Abstract
Context:
Arginine, its methylated metabolites, and other metabolites related to the urea cycle have been independently associated with cardiovascular risk, but the potential causal meaning of these associations (positive for some metabolites and negative for others) remains elusive due to a lack of studies measuring metabolite changes over time.
Objective:
To examine the association between baseline and 1-year concentrations of urea cycle metabolites and cardiovascular disease (CVD) in a case-cohort setting.
Design:
A case-cohort study was nested within the Prevención con Dieta Mediterránea trial. We used liquid chromatography–tandem mass spectrometry to assess metabolite levels at baseline and after 1-year follow-up. The primary CVD outcome was a composite of myocardial infarction, stroke and cardiovascular death. We used weighted Cox regression models (Barlow weights) to estimate multivariable-adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs).
Setting:
Multicenter randomized trial in Spain.
Participants:
Participants were 984 participants accruing 231 events over 4.7 years’ median follow-up.
Main Outcome Measure:
Incident CVD.
Results:
Baseline arginine/asymmetric dimethylarginine ratio [HR per standard deviation (SD) = 0.80; 95% CI, 0.67 to 0.96] and global arginine availability [arginine / (ornithine + citrulline)] (HR per SD = 0.83; 95% CI, 0.69 to 1.00) were significantly associated with lower risk of CVD. We observed no significant association for 1-year changes in these ratios or any effect modification by the Mediterranean diet (MD) intervention.
Conclusions:
A higher baseline arginine/asymmetric dimethylarginine ratio was associated with lower CVD incidence in a high cardiovascular risk population. The intervention with the MD did not change 1-year levels of these metabolites.
We examined the associations between urea cycle metabolites and cardiovascular disease (CVD). We found that the baseline arginine/asymmetric dimethylarginine ratio was associated with lower risk of CVD.
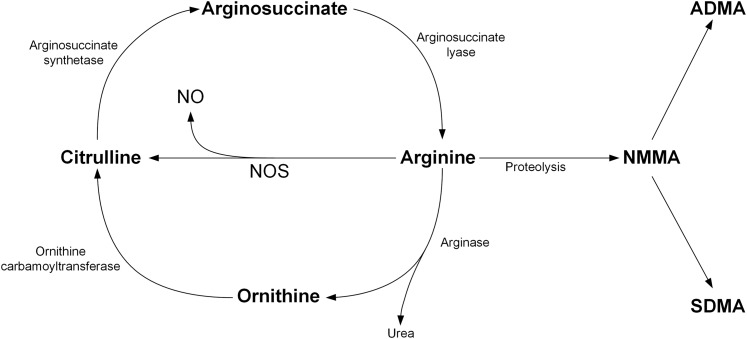
Cardiovascular disease (CVD) continues to be a major cause of death in most countries (1). Development of CVD is associated with changes in many metabolites (2), but which ones predict ischemic events have not been completely characterized. Nitric oxide (NO) is a well-studied molecule known to confer a protective effect on the vasculature system by dilating blood vessels, inhibiting leukocyte adhesion, reducing platelet aggregation, and preventing plaque formation (3). NO is synthesized via nitric oxide synthase (NOS), which converts 2 O2 + arginine + nicotinamide adenine dinucleotide phosphate into citrulline + nicotinamide adenine dinucleotide + 2 NO (4). This pathway draws arginine from the urea cycle, the details of which have been reviewed elsewhere (5). Briefly, after arginine is converted to NO and citrulline, arginosuccinate synthetase converts citrulline into argininosuccinate, which is then converted into arginine by arginosuccinate lysase (Fig. 1). The global bioavailability of arginine has been reported to be associated with lower risk of coronary artery disease and major cardiovascular events (6). Alternatively, arginine may undergo proteolysis to become asymmetric dimethylarginine (ADMA), which acts as an endogenous competitive inhibitor of NOS and causes local vasoconstriction when infused (7). ADMA has been proposed as an independent biomarker of endothelial dysfunction and a potential biomarker for higher risk of future CVD events (8). Consequentially, the arginine/ADMA ratio provides information on arginine bioavailability for production of NO (9) and has been identified as a marker for the severity of chronic heart failure (10), diastolic blood pressure (11), and recently as a predictor for atherosclerosis (12).
Figure 1.
Diagram of the urea cycle, NOS, and degradation of arginine.
A Mediterranean dietary (MD) pattern rich in unsaturated vegetable fats and whole grains and low in refined sugars and saturated fats has been shown to improve markers of vascular function (13). In the Prevención con Dieta Mediterránea (PREDIMED) trial, being allocated to a MD supplemented with extra-virgin olive oil (EVOO) or with nuts significantly decreased the risk of CVD compared with a control group (14). Furthermore, from the same trial, those consuming the MD experienced reduced 24-hour ambulatory blood pressure (15). In another smaller randomized trial, a significant increase in the arginine/ADMA ratio was observed in participants assigned to a MD compared with those in a low-fat control diet after 3 months of follow-up (16). Thus, it can be hypothesized that the beneficial effects of the MD may partially be mediated by changes in the arginine/ADMA ratio.
In the current study, we evaluated (1) the effect of the PREDIMED intervention with a MD on 1-year changes in these metabolites in a random sample of the entire PREDIMED cohort and (2) the association of both baseline levels and changes in these metabolites on incident clinical events of CVD following a case-cohort design.
Methods
Study population and design
The PREDIMED trial is a parallel-group, multicenter, randomized trial of dietary interventions of MD supplemented with either nuts or EVOO for the primary prevention of CVD, compared with a low-fat control group (www.predimed.es). The protocol, design, and primary results are detailed elsewhere (14, 17). Briefly, 7447 eligible men and women (55 to 80 years old) at high risk of CVD were randomly assigned to a MD supplemented with 1 L/wk of EVOO (MD + EVOO), a MD supplemented with 30 g/d mixed nuts (15 g walnuts, 7.5 g hazelnuts, and 7.5 g almonds; MD + nuts), or a control diet consisting of advice to reduce the intake of all types of fat (control group) from October 2003 to December 2010, a median of 4.8 years for all participants. Medical conditions and lifestyle risk factors were collected using questionnaires at the first screening visit and yearly during follow-up. Weight, height, waist circumference, and blood pressure were directly measured following a standardized protocol (17) by PREDIMED-trained personnel. The primary endpoint was a composite of CVD events, defined as nonfatal stroke, nonfatal myocardial infarction, or death from cardiovascular causes. Stroke events included all nonfatal stroke and cases of stroke-related mortality. Nonstroke events included all nonfatal myocardial infarction and all death from vascular causes not related to stroke. Information on primary endpoints was collected by study physicians, who were blinded to the intervention, and from other sources of information, such as the National Death Index. All participants provided written informed consent.
For the current study, to maintain the integrity of trial randomization, we used a case-cohort design consisting of a random sample of approximately 10% of all PREDIMED participants and all of the incident CVD cases occurring during follow-up through 1 December 2010 who had available plasma samples at baseline. A random selection of 790 participants was included at baseline (to constitute the subcohort), of which 37 individuals experienced major cardiovascular events. Incident CVD cases included 231 individuals who experienced an event during follow-up (total sample size: 790 + 231 – 37 = 984). Among the 231 cases, 118 experienced a stroke (113 ischemic and 5 hemorrhagic). In addition, 926 participants out of the 984 (i.e., 749 controls and 177 cases) also had a 1-year follow-up sample and were included in the 1-year change analyses. Data from the PREDIMED trial and previous clinical trials suggest that changes in plasma biomarkers at 1 year might be sufficient to predict later CVD (14, 18–21).
Quantification of metabolites
Fasting plasma EDTA tubes were collected for all participants, and aliquots were coded and kept refrigerated until they were stored at −80°C. Pairs of samples (baseline and first-year visits from each participant) were randomly ordered and shipped to the Broad Institute of Harvard and the Massachusetts Institute of Technology (Cambridge, MA) for metabolomics analyses. Liquid chromatography–tandem mass spectrometry on a system comprised of a Shimadzu Nexera ×2 U-HPLC (Shimadzu Corp., Kyoto, Japan), coupled to a Q Exactive hybrid quadrupole orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA), was used to quantitatively profile metabolites such arginine, ornithine, citrulline, ADMA, symmetric dimethylarginine (SDMA), and NG-monomethylarginine (NMMA) in fasting plasma collected at baseline and year 1 of the intervention. Metabolite extracts were precipitated from plasma samples (10 μL) with nine volumes of 74.9:24.9:0.2 (volume:volume:volume) of acetonitrile:methanol:formic acid–containing stable isotope-labeled internal standards [valine-d8 (Sigma-Aldrich, St. Louis, MO) and phenylalanine-d8 (Cambridge Isotope Laboratories, Tewksbury, MA)]. The samples were then centrifuged for 10 minutes at 9000 × g in 4°C, and the supernatant was injected onto a 150 × 2-mm, 3-μm Atlantis HILIC column (Waters Corp., Milford, MA). The column was eluted at a flow rate of 250 μL/min with 5% mobile phase A (10 mmol ammonium formate/L and 0.1% formic acid in H2O) for 0.5 minutes followed by a linear gradient to 40% mobile phase B (acetonitrile with 0.1% formic acid) for 10 minutes. Quantification of metabolites was carried out with the use of electrospray ionization in positive-ion mode with use of a full-scan analysis over 70–800 m/z at 70,000 resolution and a 3-Hz data-acquisition rate. Reference standards were used to confirm metabolite identities. Raw data were processed with the use of TraceFinder 3.1 (Thermo Fisher Scientific) and Progenesis CoMet v2.0 (Nonlinear Dynamics, Durham, NC).
Statistical analysis
Rank-based inverse normal transformations were used to transform the nonnormal distributions of arginine and ADMA (22). We used the arginine/ADMA ratio (by dividing the raw values and then taking inverse normal transformations), as previously described in the literature (9, 10, 12, 23), as well as a global arginine availability score (by dividing the raw values of arginine by the sum of ornithine and citrulline) as described by Tang et al. (24) We also assessed associations of CVD with alternate ratios such as arginine/citrulline and other ratios built using SDMA and NMMA including ADMA/SDMA, and ADMA/NMMA.
Baseline data by case status are presented as means [± standard deviations (SDs)] for continuous variables and N and percentages for categorical variables. Baseline characteristics were compared between cases and noncases using χ2 tests for categorical variables and one-way analysis of variance for continuous variables.
We used weighted Cox regression models with controls upweighted by their sampling fraction and used robust variance to account for correlation between observations (25). We calculated multivariable-adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) for the composite CVD endpoint, and also separately for stroke. Follow-up time was calculated from the date of enrollment to the date of diagnosis of CVD for cases and to the date of the last visit (provided that this visit took place before 1 December 2010) or death for noncases. In model 1, we adjusted for age, sex, family history of coronary heart disease (CHD), smoking status, and body mass index and stratified by the intervention group. In model 2, we additionally adjusted for baseline hypertension, dyslipidemia, and diabetes. All individual metabolites, the arginine/ADMA ratio, and the arginine availability score were also analyzed according to quartiles. Quartile cutpoints were generated on the basis of the distributions of these metabolites among controls in the subcohort. We conducted tests of linear trend by examining an ordinal score on the basis of the median value in each quartile in the multivariable models.
We conducted joint analyses and interaction tests for the arginine/ADMA ratio and the intervention groups (MD + EVOO and MD + nuts vs control group). We additionally conducted analyses stratified by sex, age (<65 vs ≥65 years old), intervention group, obesity (body mass index <30 vs ≥30 kg/m2), smoking status (current/former vs never), family history of CHD, baseline type 2 diabetes, baseline hypertension, and baseline dyslipidemia status. We used a likelihood ratio test for models without interaction terms vs models with interaction terms to assess the significance of interaction between stratifying variables and the arginine/ADMA ratio.
To assess changes in metabolites from baseline to 1 year according to intervention group, we calculated adjusted means stratified by age (<65, ≥65 years old), sex (male, female), and body mass index (<30.0 kg/m2, ≥30.0 kg/m2) in the randomly selected subcohort. If no significant changes were observed among intervention groups, we performed our primary analyses in the entire study population (i.e., combining all participants in the three intervention arms). We examined the associations between 1-year changes in the arginine/ADMA ratio and CVD risk by calculating the difference between baseline and 1-year ratio concentrations, then normalized this difference with an inverse normal transformation.
All statistical analyses were performed with SAS (v9.4, SAS Institute, Cary, NC) and R (v2.13.0, R Foundation, Vienna, Austria).
Results
The flow diagram of the current study is depicted in Supplemental Fig. 1. Among the 7447 participants recruited in the PREDIMED trial, 288 experienced a CVD before 1 December 2010. Of the 288 cases, 231 had available plasma samples at baseline and 177 had available plasma samples both at baseline and after 1-year of follow-up. The current study population consisted of 790 participants randomly selected to the subcohort at baseline and the 231 cases from the entire trial (37 of whom were in the subcohort), for a total of 984 participants at baseline with a median follow-up time of 4.7 years.
Descriptive statistics of the study population at baseline by subcohort and case status are presented in Table 1. Cases tended to be older, male, not dyslipidemic, diabetic, and more likely to smoke compared with the randomly chosen subcohort.
Table 1.
Baseline Characteristics in the Random Subcohort and Cases
| Subcohort | Cases | P Value a | |
|---|---|---|---|
| n | 790 | 231 | |
| Age, y | 67.2 ± 5.9 | 69.5 ± 6.5 | <0.001 |
| Sex, % women | 57.0 | 39.4 | <0.001 |
| BMI, kg/m2 | 29.7 ± 3.6 | 29.6 ± 3.7 | 0.70 |
| Obesity (BMI ≥30), % | 45.5 | 45.0 | 0.94 |
| Intervention group, % | 0.14 | ||
| MD + EVOO | 37.3 | 35.5 | |
| MD + nuts | 33.1 | 28.1 | |
| Control | 29.6 | 36.4 | |
| Family history of CHD, % | 25.0 | 19.1 | 0.06 |
| Hypertension, % | 83.6 | 82.7 | 0.73 |
| Dyslipidemia, % | 73.6 | 58.4 | <0.001 |
| Diabetes, % | 47.0 | 64.5 | <0.001 |
| Smoking, % | <0.001 | ||
| Never | 62.2 | 45.0 | |
| Former | 25.4 | 35.1 | |
| Current | 12.4 | 19.9 |
Data are expressed as means ± SD or percentage.
Both the subcohort (n = 790) and cases (n = 231) column include the 37 overlapping cases. Because there were individuals in both the subcohort group and cases group, we compared the observed means in the cases to the expected means calculated among the entire subcohort.
Abbreviations: BMI, body mass index; Q, quartile.
P values for trend were calculated using regression models with the median value of each quartile category as the independent variable for continuous variables, and χ2 test for categorical variables.
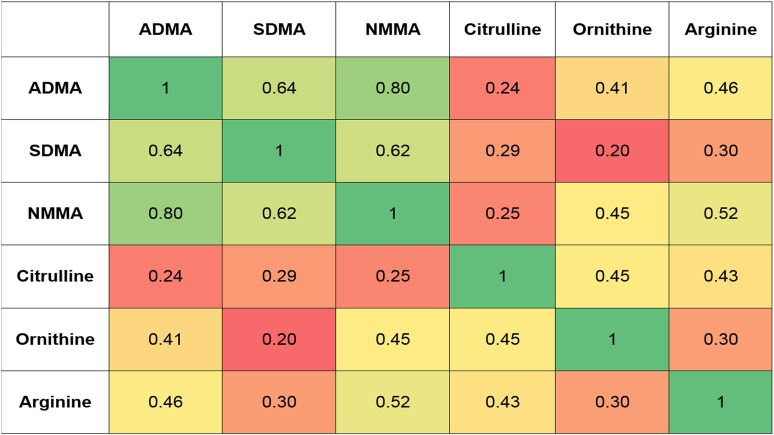
Figure 2 depicts a heat map of correlation coefficients of the metabolites included in the current study. Metabolites of the urea cycle (arginine, citrulline, and ornithine) were correlated with each other, with r = 0.30 to 0.50 for each pair of metabolites (P < 0.0001 for all correlations) Arginine metabolites were poorly correlated with citrulline, but moderately correlated with ornithine and arginine. ADMA, SDMA, and NMMA were strongly correlated (P < 0.0001 for all correlations) with each other, with r = 0.60 to 0.80 for each pair of metabolites.
Figure 2.
Correlation coefficient heat map of metabolites under study. All correlation coefficients had P values < 0.0001.
Association of individual metabolites and CVD risk
Table 2 presents the associations of both baseline and 1-year changes in urea cycle metabolites (arginine, ornithine, and citrulline) with incident composite CVD. A 1-SD increase in baseline arginine was associated with a decreased risk of CVD in model 1 (HR = 0.84; 95% CI, 0.71 to 0.99), but the association was attenuated after adjusting for baseline hypertension, dyslipidemia, and diabetes (HR = 0.86; 95% CI, 0.72 to 1.02). Compared with the bottom quartile, higher quartiles of baseline arginine appeared to be associated with lower CVD risk, but the test for linear trend was not significant in the fully adjusted model (P for trend = 0.17). Neither baseline citrulline nor baseline ornithine levels were significantly associated with CVD risk in either continuous or quartile analyses.
Table 2.
Relative risk of Incident Composite CVD by Baseline and 1-Year Changes in Urea Cycle Metabolites: HRs (95% CI) for Traits as Continuous Variables and by Quartile Levels
|
Baseline (n = 984, cases = 231)
|
1-Year Changes (n = 926, cases = 177)
a
|
|||||
|---|---|---|---|---|---|---|
| Arginine | Ornithine | Citrulline | Arginine | Ornithine | Citrulline | |
| Model 1 | ||||||
| Trait as continuous variable, per SD | ||||||
| HR (95% CI) | 0.84 (0.71–0.99) | 1.07 (0.91–1.26) | 1.00 (0.83–1.20) | 0.85 (0.72–1.01) | 0.92 (0.77–1.10) | 0.85 (0.72–1.02) |
| P | 0.04 | 0.42 | 1.00 | 0.07 | 0.37 | 0.08 |
| Trait in quartile categories, as compared with Q1 (reference) | ||||||
| Q2 | 0.58 (0.37–0.89) | 1.06 (0.66–1.71) | 0.55 (0.34–0.89) | 1.09 (0.68–1.73) | 1.04 (0.64–1.69) | 0.93 (0.58–1.48) |
| Q3 | 0.58 (0.37–0.91) | 1.10 (0.69–1.77) | 0.91 (0.58–1.40) | 0.68 (0.41–1.13) | 0.93 (0.55–1.57) | 0.68 (0.41–1.13) |
| Q4 | 0.68 (0.45–1.04) | 1.22 (0.79–1.90) | 0.90 (0.59–1.39) | 0.69 (0.41–1.16) | 0.85 (0.51–1.42) | 0.60 (0.36–0.99) |
| P trend | 0.10 | 0.37 | 0.78 | 0.06 | 0.46 | 0.02 |
| Model 2 | ||||||
| Trait as continuous variable, per SD | ||||||
| HR (95% CI) | 0.86 (0.72–1.02) | 1.06 (0.89–1.25) | 1.04 (0.86–1.25) | 0.87 (0.73–1.04) | 0.93 (0.77–1.11) | 0.87 (0.73–1.04) |
| P | 0.09 | 0.53 | 0.71 | 0.12 | 0.40 | 0.12 |
| Trait in quartile categories, as compared with Q1 (reference) | ||||||
| Q2 | 0.59 (0.38–0.92) | 1.16 (0.72–1.89) | 0.64 (0.39–1.03) | 1.20 (0.74–1.95) | 1.19 (0.72–1.98) | 0.99 (0.61–1.61) |
| Q3 | 0.63 (0.39–0.99) | 1.15 (0.71–1.86) | 0.98 (0.63–1.53) | 0.70 (0.42–1.18) | 0.92 (0.54–1.57) | 0.75 (0.44–1.26) |
| Q4 | 0.70 (0.45–1.09) | 1.20 (0.76–1.90) | 1.01 (0.64–1.58) | 0.73 (0.42–1.25) | 0.88 (0.52–1.48) | 0.60 (0.35–1.01) |
| P trend | 0.17 | 0.48 | 0.54 | 0.09 | 0.44 | 0.03 |
Model 1 was adjusted for age, sex, family history of CHD, smoking status, and body mass index and was stratified by intervention group. Model 2 was adjusted as for model 1, plus baseline hypertension, dyslipidemia, and diabetes.
Abbreviation: Q, quartile.
Fifty-eight individuals who provided blood samples at baseline but not at 1 year were not included.
When examining 1-year changes in the levels of these metabolites, increasing quartiles of citrulline were significantly associated with lower risk of CVD in the fully adjusted model (P for trend = 0.03). One-year changes of arginine as a continuous variable exhibited a similar trend for the association with CVD (HR = 0.87; 95% CI, 0.73 to 1.04).
Table 3 presents the associations of baseline and 1-year changes in arginine metabolites (ADMA, SDMA, NMMA) with CVD. None of them was significantly associated with incident CVD.
Table 3.
Relative Risk of Incident Composite CVD by Baseline and 1-Year Changes in Arginine Metabolites: HRs (95% CI) for Traits as Continuous Variables and by Quartile Levels
|
Baseline (n = 984, cases = 231)
|
1-Year Changes (n = 926, cases = 177)
a
|
|||||
|---|---|---|---|---|---|---|
| ADMA | SDMA | NMMA | ADMA | SDMA | NMMA | |
| Model 1 | ||||||
| Trait as continuous variable, per SD | ||||||
| HR (95% CI) | 1.03 (0.88–1.21) | 0.97 (0.81–1.15) | 0.93 (0.79–1.10) | 0.81 (0.65–1.02) | 0.86 (0.70–1.04) | 0.91 (0.74–1.1) |
| P | 0.74 | 0.72 | 0.40 | 0.07 | 0.13 | 0.35 |
| Trait in quartile categories, as compared with Q1 (reference) | ||||||
| Q2 | 0.92 (0.58–1.46) | 0.82 (0.52–1.30) | 0.90 (0.58–1.39) | 1.34 (0.80–2.27) | 0.76 (0.46–1.26) | 0.73 (0.42–1.25) |
| Q3 | 1.13 (0.72–1.77) | 0.86 (0.54–1.37) | 0.77 (0.49–1.22) | 1.24 (0.72–2.14) | 0.81 (0.48–1.36) | 0.86 (0.50–1.49) |
| Q4 | 0.93 (0.59–1.48) | 0.93 (0.60–1.44) | 0.86 (0.56–1.33) | 0.63 (0.33–1.20) | 0.68 (0.40–1.16) | 0.91 (0.52–1.58) |
| P trend | 0.99 | 0.81 | 0.40 | 0.15 | 0.21 | 0.89 |
| Model 2 | ||||||
| Trait as continuous variable, per SD | ||||||
| HR (95% CI) | 1.04 (0.88–1.22) | 1.03 (0.86–1.24) | 0.94 (0.79–1.11) | 0.83 (0.66–1.04) | 0.89 (0.72–1.08) | 0.92 (0.74–1.14) |
| P | 0.67 | 0.74 | 0.44 | 0.11 | 0.24 | 0.44 |
| Trait in quartile categories, as compared with Q1 (reference) | ||||||
| Q2 | 0.98 (0.61–1.56) | 0.90 (0.56–1.43) | 0.89 (0.57–1.40) | 1.30 (0.76–2.24) | 0.81 (0.48–1.37) | 0.79 (0.45–1.38) |
| Q3 | 1.22 (0.77–1.94) | 0.97 (0.61–1.55) | 0.81 (0.50–1.29) | 1.26 (0.71–2.24) | 0.87 (0.51–1.50) | 0.89 (0.51–1.56) |
| Q4 | 0.99 (0.61–1.61) | 1.14 (0.72–1.78) | 0.90 (0.57–1.41) | 0.64 (0.33–1.25) | 0.73 (0.42–1.26) | 0.96 (0.54–1.70) |
| P trend | 0.79 | 0.53 | 0.56 | 0.20 | 0.33 | 0.99 |
Model 1 was adjusted for age, sex, family history of CHD, smoking status, and body mass index, and was stratified by intervention group. Model 2 was adjusted as for model 1, plus baseline hypertension, dyslipidemia, and diabetes.
Abbreviation: Q, quartile.
Fifty-eight individuals who provided blood samples at baseline but not at 1 year were not included.
Supplemental Figs. 2 and 3 depict adjusted mean metabolite changes according to intervention group after 1 year of follow-up. No significant differences were noted for changes in levels of any metabolite between intervention groups (P = 0.32 for arginine, P = 0.09 for ornithine, P = 0.18 for citrulline, P = 0.77 for ADMA, P = 0.86 for SDMA, P = 0.60 for NMMA). Therefore, the intervention had no significant effect on changes in these metabolites or their ratios.
Association of the arginine/ADMA ratio with CVD
Associations of the baseline arginine/ADMA ratio and arginine availability score with CVD risk are presented in Table 4. A 1-SD increase in baseline arginine/ADMA ratio was associated with 20% lower risk of CVD (HR = 0.80; 95% CI, 0.67 to 0.96) in the fully adjusted model. Quartile analysis revealed independent inverse associations of quartiles 2 (HR = 0.55; 95% CI, 0.35 to 0.86) and 3 (HR = 0.56; 95% CI, 0.36 to 0.88) vs quartile 1, with risk of CVD, but the test of linear trend was not significant. Similarly, the global arginine availability score was associated with lower risk of CVD. However, this association was close to the limit of statistical significance (P = 0.048). A 1-SD increase in the score was associated with a HR = 0.83 (95% CI, 0.69 to 0.999). None of the 1-year changes in these traits was associated with incident CVD risk. These results did not appreciably differ when examining stroke or nonstroke events as separate endpoints (data not shown). Subgroup analyses (Supplemental Table 1) indicated no statistically significant interaction by variables of interest in the relationship between arginine/ADMA ratio and CVD risk.
Table 4.
Relative Risk of Incident Composite CVD by Baseline and 1-Year Changes in Arginine/ADMA Ratio and Arginine Availability: HRs (95% CI) for Traits as Continuous Variables and by Quartile Levels
|
Baseline (n = 984, cases = 231)
|
1-Year Change (n = 926, cases = 177)
a
|
|||
|---|---|---|---|---|
| Arginine/ADMA Ratio | Global Arginine Availability Score b | Arginine/ADMA Ratio | Global Arginine Availability Score | |
| Model 1 | ||||
| Trait as continuous variable, per SD | ||||
| HR (95% CI) | 0.79 (0.66–0.94) | 0.82 (0.66–0.98) | 1.04 (0.86–1.26) | 0.97 (0.81–1.15) |
| P | 0.01 | 0.03 | 0.70 | 0.70 |
| Trait in quartile categories, as compared with Q1 (reference) | ||||
| Q2 | 0.54 (0.35–0.83) | 0.49 (0.32–0.77) | 1.30 (0.78–2.16) | 1.06 (0.63–1.81) |
| Q3 | 0.54 (0.35–0.84) | 0.65 (0.43–0.98) | 0.90 (0.53–1.54) | 1.25 (0.75–2.08) |
| Q4 | 0.66 (0.44–1.01) | 0.66 (0.43–1.01) | 1.10 (0.66–1.83) | 0.95 (0.56–1.62) |
| P trend | 0.08 | 0.16 | 0.91 | 1.00 |
| Model 2 | ||||
| Trait as continuous variable, per SD | ||||
| HR (95% CI) | 0.80 (0.67–0.96) | 0.83 (0.69–0.999) | 1.05 (0.97–1.27) | 0.97 (0.81–1.16) |
| P | 0.02 | 0.048 | 0.63 | 0.72 |
| Trait in quartile categories, as compared with Q1 (reference) | ||||
| Q2 | 0.55 (0.35–0.86) | 0.48 (0.30–0.76) | 1.29 (0.76–2.18) | 1.05 (0.61–1.81) |
| Q3 | 0.56 (0.36–0.88) | 0.69 (0.45–1.05) | 0.89 (0.52–1.55) | 1.24 (0.74–2.10) |
| Q4 | 0.69 (0.45–1.06) | 0.71 (0.45–1.10) | 1.10 (0.66–1.84) | 0.96 (0.56–1.65) |
| P trend | 0.12 | 0.35 | 0.92 | 0.95 |
Model 1 was adjusted for age, sex, family history of CHD, smoking status, and body mass index and was stratified by intervention group. Model 2 was adjusted as for model 1, plus baseline hypertension, dyslipidemia, and diabetes.
Abbreviation: Q, quartile.
Fifty-eight individuals who provided blood samples at baseline but not at 1 year were not included.
Arginine availability score = arginine/(citrulline + ornithine).
Exploratory analyses involving alternate ratios (ADMA/SDMA ratio, ADMA/NMMA ratio, and arginine/citrulline ratio) are presented in Supplemental Table 2. Baseline ADMA/NMMA ratio was positively associated with incident CVD as a continuous variable (HR = 1.19; 95% CI, 1.01 to 1.39, per 1 SD). None of the 1-year changes in alternative ratios were significantly associated with CVD. We proceeded with further analysis of the arginine/ADMA ratio in light of its stronger observed inverse association and the better established biological plausibility for the association of this ratio with atherosclerosis (7–12).
Effects of dietary interventions on arginine/ADMA ratio and arginine in incident CVD
Supplemental Fig. 4 depicts the HRs of arginine/ADMA ratio stratified by intervention group. The MD + EVOO and the MD + nuts groups were combined to increase statistical power. Using participants in the lowest baseline arginine/ADMA ratio quartile who were randomized to a MD intervention as the reference group, those in quartiles 2 through 4 had a nonsignificant lower risk of CVD. Participants in the lowest quartile randomized to the control diet had a significantly higher risk than the reference group, presumably due to the differences due to the intervention, while those in quartiles 2 through 4 had a comparatively lower risk, signifying no strong effect modification by the dietary intervention. Supplemental Fig. 5 uses 1-year changes in arginine/ADMA ratio instead of baseline values. We observed no significant associations or effect modification when stratifying by dietary intervention.
Discussion
In the present case-cohort study in the framework of the PREDIMED trial, we observed an inverse association between both the baseline arginine/ADMA ratio, as well as the baseline global arginine bioavailability ratio proposed by Tang et al. (24) with incident CVD. However, we found no significant associations of changes in these ratios with incident CVD after 1 year of follow-up. Although changes in plasma metabolites may not become apparent in some participants within 1 year, several short-term randomized trials of arginine supplementation demonstrate significant differences between treatment arms within 6 months (19–21). To our knowledge, no prior studies have assessed changes in urea cycle or methylarginine metabolite concentrations in relation with the incidence of primary clinical events of CVD.
Our results are in line with previous studies investigating arginine and ADMA and their association with CVD. A study from the Framingham Offspring cohort reported a significantly lower risk of CVD associated with the arginine/ADMA ratio adjusted for other established CVD risk factors and biomarkers (26). A meta-analysis of 22 prospective cohort studies with a mean follow-up time of 7.1 years reported a robust association between ADMA concentrations and higher risk of subsequent CVD events (27), with individuals in the highest ADMA tertile experiencing a ∼40% increased CVD risk compared with those in the lowest tertile. High ADMA in concert with low NO has been reported to propagate a variety of harmful detrimental processes biologically related to atherosclerosis, including free radical generation, smooth cell proliferation, systemic inflammation, and endothelial dysfunction (8).
Regarding primary urea cycle metabolites, we found a significant inverse relationship for 1-year changes in citrulline and vascular events but not for ornithine or arginine (although we note that the inverse association for arginine was borderline). Arginine has been suggested as a molecule of therapeutic interest for its role as the precursor to NO. l-Arginine (and only l-arginine) is the required substrate for all isoforms of the enzyme NOS to produce NO. NO is acknowledged as a powerful short-life vasodilator with an important defensive role against ischemic disease through endothelial smooth muscle relaxation (28). In rodent models, chronic arginine treatment alleviates metabolic and cardiovascular complications in obese rats (29) and protects against oxidative stress and improves myocardial energetics in hypoxic rat hearts (30). Experimental studies aimed to increase the bioavailability of arginine have reported promising results. Inhibition of arginase, a competitive inhibitor of arginine, improves vascular integrity, and protects against ischemia-induced injury (31). In recent randomized supplementation trials, patients receiving arginine experienced improvements in anthropometric and biochemical indices associated with later CVD (20), and alleviations in postprandial endothelial dysfunction (32). Few studies have been published regarding the role of citrulline in the pathophysiology of heart disease, but it is known that citrulline is a product of NO synthesis and can be reconverted back to arginine in the kidneys to increase arginine bioavailability (33, 34). Joint therapy of both arginine and citrulline has also been recommended, as arginine supplementation alone appears to increase arginase expression and reduce bioavailability of arginine, whereas citrulline supplementation does not (33). In addition to the arginine pathway, it has been reported that citrulline has anti-inflammatory properties and may exert beneficial pleiotropic effects on various impaired functions due to aging (35). Thus, future investigations of citrulline as a therapeutic agent for arginine deficient diseases are warranted.
We did not observe significant effect modification by a dietary intervention on the association between the arginine/ADMA ratio and incident CVD. Previous studies have hypothesized that the cardioprotective benefit of the MD, particularly when supplemented with nuts, is in part mediated by arginine intake (36–38). This hypothesis is biologically plausible and it is in agreement with our results for nonstroke events. Dietary sources of arginine consist mainly of meat, poultry, fish, dairy, and nuts (39), and higher intake of some of these foods, especially nuts and fish, have been found to be associated with subsequently reduced CVD risk (40, 41). Studies of dietary arginine intake have been inconclusive. In a study of elderly men, Oomen et al. reported a similar nonsignificant inverse association with CHD mortality (42). More recently, Bahadoran et al. concluded that plant-derived arginine conferred a benefit for CHD events and blood pressure, whereas animal-derived arginine was a risk factor for these outcomes (43). Given the emerging evidence of arginine as a cardioprotective molecule, it will be important for future epidemiologic and clinical studies to assess the potential causal link between arginine and CVD.
We note several strengths of the current study. Our cohort assessed the incidence of major CVD clinical events as the primary endpoint, as well as stroke and nonstroke cases as secondary endpoints. The prospective assessment of urea cycle and methylarginine metabolites, as well as the adjustment for hypothesized confounders measured among individuals under close follow-up within a randomized trial, adds to the potential causal interpretation of our findings. Limitations of the current study also warrant discussion. First, residual confounding by other unknown or unmeasured variables cannot be ruled out. Second, individuals who donated blood at baseline but not at 1 year of follow-up may have been systematically different from those who donated at both time points. Third, we were assuming a determined induction period of 1-year, and perhaps a 1-year follow-up is not a sufficiently long period to observe an effect of the dietary pattern on the metabolites tested. Fourth, the PREDIMED trial consisted of high-risk participants living in the Mediterranean region, and our findings may not be generalizable to populations with different demographic distributions or exposure ranges.
Conclusion
Results from a case-cohort study nested within the PREDIMED trial indicated that the baseline arginine/ADMA ratio, as well as global arginine availability, were associated with lower risk of future CVD events. One-year changes in citrulline were inversely associated with subsequent CVD incidence, but neither the arginine/ADMA ratio nor global arginine availability was significantly associated with CVD outcomes.
Supplementary Material
Acknowledgments
We thank Monica Bullo, Cristina Razquin Burillo, and Yan Zheng for their valuable comments.
Author contributions: All authors approved of the final version of the manuscript.
Disclosure Summary: J.S.-S. has received grants from the International Nut and Dried Fruit Foundation and is a nonpaid member of the scientific advisory board of the International Nut and Dried Fruit Foundation. E.R. has received grants from the California Walnut Commission and is a nonpaid member of its scientific advisory committee. The remaining authors have nothing to disclose.
Abbreviations:
- ADMA
asymmetric dimethylarginine
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- EVOO
extra-virgin olive oil
- HR
hazard ratio
- MD
Mediterranean diet
- NO
nitric oxide
- NMMA
NG-monomethylarginine
- PREDIMED
Prevención con Dieta Mediterránea
- SD
standard deviation
- SDMA
symmetric dimethylarginine.
References
- 1. Yusuf S, Reddy S, Ôunpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–2753. [DOI] [PubMed] [Google Scholar]
- 2. Shah SH, Kraus WE, Newgard CB. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation. 2012;126(9):1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113(13):1708–1714. [DOI] [PubMed] [Google Scholar]
- 4. Wiesinger H. Arginine metabolism and the synthesis of nitric oxide in the nervous system. Prog Neurobiol. 2001;64(4):365–391. [DOI] [PubMed] [Google Scholar]
- 5. Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Z, Tang WH, Cho L, Brennan DM, Hazen SL. Targeted metabolomic evaluation of arginine methylation and cardiovascular risks: potential mechanisms beyond nitric oxide synthase inhibition. Arterioscler Thromb Vasc Biol. 2009;29(9):1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr. 2004;134(10 Suppl):2842S–2847S. [DOI] [PubMed] [Google Scholar]
- 8. Sibal L, Agarwal SC, Home PD, Boger RH. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev. 2010;6(2):82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bode-Böger SM, Scalera F, Ignarro LJ. The L-arginine paradox: importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther. 2007;114(3):295–306. [DOI] [PubMed] [Google Scholar]
- 10. Seljeflot I, Nilsson BB, Westheim AS, Bratseth V, Arnesen H. The L-arginine-asymmetric dimethylarginine ratio is strongly related to the severity of chronic heart failure: no effects of exercise training. J Card Fail. 2011;17(2):135–142. [DOI] [PubMed] [Google Scholar]
- 11. Lüneburg N, Xanthakis V, Schwedhelm E, Sullivan LM, Maas R, Anderssohn M, Riederer U, Glazer NL, Vasan RS, Böger RH. Reference intervals for plasma L-arginine and the L-arginine:asymmetric dimethylarginine ratio in the Framingham Offspring Cohort. J Nutr. 2011;141(12):2186–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Notsu Y, Yano S, Shibata H, Nagai A, Nabika T. Plasma arginine/ADMA ratio as a sensitive risk marker for atherosclerosis: Shimane CoHRE study. Atherosclerosis. 2015;239(1):61–66. [DOI] [PubMed] [Google Scholar]
- 13. Esposito K, Kastorini CM, Panagiotakos DB, Giugliano D. Mediterranean diet and metabolic syndrome: an updated systematic review. Rev Endocr Metab Disord. 2013;14(3):255–263. [DOI] [PubMed] [Google Scholar]
- 14. Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez-González MA; PREDIMED Study Investigators . Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–1290.23432189 [Google Scholar]
- 15. Doménech M, Roman P, Lapetra J, García de la Corte FJ, Sala-Vila A, de la Torre R, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Lamuela-Raventós RM, Toledo E, Estruch R, Coca A, Ros E. Mediterranean diet reduces 24-hour ambulatory blood pressure, blood glucose, and lipids: one-year randomized, clinical trial. Hypertension. 2014;64(1):69–76. [DOI] [PubMed] [Google Scholar]
- 16. Thomazella MC, Góes MF, Andrade CR, Debbas V, Barbeiro DF, Correia RL, Marie SK, Cardounel AJ, daLuz PL, Laurindo FR. Effects of high adherence to Mediterranean or low-fat diets in medicated secondary prevention patients. Am J Cardiol. 2011;108(11):1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martínez-González MA, Corella D, Salas-Salvadó J, Ros E, Covas MI, Fiol M, Wärnberg J, Arós F, Ruíz-Gutiérrez V, Lamuela-Raventós RM, Lapetra J, Muñoz MÁ, Martínez JA, Sáez G, Serra-Majem L, Pintó X, Mitjavila MT, Tur JA, Portillo MP, Estruch R; PREDIMED Study Investigators . Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol. 2012;41(2):377–385. [DOI] [PubMed] [Google Scholar]
- 18. Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Covas MI, Fiol M, Gómez-Gracia E, López-Sabater MC, Vinyoles E, Arós F, Conde M, Lahoz C, Lapetra J, Sáez G, Ros E; PREDIMED Study Investigators . Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145(1):1–11. [DOI] [PubMed] [Google Scholar]
- 19. Bednarz B, Jaxa-Chamiec T, Maciejewski P, Szpajer M, Janik K, Gniot J, Kawka-Urbanek T, Drozdowska D, Gessek J, Laskowski H. Efficacy and safety of oral l-arginine in acute myocardial infarction. Results of the multicenter, randomized, double-blind, placebo-controlled ARAMI pilot trial. Kardiol Pol. 2005;62(5):421–427. [PubMed] [Google Scholar]
- 20. Dashtabi A, Mazloom Z, Fararouei M, Hejazi N. Oral L-arginine administration improves anthropometric and biochemical indices associated with cardiovascular diseases in obese patients: a randomized, single blind placebo controlled clinical trial. Res Cardiovasc Med. 2015;5(1):e29419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, Ernst KV, Kelemen MD, Townsend SN, Capriotti A, Hare JM, Gerstenblith G. L-arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295(1):58–64. [DOI] [PubMed] [Google Scholar]
- 22. Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165–1172. [DOI] [PubMed] [Google Scholar]
- 23. Celik M, Iyisoy A, Celik T, Yilmaz MI, Yuksel UC, Yaman H. The relationship between L-arginine/ADMA ratio and coronary collateral development in patients with low glomerular filtration rate. Cardiol J. 2012;19(1):29–35. [DOI] [PubMed] [Google Scholar]
- 24. Tang WHW, Wang Z, Cho L, Brennan DM, Hazen SL. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 2009;53(22):2061–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee EW, Wei LJ, Amato DA, Leurgans S. Cox-type regression analysis for large numbers of small groups of correlated failure time observations In: Klein JP, Goel PK, eds. Survival Analysis: State of the Art. Dordrecht, The Netherlands: Springer Netherlands; 1992:237–247. [Google Scholar]
- 26. Böger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, Schulze F, Xanthakis V, Benndorf RA, Vasan RS. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119(12):1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Willeit P, Freitag DF, Laukkanen JA, Chowdhury S, Gobin R, Mayr M, Di Angelantonio E, Chowdhury R. Asymmetric dimethylarginine and cardiovascular risk: systematic review and meta-analysis of 22 prospective studies. J Am Heart Assoc. 2015;4(6):e001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pepine CJ. The impact of nitric oxide in cardiovascular medicine: untapped potential utility. Am J Med. 2009; 122(5, Suppl)S10–S15. [DOI] [PubMed] [Google Scholar]
- 29. Alam MA, Kauter K, Withers K, Sernia C, Brown L. Chronic l-arginine treatment improves metabolic, cardiovascular and liver complications in diet-induced obesity in rats. Food Funct. 2013;4(1):83–91. [DOI] [PubMed] [Google Scholar]
- 30. Ashmore T, Fernandez BO, Branco-Price C, West JA, Cowburn AS, Heather LC, Griffin JL, Johnson RS, Feelisch M, Murray AJ. Dietary nitrate increases arginine availability and protects mitochondrial complex I and energetics in the hypoxic rat heart. J Physiol. 2014;592(21):4715–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pernow J, Jung C. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovasc Res. 2013;98(3):334–343. [DOI] [PubMed] [Google Scholar]
- 32. Deveaux A, Pham I, West SG, André E, Lantoine-Adam F, Bunouf P, Sadi S, Hermier D, Mathé V, Fouillet H, Huneau JF, Benamouzig R, Mariotti F. L-arginine supplementation alleviates postprandial endothelial dysfunction when baseline fasting plasma arginine concentration is low: a randomized controlled trial in healthy overweight adults with cardiometabolic risk factors. J Nutr. 2016;146(7):1330–1340. [DOI] [PubMed] [Google Scholar]
- 33. Romero MJ, Platt DH, Caldwell RB, Caldwell RW. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc Drug Rev. 2006;24(3–4):275–290. [DOI] [PubMed] [Google Scholar]
- 34. Cynober L, Moinard C, De Bandt JP. The 2009 ESPEN Sir David Cuthbertson: Citrulline: a new major signaling molecule or just another player in the pharmaconutrition game? Clin Nutr. 2010;29(5):545–551. [DOI] [PubMed] [Google Scholar]
- 35. Breuillard C, Bonhomme S, Couderc R, Cynober L, De Bandt JP. In vitro anti-inflammatory effects of citrulline on peritoneal macrophages in Zucker diabetic fatty rats. Br J Nutr. 2015;113(1):120–124. [DOI] [PubMed] [Google Scholar]
- 36. Ros E, Núñez I, Pérez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2004;109(13):1609–1614. [DOI] [PubMed] [Google Scholar]
- 37. Eid HM, Arnesen H, Hjerkinn EM, Lyberg T, Ellingsen I, Seljeflot I. Effect of diet and omega-3 fatty acid intervention on asymmetric dimethylarginine. Nutr Metab (Lond). 2006;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D’Armiento M, D’Andrea F, Giugliano D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–1446. [DOI] [PubMed] [Google Scholar]
- 39. King DE, Mainous AG III, Geesey ME. Variation in L-arginine intake follow demographics and lifestyle factors that may impact cardiovascular disease risk. Nutr Res. 2008;28(1):21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu FB, Stampfer MJ, Manson JE, Rimm EB, Colditz GA, Rosner BA, Speizer FE, Hennekens CH, Willett WC. Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study. BMJ. 1998;317(7169):1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Järvinen R, Knekt P, Rissanen H, Reunanen A. Intake of fish and long-chain n-3 fatty acids and the risk of coronary heart mortality in men and women. Br J Nutr. 2006;95(4):824–829. [DOI] [PubMed] [Google Scholar]
- 42. Oomen CM, van Erk MJ, Feskens EJM, Kok FJ, Kromhout D. Arginine intake and risk of coronary heart disease mortality in elderly men. Arterioscler Thromb Vasc Biol. 2000;20(9):2134–2139. [DOI] [PubMed] [Google Scholar]
- 43. Bahadoran Z, Mirmiran P, Tahmasebinejad Z, Azizi F. Dietary L-arginine intake and the incidence of coronary heart disease: Tehran lipid and glucose study. Nutr Metab (Lond). 2016;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.