPs in ERS1 and insulin sensitivity. The XbaI polymorphism of ESR1 has been correlated with the onset of the metabolic syndrome in Egyptian women; however, the mechanism associated with this polymorphism and insulin resistance has not been clearly identified (92). The presence of XbaI polymorphism of ESR1 with the PvuII C allele contributes to a metabolic and reproductive phenotype that is also observed in women with polycystic ovarian syndrome (93), an endocrine disorder caused by excessive androgen production, which increases the risk of insulin resistance, impaired glucose tolerance, T2DM, obesity, and dyslipidemia, and which eventually leads to early onset of CVD (94, 95). The same polymorphisms have also been considered as potential risk factors for the development of CVD, with the XbaI and/or PvuII C polymorphisms positively correlating with the onset of cardiovascular dysfunction. Indeed, the risk of developing a more severe form of coronary artery disease is higher in postmenopausal women carrying the XbaI and/or PvuII C polymorphisms (96). A similar association was previously reported also in men with coronary artery disease (87, 89, 97). However, despite these findings, there are some data that have found opposite results that may be related to differences in genetic background as well as the age of the study cohort (98). Given these disparities, additional information is required to better understand how these polymorphisms influence cardiometabolic outcomes. Polymorphisms in ESR2 have also been associated with the onset of cardiometabolic diseases in humans. In particular, the polymorphism AluI promotes obesity and is associated with increased cholesterol levels in women (99). Lastly, there appears to be an additive and adverse effect of ESR1 and ESR2 polymorphisms, which results in compromised metabolic function (100).
Incidence of insulin resistance and T2DM increases midlife in women (101–105). However, it is unclear whether menopause per se increases insulin resistance because prospective studies that have followed women across the menopause transition have not always reported declines in insulin sensitivity (106, 107). In support of a role for estrogens in protecting against insulin resistance, bilateral oophorectomy in women nearing menopausal age (range 40 to 54 years) reduced insulin sensitivity (estimated from oral glucose tolerance testing) (108). One mechanism by which loss of gonadal function may contribute indirectly to insulin resistance is through loss of ER expression or function secondary to prolonged estrogen deficiency. Although not all prospective studies have observed an association between natural menopause and diabetes risk (109, 110), surgical menopause has been strongly linked to an increased diabetes risk (111, 112). The increased risk associated with surgical menopause may be attributable to the more abrupt loss of sex steroids, compared with the slower decline that occurs during the natural menopause transition, supporting the notion that ovarian hormone loss is the major driver of metabolic dysfunction after menopause. Indeed, large randomized clinical trials consistently support a benefit of estrogen-based HRT on reducing fasting glucose, insulin, and incidence of new-onset T2DM after menopause (113–117). Moreover, secondary analysis of large randomized clinical trials suggests that initiating estrogen-based hormonal therapy early after menopause compared with later in menopause impacts hormonal therapy benefits and disease risk (117–121). A recent study by Van Pelt and colleagues (122) demonstrated that estrogen increased insulin-stimulated glucose disposal when administered to early postmenopausal women (i.e., within 6 years of menopause), whereas estrogens reduced insulin action when administered to late postmenopausal women (i.e., >10 years past menopause) who had never previously used HRT. The underlying mechanisms conferring the discordant effects of estrogen action on glucose disposal in early and late postmenopausal women are not yet known, but are most likely related to changes in ER with increasing age or duration of estrogen deficiency. Additional well-controlled studies in women are needed to elucidate tissue-specific changes in ERs and the underlying mechanisms of estrogen action in vivo.
Conclusions and Future Directions
Due to loss of the protective effects of estrogen, menopause significantly increases cardiometabolic risk, promoting increased incidence of CVD and T2DM, leading causes of death among aging women (3). Mechanistically, the adverse cardiometabolic effects of estrogen loss are due to impaired ER action; thus, ER signaling is an area of active investigation. As discussed in this work, estrogen action goes beyond its classical signaling through receptors α and β, with the GPR30 and other membrane-bound forms of the ERs also serving as important mediators of cardiometabolic protective effects of estrogen. Nonetheless, most research has focused on the classical ERs. Research has shown that loss of ERα signaling in particular increases adiposity (23), insulin resistance, and inflammation (123), a metabolic milieu that often precedes atherosclerosis development. These added insults lead to a vicious cycle of oxidative stress and inflammation within the vascular wall that can modulate estrogen–ER signaling, impair vascular function, and initiate atherosclerosis.
As women are living longer than any other time in history, it is critically important to elucidate therapeutic agents that can prevent and mitigate the cardiometabolic dysfunction that often precedes disease onset. In this regard, ERs may serve as prime therapeutic targets. However, more work is necessary to better understand ER signaling, which is exceedingly complex. Critical gaps remain in understanding how timing of hormone loss, aging, ER density, adiposity, pharmacological factors, and dietary factors affect ER biochemistry and the results of receptor agonization. Other novel therapeutic options may involve selective ER modulators or even tissue-specific targeting of the ERs.
Acknowledgments
This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico FONDECYT Grant 1160820 (to E.M.).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- AF
activation function
- CNS
central nervous system
- CVD
cardiovascular disease
- ER
estrogen receptor
- ERE
estrogen response element
- GPR30
G protein–coupled estrogen receptor 30
- HFD
high-fat diet
- HRT
hormone replacement therapy
- KO
knockout
- PPAR
peroxisome proliferator-activated receptor
- SNP
single-nucleotide polymorphism
- T2DM
type 2 diabetes.
References
- 1. Feldman RD. Heart disease in women: unappreciated challenges, GPER as a new target. Int J Mol Sci. 2016;17(5):E760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zaydun G, Tomiyama H, Hashimoto H, Arai T, Koji Y, Yambe M, Motobe K, Hori S, Yamashina A. Menopause is an independent factor augmenting the age-related increase in arterial stiffness in the early postmenopausal phase. Atherosclerosis. 2006;184:137–142. [DOI] [PubMed] [Google Scholar]
- 3. Ouyang P, Michos ED, Karas RH. Hormone replacement therapy and the cardiovascular system lessons learned and unanswered questions. J Am Coll Cardiol. 2006;47:1741–1753. [DOI] [PubMed] [Google Scholar]
- 4. Wang C, Liu Y, Cao JM. G protein-coupled receptors: extranuclear mediators for the non-genomic actions of steroids. Int J Mol Sci. 2014;15:15412–15425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miesfeld R, Rusconi S, Godowski PJ, Maler BA, Okret S, Wikstrom AC, Gustafsson JA, Yamamoto KR. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cDNA. Cell. 1986;46:389–399. [DOI] [PubMed] [Google Scholar]
- 7. Klinge CE. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 2001;29:2905–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jensen EV, Jacobson HI, Walf AA, Frye CA. Estrogen action: a historic perspective on the implications of considering alternative approaches. Physiol Behav. 2010;99:151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. [DOI] [PubMed] [Google Scholar]
- 11. Jia M, Dahlman-Wright K, Gustafsson JA. Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab. 2015;29:557–568. [DOI] [PubMed] [Google Scholar]
- 12. Arnal JF, Fontaine C, Abot A, Valera MC, Laurell H, Gourdy P, Lenfant F. Lessons from the dissection of the activation functions (AF-1 and AF-2) of the estrogen receptor alpha in vivo. Steroids. 2013;78:576–582. [DOI] [PubMed] [Google Scholar]
- 13. Liao S. Cellular receptors and mechanisms of action of steroid hormones. Int Rev Cytol. 1975;41:87–172. [DOI] [PubMed] [Google Scholar]
- 14. O’Malley BW. Mechanisms of action of steroid hormones. N Engl J Med. 1971;284:370–377. [DOI] [PubMed] [Google Scholar]
- 15. Chen JQ, Delannoy M, Cooke C, Yager JD. Mitochondrial localization of ERalpha and ERbeta in human MCF7 cells. Am J Physiol Endocrinol Metab. 2004;286:E1011–E1022. [DOI] [PubMed] [Google Scholar]
- 16. Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. [DOI] [PubMed] [Google Scholar]
- 17. Holland K, Norell A, Micevych P. Interaction of thyroxine and estrogen on the expression of estrogen receptor alpha, cholecystokinin, and preproenkephalin messenger ribonucleic acid in the limbic-hypothalamic circuit. Endocrinology. 1998;139:1221–1228. [DOI] [PubMed] [Google Scholar]
- 18. Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis KE, D Neinast M, Sun K, M Skiles W, D Bills J, A Zehr J, Zeve D, D Hahner L, W Cox D, M Gent L, Xu Y, V Wang Z, A Kahn S, Clegg DJ. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2:227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. [DOI] [PubMed] [Google Scholar]
- 21. Faulds MH, Zhao C, Dahlman-Wright K, Gustafsson JA. The diversity of sex steroid action: regulation of metabolism by estrogen signaling. J Endocrinol. 2012;212:3–12. [DOI] [PubMed] [Google Scholar]
- 22. Stice JP, Knowlton AA. Estrogen, NFkappaB, and the heat shock response. Mol Med. 2008;14:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35:8–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes. 2010;34:989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Menazza S, Murphy E. The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ Res. 2016;118:994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Velickovic K, Cvoro A, Srdic B, Stokic E, Markelic M, Golic I, Otasevic V, Stancic A, Jankovic A, Vucetic M, Buzadzic B, Korac B, Korac A. Expression and subcellular localization of estrogen receptors alpha and beta in human fetal brown adipose tissue. J Clin Endocrinol Metab. 2014;99:151–159. [DOI] [PubMed] [Google Scholar]
- 27. Xu P, Cao X, He Y, Zhu L, Yang Y, Saito K, Wang C, Yan X, Hinton AO Jr, Zou F, Ding H, Xia Y, Yan C, Shu G, Wu SP, Yang B, Feng Y, Clegg DJ, DeMarchi R, Khan SA, Tsai SY, DeMayo FJ, Wu Q, Tong Q, Xu Y. Estrogen receptor-alpha in medial amygdala neurons regulates body weight. J Clin Invest. 2015;125:2861–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yepuru M, Eswaraka J, Kearbey JD, Barrett CM, Raghow S, Veverka KA, Miller DD, Dalton JT, Narayanan R. Estrogen receptor-{beta}-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J Biol Chem. 2010;285:31292–31303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu L, Martinez MN, Emfinger CH, Palmisano BT, Stafford JM. Estrogen signaling prevents diet-induced hepatic insulin resistance in male mice with obesity. Am J Physiol Endocrinol Metab. 2014;306:E1188–E1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mendelsohn ME. Mechanisms of estrogen action in the cardiovascular system. J Steroid Biochem Mol Biol. 2000;74:337–343. [DOI] [PubMed] [Google Scholar]
- 32. Bottner M, Thelen P, Jarry H. Estrogen receptor beta: tissue distribution and the still largely enigmatic physiological function. J Steroid Biochem Mol Biol. 2014;139:245–251. [DOI] [PubMed] [Google Scholar]
- 33. Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci USA. 2004;101:4130–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levin ER. Cell localization, physiology, and nongenomic actions of estrogen receptors. J Appl Physiol. 1985;2001(91):1860–1867. [DOI] [PubMed] [Google Scholar]
- 35. Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. [DOI] [PubMed] [Google Scholar]
- 36. Kumar R, Thompson EB. The structure of the nuclear hormone receptors. Steroids. 1999;64:310–319. [DOI] [PubMed] [Google Scholar]
- 37. Lonard DM, Nawaz Z, Smith CL, O’Malley BW. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell. 2000;5:939–948. [DOI] [PubMed] [Google Scholar]
- 38. Iwabuchi E, Miki Y, Ono K, Onodera Y, Suzuki T, Hirakawa H, Ishida T, Ohuchi N, Sasano H. In situ detection of estrogen receptor dimers in breast carcinoma cells in archival materials using proximity ligation assay (PLA). J Steroid Biochem Mol Biol. 2017;165:159–169. [DOI] [PubMed] [Google Scholar]
- 39. Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol. 2010;122:74–81. [DOI] [PubMed] [Google Scholar]
- 40. Monteiro R, Teixeira D, Calhau C Estrogen signaling in metabolic inflammation. Mediators Inflamm. 2014;2014:615917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Feldman RD, Limbird LE. GPER (GPR30): a nongenomic receptor (GPCR) for steroid hormones with implications for cardiovascular disease and cancer. Annu Rev Pharmacol Toxicol. 2017;57:567–584. [DOI] [PubMed] [Google Scholar]
- 44. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97:12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int J Obes Relat Metab Disord. 2003;27:1020–1027. [DOI] [PubMed] [Google Scholar]
- 46. Merot Y, Metivier R, Penot G, Manu D, Saligaut C, Gannon F, Pakdel F, Kah O, Flouriot G. The relative contribution exerted by AF-1 and AF-2 transactivation functions in estrogen receptor alpha transcriptional activity depends upon the differentiation stage of the cell. J Biol Chem. 2004;279:26184–26191. [DOI] [PubMed] [Google Scholar]
- 47. Bourdeau V, Deschênes J, Métivier R, Nagai Y, Nguyen D, Bretschneider N, Gannon F, White JH, Mader S. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol. 2004;18(6):1411–1427. [DOI] [PubMed] [Google Scholar]
- 48. Hinton AO Jr, He Y, Xia Y, Xu P, Yang Y, Saito K, Wang C, Yan X, Shu G, Henderson A, Clegg DJ, Khan SA, Reynolds C, Wu Q, Tong Q, Xu Y. Estrogen receptor-alpha in the medial amygdala prevents stress-induced elevations in blood pressure in females. Hypertension. 2016;67:1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. [DOI] [PubMed] [Google Scholar]
- 50. Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, Khan A. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. [DOI] [PubMed] [Google Scholar]
- 51. Hinton AO Jr, He Y, Xia Y, Xu P, Yang Y, Saito K, Wang C, Yan X, Shu G, Henderson A, Clegg DJ, Khan SA, Reynolds C, Wu Q, Tong Q, Xu Y. Estrogen receptor-alpha in the medial amygdala prevents stress-induced elevations in blood pressure in females. Hypertension. 2016;67:1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–2117. [DOI] [PubMed] [Google Scholar]
- 53. Handgraaf S, Riant E, Fabre A, Waget A, Burcelin R, Liere P, Krust A, Chambon P, Arnal JF, Gourdy P. Prevention of obesity and insulin resistance by estrogens requires ERalpha activation function-2 (ERalphaAF-2), whereas ERalphaAF-1 is dispensable. Diabetes. 2013;62:4098–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Billon-Gales A, Krust A, Fontaine C, Abot A, Flouriot G, Toutain C, Berges H, Gadeau AP, Lenfant F, Gourdy P, Chambon P, Arnal JF. Activation function 2 (AF2) of estrogen receptor-alpha is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci USA. 2011;108:13311–13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Billon-Gales A, Fontaine C, Filipe C, Douin-Echinard V, Fouque MJ, Flouriot G, Gourdy P, Lenfant F, Laurell H, Krust A, Chambon P, Arnal JF. The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17beta-estradiol. Proc Natl Acad Sci USA. 2009;106:2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Merot Y, Metivier R, Penot G, Manu D, Saligaut C, Gannon F, Pakdel F, Kah O, Flouriot G. The relative contribution exerted by AF-1 and AF-2 transactivation functions in estrogen receptor alpha transcriptional activity depends upon the differentiation stage of the cell. J Biol Chem. 2004;279:26184–26191. [DOI] [PubMed] [Google Scholar]
- 57. Saito K, He Y, Yang Y, Zhu L, Wang C, Xu P, Hinton AO, Yan X, Zhao J, Fukuda M, Tong Q, Clegg DJ, Xu Y. PI3K in the ventromedial hypothalamic nucleus mediates estrogenic actions on energy expenditure in female mice. Sci Rep. 2016;6:23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hinton AO Jr, He Y, Xia Y, Xu P, Yang Y, Saito K, Wang C, Yan X, Shu G, Henderson A, Clegg DJ, Khan SA, Reynolds C, Wu Q, Tong Q, Xu Y. Estrogen receptor-alpha in the medial amygdala prevents stress-induced elevations in blood pressure in females. Hypertension. 2016;67(6):1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhu L, Xu P, Cao X, Yang Y, Hinton AO Jr, Xia Y, Saito K, Yan X, Zou F, Ding H, Wang C, Yan C, Saha P, Khan SA, Zhao J, Fukuda M, Tong Q, Clegg DJ, Chan L, Xu Y. The ERalpha-PI3K cascade in proopiomelanocortin progenitor neurons regulates feeding and glucose balance in female mice. Endocrinology. 2015;156:4474–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saito K, Cao X, He Y, Xu Y. Progress in the molecular understanding of central regulation of body weight by estrogens. Obesity (Silver Spring). 2015;23:919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ribas V, Drew BG, Zhou Z, Phun J, Kalajian NY, Soleymani T, Daraei P, Widjaja K, Wanagat J, de Aguiar Vallim TQ, Fluitt AH, Bensinger S, Le T, Radu C, Whitelegge JP, Beaven SW, Tontonoz P, Lusis AJ, Parks BW, Vergnes L, Reue K, Singh H, Bopassa JC, Toro L, Stefani E, Watt MJ, Schenk S, Akerstrom T, Kelly M, Pedersen BK, Hewitt SC, Korach KS, Hevener AL. Skeletal muscle action of estrogen receptor alpha is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med. 2016;8:334ra354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. [DOI] [PubMed] [Google Scholar]
- 63. Ribas V, Drew BG, Le JA, Soleymani T, Daraei P, Sitz D, Mohammad L, Henstridge DC, Febbraio MA, Hewitt SC, Korach KS, Bensinger SJ, Hevener AL. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc Natl Acad Sci USA. 2011;108:16457–16462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. [DOI] [PubMed] [Google Scholar]
- 65. Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, Gustafsson JA, Unger T, Kintscher U. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet. 2008;4:e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tomicek NJ, Lancaster TS, Korzick DH. Increased estrogen receptor beta in adipose tissue is associated with increased intracellular and reduced circulating adiponectin protein levels in aged female rats. Gend Med. 2011;8:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Davis KE, Carstens EJ, Irani BG, Gent LM, Hahner LM, Clegg DJ. Sexually dimorphic role of G protein-coupled estrogen receptor (GPER) in modulating energy homeostasis. Horm Behav. 2014;66:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. [DOI] [PubMed] [Google Scholar]
- 69. Meyer MR, Fredette NC, Howard TA, Hu C, Ramesh C, Daniel C, Amann K, Arterburn JB, Barton M, Prossnitz ER. G protein-coupled estrogen receptor protects from atherosclerosis. Sci Rep. 2014;4:7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, Korach KS, Mauvais-Jarvis F. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58:2292–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lindsey SH, Carver KA, Prossnitz ER, Chappell MC. Vasodilation in response to the GPR30 agonist G-1 is not different from estradiol in the mRen2.Lewis female rat. J Cardiovasc Pharmacol. 2011;57:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hamilton DJ, Minze LJ, Kumar T, Cao TN, Lyon CJ, Geiger PC, Hsueh WA, Gupte AA. Estrogen receptor alpha activation enhances mitochondrial function and systemic metabolism in high-fat-fed ovariectomized mice. Physiol Rep. 2016;4(17):e12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Novella S, Heras M, Hermenegildo C, Dantas AP. Effects of estrogen on vascular inflammation: a matter of timing. Arterioscler Thromb Vasc Biol. 2012;32:2035–2042. [DOI] [PubMed] [Google Scholar]
- 74. Menazza S, Murphy E. The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ Res. 2016;118:994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 1994;89:1501–1510. [DOI] [PubMed] [Google Scholar]
- 76. Brown MD, Korytkowski MT, Zmuda JM, McCole SD, Moore GE, Hagberg JM. Insulin sensitivity in postmenopausal women: independent and combined associations with hormone replacement, cardiovascular fitness, and body composition. Diabetes Care. 2000;23:1731–1736. [DOI] [PubMed] [Google Scholar]
- 77. Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Women’s Health Initiative I. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. [DOI] [PubMed] [Google Scholar]
- 78. Pereira RI, Casey BA, Swibas TA, Erickson CB, Wolfe P, Van Pelt RE. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinol Metab. 2015;100:4456–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lopez-Grueso R, Gambini J, Abdelaziz KM, Monleon D, Diaz A, El Alami M, Bonet-Costa V, Borras C, Vina J. Early, but not late onset estrogen replacement therapy prevents oxidative stress and metabolic alterations caused by ovariectomy. Antioxid Redox Signal. 2014;20:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hodis HN, Mack WJ. The timing hypothesis and hormone replacement therapy: a paradigm shift in the primary prevention of coronary heart disease in women. Part 2: Comparative risks. J Am Geriatr Soc. 2013;61:1011–1018. [DOI] [PubMed] [Google Scholar]
- 81. Hodis HN, Mack WJ. The timing hypothesis and hormone replacement therapy: a paradigm shift in the primary prevention of coronary heart disease in women. Part 1: Comparison of therapeutic efficacy. J Am Geriatr Soc. 2013;61:1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Salpeter SR, Buckley NS, Liu H, Salpeter EE The cost-effectiveness of hormone therapy in younger and older postmenopausal women. Am J Med. 2009;122(1):42–52.e2. [DOI] [PubMed] [Google Scholar]
- 83. Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, Burger HG, Colditz GA, Davis SR, Gambacciani M, Gower BA, Henderson VW, Jarjour WN, Karas RH, Kleerekoper M, Lobo RA, Manson JE, Marsden J, Martin KA, Martin L, Pinkerton JV, Rubinow DR, Teede H, Thiboutot DM, Utian WH; Endocrine Society . Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:s1–s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Leitman DC, Paruthiyil S, Yuan C, Herber CB, Olshansky M, Tagliaferri M, Cohen I, Speed TP. Tissue-specific regulation of genes by estrogen receptors. Semin Reprod Med. 2012;30:14–22. [DOI] [PubMed] [Google Scholar]
- 85. Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab. 2013;98:4507–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nilsson M, Dahlman I, Ryden M, Nordstrom EA, Gustafsson JA, Arner P, Dahlman-Wright K. Oestrogen receptor alpha gene expression levels are reduced in obese compared to normal weight females. Int J Obes. 2007;31:900–907. [DOI] [PubMed] [Google Scholar]
- 87. Shearman AM, Cupples LA, Demissie S, Peter I, Schmid CH, Karas RH, Mendelsohn ME, Housman DE, Levy D. Association between estrogen receptor alpha gene variation and cardiovascular disease. JAMA. 2003;290:2263–2270. [DOI] [PubMed] [Google Scholar]
- 88. Shearman AM, Cooper JA, Kotwinski PJ, Humphries SE, Mendelsohn ME, Housman DE, Miller GJ. Estrogen receptor alpha gene variation and the risk of stroke. Stroke. 2005;36:2281–2282. [DOI] [PubMed] [Google Scholar]
- 89. Shearman AM, Cooper JA, Kotwinski PJ, Miller GJ, Humphries SE, Ardlie KG, Jordan B, Irenze K, Lunetta KL, Schuit SC, Uitterlinden AG, Pols HA, Demissie S, Cupples LA, Mendelsohn ME, Levy D, Housman DE. Estrogen receptor alpha gene variation is associated with risk of myocardial infarction in more than seven thousand men from five cohorts. Circ Res. 2006;98:590–592. [DOI] [PubMed] [Google Scholar]
- 90. Gallagher CJ, Langefeld CD, Gordon CJ, Campbell JK, Mychaleckyj JC, Bryer-Ash M, Rich SS, Bowden DW, Sale MM. Association of the estrogen receptor-alpha gene with the metabolic syndrome and its component traits in African-American families: the Insulin Resistance Atherosclerosis Family Study. Diabetes. 2007;56:2135–2141. [DOI] [PubMed] [Google Scholar]
- 91. Lo JC, Zhao X, Scuteri A, Brockwell S, Sowers MR. The association of genetic polymorphisms in sex hormone biosynthesis and action with insulin sensitivity and diabetes mellitus in women at midlife. Am J Med. 2006;119:S69–S78. [DOI] [PubMed] [Google Scholar]
- 92. Ghattas MH, Mehanna ET, Mesbah NM, Abo-Elmatty DM. Association of estrogen receptor alpha gene polymorphisms with metabolic syndrome in Egyptian women. Metabolism. 2013;62:1437–1442. [DOI] [PubMed] [Google Scholar]
- 93. Silva FS, Soter MO, Sales MF, Candido AL, Reis FM, Silva IF, Sousa MO, Ferreira CN, Gomes KB. Estrogen receptor alphalpha gene (ESR1) PvuII and XbaI polymorphisms are associated to metabolic and proinflammatory factors in polycystic ovary syndrome. Gene. 2015;560:44–49. [DOI] [PubMed] [Google Scholar]
- 94. Dumitrescu R, Mehedintu C, Briceag I, Purcarea VL, Hudita D. The polycystic ovary syndrome: an update on metabolic and hormonal mechanisms. J Med Life. 2015;8:142–145. [PMC free article] [PubMed] [Google Scholar]
- 95. Mayer SB, Evans WS, Nestler JE. Polycystic ovary syndrome and insulin: our understanding in the past, present and future. Womens Health (Lond Engl). 2015;11:137–149. [DOI] [PubMed] [Google Scholar]
- 96. Alevizaki M, Saltiki K, Cimponeriu A, Kanakakis I, Xita N, Alevizaki CC, Georgiou I, Sarika HL. Severity of cardiovascular disease in postmenopausal women: associations with common estrogen receptor alpha polymorphic variants. Eur J Endocrinol. 2007;156:489–496. [DOI] [PubMed] [Google Scholar]
- 97. Lu H, Higashikata T, Inazu A, Nohara A, Yu W, Shimizu M, Mabuchi H. Association of estrogen receptor-alpha gene polymorphisms with coronary artery disease in patients with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2002;22:817–823. [DOI] [PubMed] [Google Scholar]
- 98. Casazza K, Page GP, Fernandez JR. The association between the rs2234693 and rs9340799 estrogen receptor alpha gene polymorphisms and risk factors for cardiovascular disease: a review. Biol Res Nurs. 2010;12:84–97. [DOI] [PubMed] [Google Scholar]
- 99. Saltiki K, Mantzou E, Doukas C, Kanakakis I, Zotos P, Lazaros L, Georgiou I, Alevizaki M. Estrogen receptor beta gene variants may be associated with more favorable metabolic profile in postmenopausal women undergoing coronary angiography. Exp Clin Endocrinol Diabetes. 2009;117:610–615. [DOI] [PubMed] [Google Scholar]
- 100. Efstathiadou ZA, Sakka C, Polyzos SA, Goutou M, Stakias N, Bargiota A, Koukoulis GN. Associations of estrogen receptor alpha and beta gene polymorphisms with lipid levels and insulin resistance in men. Metabolism. 2015;64:611–617. [DOI] [PubMed] [Google Scholar]
- 101. Karvonen-Gutierrez CA, Park SK, Kim C. Diabetes and menopause. Curr Diab Rep. 2016;16:20. [DOI] [PubMed] [Google Scholar]
- 102. Heianza Y, Arase Y, Kodama S, Hsieh SD, Tsuji H, Saito K, Shimano H, Hara S, Sone H. Effect of postmenopausal status and age at menopause on type 2 diabetes and prediabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 17 (TOPICS 17). Diabetes Care. 2013;36:4007–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Di Donato P, Giulini NA, Bacchi Modena A, Cicchetti G, Comitini G, Gentile G, Cristiani P, Careccia A, Esposito E, Gualdi F, Golinelli S, Bergamini E, Masellis G, Rastelli S, Gigli C, Elia A, Marchesoni D, Sticotti F, Del Frate G, Zompicchiatti C, Marino L, Costa MR, Pinto P, Dodero D, Storace A, Spinelli G, Quaranta S, Bossi CM, Ollago A, Omodei U, Vaccari M, Luerti M, Repetti F, Zandonini G, Raspagliesi F, Dolci F, Gambarino G, De Pasquale B, Polizzotti G, Borsellino G, Alpinelli P, Natale N, Colombo D, Belloni C, Viani A, Cecchini G, Vinci GW, Samaja BA, Pasinetti E, Penotti M, Ognissanti F, Pesando P, Malanetto C, Gallo M, Dolfin G, Tartaglino P, Mossotto D, Pistoni A, Tarani A, Rattazzi PD, Rossaro D, Campanella M, Arisi E, Gamper M, Salvatores D, Bocchin E, Stellin G, Meli G, Azzini V, Tirozzi F, Buoso G, Fraioli R, Marsoni V, Cetera C, Sposetti R, Candiotto E, Pignalosa R, Del Pup L, Bellati U, Angeloni C, Buonerba M, Garzarelli S, Santilli C, Mucci M, Di Nisio Q, Cappa F, Pierangeli I, Cordone A, Falasca L, Ferrante D, Serra GB, Cirese E, Todaro PA, Romanini C, Spagnuolo L, Lanzone A, Donadio C, Fabiani M, Baldaccini E, Votano S, Bellardini P, Favale W, Monti V, Bonomo A, Boninfante CE, Pietrobattista P, Massacesi L, Donini G, Del Savio F, Palombi L, Procaccioli P, Romani A, Romagnoli G, Genazzani AR, Gambacciani M, Scarselli G, Curiel P, De Leo V, Melani A, Levi D’Ancona V, Giarre G, Di Gioia E, Ceccarelli P, Massi GB, Cosci S, Gacci G, Cascianini A, Donati Sarti C, Bircolotti S, Pupita P, Mincigrucci M, Spadafora A, Santeufemia G, Marongiu G, Lai GR, Lai R, Dessole S, D’Andrea SA, . Coppola, Chiantera A, De P, Arienzo R, Pastore AR, Tamburrino A, Cardone A, Colacurci N, Izzo S, Tesauro R, Pascarella A, De Silvio MG, Di Prisco L, Lauda N, Sirimarco F, Agrimi C, Casarella G, Senatore G, Ronzini S, Ruccia G, De Carlo G, Pisaturo G, Carlomagno F, Fasolino A, Fiorillo F, Sorrentino R, Ercolano VB, Panariello S, Brun A, Tropea P, Stigliano CM, Amoroso A, Vadala P, Coco A, Galati G, Barese G, Masciari G, Pirillo P, Gioffre T, Mastrantonio P, Cardamone A, D’Angelo N, Valentino G, Barretta R, Ferraro G, Ferruccio C, Agostinelli D, Corrado G, Scopelliti A, Schonauer S, Trojano V, Bongiovanni F, Tinelli F, Poddi ER, Scarpello F, Colonna L, Fischetti G, Doria R, Trombetta G, Cocca EB, D’Amore A, Di Masi M, Liguori R, Dimaggio A, Laneve MR, Maolo MC, Gravina G, Nacci G, Nocera F, Lupo A, Giannola C, Graziano R, Mezzatesta M, Vegna G, Giannone G, Palumbo G, Cancellieri F, Mondo A, Cordopatri A, Carrubba M, Mazzola V, Cincotta L, D’Asta S, Bono A, Li Calsi L, Cavallaro Nigro S, Schiliro S, Repici A, Gullo D, Orlando A, Specchiale F, Papotto A, Abruzzo, Basilicata, Calabria, Campania, Emilia, Romagna, Giulia FV, Lazio, Liguria, Lombardia, Marche, Molise, Piemonte, Puglia, Sardegna, Sicilia, Toscana, Adige TA, Umbria, D’Aosta V, Veneto, Massacesi A, De Aloysio P, Campagnoli C, Gambacciani A, Graziottin A, Baldi C, Corrado Tonti G, Parazzini F, Chatenoud L; Gruppo di Studio Progetto Menopausa Italia. Risk factors for type 2 diabetes in women attending menopause clinics in Italy: a cross-sectional study. Climacteric. 2005;8:287–293. [DOI] [PubMed] [Google Scholar]
- 104. Monterrosa-Castro A, Blumel JE, Portela-Buelvas K, Mezones-Holguin E, Baron G, Bencosme A, Benitez Z, Bravo LM, Calle A, Chedraui P, Flores D, Espinoza MT, Gomez G, Hernandez-Bueno JA, Laribezcoa F, Lima S, Martino M, Mostajo D, Ojeda E, Onatra W, Sanchez H, Navarro D, Tserotas K, Vallejo MS, Witis S, Zuniga MC. Type II diabetes mellitus and menopause: a multinational study. Climacteric. 2013;16:663–672. [DOI] [PubMed] [Google Scholar]
- 105. Lee JS, Hayashi K, Mishra G, Yasui T, Kubota T, Mizunuma H. Independent association between age at natural menopause and hypercholesterolemia, hypertension, and diabetes mellitus: Japan nurses’ health study. J Atheroscler Thromb. 2013;20:161–169. [DOI] [PubMed] [Google Scholar]
- 106. Abdulnour J, Doucet E, Brochu M, Lavoie JM, Strychar I, Rabasa-Lhoret R, Prud’homme D. The effect of the menopausal transition on body composition and cardiometabolic risk factors: a Montreal-Ottawa New Emerging Team group study. Menopause. 2012;19:760–767. [DOI] [PubMed] [Google Scholar]
- 107. Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring). 2010;18:604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pirimoglu ZM, Arslan C, Buyukbayrak EE, Kars B, Karsidag YK, Unal O, Turan MC. Glucose tolerance of premenopausal women after menopause due to surgical removal of ovaries. Climacteric. 2011;14:453–457. [DOI] [PubMed] [Google Scholar]
- 109. Kim C, Edelstein SL, Crandall JP, Dabelea D, Kitabchi AE, Hamman RF, Montez MG, Perreault L, Foulkes MA, Barrett-Connor E. Menopause and risk of diabetes in the Diabetes Prevention Program. Menopause. 2011;18:857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Soriguer F, Morcillo S, Hernando V, Valdes S, Ruiz de Adana MS, Olveira G, Fuentes EG, Gonzalez I, Tapia MJ, Esteva I, Rojo-Martinez G. Type 2 diabetes mellitus and other cardiovascular risk factors are no more common during menopause: longitudinal study. Menopause. 2009;16:817–821. [DOI] [PubMed] [Google Scholar]
- 111. Appiah D, Winters SJ, Hornung CA. Bilateral oophorectomy and the risk of incident diabetes in postmenopausal women. Diabetes Care. 2014;37:725–733. [DOI] [PubMed] [Google Scholar]
- 112. Lejskova M, Pitha J, Adamkova S, Auzky O, Adamek T, Babkova E, Lanska V, Alusik S. Bilateral oophorectomy may have an unfavorable effect on glucose metabolism compared with natural menopause. Physiol Res. 2014;63(Suppl 3):S395–S402. [DOI] [PubMed] [Google Scholar]
- 113. Bonds DE, Lasser N, Qi L, Brzyski R, Caan B, Heiss G, Limacher MC, Liu JH, Mason E, Oberman A, O’Sullivan MJ, Phillips LS, Prineas RJ, Tinker L. The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative randomised trial. Diabetologia. 2006;49:459–468. [DOI] [PubMed] [Google Scholar]
- 114. Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/Progestin Replacement Study: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:1–9. [DOI] [PubMed] [Google Scholar]
- 115. Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. [DOI] [PubMed] [Google Scholar]
- 116. Szmuilowicz ED, Stuenkel CA, Seely EW. Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol. 2009;5:553–558. [DOI] [PubMed] [Google Scholar]
- 117. Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8:538–554. [DOI] [PubMed] [Google Scholar]
- 118. Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt). 2006;15:35–44. [DOI] [PubMed] [Google Scholar]
- 119. Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. [DOI] [PubMed] [Google Scholar]
- 120. Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, Burger HG, Colditz GA, Davis SR, Gambacciani M, Gower BA, Henderson VW, Jarjour WN, Karas RH, Kleerekoper M, Lobo RA, Manson JE, Marsden J, Martin KA, Martin L, Pinkerton JV, Rubinow DR, Teede H, Thiboutot DM, Utian WH. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:s1–s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Manson JE, Kaunitz AM. Menopause management–getting clinical care back on track. N Engl J Med. 2016;374:803–806. [DOI] [PubMed] [Google Scholar]
- 122. Pereira RI, Casey BA, Swibas TA, Erickson CB, Wolfe P, Van Pelt RE. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinol Metab. 2015;100(12):4456–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Das SK, Ma L, Sharma NK. Adipose tissue gene expression and metabolic health of obese adults. Int J Obes. 2015;39:869–873. [DOI] [PMC free article] [PubMed] [Google Scholar]