Abstract.
Strongyloides stercoralis is the main etiological agent of human strongyloidiasis. Severe strongyloidiasis is commonly associated to alcoholism, corticostereoid use, and human T cell lymphotropic virus type 1 (HTLV-1) coinfection. Herein, we report a case of a 13-year-old boy coinfected with S. stercoralis and HTLV-1, excreting several parasitic forms in the stool. The parasitological examination of his feces showed a large amount of filariform (about 3,000 larvae per gram of feces) and rhabditiform larvae (about 2,000 larvae per gram of feces). In addition, free-living adult females (about 50 parasites per gram of feces) and eggs (about 60 eggs per gram of feces) were detected. The main laboratory findings pointed to high immunoglobulin E (IgE) levels (228 UI/mL) and eosinophila (11.6%). The patient was treated with three courses of ivermectin (200 µg/kg twice, 2 weeks apart), achieving the parasitological cure. An increase of about 19 times in interleucin (IL)-17 level was observed following the parasitological cure, in addition to a decrease in the white blood cell, eosinophil counts, and IgE levels. This is the first case report, to our knowledge, in which an S. stercoralis adult free-living female was described in human feces and where an increase in IL-17 levels after Strongyloides treatment in a HTLV-1 coinfected individual was observed. This finding raises the need for further studies about IL-17 immunomodulation in S. stercoralis and HTLV-1 coinfected patients.
Introduction
Strongyloides stercoralis is the main etiological agent of human strongyloidiasis.1 The host can develop severe forms of strongyloidiasis, as hyperinfection and/or disseminated disease, depending on alterations in the immune status and it is commonly associated with alcoholism, corticosteroid use, and human T cell lymphotropic virus type 1 (HTLV-1) coinfection.2
Human T cell lymphotropic virus type 1 is a retrovirus highly prevalent in some areas of Japan, Papua New Guinea, South America, and West Africa.3 It is transmitted through mother-to-child (mainly through breastfeeding), sexual intercourse, blood transfusions, contaminated blood products, and needle sharing.4 Infected individuals are at risk of developing a rapidly progressive malignancy, adult T-cell leukemia, and a debilitating and sometimes fatal neurologic condition, myelopathy/tropical spastic paraparesis (HTLV-I associated myelopathy [HAM]/TSP).4 Moreover, HTLV-1 acts to reduce the ability of the infected host to develop an adequate immune response to oganisms that require a T-helper 2 (Th2) response.5,6 This phenomenon is likely to be associated with an elevation in the inflammatory and regulatory cytokine levels.7 As a result, patients infected with HTLV exhibit increased susceptibility to infection with S. stercoralis and progression to severe forms of strongyloidiasis. Herein, we describe a case report of an individual coinfected with S. stercoralis and HTLV-1, excreting several parasitic forms in the stool.
Case Report
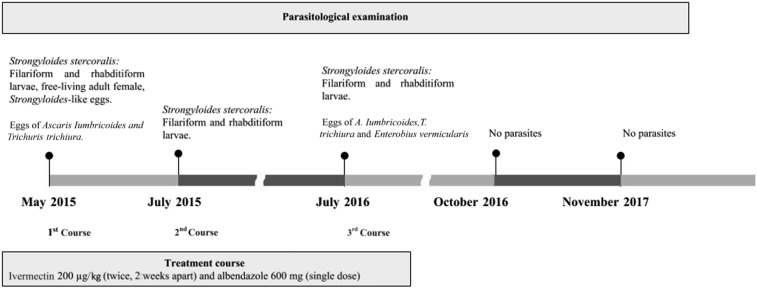
The patient, a 13-year-old boy, lives with his 16 membered family in a precarious rural settlement, in the Baía de Camamú, located 300 km off Salvador (Bahia, Brazil). His mother was diagnosed with HAM/TSP in July 2013 and has been treated at a reference center for HTLV. Subsequently, an active search was conducted in the settlement for evaluating the family (May 2015) (Figure 1). The family lived in a precarious condition with an outside adapted kitchen and no bathroom, sewage, or potable water. The peridomicile was used for bodily functions. Blood and feces samples were collected from 14 individuals (except for two children under 2 years old). Thirteen members of the family tested positive to HTLV-1 by enzyme-linked immunosorbent assay and confirmed by Western blotting. All individuals were breast fed when they were children, and nine of them were under 13 years old; the probable route of HTLV-1 transmission was through breastfeeding.
Figure 1.
A timeline of parasitological examination and treatment course of a 13-year-old boy with Strongyloidis stercoralis hyperinfection as a manifestation of human T cell lymphotropic virus type 1.
The 13-year-old boy reported alternating diarrhea and intestinal constipation, in addition to abdominal pain and dyspnea. He was in a regular general condition, and his height and weight were compatible with age. The main laboratory findings were as follows: white blood cell count of 6,100/µL (neutrophils: 28.4%, lymphocytes: 50.6%, and eosinophils: 11.6%), hemoglobin of 13.9 g/dL, platelet count of 2.2 × 104/µL, and total immunoglobulin E (IgE) of 228 UI/mL. The patient tested negative for hepatitis, Chagas’ disease, syphilis, and human immunodeficiency virus serologies.
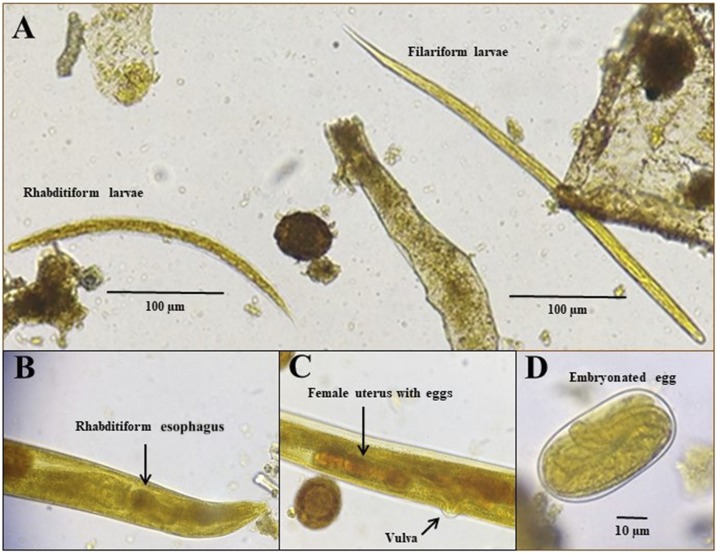
The parasitological examination was performed using Baermann–Moraes,8 spontaneous sedimentation and agar plate culture methods. It showed a large amount of filariform (about 3,000 larvae/gram of feces) and rhabditiform larvae (about 2,000 larvae/gram of feces). Free-living adult females (about 50 parasites/gram of feces) with 1-mm length and presenting rhabditiform esophagus, uterus filled with eggs and vulva were also found in the direct smear. In addition, Strongyloides-like eggs (about 60/gram of feces) (Figure 2), and eggs of Ascaris lumbricoides and Trichuris trichiura were present. The boy was then diagnosed with S. stercoralis hyperinfection as a manifestation of HTLV-1. He was then treated with ivermectin 200 µg/kg (twice, 2 weeks apart), and albendazole 600 mg (single dose).
Figure 2.
Photomicrographies of Strongyloides stercoralis stages in fecal smear stained with iodine showing rhabditiform and filariform larva (A), free-living female rhabditiform esophagus (B) and uterus (C), and an embrionated egg (D). This figure appears in color at www.ajtmh.org.
After 2 months of treatment (July 2015), he no longer complained of dyspnea. However, the parasitological examination of feces revealed a large amount of S. stercoralis rhabiditiform and filariform larvae (more than 1,000 larvae/gram of feaces). The patient was then treated with a new course of ivermectin and Albendazol at the same therapeutic regimen.
One year later (July 2016), a new parasitological examination was performed and a large amount of S. stercoralis larvae, A. lumbricoides, T. trichiura, hookworm, and Enterobius vermicularis eggs, as well as Endolimax nana cysts were present in feces. A new course of ivermectin and albendazole was given, at the same regimen. Parasitological examinations of feces performed in October 2016 and November 2017 showed no parasites, indicating parasitological cure (Figure 1).
The levels of cytokines in sera were measured by cytometric bead array during S. stercoralis infection (mean of the three points before cure), and following the parasitological cure in October 2016. T-helper 1 (IL-2, IL-6, and interferon [INF]-γ), Th-2 (IL-4), and Treg (IL-10 and tumor necrosis factor [TNF]-α) cytokine levels were similar before and after parasitological cure. IL-17 level presented a 19-fold elevation following parasitologial cure (Table 1). In addition, a decrease in the white blood cell and eosinophil counts, total IgE and specific IgE and immunoglobulin G1 (IgG1) levels were observed after parasitological cure (Table 1).
Table 1.
Biological markers in an individual coinfected with Strongyloides stercoralis and human T cell lymphotropic virus type 1 before and after parasitological cure
| Description | Before parasitological cure* | After parasitological cure |
|---|---|---|
| IL-2 (pg/mL) | 1.782 | 1.870 |
| IL-6 (pg/mL) | 2.172 | 2.263 |
| INF-γ (pg/mL) | 19.640 | 19.830 |
| IL-4 (pg/mL) | 1.866 | 1.618 |
| IL-10 (pg/mL) | 2.130 | 2.111 |
| TNF-α (pg/mL) | 1.869 | 1.648 |
| IL-17(pg/mL) | 0.973 | 19.031 |
| IgG1 anti-S. stercoralis | 0.260 | 0.178 |
| IgE anti-S. stercoralis | 0.140 | 0.106 |
| White blood cell count (/µL) | 6.100 | 5.500 |
| Eosinphils (%) | 11.6 | 5.0 |
| Total IgE (UI/mL) | 228 | 141.4 |
* Mean of three measurements before the parasitological test became negative.
The Committee of Ethics in Research of the Nursing School, Federal University of Bahia, Brazil, approved the study and a written informed consent for this case report publication was assigned by the child legal guardian.
Discussion
The case reported herein presents a typical S. stercoralis hyperinfection syndrome, with a high parasite load, and gastrointestinal and pulmonary symptoms, in a HTLV-1 coinfected individual. However, a very unusual presentation appeared when filariform larvae, eggs, and free-living adult female were found in feces. Indeed, this is the first case report, to our knowledge, presenting S. stercoralis free-living adult females in human stool. The S. stercoralis free-living and parthenogenetic females differ morphologically. The two main differences are the size and the esophageal morphology. The parasitic forms measure around 2.5 mm in length and have filariform esophagus that occupies about a third of their body size, whereas the free-living females are smaller, measuring up to 1 mm in length and with a rhabditiform esophagus occupying only 10% of the body of the parasite.9 Besides, parasitic female worms live in the small intestinal mucosa, forming tunnels in the epithelium at the bases of villi in the small intestines, being rarely found in feces. The presence of a free-living form in the individual feces is probably due to the intestinal constipation, and could indicate that both parthenogenetic and sexual reproduction may be occurring in the host, which could enhance the parasite load, as was observed in the infected individual before the treatment.
Human T cell lymphotropic virus type 1 induces a spontaneous cell proliferation, generating an exacerbated Th1 response, with high levels of INF-γ, TNF-β, and IL-2, along with a decline in IL-4, IL-5, and IL-10 production, which could leave the coinfected host more susceptible to severe strongyloidiasis.10,11 Although, increased IgE levels and a high eosinophil count have been associated with the host immune response against S. stercoralis, they are usually decreased in coinfected S. stercoralis and HTLV-1 individuals.6,10,12–14 However, in this case report, the levels of Th1, Th2, and Treg cytokines were similar before and after the parasitological cure. Along with the elevated IgE levels and eosinophils count before the treatment, it may indicate a T-cell balance and preserved immune response, which could prevent Strongyloides dissemination to other organs and a fatal outcome.
IL-17 is a pro-inflammatory cytokine associated with the induction and maintenance of inflammation, resulting in the recruitment of neutrophils to the tissue.15 Human T cell lymphotropic virus type 1 tax protein induces IL-17 gene expression in HTLV-1 infected T-cell lines, which could contribute to the pathogenesis of neurological disease related to HTLV-1 infection.16–18 On the other hand, in S. stercoralis infection, cluster of differentiation 4+ Th17 cell frequency are decreased in an Ag-specific manner.19 In this case report, an increase in IL-17 level following strongyloidiasis treatment in a HTLV-1 coinfected individual was observed for the first time, to our knowledge, which could reflect an inhibition of HTLV-1 inflammation response by Strongyloides. This could indicate a protection against inflammatory complications caused by HTLV-1, such as HAM/TSP. This finding raises the need for further studies.
The recommended treatment for Strongyloides hyperinfection syndrome is ivermectin 200 µg/kg in two doses, 2 weeks apart.19–23 However, studies reported successive failure in the treatment of patients coinfected with HTLV-1.24 In the current case, the patient was treated three times with ivermectin before achieving a parasitological cure. Because the patient lived in a high-risk condition—without basic sanitation and with the habit of walking barefoot—it is difficult to establish whether there was a treatment failure or a reinfection. In addition to the absence of larvae in the feces, after the last treatment, a decrease in specific IgG1 and IgE levels was observed, which could indicate a reduction and/or elimination of the parasite. In summary, this is the first case report, to our knowledge, where it was possible to observe an adult free-living female elimination in human feces and where an increase in IL-17 levels following Strongyloides treatment was observed in a HTLV-1 coinfected patient.
Acknowledgments:
This work was supported by Ministério da Saúde/Fundacão de Amparo à Pesquisa do Estado da Bahia (PPSUS/FAPESB), Brazil.
REFERENCES
- 1.Nutman TB, 2017. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology 144: 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Souza JN, Machado PR, Teixeira MC, Soares NM, 2014. Recurrence of Strongyloides stercoralis infection in a patient with Hansen’s disease: a case report. Lepr Rev 85: 58–62. [PubMed] [Google Scholar]
- 3.Zammarchi L, Montagnani F, Tordini G, Gotuzzo E, Bisoffi Z, Bartoloni A, Luca AD, 2015. Persistent strongyloidiasis complicated by recurrent meningitis in an HTLV seropositive peruvian migrant resettled in Italy. Am J Trop Med Hyg 92: 1257–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willems L, et al. 2017. Reducing the global burden of HTLV-1 infection: an agenda for research and action. Antiviral Res 137: 41–48. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho EM, Da Fonseca Porto A, 2004. Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis. Parasite Immunol 26: 487–497. [DOI] [PubMed] [Google Scholar]
- 6.Porto MAF, Muniz A, Oliveira Júnior J, Carvalho EM, 2002. Clinical and immunological consequences of the association between HTLV-1 and strongyloidiasis. Rev Soc Bras Med Trop 35: 641–649. [DOI] [PubMed] [Google Scholar]
- 7.Cabral AC, Iñiguez AM, Moreno T, Bóia MN, Carvalho-Costa FA, Cabral AC, Iñiguez AM, Moreno T, Bóia MN, Carvalho-Costa FA, 2015. Clinical conditions associated withintestinal strongyloidiasis in Rio de Janeiro, Brazil. Rev Soc Bras Med Trop 48: 321–325. [DOI] [PubMed] [Google Scholar]
- 8.et al. 2016. Association between Strongyloides stercoralis infection and cortisol secretion in alcoholic patients. Acta Trop 154: 133–138. [DOI] [PubMed] [Google Scholar]
- 9.Viney M, 2017. Strongyloides. Parasitology 144: 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porto AF, Neva FA, Bittencourt H, Lisboa W, Thompson R, Alcântara L, Carvalho EM, 2001. HTLV-1 decreases Th2 type of immune response in patients with strongyloidiasis. Parasite Immunol 23: 503–507. [DOI] [PubMed] [Google Scholar]
- 11.Weatherhead JE, Mejia R, 2014. Immune response to infection with Strongyloides stercoralis in patients with infection and hyperinfection. Curr Trop Med Rep 1: 229–233. [Google Scholar]
- 12.de Souza JN, Teixeira MCA, Soares NM, 2015. Associação entre hiperinfecção por Strongyloides stercoralis e HTLV-1: um relato de caso. Rev Ciênc Médicas E Biológicas 13: 427–430. [Google Scholar]
- 13.Negrão-Corrêa D, 2001. Importance of immunoglobulin E (IgE) in the protective mechanism against gastrointestinal nematode infection: looking at the intestinal mucosae. Rev Inst Med Trop São Paulo 43: 291–299. [DOI] [PubMed] [Google Scholar]
- 14.Silva MLS, et al. 2017. Influence of parasite load on the diagnosis and occurrence of eosinophilia in alcoholic patients infected with Strongyloides stercoralis. J Helminthol 28: 1–5. [DOI] [PubMed] [Google Scholar]
- 15.Domingos JA, Soares LS, Bandeira LM, Bonin CM, Vicente ACP, Zanella L, Puga MAM, Tozetti IA, Motta-Castro ARC, Cunha RV, 2017. Cytokine profile and proviral load among Japanese immigrants and non-Japanese infected with HTLV-1 in a non-endemic area of Brazil. PLoS One 12: e0174869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodon MD, Li Z, Hamaia S, Gazzolo L, 2004. Tax protein of human T-cell leukaemia virus type 1 induces interleukin 17 gene expression in T cells. J Gen Virol 85: 1921–1932. [DOI] [PubMed] [Google Scholar]
- 17.Santos SB, Oliveira P, Luna T, Souza A, Nascimento M, Siqueira I, Tanajura D, Muniz AL, Glesby MJ, Carvalho EM, 2012. Immunological and viral features in patients with overactive bladder associated with human T-cell lymphotropic virus type 1 infection. J Med Virol 84: 1809–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leal FE, et al. 2013. Expansion in CD39+ CD4+ immunoregulatory t cells and rarity of Th17 cells in HTLV-1 infected patients is associated with neurological complications. PLoS Negl Trop Dis 7: e2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett J, Broderick C, Soulsby H, Wade P, Newsholme W, 2016. Subcutaneous ivermectin use in the treatment of severe Strongyloides stercoralis infection: two case reports and a discussion of the literature. J Antimicrob Chemother 71: 220–225. [DOI] [PubMed] [Google Scholar]
- 20.Bisoffi Z, Buonfrate D, Angheben A, Boscolo M, Anselmi M, Marocco S, Monteiro G, Gobbo M, Bisoffi G, Gobbi F, 2011. Randomized clinical trial on ivermectin versus thiabendazole for the treatment of strongyloidiasis. PLoS Negl Trop Dis 5: e1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suputtamongkol Y, Premasathian N, Bhumimuang K, Waywa D, Nilganuwong S, Karuphong E, Anekthananon T, Wanachiwanawin D, Silpasakorn S, 2011. Efficacy and safety of single and double doses of ivermectin versus 7-day high dose albendazole for chronic strongyloidiasis. PLoS Negl Trop Dis 5: e1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaha O, Hirata T, Kinjo F, Saito A, Fukuhara H, 2002. Efficacy of ivermectin for chronic strongyloidiasis: two single doses given 2 weeks apart. J Infect Chemother 8: 94–98. [DOI] [PubMed] [Google Scholar]
- 23.Zaha O, Hirata T, Uchima N, Kinjo F, Saito A, 2004. Comparison of anthelmintic effects of two doses of ivermectin on intestinal strongyloidiasis in patients negative or positive for anti-HTLV-1 antibody. J Infect Chemother 10: 348–351. [DOI] [PubMed] [Google Scholar]
- 24.Kinjo T, Nabeya D, Nakamura H, Haranaga S, Hirata T, Nakamoto T, Atsumi E, Fuchigami T, Aoki Y, Fujita J, 2015. Acute respiratory distress syndrome due to Strongyloides stercoralis infection in a patient with cervical cancer. Intern Med Tokyo Jpn 54: 83–87. [DOI] [PubMed] [Google Scholar]