Abstract
We have recently identified from the avian hypothalamus a complementary DNA encoding a small secretory protein termed neurosecretory protein GL (NPGL). In chicks, NPGL increases body weight gain without affecting food intake. A database search reveals that NPGL is conserved throughout vertebrates. However, the central distribution and functional role of NPGL remains to be elucidated in mammals. In this study, we identified the precursor complementary DNA encoding NPGL from the mouse hypothalamus. Quantitative reverse transcription polymerase chain reaction and morphological analyses revealed that NPGL precursor messenger RNA is robustly expressed in the mediobasal hypothalamus with NPGL neurons specifically localized to the lateroposterior part of the arcuate nucleus in the hypothalamus. NPGL-immunoreactive fibers were observed in close anatomical contact with pro-opiomelanocortin neurons in the rostral region of the arcuate nucleus. NPGL messenger RNA expression was elevated by 24-hour fasting and reduced by feeding of a high-fat diet for 5 weeks. Furthermore, intracerebroventricular injection of mature NPGL increased food intake, pointing to an important role in feeding. Taken together, these findings report on the distribution of NPGL in the mammalian brain and point to an important role for this neuropeptide in energy homeostasis.
This report represents the characterization of precursor protein, localization, distribution, and biological action of neurosecretory protein GL (NPGL) in the mouse hypothalamus.
The discovery of leptin, an anorexigenic peptide hormone secreted from adipose tissues (1), helped to clarify the means by which body composition is communicated to central structures that regulate feeding and metabolism. Leptin exerts its effect via two major neuronal populations, that is, orexigenic neuropeptide Y (NPY)/agouti-related protein (AgRP) neurons and anorexigenic pro-opiomelanocortin (POMC) neurons in the arcuate nucleus (Arc) of the hypothalamus (2). In addition to leptin, ghrelin, an orexigenic peptide hormone secreted mainly from the gastric tissue, acts on NPY/AgRP neurons (3) to increase feeding. Feeding is further controlled by hypothalamic orexin, also called hypocretin, cells that are directly targeted by leptin and ghrelin (4). In turn, orexin cells innervate a number of neuronal phenotypes, including histaminergic, serotonergic, and dopaminergic neurons (4). Despite considerable progress in understanding the regulation of hunger and satiety during the last several decades, the neural control of feeding is not completely understood. To further understanding of the mechanism regulating feeding behavior, we sought to identify previously unknown hypothalamic neuropeptides.
To find neuropeptide precursors in the vertebrate brain, we performed a complementary DNA (cDNA) subtractive screen of the chicken hypothalamic infundibulum, which contains one of the feeding centers in this species. After sequencing 596 clones, we identified a cDNA encoding a previously unknown small protein and termed this neurochemical neurosecretory protein GL (NPGL) because the precursor protein contained a secretory signal sequence at the N terminus and the predicted C-terminal amino acids of the small protein were Gly-Leu-NH2 (5). In chicks, subcutaneous chronic infusion of NPGL induces a significant increase in body weight gain without affecting food intake, suggesting a central role for this protein in regulating growth in this species (5). A database search revealed that the gene for NPGL is conserved in vertebrates, including humans, rats, and mice. Given the role of NPGL in chick body weight regulation, we speculated that the mammalian homolog of NPGL might play an important role in feeding and/or energy metabolism. As a result, the present investigation sought to characterize whether NPGL is in a position to interact with well-established feeding circuitry and impact food intake. To accomplish this goal, we first cloned the cDNA encoding NPGL from the mouse hypothalamus and investigated the distribution of NPGL neurons throughout the brain. To explore the role of NPGL in monitoring energetic state, the expression of NPGL precursor messenger RNA (mRNA) was examined during fasting and in animals provided with a high-fat diet (HFD). Finally, the role of NPGL on feeding was explored by investigating the effect of intracerebroventricular injections of NPGL on food intake.
Materials and Methods
Animals
C57BL/6J mice were purchased from Charles River Laboratories (Kanagawa, Japan) and housed in standard conditions (23 ± 2°C under a 12-hour light/12-hour dark cycle) with ad libitum access to water and normal chow (CE-2; CLEA Japan, Tokyo, Japan). Mice fed a HFD (D12492; Research Diets, New Brunswick, NJ) for either 5 or 13 weeks beginning at 4 weeks of age were also purchased from Charles River Laboratories. All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals prepared by Hiroshima University (Higashi-Hiroshima, Japan).
Molecular cloning of cDNA encoding NPGL
Male mice (8 weeks old) were used. The methods used to clone NPGL have been described previously (6–9) and are briefly described herein. Preliminary results revealed that the NPGL precursor mRNA was highly expressed in the mediobasal hypothalamus (MBH). Therefore, cDNA synthesized from total RNA in the MBH was used as a source for cloning. Primers were designed based on the genomic sequence data (chromosome 1; accession no. AL645534). For cloning the 5′ region, cDNA was amplified with the first primer, that is, 5′-TCTAAGGAGCTGAGAATATGCA-3′ (nucleotide no. 72,675 to 72,654), and the oligo(dT) anchor primer supplied in the 5′/3′ rapid amplification of cDNA ends kit (Roche Diagnostics, Basel, Switzerland). First-round polymerase chain reaction (PCR) products were reamplified with the second primer, that is, 5′-TTAGAAACACGAGGCTTCC-3′ (nucleotide no. 68,693 to 68,675), and the anchor primer. For cloning the 3′ region, cDNA was amplified with the first primer, that is, 5′-CACAGTCAGACAGACCTGC-3′ (nucleotide no. 68,593 to 68,611), and the anchor primer. First-round PCR products were reamplified with the second primer, that is, 5′-CTGCTGACTCTTAACCAAGC-3′ (nucleotide no. 68,608 to 68,627), and the anchor primer. These second-round PCR products were subcloned into a TA cloning vector (pGEM-T Easy; Promega, Madison, WI) in accordance with the manufacturer’s instructions. The DNA inserts of the positive clones were amplified by PCR with universal M13 primers. The nucleotide sequence was determined using an ABI Prism dye terminator cycle sequencing ready reaction kit (Applied Biosystems, Foster City, CA) and a model 310 automated DNA sequencer (Applied Biosystems), and then analyzed with DNASIS Pro software (Hitachi Software Engineering, Kanagawa, Japan). Universal M13 primers or gene-specific primers were used to sequence both strands.
Real-time reverse transcription PCR
Male mice (8 weeks old) were killed to examine the distribution of NPGL mRNA throughout the brain (telencephalon, diencephalon, mesencephalon, cerebellum, and MBH). Additionally, the MBH from mice (9 and 17 weeks old) fed HFD for 5 and 13 weeks were also used. The tissues were frozen in liquid nitrogen and stored at −80°C for RNA processing. Total RNA was isolated with TRIzol regent (Life Technologies, Carlsbad, CA). RNA concentration was measured by nanodrop spectroscopy (Thermo Fisher Scientific, Waltham, MA), and cDNA was reverse transcribed using ReverTra Ace quantitative reverse transcription PCR (RT-PCR) master mix with genomic DNA remover (Toyobo, Osaka, Japan). PCR amplifications were performed with THUNDERBIRD SYBR quantitative PCR mix (Toyobo) using following conditions: 95°C for 20 seconds, followed by 40 cycles of 95°C for 3 seconds, and 60°C for 30 seconds. β-Actin (ACTB) was used as an endogenous control. The following primers were used: NPGL sense primer, 5′-GGAACCATGGCTTAGGAAGG-3′ (nucleotide no. 326 to 345 from the ATG initiation codon), antisense primer, 5′-TCTAAGGAGCTGAGAATATGCA-3′ (nucleotide no. 435 to 414); suppressor of cytokine signaling 3 (Socs3) sense primer, 5′-ACCAGCGCCACTTCTTCACG-3′ (nucleotide no. 224 to 243), antisense primer; 5′-GTGGAGCATCATACTGATCC-3′ (nucleotide no. 673 to 654); and ACTB sense primer; 5′-GGCACCACACCTTCTACAAT-3′ (nucleotide no. 257 to 276), antisense primer; 5′-AGGTCTCAAACATGATCTGG-3′ (nucleotide no. 379 to 360). The nucleotide sequences of PCR products were confirmed using DNA sequencer as described earlier. Data of real-time RT-PCR were analyzed by the 2−ΔΔCt method (10).
In situ hybridization
Male mice (8 weeks old) were transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. The brains were postfixed overnight and then put in sucrose solution (30% in 10 mM phosphate buffer) at 4°C until they sank. The brains were cut at 16-µm thickness with a cryostat at −20°C. Digoxigenin-labeled antisense and sense RNA probes were produced from a cloned PCR fragment (376 bp) with the sense primer, 5′-GTTCATTGTGGGAATATGCT-3′ (nucleotide no. 60 to 79), and the antisense primer, 5′-TCTAAGGAGCTGAGAATATGCA-3′ (nucleotide no. 435 to 414), using the digoxigenin RNA labeling kit (SP6/T7; Roche Diagnostics). Labeling was accomplished as previously described (6, 7).
Production of NPGL and antibody against NPGL
The deduced mouse NPGL peptide containing 80 amino acid residues, identical to the rat NPGL sequence, was synthesized by microwave-assisted solid-phase peptide synthesis using an automated peptide synthesizer (Syro Wave; Biotage, Uppsala, Sweden) as previously described (11). Guinea pig and rabbit antisera were produced as previously described (6, 7) using synthetic NPGL as the antigen. The antigen solution was mixed with Freund’s complete adjuvant and injected into guinea pigs and rabbits. After a booster injection, blood was collected from each animal and the optimal serum with high titer was selected by a dot blot analysis. Anti-NPGL antibody was purified on an NPGL-conjugated Sepharose 6B column.
Immunohistochemistry
Male mice (8 weeks old) were injected with colchicine into lateral ventricle (30 μg/2.5 μL). After 2 days, the brains were cut on 20- or 60-µm sections with a cryostat at −20°C following cryoprotection and freezing. The procedure using immunoenzyme and immunofluoroscence staining on floating sections was conducted as described previously (8, 9, 12). Briefly, for the enzyme-labeled antibody method, the sections were incubated in blocking buffer (1% bovine serum albumin, 1% normal goat serum, and 0.3% Triton X-100 in 10 mM phosphate-buffered saline) for 1 hour after incubating with 3% H2O2 in absolute methanol for 30 minutes at room temperature. Subsequently, sections were incubated in the guinea pig antibody against NPGL (1:500 dilution in blocking buffer) overnight at 4°C, and then with goat anti–guinea pig immunoglobulin G (IgG; 1:1000 dilution; Vector Laboratories, Burlingame, CA). Immunoreactive products were detected with an ABC kit (Vectastain Elite kit; Vector Laboratories). A control experiment was performed to examine antibody specificity by preadsorbing the working dilution of the primary antibody with a saturating concentration (10 µg/mL) of NPGL.
For the immunofluorescence method, the sections were incubated in blocking buffer (1% bovine serum albumin, 1% normal donkey serum, and 0.3% Triton X-100 in 10 mM phosphate-buffered saline) for 1 hour at room temperature before incubating with the rabbit antibody against NPGL (1:250 dilution in blocking buffer) overnight at 4°C. Cy3-conjugated donkey anti-rabbit IgG (1:400 dilution, 711-165-152; Jackson ImmunoResearch Laboratories, West Grove, PA) was used as a secondary antibody.
Interactions of NPGL-immunoreactive fibers with NPY/AgRP or POMC neurons were surveyed using double-label immunofluorescence as follows: a guinea pig antibody against NPGL (1:500 dilution) and a rabbit antibody against AgRP (1:10,000 dilution, H-003-57; Phoenix Pharmaceuticals, Burlingame, CA) were used for the detection of NPGL fibers and NPY/AgRP neurons, and a rabbit antibody against NPGL (1:250 dilution) and a guinea pig antibody against human β-endorphin (1:2000 dilution, T-5009; Peninsula Laboratories, San Carlos, CA) were used for the detection of NPGL fibers and POMC neurons. Cy3-conjugated donkey anti-rabbit IgG (1:400 dilution, 711-165-152; Jackson ImmunoResearch Laboratories) and Alexa Fluor 488–conjugated donkey anti–guinea pig IgG (1:600 dilution, 706-545-148; Jackson ImmunoResearch Laboratories) were used as secondary antibodies. Immunoreactive labeling was observed using an Eclipse E600 conventional microscope (Nikon, Tokyo, Japan) or a FV1000 confocal microscope (Olympus, Tokyo, Japan).
Intracerebroventricular injection
Male mice (8 weeks old) were used. A guide cannula (22 gauge, C313GS-5; Plastics One, Roanoke, VA) was fixed to the skull using acrylic resin (Shofu, Kyoto, Japan). The final coordinates of the guide cannula tips were 0.2 mm caudal to bregma, 1.0 mm lateral to midline, and 1.25 mm ventral to the skull surface. The injector (28 gauge, C313IS-5; Plastics One) was extended to 1.0 mm below the tip of the guide cannula. Injections were delivered by a syringe (Hamilton, Bonaduz, Switzerland) connected to polyethylene tubing using a syringe pump controller (BASi, West Lafayette, IN). The injection of reagents was performed after a postoperative period of 10 days. NPGL was diluted in 30% propylene glycol at pH 8.0 and the injection dose was 1.0 nmol per animal. The vehicle of 30% propylene glycol at pH 8.0 was used as a control. The injection was conducted at the beginning of light period and food intake was measured at 1, 2, 4, 6, 8, 10, and 24 hours after the injection.
Measurement of serum glucose concentration
Serum glucose concentration was measured by a Glucocard G+ meter (Arkray, Kyoto, Japan).
Statistical analysis
Statistically significant differences were calculated using a Student t test or a one-way analysis of variance. Differences at P values of <0.05 were considered statistically significant.
Results
Cloning of cDNA encoding the mouse NPGL precursor
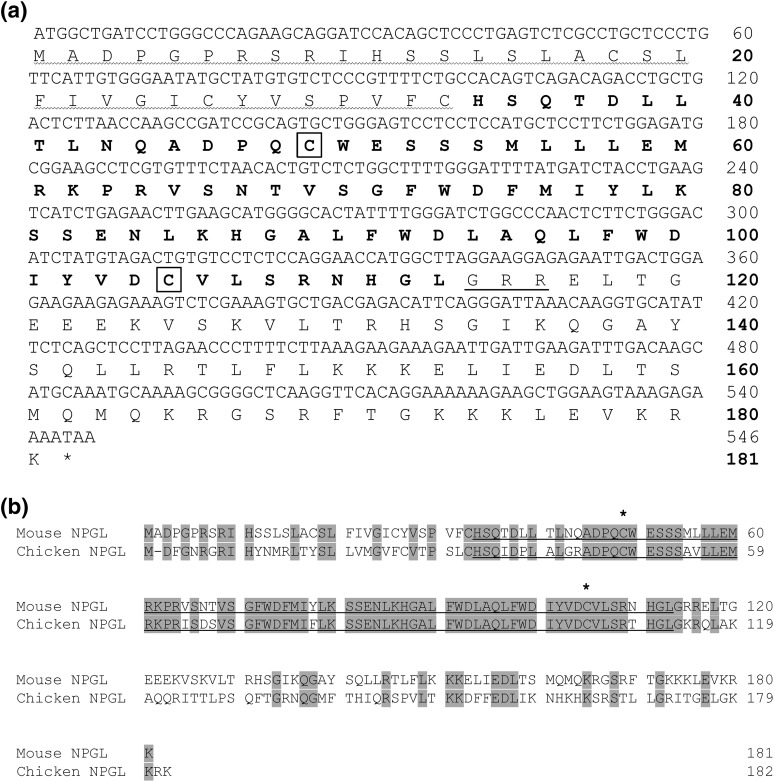
The cDNA sequence encoding NPGL was determined using total RNA isolated from the mouse hypothalamus. The open reading frame was 546 bp, and the deduced protein consisted of 181 amino acid residues [Fig. 1(a)]. The precursor protein includes a 33–amino acid signal peptide at the N terminus, an 80–amino acid residue small protein, a Gly amidation signal and a dibasic cleavage site (Arg-Arg) at the C terminus of the small protein, and an extended C-terminal sequence [Fig. 1(a)]. Additionally, NPGL contains two Cys residues, suggesting disulfide bond formation [Fig. 1(a)]. The amino acid sequence alignment of NPGL proteins deduced from the cDNA of mouse and chicken is shown in Fig. 1(b). The amino acid sequence similarity of NPGL between the mouse and chicken is 85%.
Figure 1.
(a) Nucleotide sequence and amino acid sequence of the NPGL precursor in mouse. The predicted signal peptide is denoted by a wavy line. The Gly (G) C-terminal amidation signal and the Arg (R)–Arg (R) dibasic processing site are underlined. The predicted 80–amino acid residue mature protein is present in boldface. Two Cys (C) residues are boxed. The stop codon (TAA) is indicated by the asterisk. (b) Amino acid sequence alignment of NPGL precursor deduced from mouse and chicken cDNA sequences. A hyphen was inserted in one gap to optimize the sequence alignment. Gray boxes highlight conserved amino acids. The predicted mature small proteins are underlined. The conserved two Cys (C) residues are indicated by asterisks.
Expression of the NPGL precursor mRNA in the brain
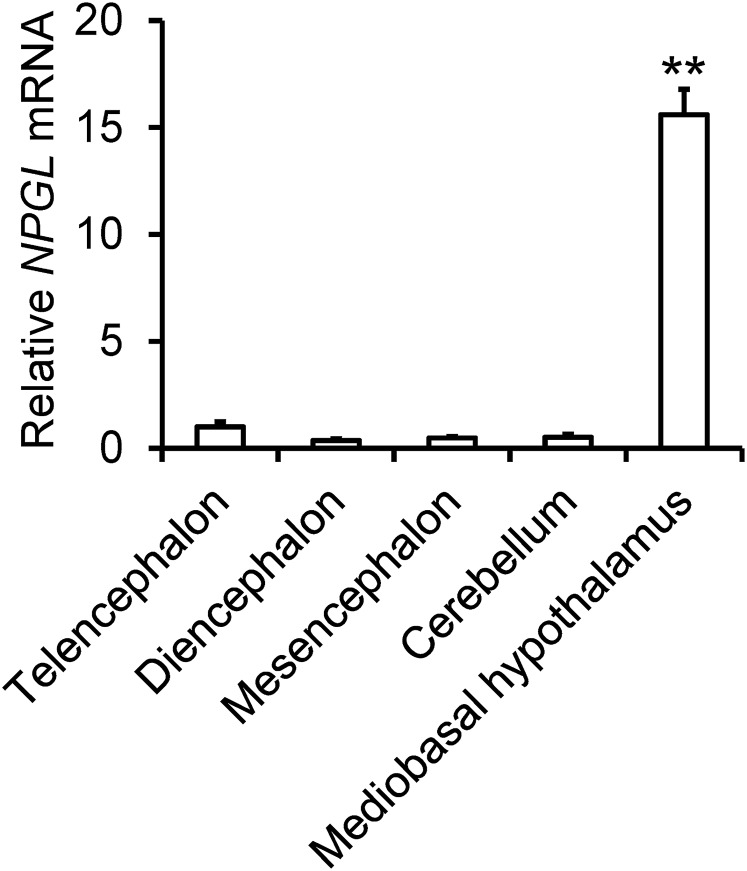
The expression levels of the NPGL precursor mRNA within different regions of the brain, including the telencephalon, diencephalon, mesencephalon, cerebellum, and MBH, were examined by real-time RT-PCR. The NPGL precursor mRNA was exclusively expressed in the MBH, whereas expression in other brain regions was around background levels (Fig. 2).
Figure 2.
Real-time RT-PCR analysis of NPGL precursor mRNA concentrations in different brain regions. NPGL precursor mRNA levels were quantified relative to the level of ACTB mRNA. Each value represents the mean ± standard error of the mean (n = 4). **P < 0.001.
Morphological analysis
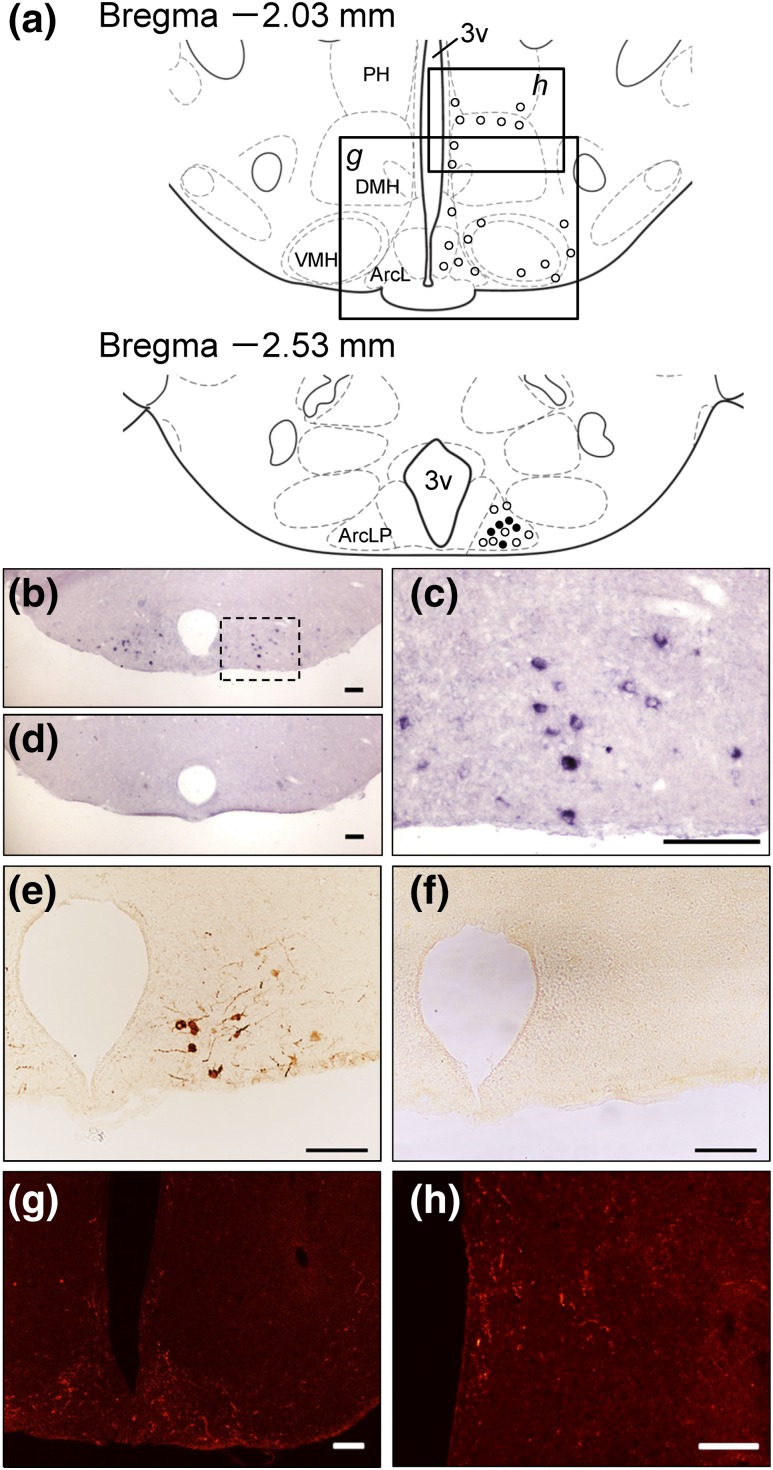
The localization of the NPGL-containing cells in the brain was further analyzed by in situ hybridization and immunohistochemistry. Fig. 3(a) schematically shows the localization of NPGL-containing cell bodies (filled circles) and fibers (open circles) in the MBH. In situ hybridization revealed that cells expressing the NPGL precursor mRNA were specifically distributed in the lateroposterior part of the Arc (ArcLP) within the MBH [Fig. 3(b) and 3(c)]. Positive signals were detected by the antisense probe [Fig. 3(b) and 3(c)], but not by the sense probe [Fig. 3(d)]. Immunohistochemistry also showed that NPGL-immunoreactive cell bodies and fibers were localized in the ArcLP within the MBH [Fig. 3(e)]. Cell and fiber labeling were absent in preadsorption experiments [Fig. 3(f)]. The colchicine treatment enabled the detection of NPGL-immunoreactive cells. NPGL-immunoreactive fibers were distributed in the lateral part of the Arc (ArcL), around the ventromedial hypothalamus (VMH), near the third ventricle [Fig. 3(g) and 3(h)], and between the dorsomedial hypothalamus (DMH) and the posterior hypothalamus [Fig. 3(a), 3(g), and 3(h)].
Figure 3.
(a) NPGL-containing cell bodies and fibers in the hypothalamus are represented by closed and open circles, respectively, in the schematic illustrations. (b–h) Photomicrographs of cells expressing NPGL precursor mRNA were obtained by in situ hybridization in the ArcLP within the MBH (b and c; c is enlarged view of b). (d) No signals were detected using the sense probe. (e–h) Photomicrographs of NPGL-immunoreactive cells and fibers were obtained by immunohistochemistry in the same region as in situ hybridization. (f) No signals were detected when the primary antibody was preadsorbed with the antigenic peptide. NPGL-immunoreactive fibers were also detected in the ArcL, around the VMH, near the third ventricle (3v), and between the dorsal part of the DMH and the posterior hypothalamus (PH) (a, g, and h). Scale bars, 100 µm.
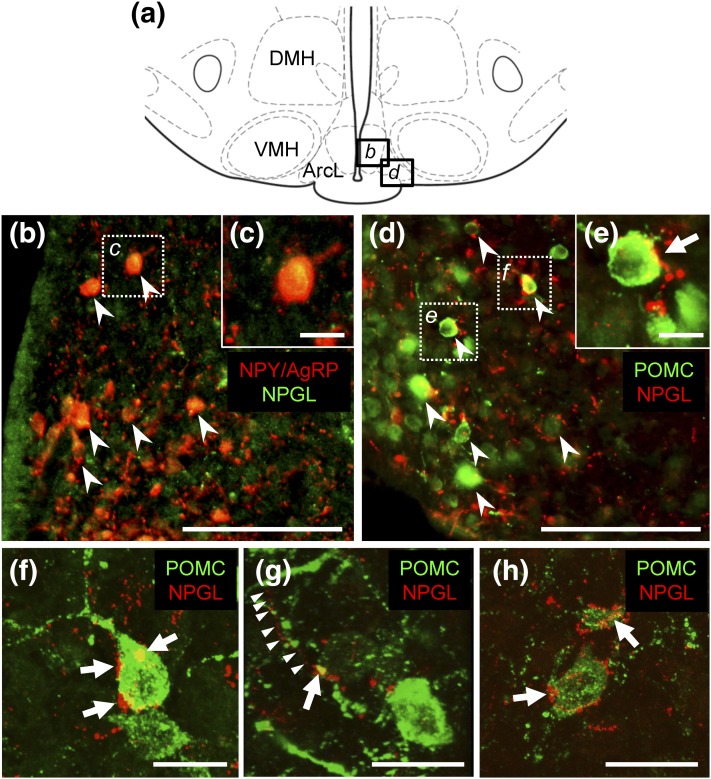
The interactions of NPGL neurons with other well-known neuropeptides regulating feeding, that is, NPY/AgRP or POMC neurons, were investigated in the rostral regions of the Arc. NPGL-immunoreactive fibers were found in close apposition to POMC neurons in the ArcL but not NPY/AgRP neurons (Fig. 4). Using confocal microscopy, NPGL-immunoreactive fibers were found to contact POMC neurons [Fig. 4(f–h)].
Figure 4.
The regions in the photomicrographs (b and d) depicted in the schematic illustration (a) are shown. Distribution of NPGL-immunoreactive fibers in the vicinity of NPY/AgRP neurons (b and c) and POMC neurons (d–h) in the rostral region of the Arc using conventional microscopy (b–e) or confocal microscopy (f–h) is shown. The dotted squares in (b) and (d) are shown magnified in (c), (e), and (f). Arrowheads in (b) and (d) indicate NPY/AgRP neurons (b) and POMC neurons (d). (e–h) Arrows show NPGL-immunoreactive fibers in contact with POMC neurons. Triangles in (g) indicate descending NPGL-immunoreactive fibers. Scale bars, 100 µm in (b) and (d), 10 µm in (c) and (e–h).
Expression of the NPGL precursor mRNA under fasting
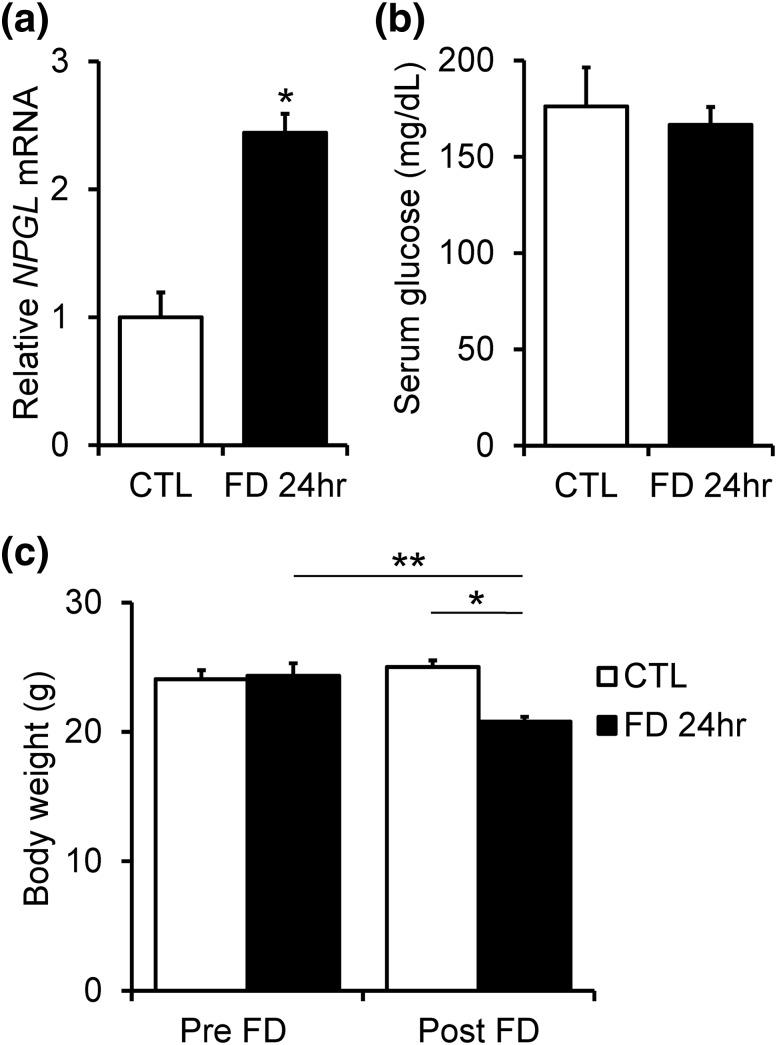
As the location of NPGL cell bodies and projections in the hypothalamus pointed to a potential role for this protein in energy metabolic regulation (13), we examined the response of NPGL to a negative energy balance. The expression of NPGL precursor mRNA was examined after 24 hours of fasting. The expression level of NPGL was significantly higher in fasted mice relative to nonfasted mice [Fig. 5(a)]. Although both fasted and nonfasted mice showed similar serum glucose levels [Fig. 5(b)], body weight significantly decreased in fasted mice [Fig. 5(c)].
Figure 5.
Effects of 24-hour of food deprivation (FD) on the expression of NPGL precursor mRNA (a), serum glucose concentration (b), and body weight (c) are shown. Each value represents the mean ± standard error of the mean (n = 6). *P < 0.05, **P < 0.001. CTL, control experiment.
Expression of the NPGL precursor mRNA under short- or long-term exposure to HFD
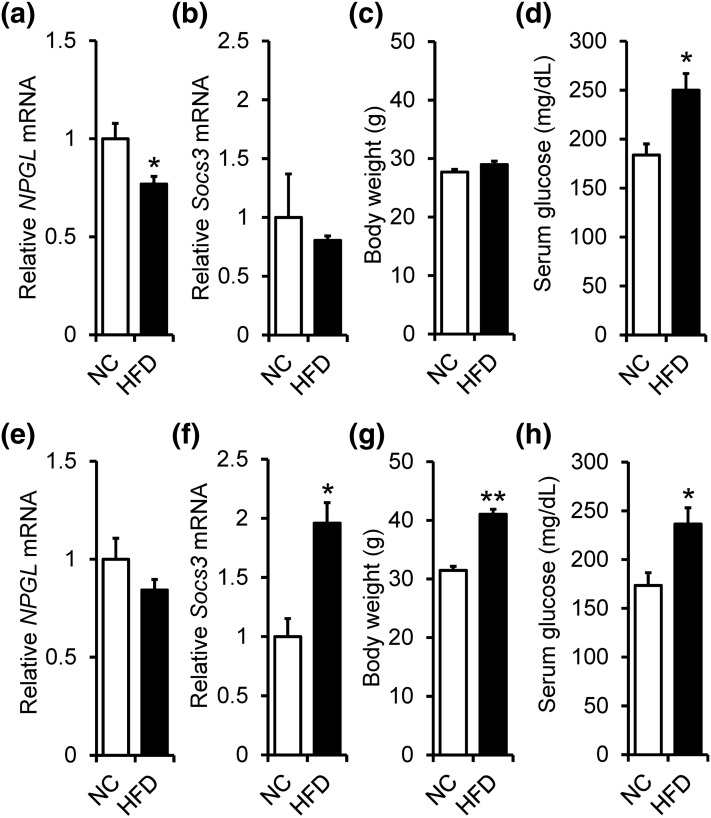
The increased expression of NPGL in fasted mice suggested that this protein may serve to signal a negative energy balance. To further examine this possibility, we explored whether NPGL expression would be decreased during a positive energy state. When mice were fed HFD for 5 weeks beginning at 4 weeks of age, the expression level of the NPGL precursor mRNA was significantly reduced in HFD-fed mice [Fig. 6(a)]. It is known that HFD intake induces an increase in a hypothalamic Socs3 that serves to inhibit leptin signaling, eventually leading to obesity in HFD-fed mice (14). Therefore, we measured the Socs3 mRNA expression level and body weight in normal chow– and HFD-fed mice. Contrary to expectation, significant differences were not detected in both parameters [Fig. 6(b) and 6(c)]. However, serum glucose levels were significantly elevated in HFD-fed mice [Fig. 6(d)].
Figure 6.
Effects of normal chow (NC) or HFD feeding for 5 (a–d) or 13 weeks (e–h) beginning at 4 weeks of age on the expression of NPGL precursor mRNA (a and e), Socs3 mRNA (b and f), body weight (c and g), and serum glucose concentration (d and h). Each value represents the mean ± standard error of the mean (n = 5 to 6). *P < 0.05, **P < 0.001.
When mice were fed HFD for 13 weeks beginning at 4 weeks of age, NPGL precursor mRNA expression levels in HFD-fed mice did not differ from those of control mice [Fig. 6(e)]. Socs3 mRNA expression levels and body weight significantly increased in HFD-fed mice [Fig. 6(f) and 6(g)]. Serum glucose levels were significantly higher in HFD-fed mice than in normal chow–fed mice, as well as mice fed for 5 weeks [Fig. 6(h)].
Effect of NPGL on food intake
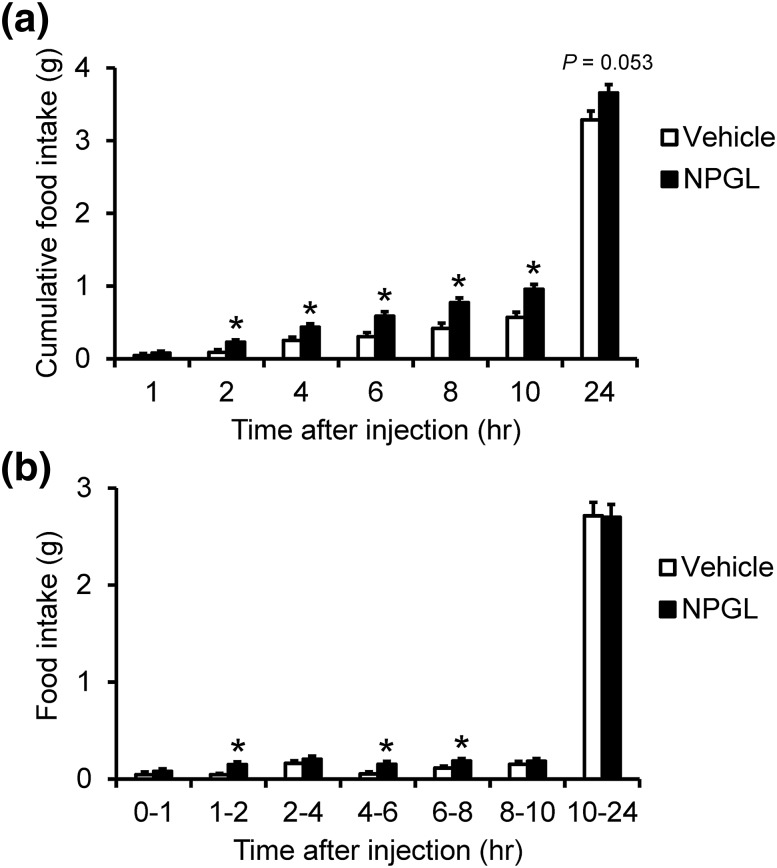
Because NPGL precursor mRNA expression levels were upregulated by fasting and downregulated by short-term HFD feeding, we predicted that NPGL acts as an orexigenic neuropeptide in mice. To examine this possibility, we administered NPGL, mature protein, into the lateral ventricle at the beginning of light period, and measured food intake at 1, 2, 4, 6, 8, 10, and 24 hours after the intracerebroventricular injection. Cumulative food intake significantly increased about 2.5-fold after 2 hours from the injection, and about twofold after 4, 6, 8, and 10 hours [Fig. 7(a)]. A significant difference was not detected after 24 hours from the injection [Fig. 7(a)]. Food intake per unit time significantly increased 3.3-fold from 1 to 2 hours, 2.9-fold from 4 to 6 hours, and 1.6-fold from 6 to 8 hours after injection [Fig. 7(b)]. This result indicates that the orexigenic effect of NPGL deteriorated over time but was sustained for at least 8 hours [Fig. 7(b)].
Figure 7.
Effect of intracerebroventricular injection of vehicle or NPGL (1.0 nmol per animal) on food intake. Data are shown as cumulative food intake after the injection (a) and per unit time (b). Each value represents the mean ± standard error of the mean (n = 8 to 10). *P < 0.05.
Discussion
We have recently reported that the mRNA encoding NPGL, a hypothalamic small secretory protein, is expressed in the chicken hypothalamus, and NPGL may participate in the growth process in chicks (5). We speculated that the NPGL precursor gene is conserved in vertebrates, including mammals, a possibility confirmed via a database search (5). The present study shows the cloning of NPGL cDNA and the identification of NPGL neurons in the mammalian brain. Furthermore, NPGL precursor mRNA expression levels are elevated by fasting and reduced by short-term HFD feeding, and central injections of NPGL increase food intake. Taken together, these results suggest that NPGL is an orexigenic neuropeptide in mice.
Quantitative RT-PCR and morphological analyses revealed that NPGL neurons are located in the ArcLP within the MBH. The rostral region of the Arc in the hypothalamus is one of the centers of energy metabolic regulation and contains two major neuronal populations (i.e., NPY/AgRP neurons and POMC neurons) (2, 15). NPY/AgRP neurons promote food intake and decrease energy expenditure, whereas POMC neurons have the opposite effect (13). mRNA expression levels of NPY and POMC change in response to energetic status to maintain energy homeostasis (13). In the present study, double-label immunofluorescence revealed that NPGL-immunoreactive fibers form close contacts with POMC neurons in the ArcL. This result suggests that NPGL may stimulate feeding behavior through inhibition of anorexigenic POMC neurons. In addition to the ArcL, NPGL neuronal fibers were also observed in the DMH and VMH. Several neuropeptidergic cell phenotypes that regulate food intake are distributed in the DMH and VMH. In particular, a population of NPY cells is found in the DMH in addition to the ArcL (16). NPY cells in this region, as well as in the ArcL, stimulate food intake (17). A few cell bodies expressing melanin-containing hormone and orexin/hypocretin are also found in the DMH, although most of these cells are located in the lateral hypothalamic area (18). Both melanin-containing hormone and orexin/hypocretin neurons promote food intake (19, 20). In the VMH, pituitary adenylate cyclase–activating polypeptide cells are expressed (21), and central injections of pituitary adenylate cyclase–activating polypeptide inhibit food intake (22). Thus, NPGL may regulate food intake by acting via these well-established mechanisms of energetic control.
A negative energy balance induced by fasting elevated the expression of NPGL precursor mRNA, suggesting that NPGL has orexigenic properties in mice. As mentioned previously, fasting increases orexigenic neuropeptides such as NPY (23, 24) and agouti-related protein (AgRP) (24, 25). Further support for an orexigenic role of NPGL comes from the fact that injection of NPGL increased food intake. Because serum glucose levels were not affected by fasting in this study, it is likely that changes in NPGL expression are not a result of changes in glucose. In contrast, HFD feeding for 5 weeks, but not 13 weeks (a positive energy balance), reduced the expression of NPGL precursor mRNA. Extended maintenance on a HFD impairs central leptin sensitivity, increases body weight, and inevitably leads to leptin resistance and obesity (26, 27), in part through increased hypothalamic Socs3 expression (14, 28). In the present study, short-term (5 weeks) HFD significantly decreased NPGL precursor mRNA without affecting body weight and Socs3 mRNA expression. This result suggests that leptin continues to play an antiobesity role in short-term HFD-fed mice to prevent diet-induced obesity. These findings suggest that NPGL neurons are responsive to leptin at this point. In contrast, in long-term (13 weeks) HFD-fed mice, NPGL precursor mRNA expression was not different from control levels, although Socs3 mRNA and body weight were increased. This result suggests that leptin resistance occurs in this stage. Taken together, these results point to the possibility that NPGL mRNA expression is reduced by leptin in short-term HFD-fed mice, and this reduction is not observed in long-term HFD-fed mice due to leptin resistance. In both short- and long-term HFD-fed mice, serum glucose levels were increased. This result suggests that serum glucose levels do not influence the expression of NPGL. Whether NPGL cells are directly responsive to leptin represents an important avenue for further inquiry.
In summary, the present findings report on the cDNA encoding NPGL in the mouse hypothalamus, representing, to the authors’ knowledge, the firstcharacterization of this neurochemical in the mammalian brain. NPGL neurons are localized to the ArcLP with fibers projecting to several areas of the hypothalamus mediating food intake and energy homeostasis, and especially POMC neurons. The mRNA expression level of the NPGL precursor is markedly, and predictably, impacted by energetic status, and central injections of NPGL increase food intake. Taken together, these findings point to NPGL as an orexigenic neuropeptide in mice and an important target for further study to fully understand the central mechanisms regulating energy homeostasis.
Acknowledgments
We are grateful to Dr. Akiko K. Satoh (Hiroshima University) for her invaluable assistance with confocal microscopy.
This work was supported by Ministry of Education, Culture, Sports, Science and Technology of Japan/Japan Society for the Promotion of Science KAKENHI Grants 22687004, 23126517, 25126717, 26291066, and 15KK0259 (to K.U.) and Grants 25440171 and 16K07440 (to E.I.-U.); Japan Society for the Promotion of Science Fellows Grant-in-Aid 15J03781 (to K.S.); the Program for Promotion of Basic and Applied Research for Innovations in Bio-oriented Industry (to K.U.); the Toray Science Foundation (to K.U.); the Mishima Kaiun Memorial Foundation (to K.U. and E.I.-U.); the Suzuken Memorial Foundation (to K.U.); the Skylark Food Science Institute (to K.U.); the Urakami Foundation for Food and Food Culture Promotion (to K.U.); and the Kao Research Council for the Study of Healthcare Science (to K.U.).
The sequence reported in this paper has been deposited in the GenBank database (accession no. LC088498) for the cDNA sequence of mouse NPGL precursor polypeptide.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- ACTB
β-actin
- AgRP
agouti-related protein
- Arc
arcuate nucleus
- ArcL
lateral part of the arcuate nucleus
- ArcLP
lateroposterior part of the arcuate nucleus
- cDNA
complementary DNA
- DMH
dorsomedial hypothalamus
- HFD
high-fat diet
- IgG
immunoglobulin G
- MBH
mediobasal hypothalamus
- mRNA
messenger RNA
- NPGL
neurosecretory protein GL
- NPY
neuropeptide Y
- PCR
polymerase chain reaction
- POMC
pro-opiomelanocortin
- RT-PCR
reverse transcription polymerase chain reaction
- Socs3
suppressor of cytokine signaling 3
- VMH
ventromedial hypothalamus.
Appendix. Antibodies Used
| Peptide/ Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| NPGL | HSQTDLLTLNQADPQCWESSSMLLLEMRKPRVSNTVSGFWDFMIYLKSSENLKHGALFWDLAQLFWDIYVDCVLSRNHGL-NH2 | Anti-NPGL | Our laboratory | Guinea pig; polyclonal | 1:500 | AB_2636992 |
| NPGL | HSQTDLLTLNQADPQCWESSSMLLLEMRKPRVSNTVSGFWDFMIYLKSSENLKHGALFWDLAQLFWDIYVDCVLSRNHGL-NH2 | Anti-NPGL | Our laboratory | Rabbit; polyclonal | 1:250 | AB_2636993 |
| Human β-endorphin | Anti–β-endorphin | Peninsula Laboratories, T-5009 | Guinea pig; polyclonal | 1:2000 | AB_518107 | |
| Agouti-related protein | Anti-AgRP | Phoenix Pharmaceuticals, H-003-57 | Rabbit; polyclonal | 1:10,000 | AB_2313909 | |
| Guinea pig IgG | Biotinylated goat anti–guinea pig IgG | Vector Laboratories, BA-7000 | Goat; polyclonal | 1:1000 | AB_2336132 | |
| Rabbit IgG | Cy3-conjugated donkey anti-rabbit IgG | Jackson ImmunoResearch Laboratories, 711-165-152 | Donkey; polyclonal | 1:400 | AB_2307443 | |
| Guinea pig IgG | Alexa Fluor 488–conjugated donkey anti–guinea pig IgG | Jackson ImmunoResearch Laboratoties, 706-545-148 | Donkey; polyclonal | 1:600 | AB_2340472 |
Abbreviation: RRID, Research Resource Identifier.
References
- 1. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. [DOI] [PubMed] [Google Scholar]
- 2. Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23(4):775–786. [DOI] [PubMed] [Google Scholar]
- 3. Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M, Kangawa K, Nakao K. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50(2):227–232. [DOI] [PubMed] [Google Scholar]
- 4. Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–181. [DOI] [PubMed] [Google Scholar]
- 5. Ukena K, Iwakoshi-Ukena E, Taniuchi S, Bessho Y, Maejima S, Masuda K, Shikano K, Kondo K, Furumitsu M, Tachibana T. Identification of a cDNA encoding a novel small secretory protein, neurosecretory protein GL, in the chicken hypothalamic infundibulum. Biochem Biophys Res Commun. 2014;446(1):298–303. [DOI] [PubMed] [Google Scholar]
- 6. Ukena K, Iwakoshi-Ukena E, Hikosaka A. Unique form and osmoregulatory function of a neurohypophysial hormone in a urochordate. Endocrinology. 2008;149(10):5254–5261. [DOI] [PubMed] [Google Scholar]
- 7. Ukena K, Tachibana T, Iwakoshi-Ukena E, Saito Y, Minakata H, Kawaguchi R, Osugi T, Tobari Y, Leprince J, Vaudry H, Tsutsui K. Identification, localization, and function of a novel avian hypothalamic neuropeptide, 26RFa, and its cognate receptor, G protein-coupled receptor-103. Endocrinology. 2010;151(5):2255–2264. [DOI] [PubMed] [Google Scholar]
- 8. Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA. 2006;103(7):2410–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ubuka T, Inoue K, Fukuda Y, Mizuno T, Ukena K, Kriegsfeld LJ, Tsutsui K. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology. 2012;153(1):373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 11. Masuda K, Ooyama H, Shikano K, Kondo K, Furumitsu M, Iwakoshi-Ukena E, Ukena K. Microwave-assisted solid-phase peptide synthesis of neurosecretory protein GL composed of 80 amino acid residues. J Pept Sci. 2015;21(6):454–460. [DOI] [PubMed] [Google Scholar]
- 12. Ukena K, Usui M, Kohchi C, Tsutsui K. Cytochrome P450 side-chain cleavage enzyme in the cerebellar Purkinje neuron and its neonatal change in rats. Endocrinology. 1998;139(1):137–147. [DOI] [PubMed] [Google Scholar]
- 13. Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet. 2002;3(8):589–600. [DOI] [PubMed] [Google Scholar]
- 14. Gamber KM, Huo L, Ha S, Hairston JE, Greeley S, Bjørbæk C. Over-expression of leptin receptors in hypothalamic POMC neurons increases susceptibility to diet-induced obesity. PLoS One. 2012;7(1):e30485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waterson MJ, Horvath TL. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab. 2015;22(6):962–970. [DOI] [PubMed] [Google Scholar]
- 16. Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1030–R1036. [DOI] [PubMed] [Google Scholar]
- 17. Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J Neurosci. 2009;29(1):179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leinninger GM. Lateral thinking about leptin: a review of leptin action via the lateral hypothalamus. Physiol Behav. 2011;104(4):572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380(6571):243–247. [DOI] [PubMed] [Google Scholar]
- 20. Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. [DOI] [PubMed] [Google Scholar]
- 21. Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci. 2009;29(47):14828–14835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mounien L, Do Rego JC, Bizet P, Boutelet I, Gourcerol G, Fournier A, Brabet P, Costentin J, Vaudry H, Jégou S. Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology. 2009;34(2):424–435. [DOI] [PubMed] [Google Scholar]
- 23. Bergen HT, Mobbs CV. Ventromedial hypothalamic lesions produced by gold thioglucose do not impair induction of NPY mRNA in the arcuate nucleus by fasting. Brain Res. 1996;707(2):266–271. [DOI] [PubMed] [Google Scholar]
- 24. Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology. 1999;140(10):4551–4557. [DOI] [PubMed] [Google Scholar]
- 25. Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1(4):271–272. [DOI] [PubMed] [Google Scholar]
- 26. Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145(11):4880–4889. [DOI] [PubMed] [Google Scholar]
- 27. El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105(12):1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thaler JP, Schwartz MW. Minireview: inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology. 2010;151(9):4109–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]