Abstract.
Recent outbreaks of Chikungunya virus (CHIKV) infection confirm the vulnerability of neonates after vertical transmission. In 2014, CHIKV was reported for the first time in the Americas, including the island of Curaçao. We describe the outcomes of symptomatic neonates with vertically transmitted CHIKV infection during the CHIKV epidemic, who were admitted in the Saint Elisabeth Hospital, Willemstad, Curaçao. There were three symptomatic neonates with serologically confirmed infection. Two neonates developed neurological complications, including convulsions and intracerebral bleeding. One newborn, in whom maternal infection occurred 7 weeks before delivery, had a fatal outcome after birth. Maternal-fetal transmission of CHIKV may cause severe neonatal complications. There is a need to share experiences and to implement protocols toward the management of perinatal CHIKV infection.
Chikungunya virus (CHIKV) is a vector-borne infectious disease–causing epidemics.1 In 2014, CHIKV was reported for the first time in the Americas, including the island of Curaçao.2 An estimated 33–50% of all inhabitants were infected, with a peak incidence from October 2014 to early 2015.3 Chikungunya virus infection is usually benign in the acute phase of the disease, with rare, life-threatening complications.1,4 Neonatal CHIKV infection can lead to sepsis-like illnesses, encephalitis, convulsions, and even death.5–13 The risk of vertical transmission is the highest close to delivery.6 In this report, we describe the outcomes of neonates with vertically transmitted CHIKV infection, who were admitted to the Saint Elisabeth Hospital (SEHOS), Willemstad, Curaçao during the CHIKV epidemic in 2014–2015.
The SEHOS is the only hospital on Curaçao with both an obstetric ward and a level IIIB neonatal intensive care unit. Curaçao has approximately 160,000 inhabitants and 2,000 annual deliveries (75% within SEHOS). Between September 2014 and March 2015, pregnant women, attending the SEHOS, tested positive for active or recent CHIKV infection were included in the study and maternal data were retrieved retrospectively from maternal records. During the epidemic, hospital guidelines for neonatal CHIKV infection were developed and it was recommended to admit neonates for 7 days of observation in the pediatric ward if the mother was suspected to have a CHIKV infection close to delivery (2 days before until 2 days postpartum). Suspicion of vertical transmission in those neonates was based on the appearance of clinical infectious symptoms. Asymptomatic neonates were not routinely tested for CHIKV infection. Neonatal data in suspected newborns were collected prospectively. Informed consent for the use of data was obtained from the patients’ mothers. Chikungunya virus–specific antibodies were detected using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Euroimmune AG, Lubeck, Germany). In mothers, infection was confirmed by CHIKV-specific serum antibodies in the presence of immunoglobulin M (IgM) and/or immunoglobulin G (IgG) seroconversion. Vertical transmission was confirmed if serum-specific IgM antibodies were detected in the neonate.
During the epidemic, 61 pregnant women were diagnosed with CHIKV infection (60 women with positive CHIKV IgM results). Median age was 30 (range 24–32) years. Thirty-seven (61%) women presented with fever, 42 (69%) were suffering from arthralgia, and 25 (41%) developed skin rash. In 35 women (57%), both fever and arthralgia was present. In three symptomatic neonates, vertical transmission of CHIKV was confirmed (Table 1).
Table 1.
Characteristics of vertically infected neonates admitted to the pediatric ward in the Saint Elisabeth Hospital, Willemstad, Curaçao
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| EGA at maternal CHIKV infection (weeks + days) | 28 + 3 | 39 + 3 | 38 + 5 |
| EGA at delivery (weeks + days) | 35 + 2 | 39 + 3 | 38 + 6 |
| Apgar score 1 minute/5 minutes | 6/7 | 9/10 | 9/9 |
| Sex | Male | Female | Female |
| Birthweight in grams (centiles) | 2535 g (50–90th p) | 2745 g (10–50th p) | 3050 g (10–50th p) |
| First day of symptoms according to DOB | 0 | 5 | 4 |
| Neonatal symptoms | |||
| Fever | No | Yes | No |
| Irritability/pain | Yes | Yes | Yes |
| Skin rash | No | No | Yes |
| Skin bullae | No | No | No |
| Convulsions | Yes | Yes | No |
| Length of hospital stay in days | 1 | 16 | 10 |
| Death | Yes | No | No |
| Neonatal CHIKV serology | |||
| IgM | Positive | Positive | Positive |
| IgG | Positive | Negative | Positive |
| Complementary brain imaging | CT scan | MRI | Not performed |
| Extensive bleeding | White matter lesions T0 | – | |
| Cerebrospinal fluid | Not taken | Normal | Not taken |
CHIKV = Chikungunya virus; CT scan = computed tomography scan; DOB = date of birth; EGA = estimated gestational age; MRI = magnetic resonance imaging.
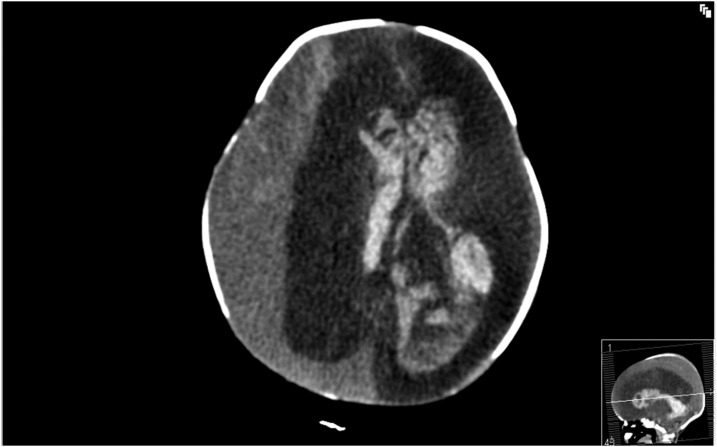
The first neonate (case 1) was born at a gestational age of 35 weeks and 2 days after an emergency caesarean section was performed due to fetal heart rhythm abnormalities suggestive of fetal distress and suspected intracerebral hemorrhages. The mother was diagnosed with CHIKV infection 7 weeks before delivery. Neurologic examination of the neonate after birth was abnormal, with absent corneal and pupil reflexes in the right eye, a tense fontanel and partial convulsions of the extremities on the left side. Cerebral computed tomography scan showed a right-sided subdural hemorrhage with a midline shift and a large intraventricular hemorrhage with dilatation of the left ventricular system (Figure 1). Prognosis was unfavorable due to extensive cerebral bleeding. In consultation with the parents, it was decided to discontinue treatment and the baby died shortly thereafter. Neonatal CHIKV serology was positive and further investigations excluded genetic, hematologic, and metabolic disorders and infectious diseases, including toxoplasmosis, rubella, and cytomegalovirus. No autopsy was performed.
Figure 1.
Computed tomography scan in a newborn (case 1) with intracerebral hemorrhage and vertically transmitted Chikungunya infection, Curaçao 2014–2015. Extensive intracerebral hemorrhage with midline shift to the left with an enlarged ventricular system and hemorrhagic choroid plexus cyst.
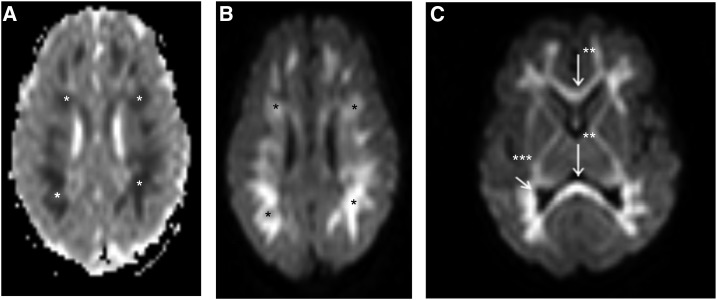
The second neonate (case 2) was admitted for observation in the neonatal ward because of maternal CHIKV infection on the day of delivery. The baby was born after an uncomplicated vaginal delivery. On day 5 the newborn developed fever and skin rash and convulsions on day 6. Convulsions were treated with phenobarbital and antibiotic treatment with amoxicillin and gentamycin was started. Blood results were positive for CHIKV IgM antibodies and bacterial cultures remained negative. The cerebrospinal fluid showed no pleocytosis and no CHIKV antibodies. Brain magnetic resonance imaging on day 8 showed diffuse white matter lesions (Figure 2), which resolved at 3 months of age. The infant did not develop epileptic encephalopathy on follow-up after 1 year.
Figure 2.
Magnetic resonance imaging in a newborn (case 2) with convulsions and vertically transmitted Chikungunya infection, Curaçao 2014–2015. *Diffusion-weighted imaging and apparent diffusion coefficient map show diffuse excessive high signal intensity. **White matter involvement is extensive (A and B) with abnormalities extending into the subcortical white matter, entire fiber tracts, corpus callosum and ***optic radiation (C).
The third neonate (case 3) was admitted in the neonatal ward because of maternal CHIKV infection on the day before delivery. Delivery was by caesarean section because of fetal heart rate abnormalities and suspected fetal distress during labor. On day 4 of life, the neonate developed a fulminant skin rash and showed irritable behavior. The chikungunya virus serology was positive for IgM and IgG antibodies. The skin rash disappeared after 24 hours but irritable behavior persisted for several days with less oral intake, requiring a nasogastric tube for feeding. On day 10, the newborn had recovered and was discharged home.
In 11 other neonates at high risk for vertical transmission, where maternal CHIKV infection occurred within 1 week of delivery, nine neonates did not develop any symptoms. One neonate developed fever, irritable behavior, and skin rash but CHIKV serology did not confirm the diagnosis. Another neonate was admitted at day 5 after delivery, due to generalized convulsions and coma. The mother had a confirmed CHIKV infection 5 days before delivery. The child’s clinical condition deteriorated rapidly and the neonate died within 24 hours after admission. Serum was tested only once in the acute phase with negative results for CHIKV infection.
The risk for CHIKV vertical transmission is the highest close to delivery, with delayed onset of symptoms in neonates, as illustrated by our cases 2 and 3. In our study, in a total of 13 neonates born when maternal CHIKV infection occurred in the perinatal period, we confirmed vertical transmission in two of them. Vertical transmission rates in the perinatal period have been described up to 50%.6,13 Studies have shown the onset of symptoms in those neonates with a mean of 5 days after birth, but the latest occurring on 9 days postpartum.13 Severe neurological complications were seen in two neonates, of whom one died within the first week of life. To our knowledge, transplacental passage of CHIKV in live-born infants has never been confirmed.5–8,10,14 Animal models have shown that the barrier function of the placenta inhibits vertical transmission.15 Exceptional cases of fetal demise attributed to in utero CHIKV infection have been reported in Reunion Island.7 Our case, where the mother was diagnosed with CHIKV infection 7 weeks before delivery may indicate placental passage of the virus and active infection in utero with fetal cerebral hemorrhage because IgM does not cross the placental barrier.
Neuropathogenesis of congenital CHIKV infection is not clearly understood and more research is needed to evaluate these neurologic abnormalities and their implications on neurocognitive outcome.5,6,16,17 In previous studies, abnormal findings on brain magnetic resonance imaging were described with white matter lesions and intraparenchymal hemorrhages.5 Furthermore, long-term neurocognitive sequelae are reported in infants with maternal-fetal CHIKV transmission.17 Unfortunately, immunohistopathological analyses, which could have ascertained vertical transmission and provided insights into the neuropathogenesis, were not performed in our fatal case.
This brief report has several limitations. First, it is likely that not all women with CHIKV infection on Curaçao were referred to our hospital, as symptoms can be absent or very mild in CHIKV infection.1 However, our pediatric department is the only one on the island, so it is unlikely that symptomatic neonates were treated elsewhere. Second, the magnitude of the outbreak on Curaçao caused an enormous strain on the entire health-care system. Reimbursement for new tests such as CHIKV-specific ELISAs and polymerase chain reaction assays lagged the actual course of the epidemic on the island of Curaçao. Serological testing for CHIKV by ELISA does not provide the titration results needed to fulfill the WHO case definition. Serological tests performed in our study are considered reliable.18 The fact that is important to be addressed is that not all patients were tested for dengue coinfection. In the second case presented, the mother also tested positive for dengue IgM antibodies, but the neonate was not tested. Therefore, we cannot exclude dengue coinfection, which shares similarities with perinatal CHIKV infection.19,20 Dengue is endemic to the island of Curaçao and may have contributed to morbidity. Finally, CHIKV serology was performed only in neonates presenting infectious symptoms. Therefore, we cannot exclude potential asymptomatic neonatal infections.
Although no vaccine or therapy is currently available to control CHIKV infection, neonates at risk for CHIKV should be monitored adequately for potentially severe manifestations. Neonatal departments in geographical regions at risk should consider the implementation of protocols toward the management of perinatal CHIKV and other arboviral infections. At the time of writing this report, Zika virus had become epidemic in the Caribbean region. As neonates appear especially vulnerable to arboviruses, it is important to share our experiences to help delineate lines to better manage vector-borne diseases in those patients.
Acknowledgments:
We would like to thank all clinicians, nurses, and staff working at the pediatric, obstetric, laboratory and archive departments of the Saint Elisabeth Hospital, Willemstad, Curaçao, and the staff working at the Analytic Diagnostic Centre, Willemstad Curaçao, for their contribution to this research. We would like to thank Jelte Elsinga and Tobias Brummaier for reviewing the concept version of this manuscript and their useful comments.
REFERENCES
- 1.Thiberville SD, Moyen N, Dupuis-Maguiraga L, Nougairede A, Gould EA, Roques P, de Lamballerie X, 2013. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res 99: 345–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staples E, Fischer M, 2014. Chikungunya virus in the Americas—what a vectorborne pathogen can do. N Engl J Med 371: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsinga J, Gerstenbluth I, van der Ploeg S, Halabi Y, Lourents NT, Burgerhof J, van der Veen HT, Bailey A, Grobusch MP, Tami A, 2017. Long-term chikungunya sequelae in Curaçao: burden, determinants, and a novel classification tool. J Infect Dis 216: 573–581. [DOI] [PubMed] [Google Scholar]
- 4.Elsinga J, Grobusch MP, Tami A, Gerstenbluth I, Bailey A, 2017. Health-related impact on quality of life and coping strategies for chikungunya: a qualitative study in Curaçao. PLoS Negl Trop Dis 11: e0005987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramful D, et al. 2007. Mother-to-child transmission of chikungunya virus infection. Pediatr Infect Dis J 26: 811–815. [DOI] [PubMed] [Google Scholar]
- 6.Gérardin P, et al. 2008. Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the island of La Réunion. PLoS Med 5: e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Touret Y, Randrianaivo H, Michault A, Schuffenecker I, Kauffmann E, Lenglet Y, Barau G, Fourmaintraux A, 2006. Transmission materno-fœtale précoce du virus chikungunya. Presse Med 35: 1664–1666. [DOI] [PubMed] [Google Scholar]
- 8.Fritel X, et al. 2010. Chikungunya virus infection during pregnancy, Réunion, France, 2006. Emerg Infect Dis 16: 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shenoy S, Pradeep GCM, 2012. Neurodevelopmental outcome of neonates with vertically transmitted chikungunya fever with encephalopathy. Indian Pediatr 49: 238–240. [PubMed] [Google Scholar]
- 10.Senanayake MP, Senanayake SM, Vidanage KK, Gunasena S, Lamabadusuriya SP, 2009. Vertical transmission in chikungunya infection. Ceylon Med J 54: 47–50. [DOI] [PubMed] [Google Scholar]
- 11.Gopakumar H, Ramachandran S, 2012. Congenital chikungunya. J Clin Neonatol 1: 155–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villamil-Gómez W, Alba-Silvera L, Menco-Ramos A, Gonzalez-Vergara A, Molinares-Palacios T, Barrios-Corrales M, Rodriguez-Morales AJ, 2015. Congenital chikungunya virus infection in Sincelejo, Colombia: a case series. J Trop Pediatr 61: 386–392. [DOI] [PubMed] [Google Scholar]
- 13.Torres JR, Falleiros-Arlant LH, Dueñas L, Pleitez-Navarrete J, Salgado DM, Brea-Del Castillo J, 2016. Congenital and perinatal complications of chikungunya fever: a latin American experience. Int J Infect Dis 51: 85–88. [DOI] [PubMed] [Google Scholar]
- 14.Ramful D, Sampériz S, Fritel X, Michault A, Jaffar-Bandjee M-C, Rollot O, Boumahni A, Gérardin P, 2014. Antibody kinetics in infants exposed to chikungunya virus infection during pregnancy reveals absence of congenital infection. J Infect Dis 209: 1726–1730. [DOI] [PubMed] [Google Scholar]
- 15.Couderc T, Lecuit M, 2009. Focus on chikungunya pathophysiology in human and animal models. Microbes Infect 11: 1197–1205. [DOI] [PubMed] [Google Scholar]
- 16.Robin S, Ramful D, Le Seach’ F, Jaffar-Bandjee M-C, Rigou G, Alessandri J-L, 2008. Neurologic manifestations of pediatric chikungunya infection. J Child Neurol 23: 1028–1035. [DOI] [PubMed] [Google Scholar]
- 17.Gérardin P, et al. 2014. Neurocognitive outcome of children exposed to perinatal mother-to-child chikungunya virus infection: the CHIMERE cohort study on Reunion island. PLoS Negl Trop Dis 8: e2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson BW, Russell BJ, Goodman CH, 2016. Laboratory diagnosis of chikungunya virus infections and commercial sources for diagnostic assays. J Infect Dis 214: S471–S474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janjindamai P, Pruekprasert P, 2003. Perinatal dengue infection: a case report and review of literature. Southeast Asian J Trop Med Public Health 34: 793–796. [PubMed] [Google Scholar]
- 20.Omarjee R, Prat CM, Flusin O, Boucau S, Tenebray B, Merle O, Huc-Anais P, Leparc-Goffart I, 2014. Importance of case definition to monitor ongoing outbreak of chikungunya virus on a background of actively circulating dengue virus, St. Martin, December 2013 to January 2014. Euro Surveill 19: 1–3. [DOI] [PubMed] [Google Scholar]