Abstract
Context:
Carotenoids have been implicated in the regulation of adipocyte metabolism.
Objective:
To compare the effects of mixed-carotenoid supplementation (MCS) versus placebo on adipokines and the accrual of abdominal adiposity in children with obesity.
Design and Setting:
Randomized (1:1), double-blind, placebo-controlled intervention trial to evaluate the effects of MCS over 6 months in a subspecialty clinic.
Participants:
Twenty (6 male and 14 female) children with simple obesity [body mass index (BMI) > 90%], a mean age (± standard deviation) of 10.5 ± 0.4 years, and Tanner stage I to V were enrolled; 17 participants completed the trial.
Intervention:
MCS (which contains β-carotene, α-carotene, lutein, zeaxanthin, lycopene, astaxanthin, and γ-tocopherol) or placebo was administered daily.
Main Outcome Measures:
Primary outcomes were change in β-carotene, abdominal fat accrual (according to magnetic resonance imaging), and BMI z-score; secondary outcomes were adipokines and markers of insulin resistance.
Results:
Cross-sectional analysis of β-carotene showed inverse correlation with BMI z-score, waist-to-height ratio, visceral adipose tissue, and subcutaneous adipose tissue (SAT) at baseline. MCS increased β-carotene, total adiponectin, and high-molecular-weight adiponectin compared with placebo. MCS led to a greater reduction in BMI z-score, waist-to-height ratio, and SAT compared with placebo. The percentage change in β-carotene directly correlated with the percentage change in SAT.
Conclusions:
The decrease in BMI z-score, waist-to-height ratio, and SAT and the concomitant increase in the concentration of β-carotene and high-molecular-weight adiponectin by MCS suggest the putative beneficial role of MCS in children with obesity.
Supplementation of mixed carotenoids with lifestyle intervention in children with obesity leads to increased β-carotene and adiponectin and lowers abdominal adiposity in the active vs placebo group.
The prevalence of abdominal obesity among U.S. children has increased by almost 70% in the past 20 years (1). This rapid accrual of abdominal adiposity places children at a particularly high risk for future metabolic and cardiovascular disease irrespective of body mass index (BMI) per se (2). Numerous cross-sectional studies in adults and children report lower serum carotenoid concentrations in individuals with obesity and features of the metabolic syndrome, after controlling for other potential confounders (3, 4). Carotenoids are lipophilic, C-40–based isoprenoid compounds that contain up to 15 conjugated double bonds. They are synthesized in plants, fungi, and other photosynthetic organisms. Their primary dietary sources for humans are fruits and vegetables. Of the more than 600 carotenoids identified in nature, about 20 are absorbed in the intestine and only six are ubiquitous in human serum: β-carotene, α-carotene, β-cryptoxanthin, lycopene, lutein, and zeaxanthin (5). Recent evidence suggests that the provitamin A (retinol) carotenoids, such as β-carotene, α-carotene, and β-cryptoxanthin, and their retinoid conversion products, retinaldehyde and all-trans-retinoic acid (at-RA), may be beneficial in the control of adipocyte differentiation, hypertrophy, thermogenesis, and dysregulated adipokine secretion, with important clinical implications for the management of obesity and obesity-related metabolic disturbances (6–8).
Adipocytes store carotenoids along with triacylglycerols in lipid droplets, but they may also be compartmentalized to the cell membrane and mitochondria (9, 10). In humans, carotenoid levels in abdominal depots show a strong association with both oral intake and plasma concentrations (11, 12). Total carotenoid concentrations are higher in abdominal adipose tissue than adipose tissue from the buttocks and thighs, and the physiologic relevance, especially related to obesity, remains unknown. Carotenoids and their retinoid conversion products are potent inhibitors of adipocyte volume (hypertrophy) and increased cell number (hyperplasia) in in vitro murine models (13, 14). The physiologic lipogenic/adipogenic effects of carotenoids on abdominal adipose tissue in childhood remain undefined.
The primary objective of this study was to determine the effects of a 6-month intervention of mixed carotenoid supplementation (MCS) versus placebo on β-carotene levels in children with obesity who were primed for weight loss through a 10-day, family-based after-school lifestyle intervention program involving cognitive-behavioral therapy, diet, and exercise. Measures of adiposity, including BMI z-scores, waist-to-height ratio (WHtR), and abdominal fat accrual (using quantitative magnetic resonance imaging [MRI]), along with changes in the concentration of other carotenoids and of adipokines (such as leptin and adiponectin) and markers of insulin sensitivity, were considered secondary outcomes.
Methods
This was a double-blinded randomized study approved by the Institutional Review Committee at Wolfson Children’s Hospital, Jacksonville, Florida. It was conducted in accordance with the Declaration of Helsinki. Otherwise healthy children aged 8 to 11 years with a BMI at or above the 90th percentile were asked to participate. Written parental informed consent and child assent were obtained for all participants upon enrollment in the study. Tanner staging based on pubic hair and genitalia was performed in all participants (15). The study was registered at ClinicalTrials.gov (registration number: NCT02060279).
Twenty children (6 male and 14 female), mean age (± standard deviation) of 10.5 ± 0.4 years, were recruited. Patients were randomly assigned to consume an MCS supplement (CarotenAll; Jarrow Formulas, Los Angeles, CA) or placebo throughout the study. The supplement contained 2000 IU of β-carotene and 500 μg of α-carotene; 10 mg of lutein; 2 mg of zeaxanthin and 10 mg of lycopene; 500 μg of astaxanthin; and 10 mg of γ-tocopherol per capsule; both the supplement and placebo capsules were dispensed in identical light-protected containers. The participants were instructed to take one capsule with meals twice daily. They were then asked to participate, along with a parent or caregiver, in a 3-hour-per-day, 10-day, intense family-based afterschool lifestyle intervention program at a local YMCA (Young Men's Christian Association) site. The program was facilitated by a clinical dietician, psychologist, and life coach and focused on improved nutrition (5 servings of fruit and vegetable daily), exercise (60 minutes of outdoor activity per day), and cognitive-behavior modification aimed at weight reduction. The families were contacted monthly by phone to encourage them to continue healthy lifestyle practices, address and record any side effects, and ensure consumption of the supplement as prescribed. At the 6-month visit they were also encouraged to enroll in our weight management clinic. Participants were recruited from the Nemours Endocrinology and Metabolism Clinic in Jacksonville, Florida, and through approved advertising sent to neighboring pediatric clinics.
Participants with a history of chronic illness or receiving long-term medications, those with cognitive or neuromuscular impairment, those with any organic cause of obesity, or those who had metal implants that would preclude them from safely undergoing MRI were excluded from the study. To avoid illness-related acute changes in the markers of interest, participants were studied only if they had no history of recent illness or bone fracture within 2 weeks of their blood draw. They were instructed not to consume any medications, including vitamins, herbal remedies, or anti-inflammatory drugs, within 15 days of the anticipated blood draw. The intention-to-treat principle was applied to 20 participants, who were randomly assigned by using a randomization scheme generated at http://www.randomization.com.
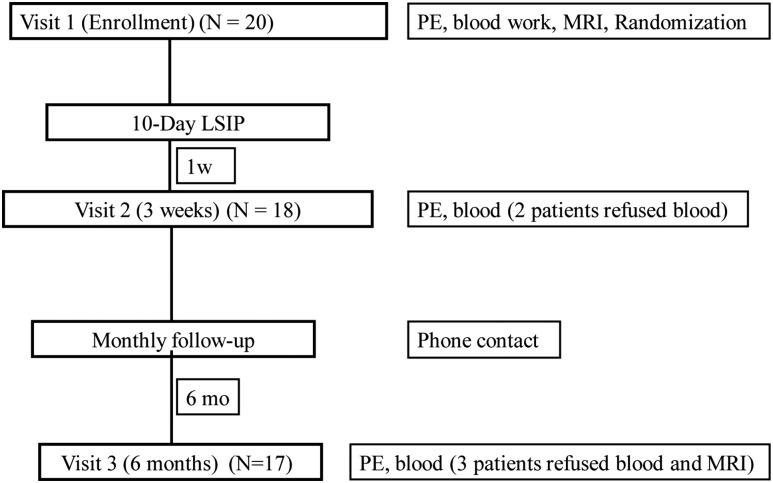
The study protocol is summarized in Fig. 1. History and physical examinations were performed, including sitting blood pressure, which was averaged from three measurements obtained with an automated sphygmomanometer. Waist circumference was measured to the nearest millimeter with a flexible steel tape while the participants were standing, after gently exhaling, at the minimal circumference measurable on the horizontal plane between the lowest portion of the rib cage and iliac crest and averaged from three measurements. A digital scale and Harpenden stadiometer were used to measure body mass and height for BMI (kg/m2), with a z-score generated according to the U.S. Centers for Disease Control and Prevention reference criteria (16). All participants fasted 8 hours before the blood collection at baseline, 3 weeks, and 6 months. Blood samples were processed under orange lights immediately after collection, and aliquots of serum and plasma were frozen in opaque tubes at −80°C until analysis.
Figure 1.
Protocol study flow. PE, physical examination; LSIP, lifestyle intervention program.
Serum carotenoids were measured by reverse-phase high-pressure liquid chromatography with photodiode array detection (Genox Corporation, Baltimore, MD); the mean intra-assay coefficients of variation (CVs) were 6.5% for α- and β-carotene, lutein, and zeaxanthin; 5.7% for β-cryptoxanthin; 6.4% for lycopene; and 10.0% for retinol. Total adiponectin, leptin, and insulin were measured by radioimmunoassay (Linco Research, St. Charles, MO); the intra-assay CVs were 7.1%, 8.0% and 11.6%, respectively. High-molecular-weight adiponectin (HMW-ADI) was measured by enzyme-linked immunosorbent assay; the intra-assay CV was 4.7% (Millipore, Billerica, MA). Glucose was measured by the hexokinase method (GM-7 Analyzer; Analox Instruments, Stourbridge, UK); the CV was 1.4%. Triglycerides and high-density lipoprotein cholesterol (HDL-C) were measured by an autoanalyzer (Roche-Hitachi, Basel, Switzerland). Homeostatic model assessment of insulin resistance-2 (HOMA-2) was calculated by using the HOMA calculator, version 2.23 (http://www.dtu.ox.ac.uk/HOMACalculator/).
The percentage change in visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) were determined by cross-sectional multislice MRI (1.5 T; GE Healthcare, Milwaukee, WI) at baseline and 6 months. Patients were imaged after administration of 1 mg glucagon intramuscularly to slow bowel motion. Briefly, a two-dimensional respiratory gated Dixon imaging sequence was used to produce fat-only images, similar to the method used in a previously described study (17). A series of five transverse images were acquired from the lumbar region, beginning at the inferior border of the fifth lumbar vertebra and proceeding toward the head; slice thickness was 10 mm, with a 2-mm gap between images to prevent crosstalk. Total fat, SAT, and VAT were segmented by using a semiautomated threshold and region-drawing methods that subtracted all nonfat tissues, based on the method of Ross et al. (18). To calculate volumes for SAT and VAT, the images were loaded into a three-dimensional processing software (AW workstation, GE Healthcare), and the segmented fat was measured by using the volume measurement tool of the software. The intraclass correlation coefficients for repeat analyses in a previous study were r = 0.98 for both SAT and VAT (19).
Statistical analysis
The intention-to-treat principle was applied to all participants in the primary analysis. Quantitative variables are presented by using either mean ± standard error of the mean or, in the case of substantially skewed distribution, median and 25%–75% interquartile range. Categorical variables are presented by using frequencies and percentages. The Pearson correlation coefficient analysis was performed to determine the relationship between baseline carotenoid concentrations and visceral anthropometric parameters. A two-sample t test was used to compare the mean percentage changes in β-carotene, total adiponectin, and HMW-ADI at 6 months between two groups. In addition, generalized linear models adjusting for baseline levels, Tanner stage, and BMI z-scores were used to compare treatment effects of carotenoids, adipokines, and HOMA-2 estimated marginal means (± standard error of the mean) between groups with Bonferroni adjustments. A multiple regression analysis was performed to evaluate the independent contribution of age; sex; BMI z-score; and percentage change in β-carotene, triglycerides, HDL-C, VAT, and SAT to the percentage change in HMW-ADI at 6 months. All tests were two tailed, and the level of significance was set at P < 0.05. SPSS software, version 22 (SPSS Inc, Chicago, IL), was used for analyses.
Results
The clinical and biochemical characteristics of the study participants by treatment groups are presented in Table 1. Ninety-six percent of the participants attended eight or more sessions of the family-based after-school program, and 85% completed the placebo-controlled MCS intervention study. Adherence, based on returned pill counts, was 72% for the placebo group and 80% for the MCS group (P = 0.522).
Table 1.
Baseline Clinical and Biochemical Characteristics of Study Participants by Treatment Group
| Characteristic | MCS (n = 10) | Placebo (n = 10) | P Value |
|---|---|---|---|
| Age, mo | 125 ± 4.9 | 126 ± 4.7 | NS |
| Sex (male:female), n | 3:7 | 3:7 | NS |
| Tanner stage ratio (I–II:III–V), n | 7:3 | 6:4 | NS |
| BMI (kg/m2) | 32.1 ± 1.5 | 27.1 ± 1.6 | 0.036 |
| BMI z-score | 2.42 ± 0.08 | 1.99 ± 0.15 | 0.021 |
| WHtR | 0.68 ± 0.02 | 0.63 ± 0.03 | NS |
| VAT per MRI, g | 302 ± 39 | 310 ± 50 | NS |
| SAT per MRI, g | 2550 ± 255 | 1625 ± 280 | 0.03 |
| β-carotene, μg/dL | 10.9 ± 0.01 | 13.8 ± 0.03 | NS |
| Glucose, mg/dL | 82 ± 2.5 | 80 ± 1.8 | NS |
| Insulin, mIU/mL | 23.0 ± 3.1 | 20.3 ± 4.2 | NS |
| HOMA-2 | 2.66 ± 0.36 | 2.59 ± 0.47 | NS |
| Triglycerides, mg/dLa | 89 (69–163) | 75 (48–105) | NS |
| Total adiponectin, μg/dL | 8.68 ± 1.49 | 7.74 ± 1.04 | NS |
| HMW -ADI, µg/dL | 4.39 ± 0.97 | 4.38 ± 0.98 | NS |
| Leptin, µg/L | 40.5 ± 5.7 | 28.9 ± 5.7 | NS |
| Leptin/adiponectin ratio | 7.32 ± 2.6 | 4.93 ± 1.4 | NS |
All values mean ± standard error of the mean except where noted.
Abbreviation: NS, not significant.
Median (interquartile range, 25%–75%).
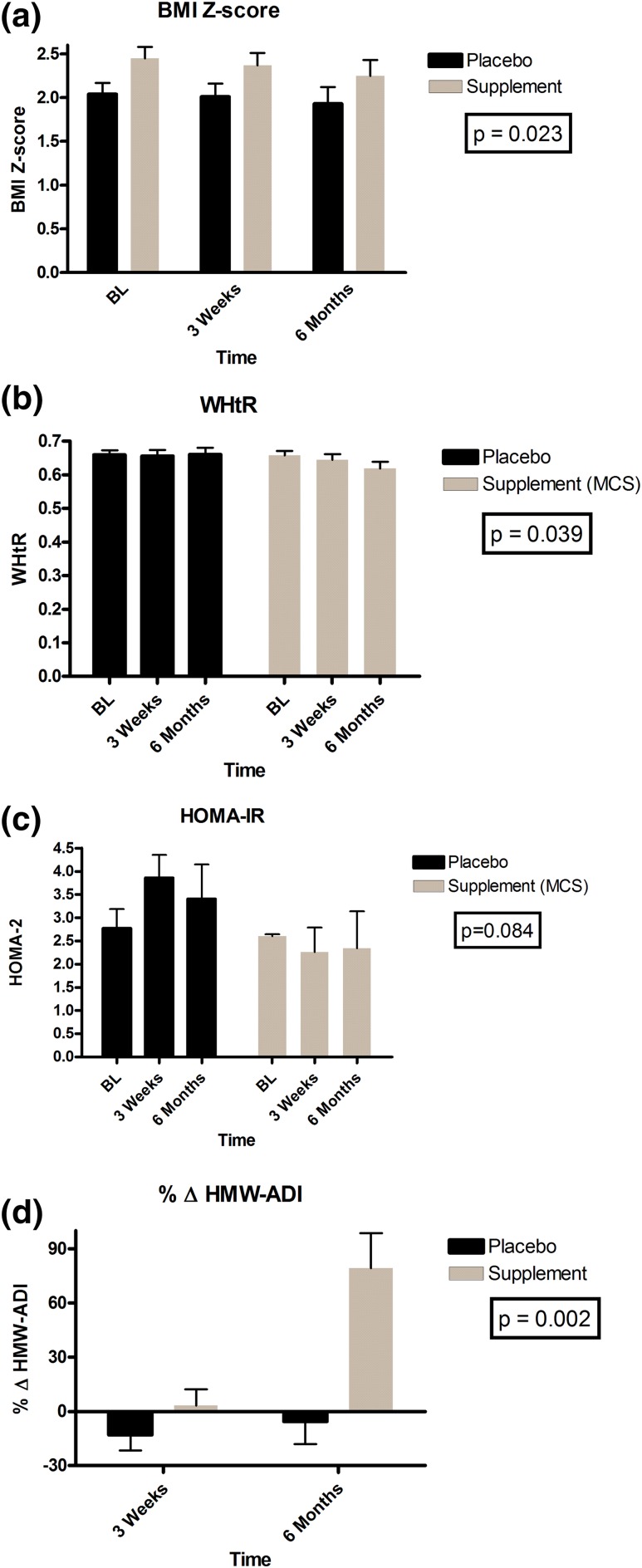
The MCS group had higher BMI z-scores (P = 0.036), WHtR (P = 0.021), and SAT (P = 0.03) than did the placebo group, but the groups did not differ in adipocytokines or individual carotenoid concentrations (data not shown). MCS induced a −0.19 ± 0.13 change in BMI z-score (P = 0.024), a 3% ± 2% change in waist circumference (P = 0.021), and a 0.03 ± 0.03 decrease in WHtR (P = 0.039) at 6 months compared with placebo [Fig. 2(a) and 2(b)].
Figure 2.
Mean treatment effects between MCS and placebo groups. (a) BMI z-score. (b) WHtR. (c) HOMA-IR. (d) Percentage change in HMW-ADI. BL, baseline.
At baseline, β-carotene was inversely related to BMI z-score (P = 0.003), WHtR (P = 0.017), VAT (P = 0.023), and SAT (P = 0.045), but none of the other carotenoids, such as α-carotene, β-cryptoxanthin, lycopene, lutein, and zeaxanthin, showed any such relationships. Generalized linear models adjusting for baseline values, Tanner stage, and BMI z-scores for treatment effects revealed significant increases in α-carotene, β-carotene, lutein, and zeaxanthin and significant reductions in vitamin A (retinol) at 6 months in the MCS group compared with the placebo group (Table 2). HOMA-2 remained stable in the MCS group but trended higher by 1.28 ± 0.7 in the placebo group (P = 0.084) for between-subject treatment effect over time [Table 3 and Fig. 2(c)]. At 6 months compared with baseline, MCS had increased β-carotene by 96% ± 23% (P < 0.001), total adiponectin by 23% ± 8% (P = 0.092), and HMW-ADI by 79% ± 19% (P = 0.001) versus placebo [Fig. 2(d)].
Table 2.
Pairwise Comparisons for Mean Treatment Effects of Carotenoid Concentrations Over Time Between MCS and Placebo Groups
| Carotenoid |
MCS (n = 8)
|
Placebo (n = 9)
|
Mean Difference | P Value a | ||||
|---|---|---|---|---|---|---|---|---|
| 0 mo | 3 wk | 6 mo | 0 mo | 3 wk | 6 mo | |||
| α-Carotene | 0.041 ± 0.013 | 0.137 ± 0.019 | 0.111 ± 0.015 | 0.030 ± 0.012 | 0.037 ± 0.018 | 0.020 ± 0.014 | 1.018 ± 0.144 | <0.001b |
| β-Carotene | 0.127 ± 0.024 | 0.221 ± 0.025 | 0.224 ± 0.024 | 0.116 ± 0.019 | 0.127 ± 0.024 | 0.107 ± 0.023 | 0.288 ± 0.049 | <0.001b |
| β-Cryptoxanthin | 0.070 ± 0.015 | 0.062 ± 0.011 | 0.093 ± 0.029 | 0.057 ± 0.014 | 0.048 ± 0.010 | 0.061 ± 0.027 | 0.129 ± 0.118 | 0.297b |
| Lycopene | 0.185 ± 0.049 | 0.262 ± 0.025 | 0.239 ± 0.045 | 0.194 ± 0.046 | 0.186 ± 0.024 | 0.227 ± 0.035 | 0.101 ± 0.072 | 0.185 |
| Lutein | 0.094 ± 0.012 | 0.423 ± 0.052 | 0.280 ± 0.055 | 0.083 ± 0.011 | 0.078 ± 0.049 | 0.089 ± 0.051 | 0.242 ± 0.052 | 0.001 |
| Zeaxanthin | 0.040 ± 0.005 | 0.122 ± 0.009 | 0.099 ± 0.016 | 0.032 ± 0.005 | 0.030 ± 0.008 | 0.034 ± 0.015 | 0.070 ± 0.010 | <0.001 |
| Retinol | 0.351 ± 0.039 | 0.274 ± 0.016 | 0.281 ± 0.023 | 0.365 ± 0.036 | 0.346 ± 0.015 | 0.373 ± 0.022 | -0.076 ± 0.015 | <0.001 |
All values presented as estimated marginal means ± standard error of the mean (μg/mL) adjusted for baseline levels, Tanner stage, and BMI z-score.
Bonferroni adjusted P value for treatment effects between MCS vs placebo.
Log-transformed values.
Table 3.
Pairwise Comparisons for Mean Treatment Effects for HOMA-2 and Adipokine Concentrations Between MCS and Placebo Groups
| Variable |
MCS (n
=
8)
|
Placebo (n
=
9)
|
Mean Difference | P Value a | ||||
|---|---|---|---|---|---|---|---|---|
| 0 mo | 3 wk | 6 mo | 0 mo | 3 wk | 6 mo | |||
| HOMA-2 | 2.60 ± 0.4 | 2.26 ± 0.5 | 2.34 ± 0.8 | 2.77 ± 0.4 | 3.86 ± 0.5 | 3.40 ± 0.7 | −1.28 ± 0.7 | 0.084 |
| Total adiponectin, μg/dL | 8.79 ± 0.9 | 7.38 ± 0.9 | 10.36 ± 0.9 | 6.26 ± 0.8 | 6.44 ± 0.9 | 6.50 ± 0.8 | 2.73 ± 0.9 | 0.611 |
| HMW-ADI | 4.65 ± 0.8 | 4.20 ± 0.9 | 7.53 ± 1.1 | 3.35 ± 0.7 | 3.04 ± 0.8 | 2.86 ± 1.1 | 1.63 ± 0.7 | 0.037 |
| HMW/total adiponectin ratio | 0.49 ± 0.09 | 0.53 ± 0.09 | 0.67 ± 0.08 | 0.46 ± 0.08 | 0.41 ± 0.09 | 0.37 ± 0.07 | 0.18 ± 0.06 | 0.005 |
| Leptin, µg/L | 36.6 ± 5.5 | 27.0 ± 7.2 | 34.6 ± 6.8 | 34.8 ± 5.2 | 33.7 ± 6.7 | 30.3 ± 6.4 | −3.13 ± 5.7 | 0.590 |
| Leptin/adiponectin ratio | 6.2 ± 2.1 | 5.9 ± 2.4 | 5.3 ± 2.0 | 7.2 ± 2.0 | 6.4 ± 2.2 | 5.8 ± 1.9 | 0.467 ± 0.6 | 0.476 |
All values presented as mean ± standard error of the mean and adjusted for baseline values, Tanner stage, and BMI z-score.
Bonferroni adjusted P value for between-subject treatment effects.
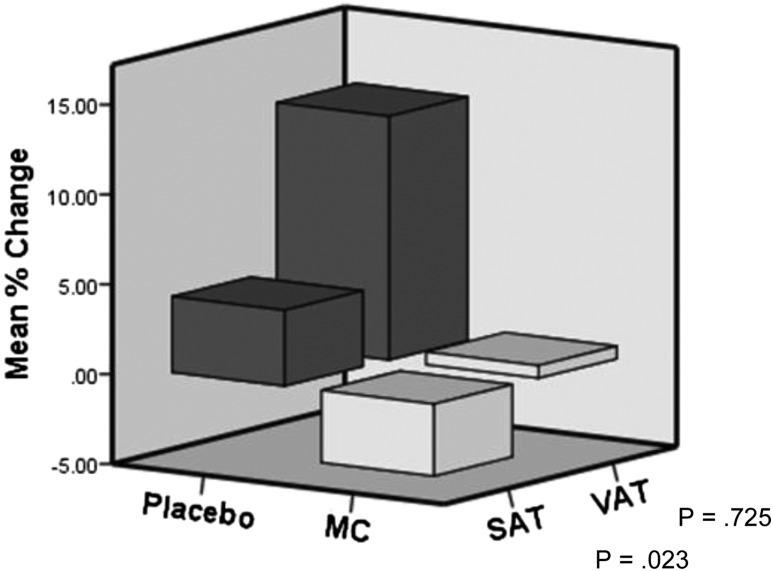
Mean percentage change in SAT, adjusted for Tanner stage and BMI z-score, increased by 4.2% ± 1.8% in the placebo group but decreased by 4.0% ± 4.1% in the MCS group (P = 0.023) (Fig. 3). There was also an attenuation of VAT accrual in the MCS group (0.7% ± 13%) as opposed to an increase in the placebo group (13.5% ± 19%) at 6 months; however, because of the large intrasubject variation, this was not statistically significant (Fig. 3). At 6 months, the percentage change in SAT was highly correlated to the percentage change in β-carotene (P = 0.004) but not to the percentage change in VAT (P = 0.549). In contrast, MCS induced an 18% drop in retinol at 6 months as opposed to an 8% increase in the placebo group (P = 0.03).
Figure 3.
Mean percentage change in SAT and VAT by MRI after 6 months of mixed carotenoid (MC) versus placebo adjusted for Tanner stage and BMI z-score.
There were no significant treatment effects in triglycerides or HDL-C in the study participants. In the MCS group, participants reached an average β-carotene level of 20.9 ± 1.9 μg/dL as compared with 12.0 ± 1.6 μg/dL in the placebo group (P = 0.007). β-Carotene showed a modest positive correlation with total adiponectin at baseline when adjusted for sex (P = 0.077). With use of age; sex; BMI z-score; and percentage change in β-carotene, triglycerides, HDL-C, VAT, and SAT in stepwise multiple linear regression analysis, we determined that the percentage change in β-carotene, triglycerides, and SAT at 6 months explained 67.8% of the variance in HMW-ADI at 6 months (P = 0.002) without violating colinearity.
Discussion
The current placebo-controlled intervention study has several findings. These include reduction in BMI z-scores and WHtR; attenuation of VAT accrual; and increase in the circulating concentrations of HMW-ADI, ratio of HMW-ADI to total adiponectin, and carotenoids compared with 6 months of MCS intervention. Similar changes were not seen in the placebo group. We also observed a unique positive association between β-carotene and total adiponectin at baseline and negative association between β-carotene and measures of abdominal adiposity, such as waist circumference, WHtR, and MRI-quantified SAT and VAT after adjustment for sex in children with obesity. In addition, following supplementation the percentage change in β-carotene correlated significantly only with the percentage change in SAT and not with the percentage change in VAT.
Accumulating evidence in animal models link adipose tissue β-carotene and its conversion products retinaldehyde and at-RA but not vitamin A (retinol) as modulators of the retinoic acid receptor, the retinoid X receptor, and the peroxisome proliferator-activated receptors (PPARs) in the control of thermogenic signaling and the fat storage capacity of both mature white and brown adipocytes (6, 20). These ligand-activated nuclear transcription factors are required to maintain the expression of genes that confer the hypertrophic characteristics of mature adipocytes (21), and they play a role in mediating high-fat diet–induced insulin resistance (22). β-Carotene reduces PPARγ activity in mature adipocytes in vitro, resulting in fat mobilization and fat browning of mature white adipocytes (21). Interestingly, mature mouse adipocytes in culture rely mainly on β-carotene by the action of retinaldehyde dehydrogenase enzymes (RALDHs/ALDH1A) for the synthesis of retinaldehyde and at-RA and its nuclear receptor signaling, as supplementation with retinol leads only to the production of retinyl esters and cannot substitute for β-carotene to induce at-RA production (23).
In a previous study, we showed that supplementation with an encapsulated fruit and vegetable juice concentrate, predominantly consisting of a mixture of carotenoids and phytonutrients, increased serum β-carotene but not retinol and reduced adiposity, with improvements in insulin resistance in overweight boys (7). The 96% increase in β-carotene as compared with the 18% decrease in retinol in the current study is remarkable because β-carotene is considered a major provitamin A carotenoid. The unique inverse relationship between measures of abdominal adiposity and β-carotene and the enhancement of β-carotene along with an attenuation of SAT accrual are important findings and may indicate a putative role for β-carotene in the regulation of adipose tissue biology in children with obesity. A previous longitudinal adult study in 2672 women over a 12-year period also showed a strong inverse association between waist circumference and serum β-carotene, similar to our study (24). In another study the concentration of β-carotene in subcutaneous adipocytes from obese and nonobese participants with diabetes was about 50% lower than in the controls (25). The metabolic implications of these results remain less clear.
High-fat diets induce the expression of the retinaldehyde dehydrogenase enzyme ALDH1A1, responsible for increased retinoic acid receptor ligands (retinaldehyde and at-RA) and reduced adiponectin expression selectively in white adipose tissue (26). Adiponectin is an adipose-specific but atypical adipokine, with well-established insulin-sensitizing, anti-inflammatory, and antiatherogenic effects (27, 28). It is paradoxically downregulated in obesity, insulin resistance, and type 2 diabetes, suggesting that its deficiency may be permissive to accelerate these processes. Hypertrophied adipocytes from obese participants produce less adiponectin, leading to a decrease in fatty acid oxidation in liver, cardiac, and skeletal muscle and subsequently inducing a decrease in insulin sensitivity (29). In a previous report among 1042 Chinese participants who were assessed for features of metabolic syndrome, at-RA was positively associated with adiponectin and inversely associated with components of metabolic syndrome, including central obesity, hypertriglyceridemia, reduced HDL-C, and hyperglycemia (30). Adiponectin exists in a wide variety of multimer complexes—from low-molecular-weight trimers, to middle-molecular-weight hexamers, to high-molecular-weight multimers (31). The high-molecular-weight form is physiologically the most active form (32) and may have better predictive power for insulin resistance and metabolic syndrome (29). Similar to our study, in 437 Japanese adult participants, both men and women, who attended a health examination, serum β-carotene was positively associated with serum HMW-ADI concentrations in both sexes, even after adjustment for possible confounding factors, including inflammatory markers (33). Exposure to β-carotene from days 4 to 8 of differentiation also induces adiponectin expression in 3T3-L1 adipocytes (34).
Several studies have reported that improving insulin resistance and reducing insulin levels with insulin-sensitizing agents, such as PPARγ agonists, and/or increased physical activity markedly increased adiponectin concentrations, even after adjustment for changes in body weight (35, 36). Little is known, however, about the association between dietary plant-derived micronutrients and plasma adiponectin concentrations in humans (37). Esposito et al. found in a randomized controlled trial that a Mediterranean-style diet and increased physical activity, aimed at reducing body weight, significantly decreased BMI and concomitantly increased plasma adiponectin concentrations in postmenopausal obese women (38). Ben-Amara et al. described a positive association between concentrations of β-carotene and adiponectin in plasma, independently of sex, age, smoking status, BMI, and waist circumference (39). Yoshida et al. reported that 12-week administration of astaxanthin, a xanthophyll carotenoid pigment found in marine animals, at doses of 12 to 18 mg/dL, significantly increased serum adiponectin levels by 26% in adults with mild hyperlipidemia (40). The combination of carotenoids in our preparation had a much lower dose of astaxanthin, with similar adiponectin effects, suggesting synergy in MCS supplementation. Interestingly, the percentage change in β-carotene, triglycerides, and SAT at 6 months explained almost 68% of the variance in HMW-ADI at 6 months in our study. Overall, the substantial increase in the concentration of HMW-ADI and carotenoids with the MCS, the stabilization of HOMA-IR, and the unique relationship between HMW-ADI and β-carotene in our study suggest the potential ability of carotenoids, predominantly β-carotene, to beneficially temper the metabolic derangements related to obesity in children.
The lack of a control group, the relatively small sample size of our cohort, and the unequal BMI z-score distribution among the groups at baseline are important limitations of this study. The strengths of this study, on the other hand, include the randomized, double-blind, placebo-controlled nature of the intervention; the measurement of various serum carotenoids along with total and HMW-ADI; and the serial quantitative imaging (MRI) to accurately assess changes in visceral adiposity accrual. Of note, adherence, as assessed by returned pill counts, did not differ significantly between groups. Furthermore, no serious adverse events were reported throughout the study, although one child reported carotenodermia during the study, which resolved with reassurance. Obviously, future studies in larger populations and accounting further for various confounders are needed to validate the results of our study. The complex nature of the supplement preclude us from assigning causality of β-carotene per se as being responsible for the beneficial changes in adiposity and increase in total and HMW-ADI. However, the unique relationship between β-carotene and abdominal adiposity found in the current study is remarkable. Future studies with individual carotenoids, such as β-carotene alone, to target specific therapeutic serum levels are needed to validate the findings from the current study.
Conclusion
The data from the current study on the increase in serum β-carotene, HMW-ADI, and the concomitant decrease in the accrual of abdominal SAT in response to mixed carotenoid supplementation in children with obesity suggest a putative beneficial role of β-carotene in the prevention and/or management of obesity and related comorbidities. Further studies are needed to confirm these findings.
Acknowledgments
The authors thank Shawn Sweeten and Karl Mann for laboratory analysis and Tina M. Ewen, MA, Robbin Seago, MA, Amy Milkes, MA, Alex Taylor, MA, Lindsay Fuzzell, MA, for research assistance. We are also deeply grateful to our study participants and their families for their interest, enthusiasm, and dedication in these studies.
Clinical trial registry: NCT02060279 (registered 16 April 2013).
This study was funded by The Players Center for Child Health at Wolfson Children’s Hospital.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- at-RA
all-trans retinoic acid
- BMI
body mass index
- CV
coefficient of variation
- HDL-C
high density lipoprotein cholesterol
- HMW-ADI
high-molecular-weight adiponectin
- HOMA-2
homeostatic model assessment of insulin resistance-2
- MCS
mixed carotenoid supplement
- MRI
magnetic resonance imaging
- PPAR
peroxisome proliferator activated receptor
- SAT
subcutaneous adipose tissue
- VAT
visceral adipose tissue
- WHtR
waist-to-height ratio.
References
- 1. Li C, Ford ES, Mokdad AH, Cook S. Recent trends in waist circumference and waist-height ratio among US children and adolescents. Pediatrics. 2006;118(5):e1390–e1398. [DOI] [PubMed] [Google Scholar]
- 2. Garnett SP, Baur LA, Cowell CT. The prevalence of increased central adiposity in Australian school children 1985 to 2007. Obes Rev. 2011;12(11):887–896. [DOI] [PubMed] [Google Scholar]
- 3. Beydoun MA, Shroff MR, Chen X, Beydoun HA, Wang Y, Zonderman AB. Serum antioxidant status is associated with metabolic syndrome among U.S. adults in recent national surveys. J Nutr. 2011;141(5):903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beydoun MA, Canas JA, Beydoun HA, Chen X, Shroff MR, Zonderman AB. Serum antioxidant concentrations and metabolic syndrome are associated among U.S. adolescents in recent national surveys. J Nutr. 2012;142(9):1693–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res 2007;55:207–216. [DOI] [PubMed] [Google Scholar]
- 6. Bonet ML, Canas JA, Ribot J, Palou A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch Biochem Biophys. 2015;572:112–125. [DOI] [PubMed] [Google Scholar]
- 7.Canas JA, Damaso L, Altomare A, Killen K, Hossain J, Balagopal PB. Insulin resistance and adiposity in relation to serum beta-carotene levels. J Pediatr 2012;161:58–64 e52. [DOI] [PubMed] [Google Scholar]
- 8. Canas JA, Damaso L, Hossain J, Balagopal PB. Fatty acid binding proteins 4 and 5 in overweight prepubertal boys: effect of nutritional counselling and supplementation with an encapsulated fruit and vegetable juice concentrate. J Nutr Sci. 2015;4:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parker RS. Carotenoid and tocopherol composition of human adipose tissue. Am J Clin Nutr. 1988;47(1):33–36. [DOI] [PubMed] [Google Scholar]
- 10. Palczewski G, Amengual J, Hoppel CL, von Lintig J. Evidence for compartmentalization of mammalian carotenoid metabolism. FASEB J. 2014;28(10):4457–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chung H-Y, Ferreira ALA, Epstein S, Paiva SA, Castaneda-Sceppa C, Johnson EJ. Site-specific concentrations of carotenoids in adipose tissue: relations with dietary and serum carotenoid concentrations in healthy adults. Am J Clin Nutr. 2009;90(3):533–539. [DOI] [PubMed] [Google Scholar]
- 12. El-Sohemy A, Baylin A, Kabagambe E, Ascherio A, Spiegelman D, Campos H. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am J Clin Nutr. 2002;76(1):172–179. [DOI] [PubMed] [Google Scholar]
- 13. Bonet ML, Ribot J, Felipe F, Palou A. Vitamin A and the regulation of fat reserves. Cell Mol Life Sci. 2003;60(7):1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amengual J, Gouranton E, van Helden YG, Hessel S, Ribot J, Kramer E, Kiec-Wilk B, Razny U, Lietz G, Wyss A, Dembinska-Kiec A, Palou A, Keijer J, Landrier JF, Bonet ML, von Lintig J. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS One. 2011;6(6):e20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39(2):43–55. [DOI] [PubMed] [Google Scholar]
- 16. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1–190. [PubMed] [Google Scholar]
- 17. Burgert TS, Taksali SE, Dziura J, Goodman TR, Yeckel CW, Papademetris X, Constable RT, Weiss R, Tamborlane WV, Savoye M, Seyal AA, Caprio S. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91(11):4287–4294. [DOI] [PubMed] [Google Scholar]
- 18. Ross R, Freeman J, Hudson R, Janssen I. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women. J Clin Endocrinol Metab. 2002;87(11):5044–5051. [DOI] [PubMed] [Google Scholar]
- 19. Pollock NK, Bundy V, Kanto W, Davis CL, Bernard PJ, Zhu H, Gutin B, Dong Y. Greater fructose consumption is associated with cardiometabolic risk markers and visceral adiposity in adolescents. J Nutr. 2012;142(2):251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Plutzky J. The PPAR-RXR transcriptional complex in the vasculature: energy in the balance. Circ Res. 2011;108(8):1002–1016. [DOI] [PubMed] [Google Scholar]
- 21. Tamori Y, Masugi J, Nishino N, Kasuga M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes. 2002;51(7):2045–2055. [DOI] [PubMed] [Google Scholar]
- 22. Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Nagai R, Tobe K, Kimura S, Kadowaki T. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4(4):597–609. [DOI] [PubMed] [Google Scholar]
- 23. Lobo GP, Amengual J, Li HNM, Golczak M, Bonet ML, Palczewski K, von Lintig J. β,β-carotene decreases peroxisome proliferator receptor γ activity and reduces lipid storage capacity of adipocytes in a β,β-carotene oxygenase 1-dependent manner. J Biol Chem. 2010;285(36):27891–27899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kabat GC, Heo M, Ochs-Balcom HM, LeBoff MS, Mossavar-Rahmani Y, Adams-Campbell LL, Nassir R, Ard J, Zaslavsky O, Rohan TE. Longitudinal association of measures of adiposity with serum antioxidant concentrations in postmenopausal women. Eur J Clin Nutr. 2016;70(1):47–53. [DOI] [PubMed] [Google Scholar]
- 25. Östh M, Öst A, Kjolhede P, Strålfors P. The concentration of β-carotene in human adipocytes, but not the whole-body adipocyte stores, is reduced in obesity. PLoS One. 2014;9(1):e85610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Landrier JF, Kasiri E, Karkeni E, Mihály J, Béke G, Weiss K, Lucas R, Aydemir G, Salles J, Walrand S, de Lera AR, Rühl R. Reduced adiponectin expression after high-fat diet is associated with selective up-regulation of ALDH1A1 and further retinoic acid receptor signaling in adipose tissue. FASEB J. 2017;31(1):203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–2326. [DOI] [PubMed] [Google Scholar]
- 28. Winer JC, Zern TL, Taksali SE, Dziura J, Cali AM, Wollschlager M, Seyal AA, Weiss R, Burgert TS, Caprio S. Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J Clin Endocrinol Metab. 2006;91(11):4415–4423. [DOI] [PubMed] [Google Scholar]
- 29. Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29(6):1357–1362. [DOI] [PubMed] [Google Scholar]
- 30. Liu Y, Chen H, Mu D, Fan J, Song J, Zhong Y, Li D, Xia M. Circulating retinoic acid levels and the development of metabolic syndrome. J Clin Endocrinol Metab. 2016;101(4):1686–1692. [DOI] [PubMed] [Google Scholar]
- 31. Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278(11):9073–9085. [DOI] [PubMed] [Google Scholar]
- 32. Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55(1):249–259. [PubMed] [Google Scholar]
- 33. Suzuki K, Inoue T, Hashimoto S, Ochiai J, Kusuhara Y, Ito Y, Hamajima N. Association of serum carotenoids with high molecular weight adiponectin and inflammation markers among Japanese subjects. Clin Chim Acta. 2010;411(17-18):1330–1334. [DOI] [PubMed] [Google Scholar]
- 34. Kameji H, Mochizuki K, Miyoshi N, Goda T. β-Carotene accumulation in 3T3-L1 adipocytes inhibits the elevation of reactive oxygen species and the suppression of genes related to insulin sensitivity induced by tumor necrosis factor-α. Nutrition. 2010;26(11-12):1151–1156. [DOI] [PubMed] [Google Scholar]
- 35. Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51(10):2968–2974. [DOI] [PubMed] [Google Scholar]
- 36. Balagopal P, George D, Yarandi H, Funanage V, Bayne E. Reversal of obesity-related hypoadiponectinemia by lifestyle intervention: a controlled, randomized study in obese adolescents. J Clin Endocrinol Metab. 2005;90(11):6192–6197. [DOI] [PubMed] [Google Scholar]
- 37. Rühl R, Landrier JF. Dietary regulation of adiponectin by direct and indirect lipid activators of nuclear hormone receptors. Mol Nutr Food Res. 2016;60(1):175–184. [DOI] [PubMed] [Google Scholar]
- 38. Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289(14):1799–1804. [DOI] [PubMed] [Google Scholar]
- 39. Ben Amara N, Tourniaire F, Maraninchi M, Attia N, Amiot-Carlin MJ, Raccah D, Valéro R, Landrier JF, Darmon P. Independent positive association of plasma β-carotene concentrations with adiponectin among non-diabetic obese subjects. Eur J Nutr. 2015;54(3):447–454. [DOI] [PubMed] [Google Scholar]
- 40. Yoshida H, Yanai H, Ito K, Tomono Y, Koikeda T, Tsukahara H, Tada N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis. 2010;209(2):520–523. [DOI] [PubMed] [Google Scholar]