Abstract.
We report here one case of Zika virus (ZIKV) infection associated with auto-immunity directed against the central nervous system in a Brazilian woman who developed acute transverse myelitis 9 days after recovery from an acute episode of fever with generalized erythema. Imaging of the spinal cord showed an elongated area on the T1–T10 level with gadolinium uptake. The diagnostic of the ZIKV infection was confirmed by cerebrospinal fluid and serum analysis. This patient had serum positivity for autoantibodies against myelin oligodendrocyte glycoprotein (MOG), a specific antibody against the myelin sheath. We propose that a direct central nervous system infection by ZIKV could lead to a specific auto-immunity against MOG protein.
In 2015, an outbreak of Zika virus (ZIKV), a flavivirus transmitted by Aedes mosquitoes, was identified in northeastern Brazil.1 Since then, numerous cases have been reported all over the country.2 The infection has demonstrated itself to be benign and self-limited in the vast majority of cases.3 However, injuries to the central and peripheral nervous systems have been associated to the infection, mainly microcephaly and Guillain- Barré syndrome. This suggests that ZIKV, similar to other flaviviruses, could be neuropathogenic.4,5 More recently, meningoencephalitis and myelitis have been reported, but without clear evidence of a direct pathogenic effect of the virus.6 We report one case of ZIKV infection associated with auto-immunity directed against the central nervous system.
CASE REPORT
We describe the case of a 38-year-old white woman who suddenly presented on February 28, 2016, with generalized erythema, arthralgia, myalgia, headache, conjunctival congestion, and fever followed by clinical improvement. After 9 days of the initial symptoms, she presented with urinary retention and intestinal constipation. On the 10th day, she presented with sudden paraparesis that progressed to flaccid paralysis with pyramidal signs within a few hours.
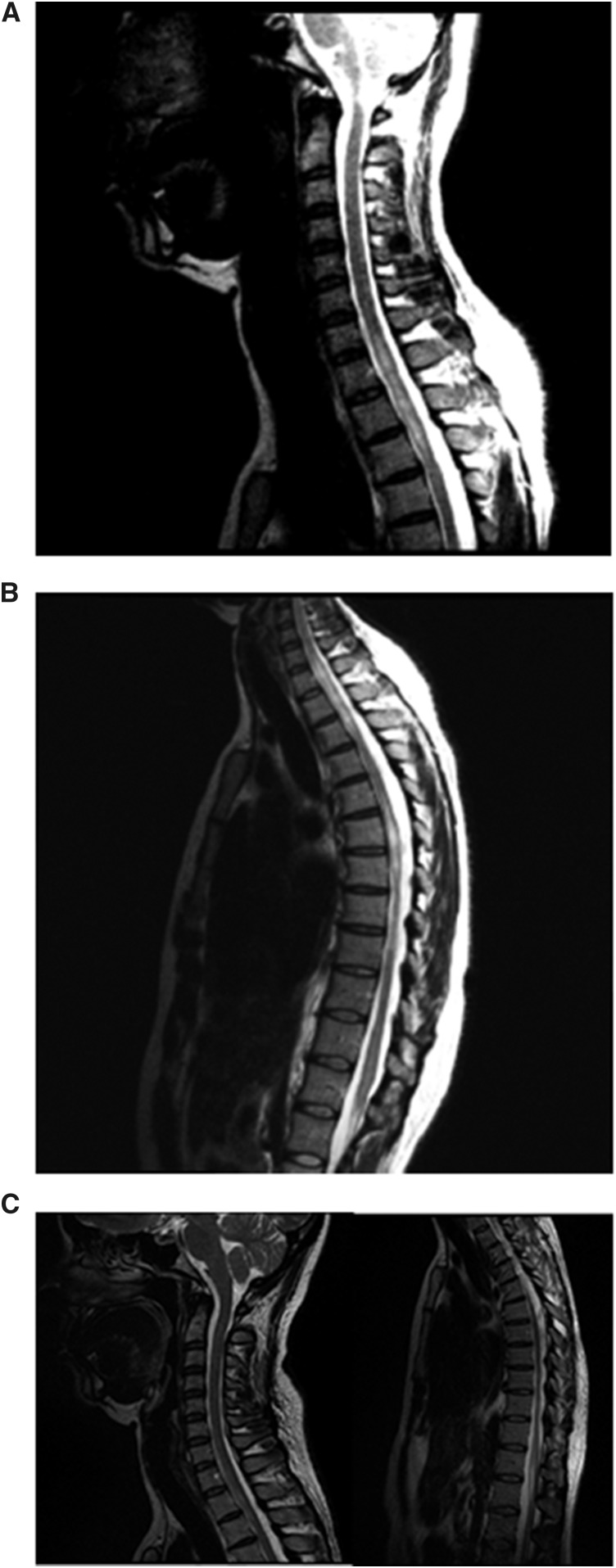
Cerebrospinal fluid (CSF) and serum samples (10 mL) were concurrently collected on the 10th day after the first infectious signal. The CSF analysis included standard routine testing, and immunological testing for infectious diseases such as cysticercosis, toxoplasmosis, and schistosomiasis (Shistossoma mansoni) and for dengue, chikungunya, and ZIKV (ELISA/reverse-transcriptase polymerase chain reaction [RT-PCR]); bacterial analysis (Gram and Ziehl–Neelsen staining methods or culture); fungal analysis (Cryptococcus latex agglutination test and culture); Treponema pallidum test (enzyme-linked immunosorbent assay [ELIZA] and fluorescent treponemal antibody absorption); and viral analysis (herpes simplex, cytomegalovirus, varicella–zoster, and human T-lymphotropic virus [HTLV]; and human immunodeficiency virus [HIV]-ELISA), were also conducted. Quantitative evaluation of intrathecal immunoproduction of immunoglobulin G (IgG) and immunoglobulin M (IgM) were also performed. Furthermore, complete blood count, erythrocyte sedimentation rate, kidney and liver assessment, angiotensin-converting enzyme dosage, and a complete serologic profile for rheumatology (anti-nuclear antibody, antibodies against anti-nuclear antibodies Sjögren’s syndrome A and anti-phospholipid) and infections (antibody tests for HIV, HTLV, syphilis, toxoplasmosis and hepatitis, cytomegalovirus, Epstein–Barr virus, varicella–zoster, herpes simplex, dengue, Chikungunya, and ZIKV [ELISA and RT-PCR]) were performed. In addition, the presence of antibodies against aquaporin-4 and antibodies against myelin oligodendrocyte glycoprotein was identified by live cell based assays (CBA).7 Vertebral magnetic resonance imaging (MRI) on March 3, 2016, showed elongated areas with increased T2 signal at the C2, C6-C7, C7-T1, and T1-T10 levels with gadolinium uptake (Figure 1A and B) and MRI of the brain was normal. After admission, she was treated with two cycles of methylprednisolone (1 g IV/day/5 days) and the neurological symptoms progressively improved. Second vertebral MRI (25 days after the onset of symptoms), revealed reduction of the lesion length and load (Figure 1C). At discharge, she was fully ambulatory with no motor deficits, but experienced muscle cramps and spasms in the lower limbs associated with reduction of touch and pain in the inferior limbs. She was treated with pregabalin (150 mg/day), carbamazepine (400 mg/day), and baclofen (20 mg/day). Considering the good outcome and the complete recovery, she was not treated and returned to her professional activities.
Figure 1.
Imaging of the spinal cord. (A) Magnetic resonance imaging T2 sequences showing hypersignal in the cervical spinal cord C2, C6-C7, and C7-T1. (B) The same widespread change is observed in the thoracic spinal cord, C7-T1, and T9-T10, determining expansion at some levels, suggestive of an inflammatory process. (C) After treatment with methylprednisolone for two cycles, important reduction of lesions in cervical and dorsal spinal cord was noted.
The diagnostic of the ZIKV infection was confirmed by the serum analysis (IgM for ZIKV) associated with high concentration of ZIKV, as detected by RT-PCR. In the CSF, immunological assessment the IgG and IgM Index were within normal values and no oligoclonal bands were detected. In the CSF and in the serum, there were no records of coinfections (Supplemental Tables 1 and 2). Antibodies against aquaporin-4, a marker of neuromyelitis optica, was negative, but serum autoantibodies against MOG, tested in a blinded way twice by CBA,7 were positive.
DISCUSSION
The main neurological complication of ZIKV infection is the congenital ZIKV syndrome, characterized by microcephaly. This syndrome is probably driven by a direct toxic effect of ZIKV on neurogenesis.1 The events related to this neurotoxic mechanism is, however, less clear in acquired neurological presentation associated to ZIKV. In the reported case, rapid and full recovery after the episode plaid against a major direct and permanent toxic effect of ZIKV on the spinal cord. In the first report of ZIKV infection–associated myelitis, although ZIKV was found in the CSF, none of the studies published so far have demonstrated a direct neurotoxicity of the virus.5 Therefore, there is the possibility that immune reaction after ZIKV infection might be a source of cross-reaction against specific protein from the central and peripheral nervous system leading to autoimmunity.8 This mechanism of cross-reactivity was highly suspected in the Guillain-Barré syndrome outbreak associated with ZIKV infection in French Polynesia.9
In our case, we question such mechanism involving ZIKV-induced antibodies able to cross-react against MOG. Myelin oligodendrocyte glycoprotein is a minor type of transmembrane component of the myelin which is only found in the CNS. Anti-MOG antibodies should exert encephalitogenic properties because this protein is located at the outer surface of the myelin lamellae and, thus, is more susceptible to the deleterious actions of antibody-dependent neurotoxic immune events.10 The cause of emergence of auto-antibodies against MOG is still unknown, but there is an increased incidence of an infectious prodrome in patients with MOG antibody–associated demyelination.11 The association of a prodrome in many children with acute demyelinating encephalomyelitis also leads one to consider the role of infection triggering immunity in MOG antibody–positive disease, as it could be the case for ZIKV infection prodrome in our case. However, there is not yet clear evidence for a direct pathogenic role of MOG-IgG and of clinical course. More studies are necessary to established whether anti-MOG antibodies are directly associated with myelin damage, or whether their presence is an epiphenomenon secondary to demyelination process. Nevertheless, another argument for considering that MOG-IgG are associated to the myelitis episode is that our report fulfilled the classical features recently associated to MOG-IgG.
Thus, we propose that a direct CNS infection by ZIKV could lead to a specific auto-immunity against MOG protein, either by be an inflammatory response associated to a breakdown of the blood–brain barrier and a subsequent leakage of MOG antigens into the peripheral circulation, or by stimulating in periphery adaptive immune responses with activation of specific B and T cells against MOG.
Supplementary Material
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1.Schuler-Faccini L, et al. Brazilian Medical Genetics Society–Zika Embryopathy Task Force , 2016. Possible association between Zika virus infection and microcephaly-Brazil, 2015. MMWR Morb Mortal Wkly Rep 65: 59–62. [DOI] [PubMed] [Google Scholar]
- 2.Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M, 2014. Current Zika virus epidemiology and recent epidemics. Med Mal Infect 44: 302–307. [DOI] [PubMed] [Google Scholar]
- 3.Brasil P, et al. 2016. Zika vírus outbreak in Rio de Janeiro, Brazil: clinical characterization, epidemiological and virological aspects. PLoS Negl Trop Dis 10: e0004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fauci AS, Morens DM, 2016. Zikavirus in the Americas—yet another arbovirus threat. N Engl J Med 374: 601–604. [DOI] [PubMed] [Google Scholar]
- 5.Mécharles S, Herrmann C, Poullain P, Tran TH, Deschamps N, Mathon G, Landais A, Breurec S, Lannuzel A, 2016. Acute myelitis due to Zika virus infection. Lancet 387: 1481. [DOI] [PubMed] [Google Scholar]
- 6.Araujo AQ, Silva MT, Araujo AP, 2016. Zika virus-associated neurological disorders: a review. Brain 139: 2122–2130. [DOI] [PubMed] [Google Scholar]
- 7.Cobo-Calvo Á, Sepúlveda M, Bernard-Valnet R, Ruiz A, Brassat D, Martínez-Yélamos S, Saiz A, Marignier R, 2016. Antibodies to myelin oligodendrocyte glycoprotein in aquaporin 4 antibody seronegative longitudinally extensive transverse myelitis: clinical and prognostic implications. Mult Scler 22: 312–319. [DOI] [PubMed] [Google Scholar]
- 8.Lucchese G, Kanduc D, 2016. Zika virus and autoimmunity: from microcephaly to Guillain-Barré syndrome, and beyond. Autoimmun Rev 15: 801–808. [DOI] [PubMed] [Google Scholar]
- 9.Cao-Lormeau VM, et al. 2016. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387: 1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Büdingen HC, Tanuma N, Villoslada P, Ouallet JC, Hauser SL, Genain CP, 2001. Immune responses against the myelin/oligodendrocyte glycoprotein in experimental autoimmune demyelination. J Clin Immunol 21: 155–170. [DOI] [PubMed] [Google Scholar]
- 11.Ramanathan S, et al. 2014. Antibodies to myelin oligodendrocyte glycoprotein in bilateral and recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm 1: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.