Abstract
Context:
Nonalcoholic fatty liver disease and elevated circulating branched-chain amino acids (BCAAs) are common characteristics of obesity and type 2 diabetes. In rodents, brain insulin signaling controls both hepatic triglyceride secretion and BCAA catabolism. Whether brain insulin signaling controls similar metabolic pathways in humans is unknown.
Objective:
Here we assessed if intranasal insulin, a method to preferentially deliver insulin to the central nervous system, is able to modulate hepatic lipid content and plasma BCAAs in humans.
Design/Setting:
We conducted a randomized, double-blind, placebo-controlled trial at the Medical University of Vienna.
Participants/Intervention:
We assessed if a chronic 4-week intranasal insulin treatment (40 IU, 4 times daily) reduces hepatic triglyceride content and circulating BCAAs in 20 healthy male volunteers.
Main Outcome Measures:
Hepatic lipid content was assessed noninvasively by 1H-magnetic resonance spectroscopy, and BCAAs were measured by gas chromatography mass spectrometry at defined time points during the study.
Results:
Chronic intranasal insulin treatment did not alter body weight, body mass index, and hepatic lipid content but reduced circulating BCAA levels.
Conclusions:
These findings support the notion that brain insulin controls BCAA metabolism in humans. Thus, brain insulin resistance could account at least in part for the elevated BCAA levels observed in the insulin-resistant state.
Chronic intranasal insulin does not affect hepatic lipid content but lowers circulating branched-chain amino acid levels in nonobese male human subjects.
Liver steatosis is closely associated with obesity and type 2 diabetes and represents the hepatic manifestation of the metabolic syndrome. Hepatic steatosis results from a net retention of lipids in the liver; thus, a key mechanism for the liver to fend off steatosis is to increase hepatic triglyceride (TG) export. The enhancement of brain insulin signaling in rodents through a chronic insulin infusion into the third ventricle increases hepatic very low-density lipoprotein secretion and within weeks leads to a reduction in hepatic lipid content (1). In humans, a single-dose administration of intranasal (IN) insulin administration, an administration mode used to deliver neuropeptides directly to the human brain (2), reduces hepatic lipid content 3 hours after IN insulin application in healthy insulin-sensitive humans (3).
The branched-chain amino acids (BCAAs) valine, leucine, and isoleucine have emerged as early markers for the future risk of type 2 diabetes (4). Vice versa, circulating BCAAs correlate with the severity of hepatic steatosis (5). Of note, brain insulin action plays a pivotal role in the regulation of circulating BCAA levels by inducing hepatic branched-chain α-ketoacid dehydrogenase expression, the rate-limiting enzyme in the BCAA degradation process, in male Sprague Dawley rats (6).
Hence, in this randomized, double-blind, placebo-controlled trial, we tested whether chronic IN insulin administered over a period of 28 days sustainably reduces hepatic TG content and/or lowers plasma BCAA levels in healthy male human subjects.
Materials and Methods
Study cohort
Twenty healthy male human subjects not taking any medication, without any history of liver illness, were recruited in this randomized double-blind placebo-controlled single-center trial. Before their inclusion into the study (NCT02164032; clinicaltrials.gov), all participants gave their written informed consent. The trial was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the local human ethics committee of the Medical University of Vienna (EK Nr.: 2024/2013). Study participants underwent a medical history, physical examination, and blood testing to exclude liver disease, diabetes or other endocrine diseases, sinusitis, nasal polyposis, infectious diseases, and potential contraindications to magnetic resonance (MR) imaging. None of the participants had clinical or laboratory signs of the previously-listed exclusion criteria. All study subjects were living a primarily sedentary lifestyle and were instructed to refrain from changing their dietary and exercise habits during the trial.
Study design
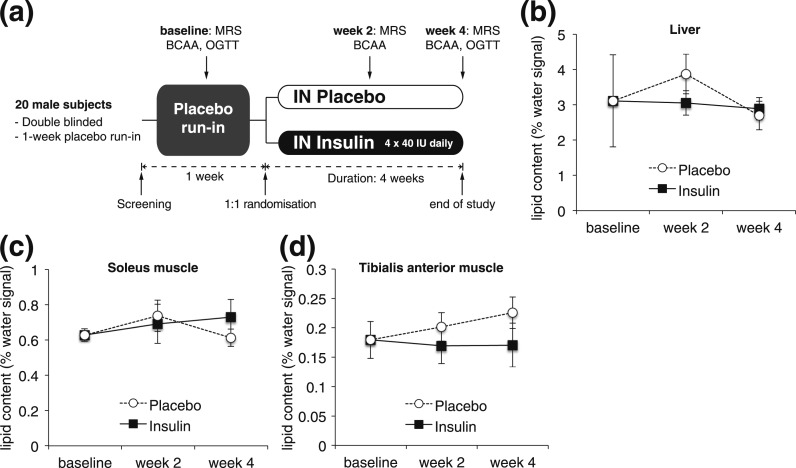
The outline of the study design is depicted in Fig. 1(a). At baseline blood samples for laboratory analyses were collected and the study subjects underwent an oral glucose tolerance test (OGTT) after an overnight fast. Subjects were then instructed to intranasally administer the study medication, and a 1-week placebo run-in phase was started. At the end of week 1, liver and muscle lipids were measured using magnetic resonance spectroscopy (1H-MRS), and plasma samples for BCAA analyses were collected after overnight fasting. Patients were then randomly assigned to either IN insulin or placebo treatment for 4 consecutive weeks. To ensure compliance, subjects were required to keep a diary on their daily IN insulin application routine. The study medication was renewed weekly. 1H-MRS and plasma BCAA levels were then determined at weeks 2 and 4. We deliberately chose a time course of 4 weeks to avoid confounding changes in body weight, which has been shown by others after 6 to 8 weeks of IN insulin application (7). To prevent potential acute effects of IN insulin, study subjects omitted the IN medication on the morning of an MR examination.
Figure 1.
IN insulin application for 4 weeks does not affect hepatic lipid content. (a) Study design. (b) Hepatic lipid content as assessed by 1H-MRS at baseline and after 2 and 4 weeks of IN insulin or placebo application. (c, d) Intramyocellular lipid content assessed by 1H-MRS at the indicated time points (baseline, week 2, and week 4) of the soleus muscle (c) and the tibialis anterior muscle (d). Values are baseline adjusted; all error bars are SEM; n = 10 per group.
IN insulin and placebo
In total, 40 IU of human insulin (100 IU/mL; Actrapid; Novo Nordisk, Mainz, Germany) was administered intranasally 4 times a day before each main meal and before going to bed using precision air pumps (Aero Pump, Hochheim, Germany) as described by others (8, 9). Each puff consisted of 0.1 mL (i.e., 10 IU of insulin). Insulin dilution buffer (Novo Nordisk), which exactly matches the carrier solution of human insulin (Actrapid; Novo Nordisk), was used as a placebo. The study medication was bottled by the in-house pharmacy of the Vienna General Hospital and handed out to the participants according to the randomization list generated with http://www.randomizer.org. Only a mild burning sensation in the nose directly following drug administration was occasionally reported for both groups. Study subjects were instructed to recognize clinical signs of hypoglycemia, but no hypoglycemic complications occurred.
Blood parameters
Blood sampling was performed after an overnight fast. To prevent potential acute effects of IN insulin, study subjects omitted the IN medication on the morning of the blood collection. All blood parameters, except for free fatty acids, listed in Table 1, were measured by routine laboratory methods at the Department of Medical and Chemical Laboratory Diagnostics, Medical University of Vienna. Detailed descriptions of the assays can be found at http://www.kimcl.at. Free fatty acids were measured using a colorimetric assay (Wako, Neuss, Germany) according to the manufacturer’s instructions.
Table 1.
Cohort Details and Blood Parameters of IN Insulin- and Placebo-Treated Subjects at Baseline and After 4 Weeks of Treatment
| Characteristic | Baseline | Week 4 | ||||
|---|---|---|---|---|---|---|
| Placebo | Insulin | P Value (vs Placebo) | Placebo | Insulin | P Value (vs Placebo) | |
| Cohort details | ||||||
| Age, y | 29 ± 3 | 36 ± 4 | 0.20 | |||
| Weight, kg | 83.4 ± 2 | 84.0 ± 3 | 0.87 | 83.2 ± 2 | 84.9 ± 3 | 0.65 |
| Waist circumference, cm | 87 ± 3 | 89 ± 2 | 0.70 | 90 ± 2 | 89 ± 3 | 0.94 |
| Waist to hip ratio | 0.84 ± 0.03 | 0.86 ± 0.03 | 0.73 | 0.88 ± 0.02 | 0.86 ± 0.03 | 0.67 |
| BMI, kg/m2 | 25.4 ± 0.5 | 24.6 ± 0.7 | 0.34 | 25.3 ± 0.5 | 24.8 ± 0.7 | 0.54 |
| Glucose metabolism | ||||||
| HOMA | 2 ± 0.3 | 1.5 ± 0.2 | 0.20 | 1.7 ± 0.3 | 1.2 ± 0.1 | 0.14 |
| Matsuda index | 6.6 ± 1.3 | 8.2 ± 1.4 | 0.40 | 7.1 ± 0.6 | 8.5 ± 1.3 | 0.38 |
| HbA1c, % | 4.9 ± 0.1 | 4.9 ± 0.1 | 0.86 | 4.9 ± 0.1 | 4.9 ± 0.1 | 0.87 |
| Insulin, µU/mL | 9 ± 1.2 | 7.6 ± 1.1 | 0.36 | 7.9 ± 0.9 | 5.9 ± 0.5 | 0.08 |
| C-peptide, ng/mL | 2.0 ± 0.2 | 2.1 ± 0.2 | 0.90 | 2.0 ± 0.2 | 1.8 ± 0.2 | 0.65 |
| Glucose, mg/dL | 85 ± 5 | 80 ± 5 | 0.51 | 85 ± 5 | 83 ± 2 | 0.66 |
| Lipid parameters | ||||||
| Triglycerides, mg/dL | 91 ± 16 | 108 ± 21 | 0.52 | 85 ± 8 | 117 ± 26 | 0.25 |
| Free fatty acids, µmol/L | 321 ± 70 | 318 ± 80 | 0.98 | 419 ± 78 | 301 ± 42 | 0.18 |
| Cholesterol, mg/dL | 159 ± 7 | 192 ± 14 | 0.05 | 161 ± 7 | 196 ± 13 | 0.03 |
| HDL, mg/dL | 46 ± 3 | 54 ± 5 | 0.19 | 49 ± 3 | 54 ± 4 | 0.35 |
| LDL, mg/dL | 95 ± 7 | 116 ± 13 | 0.17 | 95 ± 5 | 118 ± 12 | 0.08 |
| Non-HDL, mg/dL | 113 ± 7 | 138 ± 16 | 0.18 | 106 ± 8 | 142 ± 16 | 0.07 |
| Protein metabolism | ||||||
| Creatinine, mg/dL | 0.90 ± 0.03 | 0.97 ± 0.04 | 0.15 | 0.89 ± 0.04 | 0.97 ± 0.04 | 0.23 |
| Total protein, g/L | 69 ± 1 | 70 ± 1 | 0.65 | 70 ± 1 | 69 ± 1 | 0.71 |
| Albumin, g/L | 47 ± 0.6 | 46 ± 1.2 | 0.80 | 46 ± 0.7 | 47 ± 0.8 | 0.41 |
| Endocrine parameters | ||||||
| Thyrotropin, µU/mL | 2.4 ± 0.3 | 2.5 ± 0.3 | 0.81 | 2.5 ± 0.3 | 2.4 ± 0.3 | 0.86 |
| Free thyroxine, ng/dL | 1.22 ± 0.22 | 1.33 ± 0.06 | 0.13 | 1.25 ± 0.05 | 1.22 ± 0.05 | 0.68 |
| Luteinizing hormone, mU/mL | 4.0 ± 0.4 | 4.9 ± 0.6 | 0.23 | 4.4 ± 0.5 | 4.8 ± 0.5 | 0.50 |
| Follicle-stimulating hormone, mU/mL | 3.5 ± 0.7 | 4.7 ± 0.7 | 0.21 | 3.7 ± 0.6 | 4.4 ± 0.5 | 0.37 |
| Free testosterone, ng/mL | 2.5 ± 0.2 | 3.4 ± 0.8 | 0.29 | 2.7 ± 0.3 | 2.6 ± 0.3 | 0.79 |
| Prolactin, ng/mL | 11 ± 1 | 11 ± 2 | 0.70 | 12 ± 1 | 9 ± 1 | 0.16 |
| Somatotropin, ng/mL | 1 ± 0.7 | 0.8 ± 0.3 | 0.79 | 0.3 ± 0.1 | 0.8 ± 0.4 | 0.20 |
| IGF-1, ng/mL | 269 ± 25 | 234 ± 20 | 0.29 | 253 ± 16 | 244 ± 21 | 0.73 |
| Cortisol, µg/dL | 22.1 ± 2.1 | 18.0 ± 0.8 | 0.10 | 17.9 ± 2.1 | 12.2 ± 0.9a | 0.02 |
All values are mean ± SEM; n = 10 per group.
Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA, homeostatic model assessment; IGF-1, insulin-like growth factor 1.
Paired t test (baseline vs week 4), P < 0.01.
Amino acid analyses were performed using gas chromatography mass spectrometry (Agilent technology GC7890-MS5975; Agilent Technologies, Santa Clara, CA) from plasma samples collected after an overnight fast at baseline and after weeks 2 and 4. Briefly, an 8-mL EDTA plasma sample was deproteinized with 1 mL methanol and subsequently purified by ion exchange columns and derivatized using N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide (Sigma, St. Louis, MO). Plasma concentrations were determined using as internal standard a mix of 13C-labeled amino acids (Celtone; CIL, Cambridge, MA). The following amino acid concentrations were measured: aromatic amino acids, phenylalanine and tyrosine; BCAAs, leucine, isoleucine, and valine; and alanine, lysine, histidine, proline, threonine, glutamate, serine, aspartic acid, methionine, and glycine.
OGTT
The OGTT was performed after an overnight fast. Blood was drawn from a venous catheter inserted into an antecubital vein at fasting and 30, 60, 90, and 120 minutes after drinking a 75-g glucose solution for determination of plasma glucose and insulin levels. The Matsuda index was calculated as described in Matsuda and DeFronzo (10).
MR imaging and 1H-MRS
Hepatic lipid content was assessed noninvasively using 1H-MRS over the course of 4 weeks. 1H-MRS measurements were performed with a 3.0-T Tim Trio System (Siemens Healthcare, Erlangen, Germany) using a combination of multichannel spine and body-flex receiver coils from the MR manufacturer. Liver, tibialis anterior, and soleus muscle lipid content was measured at baseline and after 2 and 4 weeks of IN insulin or placebo treatment by using localized single-voxel 1H-MRS as shown elsewhere (11, 12).
Statistical analysis
Data are presented as means ± standard errors of the means (SEMs). In Table 1 and Supplemental Table 1, comparison between groups was performed by using an unpaired 2-tailed Student t test. Comparison between baseline and week 4 within the treatment group was performed by using a paired 2-tailed Student t test. In Figs. 1 and 2, baseline adjustment was achieved by individually subtracting averaged baseline values from posttreatment changes. An unpaired 2-tailed Student t test was used for comparisons. For Fig. 2(c), we used Pearson correlation and a 2-tailed t test. Areas under the curve were calculated using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA). A P value of <0.05 was considered statistically significant.
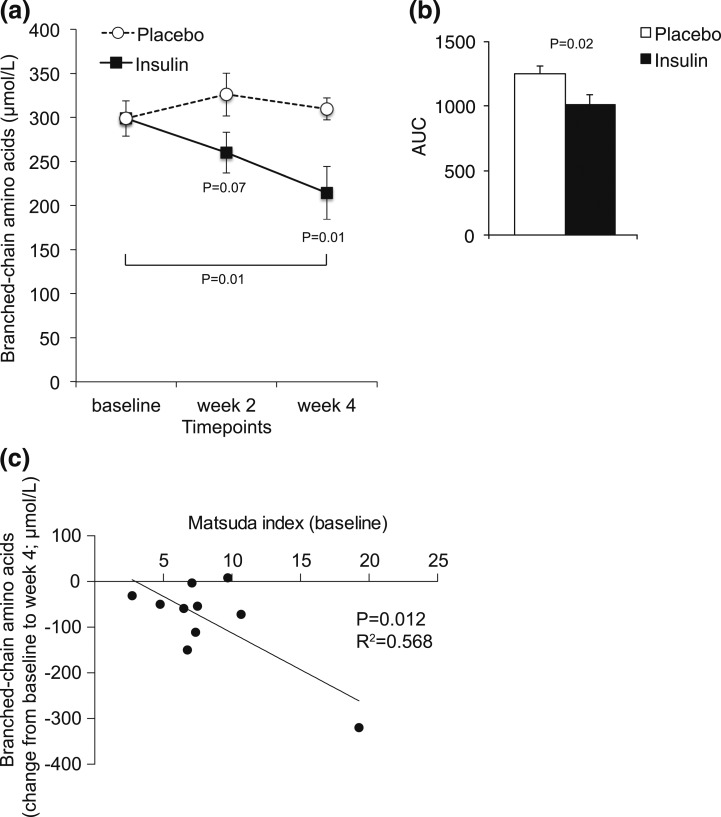
Figure 2.
Chronic IN insulin application for 4 weeks reduces circulating BCAA levels. Fasting plasma BCAA levels at baseline and weeks 2 and 4 following IN insulin or placebo treatment. (a) Baseline-adjusted plasma BCAA levels. (b) Area under the curve (AUC) of the graphs depicted in panel (a). Pearson correlation of baseline Matsuda index and absolute change in BCAA levels after 4 weeks of IN insulin treatment (c). All error bars are SEM; P values vs the control group or as indicated by the bracket; n = 10 per group.
Results
Baseline characteristics
The study participants’ body weight (83.4 ± 2 vs 84.0 ± 3 kg) and body mass index (25.4 ± 0.5 vs 24.6 ± 0.7 kg/m2) were similar between groups. Due to randomization, the mean age (29 ± 3 vs 36 ± 4 years) differs by 7 years, but this did not reach statistical significance. Baseline (week 1) parameters of glucose metabolism and insulin sensitivity were comparable between the 2 groups (Table 1). Except for a minor difference in baseline total cholesterol (18%, P = 0.052) and low-density lipoprotein cholesterol, which persisted throughout the treatment period, all other lipid parameters were similar between IN insulin and placebo-treated individuals. Furthermore, baseline thyroid function parameters, cortisol levels, and pituitary hormones were comparable in both groups (Table 1).
Chronic IN insulin treatment had no effects on hepatic lipid content
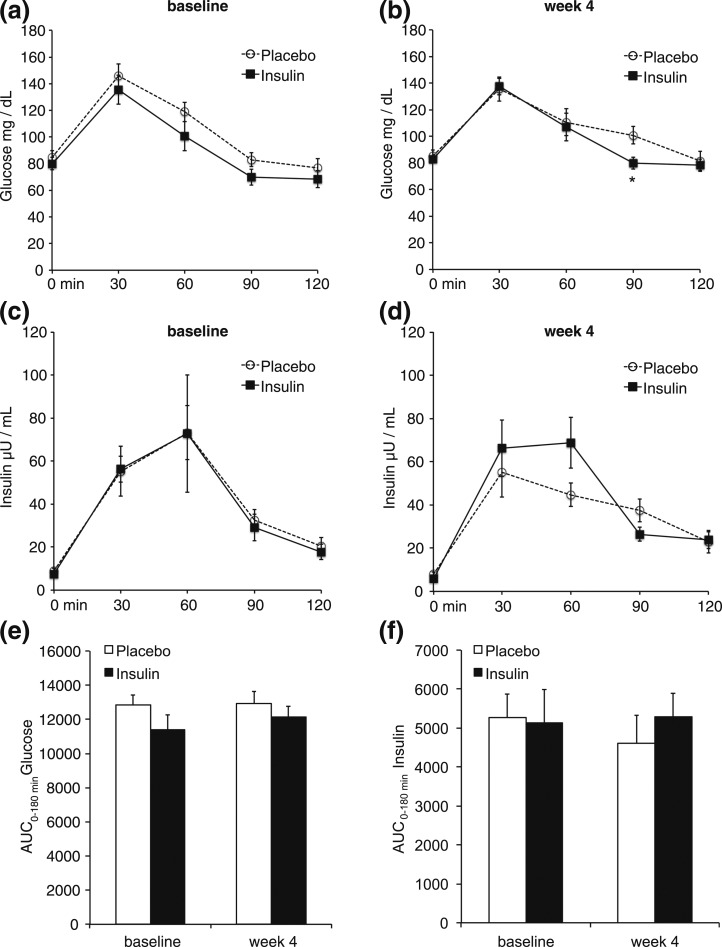
Hepatic lipid content as assessed by 1H-MRS at weeks 2 and 4 was not different during the IN insulin treatment [Fig. 1(b) and Supplemental Fig. 1(a)]. Notably, after 4 weeks of IN insulin administration, no changes in body weight and body mass index were detected (Table 1), in accordance with earlier reports (7) that show statistically significant changes occurring only after 6 to 8 weeks of IN insulin treatment. Thus, it is noteworthy that data reported herein are independent of confounding changes in body weight. Furthermore, no changes in intramyocelluar lipid content, a marker for whole-body insulin sensitivity (13), were detected [Fig. 1(c) and 1(d); Supplemental Fig. 1(b) and 1(c)]. In line with this, circulating glucose and insulin levels did not differ during an OGTT after 4 weeks of IN insulin treatment [Fig. 3(a–e)]. However, morning cortisol levels were decreased after 4 weeks of IN insulin treatment (–32%; Table 1) compared with the placebo group, which is consistent with previous reports (14). Conversely, no changes in growth hormone and insulin-like growth factor 1 levels were detected after IN insulin exposure (Table 1).
Figure 3.
IN insulin administration for 4 weeks does not change glucose tolerance. Plasma glucose levels at the indicated time points after an oral glucose load at baseline (a) and after 4 weeks of IN insulin or placebo (b). Plasma insulin levels at the indicated time points measured during the oral glucose tolerance test at baseline (c) and after 4 weeks of IN insulin or placebo (d). Corresponding areas under the curve (AUCs) of glucose (e) and insulin (f) levels depicted in panels (a–d) at baseline and after 4 weeks of IN insulin/placebo treatment. All error bars are SEM; P value vs the control group; n = 10 per group.
Chronic IN insulin treatment lowered plasma BCAA levels
Rodent studies have shown that brain insulin action lowers plasma BCAA levels by inducing BCAA catabolism in the liver (6); thus, we hypothesized that IN insulin reduces circulating BCAA. Consistent with the findings in rodents, we observed a decrease in plasma BCAA (leucine, isoleucine, and valine) levels compared with baseline after 2 weeks (–13%) and 4 weeks (–28%) [Fig. 2(a) and 2(b)] of IN insulin treatment. Analysis of aromatic amino acids, including tyrosine and phenylalanine, which have also been implicated in predicting diabetes risk, showed a similar reduction (Supplemental Table 1), whereas total protein content in plasma was unchanged. Of note, the subjects with the highest Matsuda index, a measure that correlates tightly with insulin sensitivity, displayed the strongest suppression of BCAA after IN insulin application [Fig. 2(c)].
Discussion
To our knowledge, this is the first randomized, double-blind, placebo-controlled trial using chronic IN insulin delivery to assess the role of brain insulin signaling in hepatic lipid and BCAA metabolism in humans. There are two major findings of this study: first, IN insulin treatment of 4 weeks was unable to reduce hepatic lipid content in healthy male nonobese subjects (Fig. 1). Second, IN insulin treatment lowered plasma BCAA levels as early as after 2 weeks (Fig. 2), consistent with rodent studies in which the stereotaxic infusion of insulin selectively into the mediobasal hypothalamus lowered plasma BCAA levels by increasing hepatic BCAA catabolism (6).
Interestingly, IN insulin was most effective in suppressing BCAA in subjects with a high baseline Matsuda index [Fig. 2(c)], which suggests that the more insulin sensitive a person is, the more effective is IN insulin treatment in lowering plasma BCAA levels. This observation supports the notion that brain insulin resistance contributes to the elevated plasma BCAA levels seen in patients with diabetes. Because we only measured circulating BCAAs at 3 time points, we are unable to assess whether an increase in BCAA catabolism or a reduction in proteolysis accounts for the observed drop in BCAA levels in the IN insulin-treated group, a question that should be subject of future studies. Systemic insulin levels tended to be lower in the IN insulin group (7.9 ± 0.5 vs 5.9 ± 0.5 µU/mL; P = 0.08), although a paired t test comparing baseline to week 4 did not reach statistical significance (P = 0.13), which could be due to the limited sample size. Because fasting glucose levels did not change, the same trend was also apparent for the homeostatic model assessment score (1.7 ± 0.3 vs 1.2 ± 0.14; P = 0.14) suggestive of an improvement in insulin sensitivity, which has been reported by others after acute IN insulin application (15).
A previous report demonstrated that a single IN insulin dose reduced hepatic TG content after only 3 hours, demonstrating that IN insulin reduces hepatic lipids after an acute IN insulin bolus (3). This discrepancy between our study assessing the effects of chronic IN insulin treatment with that of the acute IN insulin administration in humans and long-term intracerebroventricular insulin infusion in rodents could be explained through several mechanisms (1, 3). First, endogenous insulin signaling may be sufficient in healthy nonobese individuals to warrant a normal hepatic TG content, and any additional increase in brain insulin signaling may hence be unable to further lower hepatic lipids in the long term. Second, rats continuously gain weight and become more insulin resistant during the process of aging (16, 17). Thus, the previous rat study (1), in which a chronic brain insulin infusion lowered hepatic lipid content and prevented the increase in hepatic lipid content associated with weight gain, does not exactly mimic the current study in humans. In contrast to the rat study, where body weights increased during the 4-week infusion period (+35% in 4 weeks), in the human study, body weights and insulin sensitivity were unchanged (Table 1). Thus, endogenous brain insulin signal may have remained sufficient to maintain liver TG content at normal levels in the human study, whereas in the rat study, due to the constant increase in body weight, the brain insulin infusion counteracted the development of insulin resistance in the brain and thereby led to the improvement in liver lipids. Hence, an important future question will be to examine if enhancement of brain insulin signaling by IN insulin in insulin-resistant individuals will be sufficient to reduce hepatic TG levels. This notion is supported by the fact that insulin-resistant subjects also exhibit brain insulin resistance (18), and enhancement of brain insulin signaling in these individuals may reduce hepatic lipid content to a greater degree than in insulin-sensitive individuals.
Third, the baseline insulin sensitivity was likely different in the acute study by Gancheva et al. (3) vs our chronic IN insulin study. In fact, in our study cohort, the homeostatic model assessment score was indeed normal but twice as high as in the study by Gancheva et al. (3) (1.7 vs 0.8), suggesting that in the latter study, subjects were exquisitely insulin sensitive. Thus, in our trial, the borderline body mass index of 25 kg/m2 and higher homeostatic model assessment score may be indicative of some degree of insulin resistance, which may have decreased the overall effectiveness of the IN insulin treatment to increase hepatic TG secretion and reduce hepatic lipid content.
Fourth, it is possible that chronic IN insulin treatment induces some degree of brain insulin resistance due to brain insulin receptor desensitization as a result of recurrent brain hyperinsulinemia.
Last, the relatively short duration of the trial may have limited the effects on hepatic lipid content. However, the extended application of IN insulin would have complicated the interpretation of altered hepatic TG content, because changes in body weights would be expected after prolonged IN insulin treatment and in turn can affect hepatic TG content (7). Because in our study, no differences in body weights were detected, the reduction in circulating BCAA levels in the IN insulin group was weight independent.
Furthermore, the IN insulin formulations commonly used in the field are not pharmacologically optimized for IN application. Only a few studies have attempted to examine the uptake of intranasal peptides into the cerebrospinal fluid or the brain in humans. From these studies, it seems that IN insulin has a relatively low nose-brain uptake compared with other peptides such as vasopressin or melanocortin (2). Thus, relatively high IN doses are necessary to achieve sufficient brain delivery, and acute studies should control for transient systemic insulin peaks after IN insulin application. In the present trial, we conducted the final measurements after an overnight fast, omitting the IN insulin dose in the morning to isolate the chronic effects of IN insulin. Notably, our IN insulin dose is only one-fourth of that used in previous acute IN insulin experiments (3), which reduced hepatic lipid content independent of any potential effect from the minor systemic spillage, which was controlled for through an intravenous bolus of 0.1 IU of insulin. The spillover correction for 40 IU of IN insulin in our chronic study would translate to 0.025 IU, which represents as little as 0.04% of the estimated endogenous insulin production over 24 hours (19). Of note, a similar intravenous bolus infusion, although feasible in acute studies, is not practical in chronic studies, because the correction would amount to a total of only 2.8 IU of insulin in 4 weeks. Furthermore, because the dose that mimics a potential spillover is so low, it is likely not clinically important. Based on this and the fact that C-peptide and insulin levels were unchanged throughout the study (Table 1), minor systemic spillover of IN insulin cannot be formally ruled out but is unlikely to account for the reductions in plasma BCAAs.
In conclusion, our study demonstrates that chronic IN insulin treatment fails to affect hepatic lipids in healthy nonobese subjects but reduces circulating BCAAs. Hence, brain insulin controls circulating BCAA levels in humans, and plasma BCAA levels may represent a marker of brain insulin action. Increased plasma BCAA levels in obesity and type 2 diabetes may be a consequence of brain insulin resistance.
Supplementary Material
Acknowledgments
We thank the in-house pharmacy of the Vienna General Hospital and especially Bernadette Aretin for providing logistic support. We also thank Armin Motaabbed for helping with data administration.
This study was in part funded by grants from the Austrian Heart Foundation and the Medical Scientific Fund of the Mayor of the City of Vienna (grant 12023) to M.Kre, the Austrian Association of Endocrinology and Metabolism (ÖGES) to P.W., the Austrian National Bank (Nr. 13249 and 15363) to M.Krs, and the Austrian Science Fund (grant 26766) and the Medical Scientific Fund of the Mayor of the City of Vienna (grant 15228) to T.S.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- BCAA
branched-chain amino acid
- 1H-MRS
magnetic resonance spectroscopy
- IN
intranasal
- MR
magnetic resonance
- OGTT
oral glucose tolerance test
- SEM
standard error of the mean
- TG
triglyceride.
References
- 1. Scherer T, Lindtner C, O’Hare J, Hackl M, Zielinski E, Freudenthaler A, Baumgartner-Parzer S, Tödter K, Heeren J, Krššák M, Scheja L, Fürnsinn C, Buettner C. Insulin regulates hepatic triglyceride secretion and lipid content via signaling in the brain. Diabetes. 2016;65(6):1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5(6):514–516. [DOI] [PubMed] [Google Scholar]
- 3. Gancheva S, Koliaki C, Bierwagen A, Nowotny P, Heni M, Fritsche A, Häring HU, Szendroedi J, Roden M. Effects of intranasal insulin on hepatic fat accumulation and energy metabolism in humans. Diabetes. 2015;64(6):1966–1975. [DOI] [PubMed] [Google Scholar]
- 4. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng S, Wiklund P, Autio R, Borra R, Ojanen X, Xu L, Törmäkangas T, Alen M. Adipose tissue dysfunction and altered systemic amino acid metabolism are associated with non-alcoholic fatty liver disease. PLoS One. 2015;10(10):e0138889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shin AC, Fasshauer M, Filatova N, Grundell LA, Zielinski E, Zhou JY, Scherer T, Lindtner C, White PJ, Lapworth AL, Ilkayeva O, Knippschild U, Wolf AM, Scheja L, Grove KL, Smith RD, Qian WJ, Lynch CJ, Newgard CB, Buettner C. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab. 2014;20(5):898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes. 2004;53(11):3024–3029. [DOI] [PubMed] [Google Scholar]
- 8. Benedict C, Brede S, Schiöth HB, Lehnert H, Schultes B, Born J, Hallschmid M. Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes. 2010;60(1):114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jauch-Chara K, Friedrich A, Rezmer M, Melchert UH, Scholand-Engler HG, Hallschmid M, Oltmanns KM. Intranasal insulin suppresses food intake via enhancement of brain energy levels in humans. Diabetes. 2012;61(9):2261–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 11. Wolf P, Krššák M, Winhofer Y, Anderwald CH, Zwettler E, Just Kukurová I, Gessl A, Trattnig S, Luger A, Baumgartner-Parzer S, Krebs M. Cardiometabolic phenotyping of patients with familial hypocalcuric hypercalcemia. J Clin Endocrinol Metab. 2014;99(9):E1721–E1726. [DOI] [PubMed] [Google Scholar]
- 12. Anderwald C, Bernroider E, Krssak M, Stingl H, Brehm A, Bischof MG, Nowotny P, Roden M, Waldhäusl W. Effects of insulin treatment in type 2 diabetic patients on intracellular lipid content in liver and skeletal muscle. Diabetes. 2002;51(10):3025–3032. [DOI] [PubMed] [Google Scholar]
- 13. Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42(1):113–116. [DOI] [PubMed] [Google Scholar]
- 14. Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signaling. Int J Obes. 2007;32(2):275–282. [DOI] [PubMed] [Google Scholar]
- 15. Heni M, Wagner R, Kullmann S, Veit R, Mat Husin H, Linder K, Benkendorff C, Peter A, Stefan N, Häring HU, Preissl H, Fritsche A. Central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes. 2014;63(12):4083–4088. [DOI] [PubMed] [Google Scholar]
- 16. Barzilai N, Rossetti L. Relationship between changes in body composition and insulin responsiveness in models of the aging rat. Am J Physiol. 1995;269(3 Pt 1):E591–E597. [DOI] [PubMed] [Google Scholar]
- 17. Barzilai N, Rossetti L. Age-related changes in body composition are associated with hepatic insulin resistance in conscious rats. Am J Physiol. 1996;270(6 Pt 1):E930–E936. [DOI] [PubMed] [Google Scholar]
- 18. Tschritter O, Preissl H, Hennige AM, Stumvoll M, Porubska K, Frost R, Marx H, Klösel B, Lutzenberger W, Birbaumer N, Häring HU, Fritsche A. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci USA. 2006;103(32):12103–12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kruszynska YT, Home PD, Hanning I, Alberti KG. Basal and 24-h C-peptide and insulin secretion rate in normal man. Diabetologia. 1987;30(1):16–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.