Abstract
Background
Diseases caused by Aedes-borne viruses, such as dengue, Zika, chikungunya, and yellow fever, are emerging and reemerging globally. The causes are multifactorial and include global trade, international travel, urbanisation, water storage practices, lack of resources for intervention, and an inadequate evidence base for the public health impact of Aedes control tools. National authorities need comprehensive evidence-based guidance on how and when to implement Aedes control measures tailored to local entomological and epidemiological conditions.
Methods and findings
This review is one of a series being conducted by the Worldwide Insecticide resistance Network (WIN). It describes a framework for implementing Integrated Aedes Management (IAM) to improve control of diseases caused by Aedes-borne viruses based on available evidence. IAM consists of a portfolio of operational actions and priorities for the control of Aedes-borne viruses that are tailored to different epidemiological and entomological risk scenarios. The framework has 4 activity pillars: (i) integrated vector and disease surveillance, (ii) vector control, (iii) community mobilisation, and (iv) intra- and intersectoral collaboration as well as 4 supporting activities: (i) capacity building, (ii) research, (iii) advocacy, and (iv) policies and laws.
Conclusions
IAM supports implementation of the World Health Organisation Global Vector Control Response (WHO GVCR) and provides a comprehensive framework for health authorities to devise and deliver sustainable, effective, integrated, community-based, locally adapted vector control strategies in order to reduce the burden of Aedes-transmitted arboviruses. The success of IAM requires strong commitment and leadership from governments to maintain proactive disease prevention programs and preparedness for rapid responses to outbreaks.
Author summary
Aedes aegypti and A. albopictus are mosquito species that thrive in towns and cities and can transmit viruses to humans that cause diseases, such as dengue, Zika, chikungunya, and yellow fever. The geographic range of human infection with these viruses is rapidly expanding globally. Even when preventative or therapeutic treatments are available to fight these diseases, controlling the mosquito vector will remain an important control option. We therefore developed a framework called IAM that offers decision-making guidance based on available evidence of effective control of Aedes at different levels of infestation and virus transmission risk. Our work aims to strengthen the capacity of countries at risk of and/or affected by these diseases and vectors so they will be better prepared for existing and emerging Aedes-borne disease threats.
Introduction
During the past 50 years, Aedes-borne diseases, such as dengue, Zika, chikungunya, and yellow fever, have emerged and/or reemerged globally [1]. Dengue virus is on the rise, causing about 390 million human infections per year; chikungunya virus spread worldwide in the early 2000s; Zika virus spread worldwide in the past three years; and yellow fever has resurged in Africa and the Americas [1, 2]. The expansion of these diseases can be explained in part by an intensification of the conditions favouring the dispersal and proliferation of Aedes as a result of global trade and unplanned urbanisation; inefficient implementation of vector control programmes due to inadequate human, financial, and infrastructural capacities; erratic water supply and associated water storage practices; ineffective waste disposal; and a lack of community engagement and political will [3, 4, 5]. All of the viruses that cause these diseases are transmitted primarily by the tropical yellow fever mosquito Aedes aegypti, and to a lesser extent by A. albopictus, the Asian tiger mosquito, of which there are both temperate and tropical strains [2]. The total global economic impact of Aedes vectors and related diseases is still unknown [6], but economic losses due to dengue have been estimated to be at least US$ 9 billion annually [7].
Although there is a vaccine for yellow fever, there are currently no commercially available drugs or vaccines for Zika or chikungunya. A dengue vaccine (Dengvaxia), developed by Sanofi-Pasteur, has been approved in several countries but has safety concerns for mass administration [8]. Moreover, new viruses may potentially emerge that could be transmitted by these vectors. Preventing or reducing disease caused by currently recognised or novel Aedes-borne viruses on a global scale continues to depend largely on controlling mosquito vector populations or interrupting human–vector contact.
Historically, well-implemented vertical Aedes control programmes were successful in controlling yellow fever in the Americas (1900s to 1960s) and, more recently, dengue in Singapore (1970s to 1980s) and Cuba (1980s to 1990s) [9]. Unfortunately, the recent resurgence of Aedes-transmitted arbovirus outbreaks throughout the world highlights the limitations of vector control, as currently deployed, to reduce the incidence of disease [1, 4, 10].
Efforts to address this increasingly urgent challenge have been boosted by a renewed focus on strengthening vector control, as witnessed at the May 2017 World Health Assembly, where the Global Vector Control Response (GVCR) received strong support from member states [11]. The GVCR provides countries with high-level, strategic guidance to reduce the burden and threat of vector-borne diseases—including Aedes-borne viruses—through effective, locally optimised, sustainable vector control. It aims to strengthen 2 foundations of vector control: (i) basic and applied research, and (ii) capacity and skill development. Building on these foundations, the GVCR advocates 4 pillars of action to be undertaken by countries: (i) vector surveillance and monitoring and evaluation (M&E); (ii) integrated application of control tools and approaches; (iii) engagement and mobilisation of communities; and (iv) inter- and intrasectoral collaboration. A number of enabling factors are required to achieve the desired results, including strong country leadership for resource mobilisation.
Despite this fresh impetus, many countries are still unprepared to address the challenge of Aedes-borne diseases and lack practical guidance on how and when to deploy vector control interventions in different entomological and epidemiological settings. This review was conducted by members of the Worldwide Insecticide resistance Network (WIN) [10] with the aim to support implementation of the GVCR by offering detailed recommendations for: (i) integrated vector and disease surveillance, (ii) vector control strategies, (iii) social mobilisation, and (iv) multisectoral approaches, providing a framework targeted to Aedes distribution and level of disease risk. We offer evidence-based guidance to implementing Integrated Aedes Management (IAM) systems and for strengthening national capacities so that public health programmes are better prepared for the emerging threat of Aedes-borne diseases.
Decision-making based on transmission risk and Aedes distribution scenarios
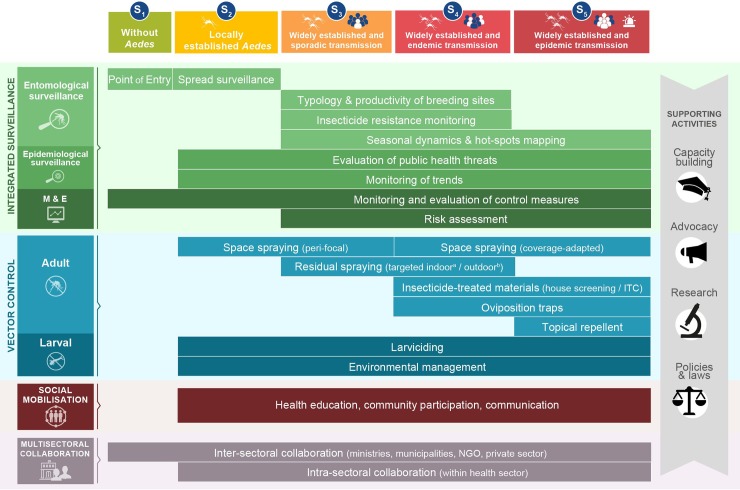
IAM proposes to tailor vector control responses according to the following 5 scenarios based on the local stage of Aedes distribution and level of virus transmission risk: Scenario 1 (or S1), no Aedes present (and no transmission); S2, Aedes locally established and no transmission; S3, Aedes widely established and sporadic transmission; S4, Aedes widely established and endemic transmission; and S5, Aedes widely established and epidemic transmission (Fig 1; Box 1). Risk scenarios are not fixed in space (at country, province, or district levels) or time and are likely to evolve based on updates in entomological and epidemiological risk assessment. The IAM aims to provide ‘graduated’ responses according to the risk level, but the switch from one scenario to another does not systematically follow a ‘linear transition’. For example, Key West, Florida, transitioned directly from S3 to S5 during the 2017’s Zika outbreak. Similarly, La Reunion Island and Italy switched from S3 to S5 during the chikungunya outbreak in 2007. Note that S4 (‘endemic transmission’) will typically be applied to viruses that have been established in a given location of some time, e.g., endemic dengue. A novel introduction and spread of a new arbovirus (e.g., Zika or chikungunya) or a new dengue serotype can rapidly produce a transition to an outbreak (S5).
Fig 1. Conceptual framework of the IAM system.
IAM builds on 4 pillars of activities (integrated surveillance, vector control, social mobilisation, and multisectoral collaboration) and 4 supporting activities (capacity building, advocacy, policies and laws, and research). Activities are tailored to local scenarios of Aedes distribution and virus transmission risk (see Box 1 for definition of terms). aA. aegypti; bA. albopictus. IAM, Integrated Aedes Management; ITC, insecticide treated curtain; M&E, monitoring and evaluation; NGO, nongovernmental organisation; S, scenario.
Box 1. Definition of key terms
Aedes: We use the term Aedes for those members of the Aedes subgenus Stegomyia, the principal arbovirus vector A. aegypti and the secondary vector A. albopictus, although our approach may be equally applicable to other container-inhabiting vectors, such as A. polynesiensis, A. japonicus, or even other species.
Locally established: Indicates a situation in which the species has colonised an area of less than 25 km2 [52].
Widely established: Indicates a situation in which the species has colonised an area of more than 25 km2. European guidelines recommend an area greater than 25 km2 for the ‘widely established’ surveillance of invasive mosquitoes [52].
Sporadic transmission: Refers to a situation in which autochthonous transmission occurs locally, irregularly, and unpredictably through viraemic travellers returning from disease-endemic countries [21, 53].
Endemic transmission: Refers to the constant occurrence of cases of Aedes-borne disease limited in space but not in time (sustained temporal transmission) and where the highest number of cases is reported during a particular season [53, 54].
Epidemic transmission: Refers to the occurrence of human cases of Aedes-borne viruses limited in space and time and clearly in excess of the number of cases normally expected [21, 53].
Integrated surveillance and M&E
Integrated surveillance is an ongoing systematic collection, recording, analysis, interpretation, and dissemination of data to aid control efforts for initiating suitable public health interventions for prevention and control, including the M&E of the implemented control measures [12]. Collecting and using data in this way is intended to support assessment of risk for introduction and spread of vectors and viruses and to monitor and evaluate the control efforts followed by adjustments over time if necessary in accordance with predefined indicators (Table 1 and S1 Table). Entomological and epidemiological surveillance data should be promptly integrated, which will require efficient collaboration between vector-control and public health programs, and made available on a shared, easily accessible platform (e.g., the DHIS2, https://www.dhis2.org/overview).
Table 1. Integrated surveillance and M&E of Aedes and Aedes-borne diseases.
| Tasks | Objectives | Description | Methodology/specifications | References | |
|---|---|---|---|---|---|
| Entomological surveillance | Surveillance of routes of entry and monitoring the spread | The main routes of Aedes introduction and spread are harbours, international airports, accumulation in imported goods (such as tyres or lucky bamboo plants), borders, ground crossings, and traffic corridors (such as highways). | Ovitraps, oviposition traps (AGOs and GATs) and BG-Sentinel traps are recommended for points and/or routes of entry. These can be complemented by larval collection at relevant sites. Active monitoring of the spread is implemented with a network of oviposition traps. Passive surveillance is based on citizens’ reports. Mobile and web applications recommended for data entry and recording. |
[52, 55] | |
| Seasonal dynamics and mapping (hot-spot identification) | Identification of suitable periods and areas of mosquito activity and abundance. |
Weekly, biweekly, or monthly sampling all year round and in the same sites preferably with adult traps (BG-Sentinel) or ovitraps and in particular cases other methods (sticky traps, GAT traps, aspirator). Data can be related to climatic, landscape, demographic, and epidemiological variables. This task can be used to measure seasonal variations on vector density and better address relationships between entomological and epidemiological outcomes. GIS and mobile apps can be useful for mapping spatial heterogeneity of mosquito populations. Mathematical models can be used to predict potential expansion of vectors and location of hot-spots. | [14, 16, 22, 34] | ||
| Typology and productivity of habitats suitable for mosquito larval development (breeding sites) | Identification of main larval development habitats and most productive containers to guide larval control actions. | Larval surveys are useful to investigate the presence and abundance of immature stages and to characterise the typology of larval development habitats. Pupal surveys are relevant for measuring productivity indices. This helps to identify the most productive containers for targeting larval control measures. | [13, 16, 56] |
||
| Insecticide resistance monitoring | Monitoring the susceptibility of Aedes vector populations to public health insecticides. | WHO cylinder tests and CDC bottle assays are recommended for monitoring insecticide resistance at adult stages. WHO larval bioassays are recommended for immature stages. Resistance phenotype assays (frequency of resistance) and intensity bioassays (levels) are both recommended. | [18, 26, 49, 52] | ||
| Epidemiological surveillance | Evaluation of public health threats | Passive and active case detection depending on human and financial resources. | In addition to national routine surveillance system(s), enhancement strategies such as sentinel networks (hospitals, physicians, clinics, etc.) should be established for rapid laboratory diagnosis (RDT, molecular and serological tests, etc.) and to give notification of cases to vector control programs. Laboratory-based surveillance can contribute to distinguishing between imported and autochthonous cases. Laboratories with strong diagnostic capacity are needed to rapidly identify strains and/or serotypes and assess the potential for outbreaks. | [12, 13, 20, 57, 58] | |
| Guiding public health actions | Epidemiological data (incidence, morbidity, mortality) are used to prioritise and target public health actions, such as vector control, communication, and vaccination. In the case of an epidemic alert, passive surveillance can be enhanced to active surveillance with strengthen case detection. |
Epidemiological data are obtained from surveillance, as described above. Active case detection (through door-to-door surveys or questioning by public health professionals) can be used to identify infected people who have not sought treatment, i.e., undiagnosed and under-reported cases. Monitoring the movements of population (i.e., seasonal migration) between different environments (from forest or rural to urban areas or cities) can be crucial to respond to virus introduction. |
[12, 13, 19, 20, 21, 27, 58] | ||
| Monitoring trends in public health burden | The health burden can be assessed at different levels (from local to national to regional) by recording clinical manifestations and by sorting cases according to relevant criteria, e.g., sex and age group. Results can facilitate the assessment of the disease and associated risk factors. | Although active surveillance provides more accurate data, monitoring can be used to assess health impact. Mandatory reporting of high quality data is crucial to support collection of information on demographics and transmission risks. |
[13, 27, 58] | ||
| Risk assessment | Risk assessment based on adequate entomological and epidemiological surveillance is key to identifying areas of potential risk of Aedes-transmitted virus outbreaks and/or Aedes introduction and spread. Risk assessment will be used to define a preparedness and response programme tailored to the local situation and to monitor and evaluate the vector control programme. It is also useful for raising the different stakeholders’ awareness of the plausibility and extent of the expected risks. If the situation changes, the risk assessment should be reexamined. | Five steps are required: (1) identification of hazard(s), (2) likelihood of the virus and/or vector being introduced into a specific location, (3) spread or probability of transmission in the at-risk area, (4) probability the virus and/or vector will persist over a prolonged period, and (5) effects on health and the economy. | [52] | ||
|
M&E of control measures |
Monitoring refers to continuous tracking of programme performance. It makes it possible to find out whether activities have been implemented as planned, ensures accountability, and brings to light any problems or constraints so that corrective action can be instigated. Evaluation refers to the periodic measurement of the programme’s outcomes (e.g., vector populations) and impacts (e.g., reduced infection or disease) using data from the entomological and epidemiological surveillance systems. |
Programmes should set up a logical framework that include predetermined indicators with defined data sources to measure programme performance through M&E. Data sources are typically entomological and epidemiological surveillance systems as well as programme reports and other records on delivery of interventions and behaviour change activities. These data should be available to entomology and public health programmes on a shared, integrated, easily accessible platform. | [13, 25, 29, 30, 31, 41, 48, 58] | ||
Abbreviations: AGO, autocidal gravid oviposition traps; app, application; BG-Sentinel, BioGents sentinel traps; CDC, Centers for Disease Control and Prevention; GAT, gravid Aedes traps; GIS,Geographic Information System; M&E, monitoring and evaluating; RDT, Rapid Diagnostic Tests; WHO, World Health Organisation.
Entomological surveillance
Surveillance of Aedes vectors is important for identifying changes in geographic distribution, to obtain relative measurements of variation in vector density over time, to facilitate appropriate and timely decisions regarding interventions, and to assess the entomological impact of mosquito control programs to see whether the intervention had the expected effect on the target mosquito population [13]. For routine surveillance, entomological measurements have to be done in the same location (sentinel sites) at regular time intervals in order to establish a baseline to follow variation over time (seasonal dynamics). The frequency of data collection should be based on programme capacity and the need to generate reliable data in an appropriate format. Given that the geographic distribution of Aedes is increasing globally, a systematic surveillance for Aedes in every country is needed. Surveillance at points and/or routes of entry, such as sea ports, airports, and land country borders is important for early detection of the introduction of invasive Aedes species (S1, Table 1). If mosquitoes are introduced into suitable habitats, they may become established locally (S2) or more widely (S3, S4, S5). At this stage, surveillance consists of monitoring the spread of the mosquitoes (e.g., using ovitrap networks) in order to identify areas and/or periods of high transmission risk based on vector infestation, e.g., mapping seasonal dynamics and disease hot-spot identification [14]. The presence and abundance of Aedes species are estimated from measures of different entomological indices (e.g., larvae, pupae, adult), each with their strengths and weaknesses, as summarised in S2 Table. It should be mentioned, however, that cross-sectional studies have failed to find good correlations between entomological indices and episodes of dengue [15], and no larval entomological thresholds have proven effective in predicting Aedes-borne virus epidemics [16]. This can be explained by the fact that dengue virus transmission is complex and varies through time and space, and the relationship between vector density and risk of human infection is not static nor adequately characterised through periodic entomological surveillance [15].
New technologies (i.e., geo-informatics tools, remote sensing, and mathematical and simulation models) can be helpful in mapping the spatial distribution of vectors and/or in predicting their spread and seasonal dynamics using climatic (e.g., temperature, rainfall), social (e.g., rent value or education level), demographic (e.g., population density or distance to urban habitats), and landscape (e.g., vegetation cover or type of urbanisation) variables and can be less expensive than field surveillance [17]. A good understanding of the typology and productivity of habitats suitable for mosquito larval development (S3, S4) is essential in order to target larval control operations. Due to the possibility of introduction or selection of resistant individuals, insecticide resistance should be monitored regularly, preferably during nonepidemic periods (S3 and S4) to guide the choice of insecticides used for mosquito control. A combination of biological, biochemical, and molecular tools can be used to measure the frequency, intensity, and mechanisms of insecticide resistance in natural populations. Each resistance testing tool has its own advantages and weakness [18].
In areas with virus transmission (S3, S4, S5), if resources permit, it can be helpful to screen vectors for virus infection in order to confirm the role played by suspected species in local transmission, to monitor spatial and temporal patterns in virus transmission dynamics, and to evaluate interventions. Because virus detection in mosquitoes is costly and time-consuming and finding infected mosquitoes in natural populations is often challenging, it is not regularly done for surveillance of Aedes-transmitted viruses [19].
Epidemiological surveillance
In the field of Aedes-borne viral diseases, the objectives of epidemiological (or human and possibly animal) surveillance are to (1) evaluate potential public health threats, carrying out risk assessments and detecting outbreaks early; (2) select and evaluate the effectiveness of control activities; and (3) monitor trends in public health burden to obtain data for assessing the social and economic impact on the affected community (Table 1) [20].
The threat to public health is assessed by identifying recent introductions of a virus (S3), monitoring travellers returning from areas where target viruses circulate (S3, S5), and mapping local spread of the virus (S4, S5). This kind of surveillance system requires robust indicators and action plans to be defined in order to stratify epidemiological risk and guide decision-making to facilitate switching from one epidemic scenario to another [21].
Epidemiological data play a key role for guiding and prioritising vector control responses, which will be graduated according to transmission risk (Fig 1). Firstly, attention should be focussed on timely detection of imported viraemic people (S3), followed by identifying hot-spots of virus transmission (S4, S5). Monitoring geographical and temporal trends in human cases is common to all scenarios. The main differences lie in the targets of surveillance—trends in virus importation (mainly for S3 and S5), trends in circulating strains or serotypes, disease incidence, morbidity and mortality (S4 and S5). Evidence of non−vector-borne transmission (e.g., sexual transmission and blood transfusions) should be investigated where appropriate (e.g., Zika infections) as well as potential sylvatic transmission of the viruses, e.g., yellow fever. In geographical areas where local virus circulation is already established (S4, S5), a significant proportion of all suspected cases should be confirmed by specific laboratory diagnostics, depending on local resources [13].
Epidemiological surveillance is most often based on a combination of active and passive surveillance in order to reconcile cost, sensitivity, response time, and geographical coverage. Depending on the purpose of the epidemiological surveillance system and the risk scenario, certain considerations—such as available resources (e.g., human and diagnostic capacities) or strength of the healthcare system (e.g., public and/or private and accessibility), and sensitivity or response capacity—will be critical for guiding stakeholders in their choice of design of the overall disease management system (Table 1).
Epidemiological data, however, have several limitations. The most notable challenges are a high proportion of people with asymptomatic and/or mild infections, or differential diagnosis with low specificity of symptoms; a broad range of disease manifestations, from no detectable illness to death; lack of standardisation in case definitions; limited or low diagnostic capacity; underreporting; and variation in treatment-seeking behaviour by infected people [22]. Active case detection in the surroundings of a person with a confirmed infection may help identify additional cases or clusters, which often go unreported or undiagnosed. Where there is an epidemic alert, passive surveillance can be enhanced to reduce delays in reporting cases or to extend the area of surveillance. In areas at risk of sporadic transmission (S3), healthcare personnel usually have to report cases of imported and autochthonous arboviral disease, such as dengue, chikungunya, or Zika, to public health authorities. Increasing awareness among clinicians and travellers returning from endemic areas combined with good laboratory capacity has greatly improved case reporting. Laboratory-based surveillance has been shown to play a role in monitoring the introduction of a novel dengue virus serotype, a switch of virus strains between vector species and cocirculation of different arboviruses [22]. In nonendemic and/or nonepidemic areas (S2, S3), surveillance can target imported cases because these represent the main threat for introduction into immunologically naive populations. This can be achieved by health professionals notifying the relevant authorities of suspected or confirmed imported cases [23] or by fever screening travellers at points of entry [24].
Risk assessment and M&E outcomes
Evidence-based risk assessment should be carried out within all risk scenarios (S1−S5) and should be conducted by national and international health (and environmental) agencies. The assessment should form the basis for developing guidelines for the actions needed to keep risk to a minimum. To our knowledge, there is no global framework for conducting risk assessment for Aedes-borne diseases, but several regional documents have been drawn up (see Table 1). Regular M&E of the delivery of dengue prevention and control services and of the impact of interventions (this one being a critical one) are important IAM activities in all scenarios. Suitable indicators for measuring the progress of implementation (e.g., intervention coverage) and the outputs and outcomes (e.g., reductions in vector density or disease) should be identified [13].
Vector control
Vector control efforts need to be sustained over time, which requires well-structured administration, coordination with the public health programme that is diagnosing cases, political will, skilled staff, funding, and, crucially, community engagement and mobilisation from the outset [4]. Vector control can be undertaken either as a ‘routine’ activity (i.e., a preemptive sequence of actions regularly carried out) or as an ‘emergency’ measure (i.e., a response to an excess of vectors and/or an unusual increase in the human disease incidence calling for immediate action). Both types of measures should be prepared for, but vector control is most cost-effective if it is ‘proactive’ (preventive) rather than implemented ‘in response mode’ (after the start of epidemic) [25]. Because programmes move from areas prone to virus introduction (S3) to endemic-epidemic (S4−S5) scenarios, a shift in the allocation of resources from ‘reactive’ to ‘proactive’ vector control should be considered. Targeting immature mosquitoes has been a prevalent paradigm for Aedes control, but far more attention should be directed at methods targeting both larvae and adults to maximise impact on adult Aedes density, longevity, and role in virus transmission [9]. Strategies and interventions should be adapted to local vector ecology and available resources, guided by results from operational research and subject to routine M&E (see the ‘Risk assessment and M&E outcomes’ section).
In order to prolong the life span of existing insecticides, it is imperative that noninsecticide-based tools are used whenever possible. When chemicals have to be deployed, they should be used rationally and preferably not as ‘monotherapies’ [10, 26]. Several insecticide resistance monitoring strategies exist in vector control, which are based on rotations of insecticides, mixtures of unrelated insecticides, use of interventions in combination, and mosaic spraying. Resistance management is not a ‘stand-alone’ strategy and should be implemented in the broad context of integrated vector management and be carefully monitored and evaluated. Activities to control transmission should target homes and outdoor areas in their immediate vicinity (i.e., in the place of residence as well as in neighbouring houses). Treating nonresidential areas—i.e., places where human–vector contact occurs during the daytime, such as schools, hospitals and workplaces, especially their surroundings, such as outside lunch gathering areas—can provide measurable impacts [27]. Restricting control to residences within a certain radius of a case’s home is not as effective as uniform treatment of broad geographic areas. By the time a case is detected, human movement has taken the virus beyond a radius of 100 m to 200 m [27, 28].
Evidence supporting Aedes vector control
The evidence base for the public health value of Aedes vector control is unfortunately weak. There are little data demonstrating reduction in human infection or disease for many tools currently in widespread use [29]. Epidemiological outcomes are needed to demonstrate the public health benefit of a vector control intervention and are the basis of evidence-based policy [30]. In order to provide more robust guidance on preferred Aedes vector control tools and those that should be avoided, we summarised existing evidence based on recent systematic reviews. We categorised the hierarchy of evidence according to whether there was epidemiological or entomological evidence and by study design, with randomised controlled trials providing the highest quality of evidence and nonrandomised or observational studies providing the lowest quality evidence [30]. Results and specified details for different interventions are shown in S2 Table.
Adult mosquito control and avoidance
The strengths and limitations of current adult mosquito control methods and the strength of evidence for their entomological and epidemiological effects are summarised in Table 2. Despite widespread use, there is limited entomological and epidemiological evidence for ultra-low volume (ULV) space spraying [31, 32]. In the case of virus transmission (S3, S4, S5), ‘peridomestic’ or ‘perifocal’ space spray treatments with insecticides can be carried out in and around households where human infection is suspected or has been reported. Space sprays can also be adequate in specific situations, for example, (i) to prevent local establishment of invasive mosquito species, such as A. albopictus (small area < 25 km2, S2), (ii) to halt an incipient outbreak (S3), and (iii) to curtail an ongoing epidemic and/or endemic situation (S4 and S5). Different treatment methods (house-to-house application using portable equipment, vehicle-mounted fogging, and cold or thermal fogging) are available, but they must be tailored to the risk scenario, the area to be covered, accessibility, and the Aedes species.
Table 2. Vector control tools for Aedes mosquito control.
Strength of evidence is based only on recent systematic reviews and meta-analysis studies carried out in the last 5 years. We used scores to rank the ‘strength of evidence’ based on study designs used for assessing the efficacy of vector control interventions as proposed by Wilson and colleagues (2015) for epidemiological trials (1, 2a, 2b). We created 2 new ‘levels of evidence’ (3a and 3b) to distinguish randomised versus nonrandomised (observational) ‘entomology’ trials.
| Stage/scenario | Methodology | Type of intervention/product | Strength of evidence* | Constraints/advantages | Specifications | References |
|---|---|---|---|---|---|---|
|
Adult control in emergency S2, S3, S4, and S5 |
Insecticide spraying | Space spraying (indoors, outdoors) | Epidemiological evidence for ISS based on observational studies (level 2b). Several entomological studies (level 3a and 3b) for ISS and OSS. | Insecticide resistance Low acceptability and limited sense of security in the community Poor persistence Regulatory and environmental constraints Needs skilled, experienced staff |
Thermal fogging or cold fogging (ULV spray) using WHO-recommended insecticides Indoor house-to-house application using portable sprayer. Outdoor applications (i.e., vehicle-mounted fogger) if mosquitoes are exophilic and exophagic. Applications should be carried out at the right time, in the right place and according to prescribed instructions. |
[13, 29, 31, 32, 34, 48, 58, 59] |
| Residual spraying (indoors or outdoors) | Epidemiological evidence of IRS (level 2a). Entomological evidence (level 3b) for IRS for A. aegypti and ORS for A. albopictus (level 3b). | Insecticide resistance Costly and time-consuming Requires high coverage Needs skilled, experienced staff |
TIRS for indoor resting A. aegypti ORS on the vegetation against A. albopictus Application by portable compression sprayers |
[29, 31, 34, 35, 59, 60, 61] |
||
| Adult control for routine and emergency S4 and S5 | Mass trapping | Gravid traps (AGO or GAT) | Epidemiological evidence based on observational studies (level 2b). Entomological evidence (level 3b) for A. aegypti. | Low cost Possible to combine with community participation Sustainable, able to be reused for several seasons |
Need for a coverage of greater than 80% Use large autocidal gravid traps, as AGO or GAT, to maximise visual and olfactory attraction using grass or hay infusion |
[36, 62, 63] |
| Adult control for routine and emergency S4 and S5 | Personal protection | Topical repellents (applied directly onto the skin) | Absence of epidemiological and entomological evidence as a part of control campaigns. | Individual-based action (requires high degree of compliance) No residual activity |
DEET, the longest-lasting; IR3535 or picaridin, medium-long lasting protection; plant-derived oils (eucalyptus, citronella, or geranium), short-term (frequency of applications according to national legislation and/or manufacturer’s recommendations) | [29, 64] |
| Insecticide-treated materials (clothes, curtains, house screens, water container covers, etc.) | Epidemiological evidence for house screening (level 2b). Entomological evidence for ITCs, house screening, and water container covers (level 3a and 3b). No evidence for bed nets. | Individual- and community-based action Residual activity with long-lasting technology Insecticide resistance Low protection against UV Degradation of insecticide |
Clothes, curtains, and bed nets treated with WHO-recommended insecticides. Most evidence supports house screening for preventing dengue transmission |
[29, 65, 66] | ||
|
Larval control for routine S2, S3, S4, and S5 |
Environmental management | Source reduction and educational outreach visits (door-to-door) | Epidemiological evidence (level 1) of community based campaigns. Entomological evidence (level 3a and 3b). | Labour intensive. Larval development habitats need to be accurately identified. Must be done diligently and conscientiously and with access to a high number of dwellings |
Requires a high level of education and community participation. Difficult to sustain over time. Need to characterise larval development habitats, including urban cryptic habitats. Essential to reduce mosquito larval development habitats in the long-term in private and public domains |
[29, 40, 41, 43, 48, 58, 67] |
| Larviciding | Organophosphates (Temephos, Chlorpyrifos, Pirimephos methyl, Fenthion) | Entomological evidence for Temephos (level 3b). | Affordable Not acceptable for treating drinking water containers and sources (except Temephos) Temephos resistance in several areas Regulatory constraints (e.g., OPs are not notified in the EU for mosquito control) |
Cholinesterase inhibitors Different formulations (EC, GR) and application methods (manual or with hand sprayers) |
[41, 68] | |
| Insect growth regulators (pyryproxifen, diflubenzuron, novaluron) | Epidemiological evidence for pyryproxifen as part of community base (level 2b). Entomological evidence (level 3b). | More expensive Late acting effect (pupae) on juvenoids Acceptable for treating drinking water sources and containers Constraints for the treatment of cryptic breeding sites |
Disruption of endocrine system for juvenoids (pyriproxyfen) and chitin synthesis inhibitor for ecdysoids (novaluron and diflubenzuron) Different formulations (WG, GR, DT) and application methods (manual or with hand sprayers) |
[29, 39] | ||
| Bti | Entomological evidence (level 3a and 3b) for Bti. | No resistance Selective and safe Acceptable for treating drinking water sources and containers Low residual action in polluted habitats |
Bacterial toxins targeting midgut epithelium cells Different formulations (WG, GR) and application methods (manual or with hand sprayers and fogging). |
[34, 69] |
||
| Biological control | Fish (Gambusia, etc.) | Limited entomological evidence (level 3b) for fish. | Well accepted in several countries, needs a delivery mechanism and maintenance. Adequate for treating large and/or permanent mosquito habitats, not generally accepted for drinking water storage containers. | Predators of mosquito larvae (kill all stages). Controversial, harmful impacts of nonnative species, such as Gambusia. | [34, 41, 70, 71, 72, 73, 74] | |
| Copepods (Mesocyclops) | Limited epidemiological (level 2b) and entomological evidence (level 3b) for copepods depending on settings. | Predators of mosquito larvae (kill young instar larvae). |
*Details are available in S2 Table.
Abbreviations: AGO, autocidal gravid oviposition traps; Bti, Bacillus thuriengensis serotype israliensis; DEET, N,N-diethyl-3-methylbenzamide; DT, Tablet; EC, Emulsifiable concentration; EU, European Union; GAT, gravid Aedes trap; GR, Granules; IRS, indoor residual spraying; IR3535, Ethyl butylacetylaminopropionate; ISS, indoor space sprays; ITC, insecticide-treated curtain; OP, Operational procedures; ORS, outdoor residual sprays; OSS, outdoor space spray; S, scenario; TIRS, targeted indoor residual spraying; ULV, ultra-low volume; UV, Ultraviolet; WG, Water dispersible granule; WHO, World Health Organisation.
Indoor space spray (ISS) should be distinguished from outdoor applications. Because A. aegypti tends to be endophilic and endophagic [33], only in cases in which A. albopictus (or A. aegypti) populations are primarily outdoors are outdoor applications likely to be effective. Outdoor space spraying (OSS) and outdoor residual spraying (ORS) on vegetation have been used for controlling the exophilic species A. albopictus [34], with some entomological evidence of efficacy (Table 2, S2 Table). The efficacy of ORS and its impact on the environment is still controversial, facing the same challenges as described above for ULV space spraying.
Indoor residual spraying (IRS), in particular targeted IRS (TIRS, in which IRS is performed on exposed low walls, under furniture and inside closets) has not been widely used for Aedes control so far, although it may be a promising tool for controlling Aedes-borne arboviral transmission (S3 and S4) in areas where the endophilic mosquito A. aegypti is responsible for transmission [35]. The costs, human resources, and logistics needed for suitable coverage with IRS may represent a challenge for their rapid and broad-scale deployment during outbreaks. The use of contact tracing technologies to deploy IRS and/or TIRS can be an option to overcome those limitations [35].
In S4 and/or S5, local health authorities can promote or subsidise the use of insecticide-treated materials (e.g., insecticide house screening and treated curtains) that have been proven effective (S2 Table) and, in emergency situations (S5), promote the use of topical repellents that provide protection against mosquito bites (Table 2, Fig 1).
Finally, epidemiological and entomological evidence exist with regards to the mass deployment of gravid oviposition traps to reduce Aedes mosquito density (S2 Table) that can be a low-cost, community-based, and sustainable participation complementary strategy (Table 2) [36].
Furthermore, there is currently considerable innovation in vector control for prevention of Aedes-borne viral disease [37]. Novel approaches—such as the sterile insect technique, Wolbachia, genetically modified mosquitoes, removal trapping, and spatial repellents—gather relevant entomological data, and many are engaged in well-designed field trials that will generate the epidemiologic data necessary to develop public health policy for their deployment.
Larval control
Methods of larval control and their strengths, limitations, and evidence base are described in Table 2. The aim of targeting mosquitoes at immature life stages (i.e., larvae and pupae) is to reduce adult Aedes emergence and to reduce adult population densities. Control may be intensified during the early mosquito season but necessary year-round in tropical regions and requires high coverage because there may be sufficient temporary larval habitats to maintain high mosquito adult densities and virus transmission. Larval control (i.e., environmental management, source reduction, larviciding, or biological control [community-based and/or top-down approaches]) is more effective when it is consistent and routine (S4) rather than in a periodic emergency response (S5). Larval control needs to be sustained in order to reduce the size of the adult vector population and to keep the population density below certain, currently still undefined thresholds to minimise the risk of virus transmission.
Source reduction has been, and continues to be, a key component of dengue, Zika, and chikungunya control programmes [9, 38]. It should primarily target artificial containers in private and public spaces, although some natural containers, such as bamboo and bromeliads, can also harbour Aedes larvae. Larvicides are generally long-lasting and moderately costly. Unfortunately, the evidence supporting larviciding as part of control programmes is mixed, with some studies showing a beneficial effect of pyryproxifen as part of a community-based strategy reducing dengue rates and entomological indices [39], whereas others such as Temephos or Bacillus thuriengensis (Bti) do not have strong evidence in the review of evidence (S2 Table and Table 1).
Biological control methods (fish, copepods, and others) are relatively acceptable and can be used for treatment of large and permanent breeding sites, but existing evidence is inadequate for assessing the impact of this strategy for dengue control (S2 Table). Community-based source reduction (as clean-up campaigns or the use of water container covers) can reduce Aedes vector populations and is supported by epidemiological cluster randomised controlled trials results of lower infection with dengue virus in children and fewer reports of dengue illness from a trial in Mexico and Nicaragua (Table 2 and S2 Table) [40, 41].
Social mobilisation
Human behaviour is the common denominator of all Aedes-borne virus epidemic risk scenarios and therefore of prevention and control strategies. Social mobilisation is a key factor in the success of Aedes mosquito control strategies and in preventing outbreaks. There is evidence (S2 Table) that community participation is effective in reducing larval indices and disease prevalence [40, 41, 42, 43]. Community participation and education (e.g., door-to-door visits, workshops, and webinars) can inform the population on how to reduce Aedes populations by emptying or eliminating nonpermanent water containers and covering permanent water storage containers with untreated or insecticide-treated covers. Other actions can be carried out by health education programmes, such as distribution of printed materials, educational meetings, involvement of local opinion leaders, sensitisation at schools, and the use of mass media (radio, television, newspapers, leaflets, posters) [42]. Health education efforts should be carried out routinely and intensified before peak periods of virus transmission (S4 and S5). Sex education is also important for Zika prevention because the virus can be transmitted sexually.
WHO recommends the use of communication for behavioural impact (COMBI), an approach that integrates behavioural and social communication to reduce risk and prevent disease. COMBI is used in an increasing number of countries for dengue control [44], and a toolkit has been developed to deliver more effective outbreak response measures [45]. In practice, education and communication strategies are often implemented too late, that is, after the outbreak has begun to decline (S4 and S5). Social communication is more likely to be successful when information is disseminated early, which means before the introduction of vectors or virus (S1), when transmission has recently been established (S2), or before transmission has peaked (S1, S2, S3 and S4). Activities to promote behavioural change should produce measurable and visible results and should be monitored using appropriate indicators [44]. It is important to note that social mobilisation is not a ‘stand-alone’ strategy and that community-based control campaigns are carried out in combination with other vector control interventions [29, 41, 42, 43].
Intra- and intersectoral collaboration
Aedes control cannot be successful without effective and sustained intra- and intersectoral collaboration [4, 13, 46]. Within the health sector, Aedes control should not be the responsibility of a single department. Interagency collaboration is fundamental for a successful programme. The vector control unit should, therefore, establish strong links with other vector-borne disease programmes (e.g., malaria vector control), epidemiological surveillance, clinical diagnosis and management, vaccine delivery (when appropriate), maternal and child health (e.g., integrated management of childhood illnesses), health education, veterinary surveillance, and environmental health [46]. Intersectoral actions for vector control (Table 3) should be guided by site-specific knowledge of larval aquatic habitats, locations where risk of infection is highest (i.e., inside homes for endophilic and endophagic A. aegypti or outside homes for exophilic and exophagic A. albopictus), and current and historical hot-spots of reported illness. The specific actions that can be taken in collaboration with other sectoral actors will depend on the setting and feasibility.
Table 3. Framework for promoting intra- and intersectoral collaboration.
| Ministry or organisation | Scenario | Activity | Rationale |
|---|---|---|---|
| Ministry of Public Works and municipal authorities | S1, S2, S3, and S4 | Provision of reliable piped water to households. Provision of sewage connections. Solid waste management and disposal. Design and maintenance of street storm water drainage systems that do not harbour immature vectors. | Removal of potential Aedes mosquito larval development habitats can reduce adult numbers and virus transmission rates. |
| Ministry of Housing | S3 and S4 | Develop and enforce housing and building codes mandating installation of screening, dependable water supply, waste management, and disposal and rainwater runoff control in new housing developments. | Reduce biting of humans by installing screens to prevent entry to houses and buildings. Reduce standing water to prevent immature development. |
| Ministry of Education | S3 and S4 | Incorporate information on Aedes-borne diseases, vectors, transmission, and prevention into school curricula and teach hygienic behaviour. | Empower children with knowledge and skills to reduce mosquito populations and virus transmission. |
| S3, S4, and S5 | Participation of school children in larval surveys, source reduction, and larviciding. | ||
| Ministry of Tourism | S3 and S4 | Reduce aquatic habitats in and around hotels, community gathering places, markets, etc. | Reduce virus transmission. |
| S5 | Communicate rapidly to holiday accommodation providers and tourists if there is evidence of an outbreak. | Supports rapid implementation of vector control measures. | |
| Ministry of Agriculture | S3 and S4 | Encourage livestock farmers to empty, clean, and scrub animal drinking containers weekly. | Reduces aquatic habitats for Aedes mosquito development. |
| Department of Agriculture or Customs | S1 | Involved in surveillance of invasive mosquito species at PoEs. | Supports early detection of Aedes species introductions. |
| NGOs | S3 and S4 | Promote and implement environmental management, health communication on source reduction and improvement of housing. | Mobilising community action supports for more effective control. |
| S5 | Strengthen mobilisation of communities during outbreaks. | ||
| NGOs and UN agencies | S3, S4, and S5 | Deliver vector control interventions in humanitarian crises. | Prevent outbreaks and reduce impact on vulnerable populations |
| Private sector | S3 and S4 | Develop new tools to prevent transmission, e.g., mosquito-proof water containers, door and window screens. | Stewardship, particularly in industrial and manufacturing sectors, can stimulate innovation in vector control and help reduce virus transmission. |
| Recycle containers, e.g., plastic receptacles and tyres, to reduce aquatic habitats. | |||
| Involve architectural practices in design and building of mosquito-proof housing, schools, and workplaces. | |||
| Include private health facilities in epidemiological surveillance reporting systems. | |||
| S2, S3, and S4 | Conduct health impact assessments on large-scale industrial projects and commercial agriculture. | ||
| S2, S3, and S4 | Implement control measures in large-scale industrial projects and commercial agriculture. | ||
| S4 and S5 | Increase access to subsidised personal protection measures. | ||
| Academic and research institutions | S1−S4 | Provide training and a career path for vector control specialists in the Ministry of Health, and other vector control personnel. | Support innovation and research expertise to improve and sustain surveillance and vector control. |
| S1−S4 | Share infrastructure, such as entomology laboratories, insectaries, and equipment, with the Ministry of Health. | Resource sharing reduces costs for the Ministry, supports access to specialised facilities, and fosters collaboration between academic and public health sectors. |
Abbreviations: NGO, nongovernmental organisation; PoE, point of entry; S, scenario.
Outside the public health sector, collaborations should be forged with, for example, ministries of education, environment, water, and urban planning and housing [5], and with the private sector, nongovernmental organisations (NGOs), and town councils (Table 3). For example, provision of reliable piped water should be encouraged to prevent storage of water in containers in and around the home, which can harbour Aedes. Solid waste management should be improved to remove rubbish from the peridomestic environment, which can accumulate water and provide habitats for Aedes to lay their eggs. A pilot study of recycling of plastic materials in Merida, Mexico, was able to reduce entomological indices by incentivised recycling. Bonus points were given for large volumes of reusable materials in exchange for commodities and targeted at the most at-risk neighbourhoods [47]. The scheme was organised by the local government through the Health Services and in coordination with the Ministries of Social Development, Urban and Environmental Development, and Education. The Ministry of Housing and Infrastructure can develop and enforce housing and building codes, for example, to mandate installation of screened doors and windows on properties and rainwater runoff control for new housing developments as well as prohibit construction of open groundwater wells. The Ministries of Education and Health can work together to disseminate behaviour change communication on prevention of Aedes population and disease proliferation (see the ‘Vector control’ section). Information on prevention of Aedes-borne diseases should be integrated into school curricula for long-term sustainability. NGOs, including community groups, such as neighbourhood women’s, religious, environmental, and social action organisations, should be engaged. Local community groups can be involved in promoting and implementing environmental management as well as delivering behaviour change communication. NGOs can be influential in mobilising communities and encouraging acceptance of routine and outbreak vector control methods (S5).
The private sector can be engaged. For example, commercial companies can support recycling, e.g., disposal and recycling of discarded tyres. In Brazil, a partnership between the Ministry of the Environment and the National Association of the Tyre Industry encourages consumers to return used tyres to collection points at which point they are used as alternative fuel or recycled into flooring and other products [13]. Private health facilities can be incorporated into the epidemiological surveillance system. Academic and research institutions can cooperate with the Ministry of Health to train personnel and carry out surveillance through the sharing of facilities, i.e., entomology laboratories, insectaries, and human resources. Development projects and commercial agriculture can undertake assessments of the health impact of Aedes-borne diseases and implement mitigation strategies. NGOs, UN agencies, and bilateral or multilateral donors can be engaged to implement control measures to prevent virus transmission in conflict zones.
All these activities should be coordinated through an interministerial steering committee with broad representation that seeks regular input from nonministerial stakeholders, such as NGOs, research and educational establishments, community organisations, and the private sector [13]. The committee should have clearly defined terms of reference and meet regularly, not just during outbreaks. Working in an integrated fashion has the potential to increase efficiency and public health impact more than narrowly focused, uncoordinated actions from the health sector alone.
Supporting activities
Additional important complementary activities for achieving effective vector control and Aedes-borne disease prevention include capacity building, advocacy, policies and laws, and research and innovation (Fig 1). Supporting activities are briefly summarised in S3 Table.
Conclusion
During the past 50 years, the world has seen the emergence and dramatic spread of Aedes-transmitted arboviral diseases. Social, environmental, and demographic changes have facilitated the proliferation of existing transmission systems and the spread of viruses and vectors into new ecological settings [4, 46]. Notable deficiencies in the planning and implementation of vector control programmes were reported and include the following:
Lack of commitment and leadership from governments to maintain preparedness and deliver rapid response against Aedes-borne diseases [46].
Ineffective programmatic implementation due to the difficulties in eliciting sustainable community engagement and the challenges of applying large, top-down approaches [4].
A weak evidence base for the public health effectiveness of Aedes vector control strategies due to the small number of robust trials that have been carried out and the difficulty in measuring the impact of interventions on human infection and disease [29, 30, 48].
Insufficient funding, human resources, and limited capacity building in low-income countries stifles development of innovative control tools and strategies [3, 37].
Absence of a global and coordinated plan to monitor and manage insecticide resistance in Aedes to guide decision-making for vector control [10, 49].
Increasing aversion of citizens to strategies based solely on insecticides because of their potential impacts on the environment and inadequate application coverage [26, 31].
The call for a global response and preparedness for vector borne diseases (i.e., GVCR) is, therefore, timely [11], and implementing and sustaining integrated surveillance and locally adapted Aedes control measures should be a priority [50]. Unfortunately, there have been very few well-conducted epidemiological field trials of Aedes vector control (S2 Table), which means that prioritisation of control strategies is difficult. Limited epidemiological evidence supports the deployment of community-based source reduction, ISS, TIRS, and house screening and larviciding, but evidence is lacking for most other vector control tools (S2 Table, Table 2). Promising new interventions targeting adults—such as Wolbachia for population replacement and/or population suppression, genetically modified mosquitoes, sterile insect techniques, community-based mass trap deployment, spatial repellents, and attractive targeted sugar baits—are currently being considered for dengue prevention, but their public health efficiency in field trials (i.e., effectiveness trials) has yet to be determined, and large-scale roll-out (effectiveness studies) of these programmes will take years [37, 51].
There are no magic bullets for Aedes-borne diseases control, including vaccines. The desire to find easy or rapid solutions has been tried for decades without success and is likely to continue to lead to disappointment. The most practical and productive path forward is to intensify surveillance and to strengthen the evidence base so that, when needed, ‘a box’ of effective tools can be deployed in an integrated manner that takes into account the local situation and available resources.
The IAM provides a technical guidance on how and when to implement integrated management for Aedes, tailored to location-specific entomological and epidemiological risk scenarios based on current evidence. To be effective and sustainable, IAM must be fed with robust entomological and epidemiological data collection at different time and spatial scales (country, district, city, and neighbourhood) and in particular for local scenarios in endemic and epidemic settings with randomised controlled trials of combinations of several tools, to guide-decision making for Aedes-borne disease control. The IAM needs be supported by human and financial resources and must be carefully monitored and evaluated. Increased funding is crucial given the growing threat to public health and the need for evidence that innovation makes disease prevention more effective. We hope that the measures outlined here will help promote and implement the WHO GVCR and will be used to guide actions that improve Aedes-borne disease prevention and outbreak response. The ultimate goal is to use available resources as effectively and efficiently as possible to safeguard global health.
Key Learning Points
Aedes-borne viral diseases are rapidly spreading globally with increasing health and economic impacts.
Many countries are still unprepared to address the challenge these diseases present and lack guidance on how and when to deploy available options for vector control intervention.
In the absence of easy, rapid, effective solutions, the optimal approach is to intensify surveillance and to strengthen the evidence base for which tools can be best integrated for effective and proactive deployment, together with adequate support and implementation.
IAM aims to offer practical vector control guidance on measures to prevent Aedes-transmitted viral diseases tailored to local entomological and epidemiological settings and available resources.
IAM aims to promote and support implementation of the WHO GVCR and to offer guidance on improving measures to combat the emerging threat of Aedes-borne diseases with positive impacts on health, the economy, and the global environment.
Top Five Papers
Gubler DJ. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21st Century. Trop Med Health. 2011; 39 (4): 3–11.
Achee NL, Gould F, Perkins TA, Reiner Jr RC, Morrison AC, Ritchie SA, Scott TW. A critical assessment of vector control for dengue prevention. PLoS Negl Trop Dis. 2015; 9(5): e0003655.
World Health Organization. Comprehensive guidance for prevention and control of dengue and dengue haemorrhagic fever. Revised and expanded edition. 2011. World Health Organization, Regional Office for South-East Asia. 212 pp.
Bowman LR, Donegan S, McCall PJ. Is dengue vector control deficient in effectiveness or evidence? Systematic review and meta-analysis. PLoS Negl Trop Dis. 2016; 10(3): e0004551.
Runge‐Ranzinger S, McCall PJ, Kroeger A, Horstick O. Dengue disease surveillance: an updated systematic literature review. Trop Med Int Health. 2014; 19 (9):1116–60.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Claire Durot (WIN project manager) for her support in this work.
Funding Statement
This review was funded by an award to VC and the WIN network from the World Health Organization's Special Programme for Research and Training in Tropical Diseases (http://www.who.int/tdr/). DR was partially supported by the ANR grant INVACOST. The funders had no role in the study design, data collection and analysis, nor the writing of the manuscript, nor the decision to publish.
References
- 1.Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017; 17(3): e101–e106. 10.1016/S1473-3099(16)30518-7 [DOI] [PubMed] [Google Scholar]
- 2.Mayer SV, Tesh RB, Vasilakis N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017; 166: 155–163. 10.1016/j.actatropica.2016.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horstick O, Runge-Ranzinger S, Nathan MB, Kroeger A. Dengue vector-control services: how do they work? A systematic literature review and country case studies. T Roy Soc Trop Med H. 2010; 104 (6): 379–386. 10.1016/j.trstmh.2009.07.027 [DOI] [PubMed] [Google Scholar]
- 4.Gubler DJ. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21st Century. Trop Med Health. 2011; 39 (4): 3–11. 10.2149/tmh.2011-S05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindsay SW, Wilson A, Golding N, Scott TW, Takken W. Improving the built environment in urban areas to control Aedes aegypti-borne diseases. Bull World Health Organ. 2017; 95(8): 607–608. 10.2471/BLT.16.189688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradshaw CJ, Leroy B, Bellard C, Roiz D, Albert C, Fournier A, et al. Massive yet grossly underestimated global costs of invasive insects. Nat Comm. 2016; 7:12986 10.1038/ncomms12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016; 16 (8): 935–941. 10.1016/S1473-3099(16)00146-8 [DOI] [PubMed] [Google Scholar]
- 8.Aguiar M, Stollenwerk N, Halstead SB. The impact of the newly licensed dengue vaccine in endemic countries. PLoS Negl Trop Dis. 2016; 10(12): e0005179 10.1371/journal.pntd.0005179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008; 5(3):e68 10.1371/journal.pmed.0050068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbel V, Achee NL, Chandre F, Coulibaly MB, Dusfour I, Fonseca DM, et al. Tracking insecticide resistance in mosquito vectors of arboviruses: The Worldwide Insecticide resistance Network (WIN). PLoS Negl Trop Dis. 2016; 10(12): e0005054 10.1371/journal.pntd.0005054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Global Vector Control Response 2017–2030 53 pp. Geneva: World Health Organization; 2017. Available from: http://www.who.int/vector-control/publications/global-control-response/en/. [cited 2018 July 12]. [Google Scholar]
- 12.World Health Organization. Handbook for integrated vector management World Health Organization; 2012. Available from: http://apps.who.int/iris/bitstream/10665/44768/1/9789241502801_eng.pdf. [cited 2018 July 12]. [Google Scholar]
- 13.World Health Organization. Comprehensive guidance for prevention and control of dengue and dengue haemorrhagic fever Revised and expanded edition. World health Organization, Regional Office for South-East Asia; 2011. 212 pps. Available from: http://apps.searo.who.int/pds_docs/B4751.pdf. [cited 2018 July 12]. [Google Scholar]
- 14.Lessler J, Azman AS, McKay HS, Moore SM. What is a Hotspot Anyway? Am J Trop Med Hyg. 2017; 96 (6): 1270–1273. 10.4269/ajtmh.16-0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cromwell EA, Stoddard ST, Barker CM, Van Rie A, Messer WB, Meshnick SR, et al. The relationship between entomological indicators of Aedes aegypti abundance and dengue virus infection. PLoS Negl Trop Dis. 2017; 11(3): e0005429 10.1371/journal.pntd.0005429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowman LR, Runge-Ranzinger S, McCall PJ. Assessing the relationship between vector indices and dengue transmission: a systematic review of the evidence. PLoS Negl Trop Dis. 2014; 8(5): e2848 10.1371/journal.pntd.0002848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reisen WK. Landscape epidemiology of vector-borne diseases. Annu Rev Entomol. 2010; 55: 461–83. 10.1146/annurev-ento-112408-085419 [DOI] [PubMed] [Google Scholar]
- 18.Dusfour I, Vontas J, David JP, Weetman D, Fonseca DM, Corbel V, et al. Management of insecticide resistance in the major Aedes vectors of arboviruses: advances and challenges. PLoS Negl Trop Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon IK, Getis A, Aldstadt J, Rothman AL, Tannitisupawong D, Koenraadt CJ, et al. Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl Trop Dis. 2012; 6(7): e1730 10.1371/journal.pntd.0001730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.M'ikanatha NM, Lynfield R, Julian KG, Van Beneden CA, de Valk H. Infectious disease surveillance: a cornerstone for prevention and control John Wiley and Sons Ltd, 1–20. 2013. [Google Scholar]
- 21.Brady OJ, Smith DL, Scott TW, Hay SI. Dengue disease outbreak definitions are implicitly variable. Epidemics. 2015. June; 11: 92–102. 10.1016/j.epidem.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubler DJ, Ooi EE, Vasudevan SG, Farrar J, editors. Dengue and dengue hemorrhagic fever. Oxfordshire: CAB International; 2014. [Google Scholar]
- 23.La Ruche G, Souares Y, Armengaud A, Peloux-Petiot F, Delaunay P, Desprès P, et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill, 2010; 15 (39): 19676 [PubMed] [Google Scholar]
- 24.Shu PY, Chien LJ, Chang SF, Su CL, Kuo YC, Liao TL, et al. Fever screening at airports and imported dengue. Emerg Infect Dis. 2005; 11(3): 460–2. 10.3201/eid1103.040420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisen L, Beaty BJ, Morrison AC, Scott TW. Proactive Vector control strategies and improved monitoring and evaluation practices for dengue prevention. J Med Entomol. 2009; 46(6): 1245–55. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Monitoring and managing insecticide resistance in Aedes mosquito populations Interim guidance for entomologists. WHO, Geneva: 2016. Available from: http://apps.who.int/iris/bitstream/10665/204588/2/WHO_ZIKV_VC_16.1_eng.pdf. [cited 2018 July 12]. [Google Scholar]
- 27.Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, Astete H, et al. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci USA. 2013. January 15; 110(3):994–9. 10.1073/pnas.1213349110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ten Bosch QA, Clapham HE, Lambrechts L, Duong V, Buchy P, Althouse BM, et al. Contributions from the silent majority dominate dengue virus transmission. PLoS Pathog. 2018; 14(5): e1006965 10.1371/journal.ppat.1006965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowman LR, Donegan S, McCall PJ. Is dengue vector control deficient in effectiveness or evidence? Systematic review and meta-analysis. PLoS Negl Trop Dis. 2016; 10(3):e0004551 10.1371/journal.pntd.0004551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson AL, Boelaert M, Kleinschmidt I, Pinder M, Scott TW, Tusting LS, et al. Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol. 2015; 31(8): 380–90. 10.1016/j.pt.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 31.Esu E, Lenhart A, Smith L, Horstick O. Effectiveness of peridomestic space spraying with insecticide on dengue transmission; systematic review. Trop Med Int Health. 2010. May; 15(5): 619–31. 10.1111/j.1365-3156.2010.02489.x [DOI] [PubMed] [Google Scholar]
- 32.Stoddard ST, Wearing HJ, Reiner RC Jr, Morrison AC, Astete H, Vilcarromero S, et al. Long-term and seasonal dynamics of dengue in Iquitos, Peru. PLoS Negl Trop Dis. 2014; 8(7): e3003 10.1371/journal.pntd.0003003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott TW, Takken W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 2012; 28(3): 114–21. 10.1016/j.pt.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 34.Faraji A. Unlu I. The Eye of the Tiger, the Thrill of the Fight: Effective larval and adult control measures against the Asian Tiger Mosquito, Aedes albopictus (Diptera: Culicidae), in North America. J Med Entomol. 2016; 53(5): 1029–47. 10.1093/jme/tjw096 [DOI] [PubMed] [Google Scholar]
- 35.Vazquez-Prokopec GM, Montgomery BL, Horne P, Clennon JA, Ritchie SA. Combining contact tracing with targeted indoor residual spraying significantly reduces dengue transmission. Sci Adv. 2017; 3(2): e1602024 10.1126/sciadv.1602024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson BJ, Ritchie SA, Fonseca DM. The state of the art of lethal oviposition trap-based mass interventions for arboviral control. Insects. 2017; 8(1): 5 10.3390/insects8010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Achee NL, Gould F, Perkins TA, Reiner RC Jr, Morrison AC, Ritchie SA, et al. A critical assessment of vector control for dengue prevention. PLoS Negl Trop Dis. 2015; 9(5): e0003655 10.1371/journal.pntd.0003655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fonseca DM, Unlu I, Crepeau T, Farajollahi A, Healy SP, Bartlett‐Healy K, et al. Area‐wide management of Aedes albopictus. Part 2: Gauging the efficacy of traditional integrated pest control measures against urban container mosquitoes. Pest Manag Sci. 2013. December; 69(12):1351–61. 10.1002/ps.3511 [DOI] [PubMed] [Google Scholar]
- 39.Maoz D, Ward T, Samuel M, Müller P, Runge-Ranzinger S, Toledo J, et al. Community effectiveness of pyriproxyfen as a dengue vector control method: A systematic review. PLoS Negl Trop Dis. 2017; 11(7): e0005651 10.1371/journal.pntd.0005651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersson N, Nava-Aguilera E, Arosteguí J, Morales-Perez A, Suazo-Laguna H, Legorreta-Soberanis J et al. Evidence based community mobilization for dengue prevention in Nicaragua and Mexico (Camino Verde, the Green Way): cluster randomized controlled trial. BMJ. 2015. July 8;351:h3267 10.1136/bmj.h3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarado-Castro V, Paredes-Solís S, Nava-Aguilera E, Morales-Pérez A, Alarcón-Morales L, Balderas-Vargas NA, et al. Assessing the effects of interventions for Aedes aegypti control: systematic review and meta-analysis of cluster randomised controlled trials. BMC Public Health. 2017. May 30; 17(Suppl 1):384 10.1186/s12889-017-4290-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heintze C, Garrido MV, Kroeger A. What do community-based dengue control programmes achieve? A systematic review of published evaluations. Trans R Soc Trop Med Hyg. 2007; 101(4): 317–25. 10.1016/j.trstmh.2006.08.007 [DOI] [PubMed] [Google Scholar]
- 43.Vanlerberghe VE, Toledo ME, Rodriguez M, Gomez D, Baly A, Benitez JR, et al. Community involvement in dengue vector control: cluster randomised trial. BMJ. 2009; 338: b1959 10.1136/bmj.b1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parks W, Lloyd LS. Planning social mobilisation and communication for dengue fever prevention and control: a step-by-step guide Geneva, World Health Organization, 2004. Available from: http://www.who.int/immunization/hpv/communicate/planning_social_mobilization_and_communication_for_dengue_fever_prevention_and_control_who_cds_wmc_2004.pdf. [cited 2018 July 12]. [Google Scholar]
- 45.World Health Organization, & UNICEF. Communication for behavioural impact (COMBI): A toolkit for behavioural and social communication in outbreak response. 2012. Available from: http://apps.who.int/iris/bitstream/handle/10665/75170/WHO_HS?sequence=1. [cited 2018 July 12].
- 46.Halstead SB. Successes and failures in dengue control—global experience. Dengue Bull. 2000; 24: 60–70. [Google Scholar]
- 47.Barrera-Pérez MA., Pavía-Ruz N, Mendoza-Mezquita JE, Torres-Arcila N, Hernández-Hernández R, Castro-Gamboa F et al. Control of Aedes aegypti breeding sites with the program Recicla por tu bienestar in Merida, Mexico. Salud Publica Mex. 2015; 57 (3): 201–210. [PubMed] [Google Scholar]
- 48.Bouzid M, Brainard J, Hooper L, Hunter PR. Public Health Interventions for Aedes Control in the Time of Zikavirus–A Meta-Review on Effectiveness of Vector Control Strategies. PLoS Negl Trop Dis. 2016. December 7;10(12):e0005176 10.1371/journal.pntd.0005176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moyes CL, Vontas J, Martins AJ, Ng LC., Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis. 2017;11(7):e0005625 10.1371/journal.pntd.0005625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fitzpatrick C, Haines A, Bangert M, Farlow A, Hemingway J, Velayudhan R. An economic evaluation of vector control in the age of a dengue vaccine. PLoS Negl Trop Dis; 2017; 11(8):e0005785 10.1371/journal.pntd.0005785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Achee N, Grieco JP, Vatandoost H, Pinto J, Fiocruz A, Waraporn JV. Alternative methods for the control of mosquito vectors of arboviruses. PLoS Negl Trop Dis. 2018; In press. [Google Scholar]
- 52.European Centre for Disease Prevention Control (ECDC). Guidelines for the surveillance of invasive mosquitoes in Europe. Technical Report. Stockholm: ECDC, 2012. Available from: http://ecdc.europa.eu/en/publications/Publications/TER-Mosquito-surveillance-guidelines.pdf. [cited 2018 July 12].
- 53.Porta M. A dictionary of epidemiology Oxford University Press; 377 pp. 2014. [Google Scholar]
- 54.Bartley LM, Donnelly CA. Garnett GP. The seasonal pattern of dengue in endemic areas: mathematical models of mechanisms. Trans R Soc Trop Med Hyg. 2002; 96(4): 387–97. [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization. Vector surveillance and control at ports, airports, and ground crossings. 2016. Available from: http://apps.who.int/iris/bitstream/10665/204660/1/9789241549592_eng.pdf. [cited 2018 July 12].
- 56.Focks DA. A review of entomological sampling methods and indicators for dengue vectors Special Programme for Research and training in Tropical Diseases (TDR). 2003. TDR/IDE/DEN/03.1. Geneva: World Health Organization. [Google Scholar]
- 57.Runge‐Ranzinger S, McCall PJ, Kroeger A, Horstick O. Dengue disease surveillance: an updated systematic literature review. Trop Med Int Health. 2014; 19(9): 1116–60. 10.1111/tmi.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erlanger TE, Keiser J, Utzinger J. Effect of dengue vector control interventions on entomological parameters in developing countries: a systematic review and meta‐analysis. Med Vet Entomol. 2008; 22(3): 203–21. 10.1111/j.1365-2915.2008.00740.x [DOI] [PubMed] [Google Scholar]
- 59.Samuel M, Maoz D, Manrique P, Ward T, Runge-Ranzinger S, Toledo J. Community effectiveness of indoor spraying as a dengue vector control method: A systematic review. PLoS Negl Trop Dis. 2017; 11(8): e0005837 10.1371/journal.pntd.0005837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muzari MO, Devine G, Davis J, Crunkhorn B, van den Hurk A, Whelan P, et al. Holding back the tiger: Successful control program protects Australia from Aedes albopictus expansion. PLoS Negl Trop Dis. 2017. 11(2): e0005286 10.1371/journal.pntd.0005286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Organization. Manual for indoor residual spraying: application of residual sprays for vector control Geneva: World Health Organization; 2007. Available from: http://white-fog.com/WHO/WHO_CDS_NTD_WHOPES_GCDPP_2007.3_eng.pdf. [cited 2018 July 12]. [Google Scholar]
- 62.Barrera R, Acevedo V, Felix GE, Hemme RR, Vazquez J, Munoz JL, et al. Impact of Autocidal Gravid Ovitraps on Chikungunya Virus Incidence in Aedes aegypti (Diptera: Culicidae) in Areas With and Without Traps. J Med Entomol. 2017; 54(2):387–395. 10.1093/jme/tjw187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lorenzi OD, Major C, Acevedo V, Perez-Padilla J, Rivera A, Biggerstaff BJ, et al. Reduced Incidence of Chikungunya Virus Infection in Communities with Ongoing Aedes Aegypti Mosquito Trap Intervention Studies—Salinas and Guayama, Puerto Rico, November 2015-February 2016. MMWR Morb Mortal Wkly Rep. 2016; 65(18): 479–80. doi: 10.15585/mmwr.mm6518e3 [DOI] [PubMed] [Google Scholar]
- 64.Lupi E, Hatz C, Schlagenhauf P. The efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp.–A literature review. Travel Med Infect Dis. 2013; 11(6): 374–411. 10.1016/j.tmaid.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 65.Wilson AL, Dhiman RC, Kitron U, Scott TW, Van den Berg H, Lindsay SW. Benefit of insecticide-treated nets, curtains and screening on vector borne diseases, excluding malaria: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014; 8(10): e3228 10.1371/journal.pntd.0003228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kittayapong P, Olanratmanee P, Maskhao P, Byass P, Logan J, Tozan Y, et al. Mitigating diseases transmitted by Aedes mosquitoes: a cluster-randomised trial of permethrin-impregnated school uniforms. PLoS Negl Trop Dis. 2017; 11(1): e0005197 10.1371/journal.pntd.0005197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bartlett-Healy K, Hamilton G, Healy S, Crepeau T, Unlu I, Farajollahi A, et al. Source reduction behavior as an independent measurement of the impact of a public health education campaign in an integrated vector management program for the Asian tiger mosquito. Int J Environ Res Public Health. 2011; 8(5): 1358–67. 10.3390/ijerph8051358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.George L, Lenhart A, Toledo J, Lazaro A, Han WW, Velayudhan R, et al. Community-Effectiveness of Temephos for Dengue Vector Control: A Systematic Literature Review. PLoS Negl Trop Dis. 2015; 9(9): e0004006 10.1371/journal.pntd.0004006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boyce R, Lenhart A, Kroeger A, Velayudhan R, Roberts B, Horstick O. Bacillus thuringiensis israelensis (Bti) for the control of dengue vectors: systematic literature review. Trop Med Int Health. 2013; 18(5): 564–77. 10.1111/tmi.12087 [DOI] [PubMed] [Google Scholar]
- 70.Han WW, Lazaro A, McCall PJ, George L, Runge-Ranzinger S, Toledo J, et al. Efficacy and community effectiveness of larvivorous fish for dengue vector control. Trop Med Int Health. 2015; 20(9): 1239–1256. 10.1111/tmi.12538 [DOI] [PubMed] [Google Scholar]
- 71.Lazaro A, Han WW, Manrique-Saide P, George L, Velayudhan R, Toledo J, et al. Community effectiveness of copepods for dengue vector control: systematic review. Trop Med Int Health. 2015; 20(6): 685–706. 10.1111/tmi.12485 [DOI] [PubMed] [Google Scholar]
- 72.Kay B, Nam VS. New strategy against Aedes aegypti in Vietnam. The Lancet. 2005; 365(9459): 613–7. [DOI] [PubMed] [Google Scholar]
- 73.Benelli G, Jeffries CL, Walker T. Biological Control of Mosquito Vectors: Past, Present, and Future. Insects. 2016; 7 (4), 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Azevedo-Santos VM, Vitule JR, Pelicice FM, García-Berthou E, Simberloff D. Nonnative fish to control Aedes mosquitoes: a controversial, harmful tool. BioScience. 2016; 67(1): 84–90. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)